Abstract
This chapter covers the physics theory underlying iodine contrast enhanced digital mammography. We cover two methods of imaging iodine, baseline subtraction and dual energy subtraction. The chapter discusses the x-ray absorption properties of iodine, and imaging x-ray filters and x-ray tube kilovoltages needed for the dual energy subtraction method, as well as typical radiation doses for the procedure. The imaging workflow and time sequence of the procedure are shown. The known clinical performance of iodine breast imaging is briefly reviewed, and the procedure is compared to the common gadolinium breast MRI method. The differences between 2D and 3D iodinated contrast mammography are discussed. Finally, example images are shown that illustrate the subtraction process, and imaging in dense breasts.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Contrast mammography comprises a clinical and acquisition protocol that images the distribution of iodine in the breast following the injection of an iodinated contrast agent. The imaging can be done either in 2D or tomosynthesis modes. Confusingly, there are many commonly used acronyms for this method. Contrast-enhanced spectral mammography (CESM), contrast-enhanced digital mammography (CEDM), contrast-enhanced mammography (CEM), and contrast-enhanced 2D mammography (CE2D) all mean essentially the same thing. These all refer to a 2D iodine imaging procedure. If the imaging is done in 3D, such as tomosynthesis, the procedure is known as contrast-enhanced 3D mammography (CE3D). In this book, we shall refer to the procedure as CEDM.
2 Physics Theory
Iodine in the breast is usually administered in the form of an iodinated contrast agent. Iodine is a metal that has high X-ray absorption due to its heavy atomic weight; however, the actual physical amount of iodine that is present in breast tissue, in grams, is small, relative to the mass of other absorbing materials in the breast such as fibrous and glandular tissue, so special methods of imaging are needed to be able to visualize the iodine.
Historically, one of the earlier proposals for imaging iodine in the breast used a method known as background subtraction [1]. In this method, a first image of the breast (background image) was acquired, prior to the administration of iodine and therefore without any iodine present in the breast, using standard mammography equipment and techniques. Then the iodine was injected, and a second image was acquired. The subtraction of the two images subtracts out all the adipose and fibroglandular tissues, which are unchanged in the two acquisitions, leaving an image that consists solely of the changes between the two exposures, which is the iodine uptake. This method suffers from a practical issue that requires that the breast positioning remain completely unchanged between the two images which typically occur minutes apart. Even the smallest motion will cause the subtraction to create large artifacts. Due to this problem, the method of background subtraction is not used in any commercially available mammography systems.
A second method overcomes this limitation and is known as dual-energy subtraction [2]. With this method, two images are taken in rapid succession (seconds apart), at different X-ray energies, and the subtraction yields an image of the iodine. Due to the short time interval between the two exposures, patient motion artifacts are minimized. This imaging method is available on several commercial mammography systems and can be performed in either 2D or 3D imaging modes.
The physics behind the dual-energy subtraction method is based on what is known as k-edge imaging. All materials have X-ray absorption properties that vary based on X-ray energy; however these absorptions change slowly for adipose and fibroglandular tissues in the mammographic energy range, whereas the absorption changes very rapidly for iodine for X-rays having energies near 33 kilo-electron volts (keV), which is in the range of typical mammography exposures. The k-edge of iodine is approximately 33 keV. An X-ray having an energy just above the k-edge is easily absorbed by one of the iodine atom’s electrons, and an X-ray having an energy below the k-edge is less easily absorbed.
The behavior is illustrated schematically in the following figure (Fig. 3.1) [3].
Two images are acquired, ideally one with X-ray energies just above the k-edge at 33 keV and the other just below 33 keV. When one of these images are subtracted from the other, the visibility of the tissue and fat disappears, because the absorption of these two components and hence their visual appearance are almost identical in the two acquisitions. However, the amount of iodine seen in the two images varies considerably, due to the large difference in absorption, and results in an image of the breast’s iodine distribution following the subtraction. If the technique is working well, the subtracted image will have no tissue or fat visible, and the sole component in the image represents the iodine.
This theory is a little more complicated in practice, because X-ray tubes and X-ray filters do not produce X-rays of a single energy. Rather, they generate X-rays with a range of energies, and the resultant subtracted image does not completely subtract out the fibroglandular tissue. By careful selection of the X-ray filters and of the X-ray tubes operating voltage kVp, however, one can produce X-rays that are reasonably monoenergetic, enough so for the procedure to work.
Figure 3.2 illustrates the low- and high-energy X-ray spectra from a commercial CEDM system. The blue curve in the graph shows the distribution of X-rays that are produced in a standard mammography exposure using 28 kVp on a tungsten anode X-ray tube, and a rhodium (Rh) X-ray filter, and one can see that X-rays are generated having energies from about 10 to 25 keV, with most of them in the range of 20 keV. An image taken using this X-ray energy spectrum is known as the low-energy exposure. The red curve shows the X-ray spectrum that comes from a tungsten anode X-ray tube at 45 kVp using a copper (Cu) X-ray filter, and it has X-rays that range from 25 to 45 keV. This is known as the high-energy exposure. The high-energy spectrum has X-rays that are mostly above the iodine k-edge of 33 keV, and the low-energy X-rays are mostly below the 33 keV energy. Therefore, a subtraction of the two images can take advantage of iodine’s rapidly changing absorption near its k-edge (Fig. 3.2).
In a practical commercial system, these two exposures happen automatically in rapid sequence during a dual-energy exposure, with the first exposure being a more-or-less typical mammogram using standard X-ray filters, and following that exposure a different filter, commonly copper, is introduced into the system, the kilovoltage is raised to 45–49 kVp, and the second, high-energy exposure occurs. The short time between the low- and high-energy exposure minimizes the likelihood of patient motion, and hence subtraction artifacts, between the two images.
3 Generating the Iodine Image
The dual-energy acquisition results in two raw images—the low- and high-energy mammograms. There is iodine present in both images; however it is hard to visually discern this due to the overwhelming structure noise caused by normal fibroglandular tissues. The low-energy image is typically taken using the same X-ray filters, kVp ranges, and dose levels, as normal mammograms, and can be substituted for a normal 2D mammogram during the diagnostic process [4].
The iodine image is created as a subtraction of one image from the other. Normally, the difference is made from the logarithm of the two images, as the logarithm image is proportional to the X-ray absorption in the breast, as seen in the following equation:
The constant “k” is determined by the equipment manufacturer so that the adipose and fibroglandular tissues subtract out as fully as possible in the resultant iodine image.
The following figure shows an example of the low- and high-energy images, and of the final subtracted iodine image. The low-energy image appears similar to a normal mammogram. The high-energy image is relatively low contrast due to the high kV used for the exposure, and one can faintly see the regions where there is uptake of the iodine. In the subtracted image, one mainly only sees the iodine in the lesion, and in this example also some blood vessels that had iodine remaining in them. The images in this example were acquired at about 2 minutes postinjection, early in the imaging procedure, which could explain the presence of iodine in the vessels.
Typically, only the low-energy and the subtracted images are viewed and saved by the system (Fig. 3.3).
4 Exposure Techniques and Radiation Dose
Exposures are commonly made using automatic exposure control. The kVp range for the low-energy mammogram is the typical one used in mammography—25–35 kVp—whereas the high-energy image uses a much higher tube voltage, typically 45–49 kVp. The X-ray filters for the low-energy image are the common ones used in mammography, and the X-ray filter for the high-energy exposure is most commonly copper.
The dose for the low kV image is within the range used in a normal mammogram, and the dose for the high-energy exposure is commonly around 25–50% of the dose for the low-energy exposure. Thus, the patient radiation exposure for one iodine image is under about 1.5 times that for a normal screening exposure. Because this type of imaging is most commonly performed on symptomatic patients, the small additional radiation may not be of great concern.
5 Dense Breast Imaging
One of the advantages of contrast-enhanced mammography is that it is primarily insensitive to breast density. The fibroglandular tissues subtract out during the image processing, leaving visibility of the iodine lesions unaffected, even in dense breasts.
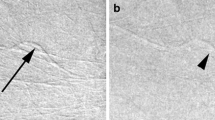
Figure 3.4 shows an example of this. The 2D LCC and LMLO images reveal areas of dense tissue, but the CEDM images easily show the lesion located in these areas.
6 Imaging Procedure Workflow
The basic procedure of a contrast examination is:
-
Evaluate the patient for renal sufficiency and allergies to iodine.
-
Perform the injection of the iodine.
-
Wait approximately 2 minutes for the iodine to distribute throughout the breast.
-
Perform the contrast imaging, in a 6-minutes imaging window.
Figure 3.5 shows the steps commonly performed during the contrast agent administration.
In terms of evaluation of the suitability of the patient for the procedure, refer to contrast media guidelines where appropriate [5, 6]. The injection is commonly performed using a power injector, to facilitate delivery of a bolus of the iodine. Refer to the datasheets for the pharmaceutical, for dosage information and any warnings. The literature reports that typical doses of iodine are 1.5 cc/kg of body weight, and the injection can be done at 3 cc/s [7]. One typically waits approximately 2 minutes postinjection before beginning the imaging sequence [8]. The patient’s breast is not compressed during the injection or during the 2-minutes wait, to allow the iodine to flow into the breast, and the patient can be seated to help minimize vasovagal reactions. The iodine imaging is commonly performed within a 6-minutes imaging window, before significant redistribution of the iodine and its background occurs.
During the imaging window, multiple images can be acquired, depending upon the clinical protocol. One must separately perform imaging on both the left and right breast if both breasts are needed for evaluation, unlike MRI where both breasts are imaged during the one scan. Figure 3.6 illustrates an example of the time sequence of the procedure, showing four projections taken. These can be, for example, CC and MLO of both breasts.
7 2D and 3D Iodine Imaging
In addition to 2D iodine imaging, it is possible to perform the procedure as part of a tomosynthesis examination.
There are two methods that have been proposed to accomplish this. One is performing the 2D iodine as part of a 2D/3D combo examination. In this method, there are three exposures taken in rapid sequence: the tomosynthesis scan followed by the low and high 2D exposures. The result of this is a 2D regular mammogram and a 2D iodine image, as described previously, and additionally a tomosynthesis volume. The acquisition sequence is illustrated in Fig. 3.7.
The iodine contrast agent is present in the tomosynthesis image, but cannot be easily detected, just as was seen with the 2D regular mammogram, because the tomosynthesis image is not a subtraction image. Because the 2D and the tomosynthesis images are taken in the same compression, they are co-registered, and if the lesion with iodine uptake is correlated to a tomosynthesis-detected lesion, the iodine lesion can be localized in three dimensions using the tomosynthesis images. This combo iodine imaging procedure has higher radiation than either a normal combo examination or a normal 2D contrast examination because it involves three exposures: the low- and high-energy 2D images and the tomosynthesis scan. If the tomosynthesis image uses about the same dose as the low-energy mammogram, then the radiation of this combo procedure is about 25 % = (1 + 1 + 0.5)/(1 + 1) times higher than a normal combo exam, which has a dose of two times relative to a 2D mammogram.
Another method of acquiring 3D contrast information is to acquire a 3D dual-energy image directly. This can be done by acquiring, for each of the tomosynthesis projection angles, two exposures, one being the low energy and the other the high energy. The final 3D contrast image can be calculated either by directly reconstructing separately the low- and high-energy 3D exams and subtracting the two volume reconstructions or by an angle-by-angle subtraction of the low- and high-energy projections and then reconstructing the subtracted projections. This method results in two image sets: the standard low-energy 3D image and the subtracted contrast 3D image. This allows localization of any 3D-visible contrast lesion, and it accomplishes this at lower radiation exposure than the combo iodine imaging, as it requires only two raw images: the low- and high-energy 3D images.
The clinical performance of contrast-enhanced tomosynthesis has not been shown to be significantly different from contrast-enhanced mammography [8]. This is perhaps not surprising. The advantage of normal tomosynthesis compared to digital mammography is due to the reduction in overlapping structures. With contrast mammography, there are essentially no overlapping structures, which would represent overlapping contrast-enhancing lesions. The lesions can be seen directly in either 2D or 3D contrast images. However, there still potentially are advantages to contrast-enhanced tomosynthesis and a contrast combo study, compared to contrast-enhanced mammography, and these relate to the ability to localize and potentially biopsy a lesion detected on the contrast image.
8 Technical Comparison to Gadolinium MRI Breast Imaging
Initial comparisons of the clinical efficacy of CEDM to gadolinium-contrast breast MRI indicate similar sensitivity and specificity in the detection of breast cancer, although there are uncertainties in these comparisons due to relatively small numbers of cases [9].
The technical differences are more easily enumerated. Some advantages of CEDM over MRI are that the procedure can sometimes be performed in women contraindicated for MRI due to claustrophobia, women unable to lie prone, and women who have metallic implants that preclude MRI. The CEDM procedure is also faster, and less costly. In preference comparisons, women preferred CEDM compared to MRI [10]. It can also be an advantage that the CEDM procedure can be performed on the same X-ray machine that is used for the evaluation of symptomatic patients and for upright tomosynthesis-guided biopsy. Advantages of MRI compared to CEDM are the ability to image both breasts at one time, ease of acquiring dynamic wash-in/wash-out information, and no X-ray radiation exposure. Gadolinium also has, compared to iodine, a lower incidence of allergic reactions to the contrast agent, although the observed accumulation of gadolinium in brain tissue is of possible concern due to its unknown long-term health consequence [11].
References
Jong RA, Yaffe MJ, Skarpathiotakis M, et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology. 2003;228(3):842–50.
Lewin JM, Isaacs PK, Vance V, et al. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229(1):261–8.
Figure courtesy of John M Lewin, MD. The Women’s Imaging Center, 3773 Cherry Ck N Dr, Suite 101, Denver CO 80209, john.lewin@thewomensimagingcenter.net.
Francescone MA, Jochelson MS, Dershaw DD, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol. 2014;83(8):1350–5.
See, for example. https://www.acr.org/Clinical-Resources/Contrast-Manual.
Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Contrast media safety committee of European Society of Urogenital Radiology (ESUR). Eur Radiol. 2011;21(12):2527–41.
Lewis TC, Pizzitola VJ, Giurescu ME, et al. Contrast-enhanced digital mammography: a single-institution experience of the first 208 cases. Breast J. 2017;23(1):67–76.
Chou CP, Lewin JM, Chiang CL, et al. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—comparison to contrast-enhanced breast MRI. Eur J Radiol. 2015;84(12):2501–8.
Patel BK, Lobbes MBI, Lewin J. Contrast enhanced spectral mammography: a review. Semin Ultrasound CT MR. 2018;39(1):70–9.
Phillips J, Miller MM, Mehta TS, et al. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging. 2017;42:193–7.
McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Smith, A.P. (2018). Physics and Practical Considerations of CEDM. In: Nori, J., Kaur, M. (eds) Contrast-Enhanced Digital Mammography (CEDM). Springer, Cham. https://doi.org/10.1007/978-3-319-94553-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-94553-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94552-1
Online ISBN: 978-3-319-94553-8
eBook Packages: MedicineMedicine (R0)