Abstract
This chapter deals with the preparation of products from material based on plastic polymers from fossil origin. The production route of traditional plastic polymers starts from available monomers, followed by reaction for converting them into polymers and successive processing to obtain objects. This synthetic description is considered necessary for the general objective of the book devoted to the comparison between plastics from fossil origin and from renewable sources. Indeed, the alternative use of plastics from renewable source is first of all depending on the evaluation of the real capacity of the bio-related plastics to be produced in adequate amount with sustainable processes. Clearly, this problem arises when monomers with different structures and new polymers are made available from bio-related starting compounds. On the other side, the bio-related monomers and polymers will be clearly submitted to the same production technology as the corresponding fossil products, the problem becoming obvious when the monomer structure is the same as in the case of ethylene from oil or from fermentation. Then, this chapter has the role to resume the state of the art in polymer technology and the scenario of polymerization reactions and material processing encountered by monomers and polymers from renewable sources to be converted in the so-called bioplastics .
Access provided by Autonomous University of Puebla. Download chapter PDF
Keywords
- Polymer synthesis
- Chain polymerization
- Stepwise polymerization
- Catalytic polymerization
- Copolymerization
- Materials processing
- Extrusion
- Injection extrusion
- Blow moulding extrusion
- Thermoforming
- Additives for processing
- Blending
3.1 Introduction
This chapter is devoted to discuss the role of the molecular structure of the macromolecules forming the polymer in order to reach the requirements for achieving the appropriate properties necessary for plastic behaviour (Billmeyer 1984). Clearly being plastics materials formed by a molecular assembly, the isolated macromolecular structure is not the only parameter to design a final property. Then the macromolecular structure is related to the material characteristics determined by a complex relation, which depends on a series of additional parameters (Callister 2007).
The Hierarchy of Structural Order in polymers affecting the ultimate properties:
-
1.
Primary Structure: Determined by the preparation method dictates the molecular arrangement of atoms in the macromolecule deriving from type and number of monomeric units, their number (molecular weight) and sequence distribution, and their stereochemistry leading to macromolecular stereoregularity . Ordered structures are referred to chains consisting of identical repeating units (one or two monomeric units).
-
2.
Secondary Structure: Indicates the spatial three-dimensional disposition of the repeating units composing the macromolecular chain. An ordered secondary structure is that where the repeating units are superimposable by translation or by translation of a fixed entity and rotation around a fixed angle.
-
3.
Tertiary Structure: Indicates the spatial three-dimensional disposition of a macromolecular chain consisting of blocks with various, ordered and/or disordered secondary structures.
-
4.
Quaternary Structure: Describes the supramacromolecular arrangement as a combination of ordered chain blocks with the same secondary structure (leading to crystalline phase) and of different conformations (leading to the amorphous state). The combination of quaternary structures is indicated as morphology of the material.
Thus the chapter has the objective of providing indication on how the molecular structure must be selected in order to control the above parameters. It follows from a practical viewpoint the general related concepts reported in the previous Chap. 2: the objective is to give a description of the available polymers that can be considered having plastic properties in the broad general sense accepted today. However, as it has been anticipated, the use of the plastic materials is very complex in the present society. To contribute to better understanding this concern, the focus will be on the correlation between the quantitative value of the basic entities described in Chap. 2 and the corresponding application. Again the need of both structural and functional properties will be stressed in relation to the final use to which each plastic material is directed.
The presentation includes, according to the aim of the book, both polymers from fossil source and those related to the biological word and derived from renewable sources (Gandini 2008). The molecular structure as reference parameter is clearly derived from the background of the authors, but it has to be regarded as the lecture key for a more realistic understanding of the possibility offered from man-made and nature-made polymers in solving life problems in the area of plastic materials . This brings to the evidence that final performances are clearly determined by the formulation of the basic molecular material with appropriate additives in a blend where molecular differentiation and structural complexity are combined and optimized to the applicative objective.
The present situation is, indeed, aimed to attempt the replacement of man-made polymers from fossil with bio-related polymers and polymers derived from renewable sources. Indeed, the polymer world has reached high technology level with material from fossil origin that today consists of a variety of molecular structures resulting from man-made synthetic approaches. Now the need of saving resources and reducing environmental impact requires a more extended involvement of material from renewable sources and the need of biodegradability in order to reduce waste disposal problems. Attempts in this direction are reported here after having described what man-made plastics from fossil can offer in order to have a picture of the problem at molecular and performance level.
In particular, the present world is characterized by a large number of items that are produced from a variety of plastic polymers from fossil origin for an increasing number of very basic to highly sophisticated applications. Molecular differentiation of the constituting macromolecules plays a remarkable role in addition to structural complexity of blends and composites .
While the structural complexity approach, blending, plasticization and filling can clearly be used for extending the property range of bioplastics , the molecular differentiation of the basic polymer is more limited for this last group. Indeed, only few bio-originated macromolecules, such as bacterial polyalkanoates are produced by nature and can be used as starting materials for producing plastics through the processing already established for plastics from fossil origin. Additional routes to bio-based plastics can arise from the use of monomers from natural renewable sources, which can be further converted into macromolecules according to polymerization procedures invented by man and used for traditional synthetic monomers. Interestingly, two different situations can arise. In the former case, the monomers from natural sources are new for traditional plastic industry, even if chemically polymerizable as the ones already existing. Consequently, also the polymers obtained are new and can keep some specific properties connected to the origin from natural sources, such as, for instance, biodegradability. A typical established example is the monomer lactic acid produced from corn and converted by already known polymerization processes into a valuable biodegradable plastic material . In the latter case, the monomers from renewable sources are molecularly identical to monomers from oil and then by polymerization they provide the same identical polymer with the advantage of reducing the environmental impact . The first example of this latter class is provided by ethylene from fermentation of ethanol that can be polymerized to a broad class of polyethylene plastics with the same properties of traditional polyethylene, but saving oil resources.
In the following pages, Sect. 3.2 is then devoted to present the molecular structure and related basic properties of plastic from fossil origin that have originated many large-scale application opportunities being now a reality of the acquired style of life that cannot be given up. The creation of these plastics was made possible by man through the synthesis from oil of different monomers that are converted into plastics according to invented polymerization processes that lead to the molecular structure designed for determined ultimate applications . The successive Sect. 3.3 reports examples of various bio-related plastic materials showing improved environmental properties as derived from renewable sources and in some cases with significant biodegradability. The comparative analysis of these two groups of plastics with different origins with reference to their availability and properties is of basic relevance to help understanding how and where traditional plastics from fossil origin can be conveniently replaced by plastic from renewable sources, which are known to the general public as bioplastics .
Two successive sections of this chapter are then devoted to describe the former (plastics) and the latter (bioplastics ) group, respectively. The description will be at the same time informative about structure, properties and application of the available materials in the two classes with critical comments aimed to evidence essential features connected to molecular structure differentiation in relation to the opportunities offered by man and nature.
3.2 Fossil Resources Derived Polymers
The chemical structure of most common plastic polymers from fossil origin is here described as examples for relating structure to plastic properties and consequently to number and type of uses.
3.2.1 Polyolefins
-
(a)
Ethylene Polymers
Polymers of ethylene were produced in 2018 in the impressive amount of more than 100 million tons. The original homopolymer, the polyethylene (abbreviated PE) or polyethene (IUPAC name polyethene or poly(methylene)), is actually containing in addition to the predominant dimethylene units derived from the opening of the double bond, which would bring only to –CH2 groups, also substituted carbon atom units depending on the polymerization process. Therefore, different material properties are obtained by selecting the initiator and then the polymerization process, even if starting from the same monomer ethylene. As the consequence, the molecular structure of the constituent macromolecules of ethylene polymers is not univocally defined even if the predominant section of the chains blocks is formed by sequences of different length of methylene groups (Fig. 3.1a). The structurally regular blocks with an adequate length (more than 100 –CH2 groups) can be organized in a regular three-dimensional arrangement giving crystalline parts with the highest melting point. However, sequences with short blocks of methylenes connected by units with short branching (Fig. 3.1b) can be also found. The accommodation of the branched units in the three-dimensional structure of crystals results in defects in the regular three-dimensional structure with lowering of the melting point. Chain portion with a high content of branches can suppress crystallinity as they cannot be organized in some regular spatial arrangement.
In the regular methylene chains, the low steric hindrance of the H-atom substituents on each backbone carbon atom generates a zig-zag planar conformation of the linear chains. Macromolecules in such conformation can be packed in a very dense crystalline structure (Fig. 3.2), thus providing a material with the highest density among ethylene polymers.

(reprinted from Olsson et al. 2017, with permission from Elsevier)
Illustration of linear polyethylene orthorhombic crystal structure
The most linear polyethylene prepared by Ziegler–Natta catalytic processes can reach high crystallinity degree (around 60–70%) and then a density of about 0.96 g/cm3. Such ordered packed structure provides a large number of optimized hydrophobic interactions thus making energy expensive the separation of the chains to obtain a disordered system; therefore, a significant melting enthalpy is characteristic of linear polyethylene. This high enthalpy is flanked by a high entropy increase going from the rigid packed crystals to the linear individual chain, where the low steric hindrance along the chain provides high conformational freedom and results in a Tg as low as −80 °C. Thanks to the high enthalpy, the melting point (Tm) for well-crystalline material is about 140 °C allowing polyethylene to display an ideal plastic behaviour in a very broad (220°) temperature range (that is between Tg and Tm).
Within the above plastic range, the material derived from ethylene polymerization shows a complex structure with more or less regular crystalline domains connected by chain sections that are in a random non-crystalline arrangement. The two kinds of domains cannot be separated by any method as the polymer chain is long enough to occupy different domains as reported in Fig. 3.3, which shows the complexity provided by the macromolecular structure in spite of the simple monomeric unit. The material is therefore not completely crystalline, but semicrystalline.
The homopolymerization and copolymerization of ethylene monomer (see Chap. 4) give a number of different plastics where the macromolecules are characterized by saturated aliphatic hydrocarbon structure and contain a variable amount and type of chain branching. Starting with pure ethylene two different homopolymers depending on the type of polymerization process can be obtained: catalytic polymerization provides a highly linear polymer with a high crystallinity degree and high density (high-density polyethylene, HDPE, Table 3.1); on the other side, free radical initiated polymerization, performed at high temperature and pressure, yields macromolecules with both long and short branching that reduce the extent of crystallization in packed materials and then result in low density of final polymer (low-density polyethylene LDPE, Table 3.1). The structure of the different kinds of polyethylenes is schematically reported in Fig. 3.4.
Low-density polymers can also be obtained by synthesizing copolymers of ethylene with 1-olefins (not more than 10% by mol) that gives rise to macromolecules with a linear backbone, but a consistent number (up to 10%) of short branching derived from the alkyl substituent of 1-olefins. These last monomers are able to reduce crystallinity and then the density with the effect to give the linear low-density polyethylene (LLDPE, Table 3.1).
When the content of the co-monomer and thus of the short branching increases up to 25–30%, the copolymer macromolecules contain very short methylene sections in the backbone that cannot crystallize. The copolymer becomes completely amorphous and loses the plastic features. Indeed, ethylene–propylene copolymers (EPM) having more than 30 to 60–65% by weight of propylene are characterized by a rubbery rather than a plastic behaviour.
An additional type of ethylene-derived polymers is the so-called ultra-high-molecular-weight polyethylene (UHMWPE) which is characterized by a very large molecular weight of several millions (from 3 to 6). As the ethylene repeating units have molecular weight 28, in this polymer, the macromolecular chains contain an average number of 100,000 units. Because of this impressive molecular weight to which very long chains correspond, the polymer is a very tough material, but the high probability of defects along the long chains results in difficulty to the three-dimensional packing and the polymer density is 0.930–0.935 g/cm3 that is lower than the one of HDPE. The properties of UHMWPE make it useful for bottle-handling machine parts, moving parts on weaving machines, artificial joints and butchers’ chopping boards. Also, it is conveniently used for articular implants in hip and knee replacements. Excellent and resistant fibres can be prepared by processing UHMWPE whose properties are competitive with those from aramids that are used in bulletproof vests.
As above illustrated, different polyethylenes are characterized by different crystallinity degree and this also affects their ultimate properties and applications.
High crystallinity is responsible for high density, good mechanical properties , high chemical stability and low solubility. For these reasons, HDPE that consists of very linear chains with a low degree of branching and consequently has a significant percentage of crystallinity making it suitable for making bottles jugs, various containers for liquids and pipes.
As mentioned above, the actual features of polyethylene depend on the polymerization process used for its preparation. For the preparation of HDPE, the polymerization process starts from pure ethylene and uses organometallic catalysts (typically titanium compounds with aluminium alkyl as co-catalyst) or Phillips-type catalysts such as chromium oxide supported on silica, which grants a low degree of branching formation. The controlled introduction of a slightly higher content of branching through the use of a minor amount (few percent units) of a substituted olefin co-monomer provides medium-density polyethylene (MDPE) with density range of 0.926–0.940 g/cm3. MDPE can be produced by chromium/silica catalysts, Ziegler–Natta catalysts or metallocene catalysts. Thanks to the molecular defects caused once again by the higher branching degree than HDPE, MDPE has better impact and drop resistance, it is less sensitive to cuts and it has better breaking strength. Because of these characteristics it can be conveniently used for producing gas pipes, sacks, shrink and packaging films, and carrier bags. A further increase of the fraction of substituted olefins in the feed monomer and then in the macromolecules originates a further decrease in the density to 0.915–0.925 g/cm3 (the LLDPE above mentioned). This polymer prepared by catalytic copolymerization of ethylene with small 1-olefins (1-butene, 1-hexene and 1-octene) is used predominantly in thin film production owing to the good toughness, flexibility and relative transparency.
The LDPE with density in the range of 0.910–0.940 g/cm3 having a high degree of short- and long-chain branching (Fig. 3.4) is produced by free radical polymerization under very high pressure and temperature. The process is initiated by a species that easily forms a radical and reacts with a monomer that forms the polymer chains by successive addition to other monomers. However, the free radical on the primary carbon of the methylene at the end of the growing chain can give, with comparable probability, propagation or re-arrangement to more stable secondary and tertiary carbon atom free radicals. The disordered occurrence of these reactions is responsible for the much branched macromolecules and the low crystallinity of LDPE. The resulting plastic material is suitable for the production of both rigid containers and plastic films such as plastic bags and film wrap.
The different physical properties of the three main types of ethylene polymers, namely, HDPE, LLDEPE and LDPE, are summarized in Table 3.1.
As mentioned above, this class of polymers has generally excellent chemical resistance to strong acids and bases, as well as to weak oxidants and reducing agents thanks to paraffin structure . This resistance to liquid penetration including to water results in quite high resistance to degradation in the environment. However, after short time use they preserve almost the same original properties and can be easily recycled.
-
(b)
Ethylene polymers with heteroatoms
Ethylene can also be copolymerized not only with 1-olefin (like propylene in the case of EPM family) or other small 1-olefin as in LLDPE and MDPE but also with several monomers containing heteroatoms such as vinyl acetate to give ethylene–vinyl acetate copolymer (EVA). This last is widely used in paints and to make foams in particular for shoe soles. In addition, it is hydrolyzed to yield formally the ethylene–vinyl alcohol copolymer (EVOH), which cannot be prepared by direct copolymerization of ethylene and vinyl alcohol. Several acrylates can be copolymerized also with ethylene. The copolymerization process occurs by free radical processes as the mentioned functional co-monomers would deactivate the catalytic organometallic sites. These copolymers have then a random non-regular structure and are generally amorphous; therefore, they cannot be rigorously classified as plastics. Exceptions are the copolymers with low content of co-monomers (2–3%) which have properties similar to LDPE thanks to the predominant content of ethylene units. However, the presence of the polar co-monomer and then of functional groups with heteroatoms provides a more polar nature to these polymeric materials which find use as adhesives and as compatibilizers in alloys and blends (see Chap. 4).
-
(c)
Propylene (PP)
The relevant propylene homopolymer, named polypropylene or less frequently polypropene (PP), is constituted by macromolecules deriving from the head-to-tail binding of propylene molecules. These macromolecules have a linear structure of the backbone, as reported in the figure below with methyl groups as side chains attached to alternate tertiary carbon atoms (Fig. 3.5).
The tertiary carbon atom bearing a bonded methyl group is formally asymmetric and a regular structure can be claimed only if their absolute configuration is regularly sequenced along the chain. In the case of the regular macromolecules, they can pack together in a regular array to give a polymer with high degree of crystallinity. The only interesting material from applied viewpoint is the one formed of macromolecules containing long chain blocks where the above asymmetric carbon atoms all have the same absolute configuration (isotactic polymer: see Chap. 2 and Fig. 3.6). The process used for the industrial production of isotactic polypropylene is based on the catalytic process where the active sites have a high stereoselectivity and allow attaching propylene units in a controlled steric manner.
These macromolecules cannot assume the reality the zig-zag planar conformation reported in Fig. 3.6 that is instead typical of polyethylene. The methyl groups in the isotactic chain would stay all by the same side of the plane containing the backbone resulting in too close vicinity considering their steric dimension. The repulsion of this group is discharged by rotation of the monomeric units around the backbone carbon–carbon bonds. As a whole, the rotation of the same angle of all units lead to a helical conformation of the main chain, as shown by Natta and Corradini (1959). This conformation is found in the polypropylene crystals and leaves more empty space if compared to the planar arrangement of linear polyethylene. As the consequence, the density of isotactic polypropylene is between 0.895 and 0.92 g/cm3, lower than that of HDPE. The less regular and less compact packing originates a lower melting enthalpy than polyethylene, but the conformational freedom, rotation of bounds along the backbone is lower thus the entropy variation during transition from crystal to melt is much lower than for linear polyethylene.
In spite of lower enthalpy, the lowering of the melting entropy provides the isotactic (or mainly isotactic) polypropylene with a melting point at around 171 °C. Notice that the commercial isotactic PP has a melting point in the range 160–166 °C due to the presence of defects in the crystals. In any case, Tm is higher than that of polyethylene. Furthermore, the Tg of PP also is higher than that of polyethylene. The range of typical plastic behaviour of isotactic polypropylene (from −10 to 160 °C) is therefore moved towards higher temperature with respect to the one of polyethylene allowing applications not accessible for polyethylene, particularly for items subjected to medium-high temperature (as for example hot water pipes). For such characteristic, it has replaced polyethylene in applications as in medical or laboratory use because it can withstand the temperature in a sterilizing autoclave. Most plastic containers for dairy products are made of polypropylene and sealed with aluminium foil (both heat-resistant materials). PP can also replace HDPE for the production of car batteries, waste bins, pharmacy bottles, cooler containers, plates and pitchers. The properties of polypropylene, as in most synthetic polymers, are depending on the molecular weight, molecular weight distribution, crystallinity degree and stereoregularity ; in copolymers, it is clearly also important the type and content of co-monomer.
The industry produces three different main types of propylene polymers: the homopolymers (mostly isotactic), the random copolymers and the block copolymers with ethylene and other 1-olefins. The ethylene–propylene random copolymer with comparable amount of the two monomers (EPM) has very low or no crystallinity and is used as a rubber rather than as a plastic. The block copolymers are particularly tough and used for high-impact materials. Among the homopolymers, the syndiotactic polypropylene has a lower melting point of 130 °C and below 0 °C it becomes brittle, thus its use would be very limited and for the moment it is considered not-industrially interesting. The most commercial polypropylene is isotactic, with a significant percent of crystallinity and it is used in a wide variety of applications, such as packaging, labelling, textiles containers of various types, laboratory equipment, loudspeakers, automotive components and polymer banknotes.
An additional popular application for polypropylene is as biaxially oriented polypropylene films and sheets (BOPP), which are used to make clear bags as an excellent packaging material. In this case, a low percentage of ethylene is used as co-monomers to reduce the crystallinity and improve the transparency of films. Polypropylene is also a good starting material for the production of fibres as it costs less than most other synthetic fibres, but can give very competitive properties. It is even used as a more ecocompatible substitute of polyvinyl chloride (PVC) as insulation for electrical cables in houses, public buildings and low-ventilation tunnels as it emits less smoke and no toxic halogens when burning. However, because of larger flammability with respect to PVC flame-retardant halogen-free additives have to be inserted in the formulated.
Commercial PP is characterized by high elastic modulus, toughness, but flexible response to mechanical stresses; these features make it a very typical plastic material . The elastic modulus values ranging from 1300 to 1800 N/mm2 are comparable to those of the more expensive acrylonitrile butadiene styrene (ABS) terpolymer and render PP useful even as engineering plastic.
Polypropylene is also used to prepare expanded foam (EPP) with very good impact characteristics due to the low stiffness that allows EPP to resume its shape after deformation. EPP is extensively used for reducing the weight of the final object and provides good impact properties. Fibres PP-based are largely employed in nonwovens for diapers or sanitary products; the empty space provided by the nonwoven structure helps in making these products to absorb water (hydrophilic) opposite to its natural hydrophobic characteristic. The nonwoven materials with the possibility to obtain fibres of very small diameters down to nanometers are used for the design and the production of filters with selected porosity for air, technical gases and liquids.
As drawback, we need to underline that the particular molecular structure with a high number of tertiary carbon atoms in the backbone makes PP liable to chain degradation or fragmentation according to a free radical mechanism during exposure to heat and UV radiation such as that present in sunlight. By ageing under these environmental stressing conditions, it shows a network of thin cracks and splits that become deeper and more severe with the exposure time. The application of PP in items for outdoor environments (automotive, gardening) needs the formulation with UV-absorbing additives; a simple example is provided by carbon black which protects the material (bumpers) against UV-activated degradation .
In addition, PP is more sensitive than polyethylene to chemical attack particularly if used in an aggressive environment, but cannot be considered biodegradable. If subjected to an extensive recycling it loses properties but this drawback can be alleviated by blending with virgin materials and specific additives.
PP is chemically similar to PE having also a saturated hydrocarbon structure . However, it is remarkable that it offers application possibility not achievable with PE. Indeed, it is a perfect plastic material in a higher temperature region than PE does, even if it loses its plastic behaviour if the temperature drops substantially below 0 °C where it becomes almost a glass. This is an additional example of the importance of a sustainable molecular differentiation to respond to the many society needs. Just to be very understandable we can conclude this comparison between these important and absolutely useful plastic materials by saying that PP is a more convenient plastic than PE in the hot Equatorial Regions, whereas the contrary holds in the Polar Regions. Clearly, both are excellent plastics in our areas with mild temperatures falling well inside the Tg ÷ Tm range of the two polymers.
3.2.2 Polystyrenes
Styrene is a synthetic monomer from fossil origin and can be polymerized with the same processes used for aliphatic olefins. The polymerization of this monomer gives macromolecules along with alternating carbon atoms attached to a phenyl group (Fig. 3.7). These macromolecules form polystyrene (PS) and can contain up to a few thousand monomers giving a molecular weight of 100,000–400,000.
As for PP, polystyrene can have isotactic, syndiotactic and atactic structural conformation. Indeed, the carbon atoms bearing the phenyl group are formally chiral as bound to a phenyl group in addition to a hydrogen atom and two chain sections of different lengths. The macromolecules where these chiral centres have all the same configuration with the phenyl groups on the same side as in isotactic polypropylene (see Fig. 3.6) are also called isotactic. Isotactic polystyrene can be obtained by catalytic polymerization of styrene, but it does not have very interesting properties and therefore is not commercially produced. The syndiotactic polystyrene, in which the asymmetric backbone carbon atoms have alternate opposite configuration, is instead industrially produced by stereospecific polymerization with metallocene catalyst. This last polystyrene is highly crystalline with Tm of 270 °C and Tg at around 90 °C. Syndiotactic polystyrene has limited applications as rigid material acting as a real plastic only over 90 °C. Differently from PP, where the isotactic polymer is the only important product, among polystyrenes the atactic one is the most commercially popular material. The random sequential distribution of the asymmetric carbon atoms with opposite configuration implies a random orientation of the phenyl groups attached to the chain backbone thus preventing the chains from aligning with sufficient regularity. Therefore, it cannot achieve any crystallinity and the polymer is amorphous and characterized by only the glass transition temperature at about 90 °C and cannot be considered a typical plastic material . Non-crystalline atactic polystyrene can instead be used as a ‘solid’ at room temperature being well below its Tg of 90 °C so to behave as a rather rigid material. Close or over 90 °C atactic polystyrene flows as a viscous liquid. Therefore, its uses are for disposable plastic cutlery, dinnerware, frames, plastic model assembly kits and many other rigid objects. These items are usually produced by thermoforming (vacuum forming). It is even conveniently used for laboratory containers almost always made by injection moulding generally employed in biomedical research apparatus. A further very popular application of the subject polymer is as expanded polystyrene (EPS). The closed-cell foam obtained starting with pre-expanded polystyrene beads is rigid and tough and it is mainly used for packing material to protect items from impacts and crashes.
Polystyrene is very stable and not biodegradable in the environments. Used items must be disposed correctly at their end life. Because of its not optimal plastic properties it is often mixed with different additives or even partially cross-linked. As a result, PS items present on the market cannot be easily recycled as for polyolefins (Pan 2009). This is certainly an important concern for reducing the environmental impact of disposed plastic material .
3.2.3 Polyesters
-
(a)
Polyethylene terephthalate (PET)
The most popular polyester is certainly PET with repeating units formed by the polycondensation of the diacid, terephthalic acid (IUPAC, benzene-1,4-dicarboxylic acid) and the diol ethylene glycol (IUPAC, ethane-1,2-diol) (Fig. 3.8). Clearly, the hypothetical bifunctional monomer is formed only in the polymerization system and converted into longer polyester chains.
A low molecular weight precursor of the above repeating unit is synthesized by the esterification of a terephthalic acid with two molecules of ethylene glycol to give a dihydroxy compound, the bis(2-hydroxyethyl) terephthalate. This last compound can also be prepared by transesterification reaction between ethylene glycol and dimethyl terephthalate and removal of two methanol molecules. This well-defined precursor can then be polymerized to PET through elimination of the exceeding ethylene glycol.
The molecular structure of PET is very regular as derived from alternating segments from terephthalic acid and ethylene glycol, respectively, connected by elimination of a water molecule. Thanks to this regular macromolecular structure this polymer can crystallize to give a semicrystalline material. Therefore, it is a typical plastic material in its Tg ÷ Tm range (from about 60–70 °C to 250–260 °C). However, depending on processing and thermal history, it may exist also as an amorphous (transparent) material.
The slow cooling of the molten polymer forms a crystalline material with small crystallites dispersed in the amorphous matrix. For this reason, the semicrystalline material tends to scatter light and the polymer becomes opaque and white in most cases. The semicrystalline polymer shows mechanical properties better than the ones of polyolefins and can be used for applications where a good resistance to stress is requested. It can be used in industrial production of relatively strong and rigid objects such as bottles and sheets for structural/building application. It is used also as the carrier for magnetic tape or backing for adhesive tapes. Films and sheets of PET are characterized by significant gas and moisture barrier properties, as well as to alcohol and solvents. Accordingly, plastic bottles made from PET are widely used for water and overall carbonated drinks. Biaxially oriented PET film is useful in flexible food packaging and other applications where thermal insulation is required. Non-oriented PET sheet can be processed to form packaging trays, which can withstand both freezing and oven baking temperatures. PET-based films packing row food (meat) that can be directly introduced in a traditional oven or in a microwave to cook the food till 270 °C have been recently introduced on the market. An additional important application of PET is based on fibre drawing which is widely used in textile production. Notice that the fibre drawing produces a nearly single-crystal product.
Recycling of PET is easier and wider than in case of many other plastics because of the high value of the material and the very large use of PET for bottles.
Obviously, as many plastics, PET can be submitted conveniently to thermal incineration, being composed of carbon, hydrogen and oxygen, with only trace amounts of catalyst residues and no sulphur. Moreover, the chemical recycling or the mechanical recycling is particularly convenient, especially for objects such as bottles for water and beverages, which are very widespread. The chemical recycling mainly occurs by esterolysis mechanism returning to the initial raw chemicals, terephthalic acid (PTA) or dimethyl terephthalate (DMT) and ethylene glycol (EG,) and can be convenient when the final products are used as feedstock by industry. It can be cost-efficient only applying high-capacity recycling lines with size larger than 50,000 tons/year. This approach results quite limited, because of the difficulty related to keeping the working plant of this size as far as running expensive material collection/purification. Instead, the mechanical recycling is today operating in several diverse variants depending on the kinds of recycled-material feedback. The related processes are typical of small- and medium-sized industry, as the cost-efficiency requires capacities within a range of 5000–20,000 tons/year. Collected PET bottles after use may contain contaminants and the polymer can present some degradation extent occurred during the first processing and usage. Therefore, the recycled PET bottles must be submitted to washing for removing minor impurities and dirt and to be converted into pellets by extrusion of the melt material. Some of the original properties when necessary can be restored by reactive additives to repair for chain breaking, which is responsible for molecular weight decrease owing to degradation effect. Efficient sorting, separation and cleaning processes become most important for high-quality recycled polyester, but when the separation is not possible compatibilization with foreign polymer eventually present can be performed by addition of macromolecular compatibilizer. Recycling industry is mainly focused on bottles, which are easy to distinguish and separate because of shape and consistency from waste plastic routes either by automatic or by hand-sorting processes. The recycling process of post-consumer PET consists of three major activities:
-
(a)
collection, separation and washing/cleaning of bottles from plastic wastes,
-
(b)
production of PET-based flakes by bottles grinding,
-
(c)
processing of flakes to provide the final products (generally obtained as pellets to be melt-processed into different items).
The intermediate product of the first section is packed with a PET content of more than 90%. The second step involves the conversion of bottles into flakes and this process can be more or less complex depending on the final PET quality of the scale required. During the third phase, the flakes of PET bottles are processed to obtain any type of product such as films, bottles, fibres or intermediates such as pellets for further melt processing .
Besides to the post-consumer polyester recycling, pre-consumer recycling processes exist: they are realized directly internally by the company as and when the polymer material scraps are reused in the same production circuit.
-
(b)
Linear aliphatic polyesters
The man-made biodegradable polyester polycaprolactone (PCL) is a plastic material with low melting point of around 60 °C and a Tg of about −60 °C. PCL because of the low melting temperature is often used as polymeric plasticizer to improve the processing characteristics and the ultimate properties of rigid materials such as PVC (for instance, to improve its impact resistance) and starch. Thanks to this characteristic and due to the presence of the CO groups, which can act for hydrogen bonding and dipole–dipole interaction, PCL is compatible with a range of other materials, as starch thus lowering its cost and providing plastic behaviour. This sustainable and environment-friendly material is actually prepared by ring-opening polymerization of ε-caprolactone using a catalyst such as stannous octoate (Fig. 3.9). Indeed, to underline once again what we want to evidence about history and nature of polymers from fossil or renewable resources and eventually biodegradable, PCL is a biodegradable plastic but the monomer is of clear fossil origin and the catalytic process belong to those invented by man.
Learning from PCL example several linear aliphatic glycols, butanediol, hexanediol and linear aliphatic diacids such as succinic and adipic acids (Fig. 3.10) were used to obtain linear biodegradable polyesters .
Similarly, to PCL, these polymers are fully man-made and biodegradable when left in the environment. They also may have useful application properties; polybutylene succinate (PBS) (sometimes written polytetramethylene succinate, Fig. 3.10) has properties comparable to polypropylene and some grades of polyethylene (Tg = −30 °C and Tm = 120 °C) particularly for some mechanical features (strain and elongation at break). For such characteristics, PBS can be processed into films, bags, boxes and packaging for food or cosmetics under conditions and in industrial plants designed for polyolefins. Owing to its added characteristic of biodegradability PBS finds interest in agriculture for the fabrication of delayed release materials for pesticide and fertilizer as well as in other fields in which recovery and recycling of materials after use is problematic. PBS is also used in medicine as biodegradable drug encapsulation systems and for implants.
3.2.4 Polyamides
Linear polyamides, fully aliphatic or semi-aromatic, have the trivial name nylons and are very popular as can be melt-processed into significant products for several commercial applications such as fibres, moulded parts for cars, electrical equipment and films (mostly for food packaging). Nylons are first obtained either by reacting linear di-functional carboxylic acids with equal number of molecules of a linear, preferably aliphatic, diamine according to a polycondensation process. During this last process, the reaction of the carboxylic groups with amino groups forms an amide functionality that binds the two reacting molecules (e.g. PA66) (see Fig. 3.11) plus water. A similar reaction can be performed starting with aliphatic linear amino acids. A completely different process for nylon preparation starts with a lactam, obtained by cyclization of an amino acid. This last is then submitted to a process of chain polymerization started with catalysts able to open the lactam ring.
Nylon polymers are identified by a nomenclature that uses numbers corresponding to the number of carbons between acid and amine functions (including the carbon of the carboxylic acid). The successive use of cyclic and aromatic monomers required the use of letters or sets of letters. Only one number after the ‘PA’ for the so-called homopolymers obtained from an amino acid or a lactam, and two numbers or sets of letters when the nylon (PA) derives from the polycondensation of a dicarboxylic acid with a diamine. Nomenclature of copolymers consists of the same numbers for each monomer separated by slashes, as shown in the examples below:
-
homopolymers:
-
PA 6: [NH−(CH2)5−CO]n made from ε-caprolactam;
-
PA 66: [NH−(CH2)6−NH−CO−(CH2)4−CO]n made from hexamethylenediamine and adipic acid;
-
PA 612: [NH−(CH2)6−NH−CO−(CH2)10−CO]n is the polymer of hexamethylenediamine and hexadecanedioic acid;
-
-
copolymers:
-
PA 6/66: [NH-(CH2)6−NH−CO−(CH2)4−CO]n−[NH−(CH2)5−CO]m made from ε-caprolactam, hexamethylenediamine and adipic acid;
-
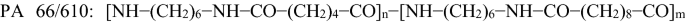
made from hexamethylenediamine, adipic acid and sebacic acid.
-
The prefix ‘PA’ or the name ‘Nylon’ are both used and have the same meaning.
The preparation method and the large number of available starting monomers allow to design macromolecules with a broad number of properties while maintaining the basic chemical nature of polyamide in a sort of natural inspiration derived from natural polyamides, which are obviously the proteins . Even subtle variation of the molecular structure of a monomer may be responsible for significant properties change and then solves specific application problems. As a single evident example, we can notice that the melting point of Nylon 6 (220 °C) is 45 °C lower than the melting point of Nylon 66 (265 °C) in spite of the same CO/NH/CH2 ratio. The difference is ascribed to the different position along the chain of the NH and CO groups: perfectly alternating in Nylon 6 and with –NH–NH–CO–CO– order in Nylon 66. Other nylons include copolymerized dicarboxylic acid/diamine products that are not based upon the monomers listed above and some can be obtained from natural sources rather than from fossil oil. While nylons are in general fully aliphatic, some fully aromatic PA (known as ‘aramids’) are prepared from aromatic diacids like terephthalic acid or isophthalic acid with aromatic diamines (the renowned Kevlar and Nomex) used in protective clothing for motorcyclists and for sports activities. Linear polymers are the most useful, but it is possible to introduce branches in nylon by the condensation of dicarboxylic acids with polyamines having three or more amino groups.
3.2.5 Polycarbonates (PC)
Polymers under the name of polycarbonates are formed by carbonate links connecting hydrocarbon groups. If these last are aromatic the polymer is strong and tough, and some grades are optically transparent as the material is not able to crystallize. The most common polycarbonate is produced by the reaction of bisphenol A (BPA) (IUPAC, 4,4′-(propane-2,2-diyl) diphenol) and phosgene COCl2. The scheme of the formal reaction is shown in Fig. 3.12.
Actually, the first step of the synthesis involves the reaction of bisphenol A with sodium hydroxide, to yield the disodium salt of the bisphenol A. The condensation reaction with phosgene produces sodium chloride as by-product and a chloroformate, which subsequently is attacked by another phenoxide propagating the growth of the chain by condensation reaction. The resulting polycarbonate is an amorphous material with glass transition temperature of about 147 °C that can be submitted to extrusion into tubes, rods and other profiles including multiwall.
As already evidenced for atactic polystyrene even polycarbonate is not formally a real plastic polymer as it has only a single transition temperature, the Tg , and can be used as a solid material only below the Tg itself. This last is, however, about 120 °C higher than room temperature and therefore at ambient conditions PC behaves as a very strong rigid material. In addition, the lacking of any crystalline fraction provides items with high transparency to visible light, and makes this polymer convenient for applications where transparent or electrically non-conductive parts are needed with high mechanical resistance to fracture.
3.2.6 Polyvinylchloride (PVC)
Vinyl chloride is a monomer of pure fossil origin that can be polymerized to PVC by free radical polymerization process; by opening of the double bond, it gives rise to macromolecules where the monomer units are enchained head to tail to minimize repulsion between chlorine atoms (Fig. 3.13).
No other initiator was discovered which could replace the free radical one. Because the free radical process lacks efficient stereochemical control, the Cl bearing three substituted carbon atoms is distributed randomly along the chain. This provides the polymer with an irregular structure that can be considered as atactic, even if the significant repulsion between the chlorine atoms on the last inserted unit and the incoming monomer favours the syndiotactic sequence over the isotactic sequence, and the former is present at a major extent than expected for a complete casual distribution. In spite of the presence of short syndiotactic chain segments, the polymer cannot crystallize to an amount which can be really significant for the material application. The commercially available PVC is then an amorphous polymer with Tg of about 90 °C. Then, as polystyrene and polycarbonate, PVC is not a true plastic material , but can be shaped with temperature by heating over 90 °C and then used as a solid rigid material near room temperature. Application of PVC includes also soft material obtained by using plasticizers with can derive from low or high molecular weight molecules. This molecules are characterized by a very low glass transition temperature and are compatible with the polymer. Rigid PVC is a very durable and long-lasting material also known as PVC-U (the U means ‘unplasticised’) used extensively as product/material for construction such as in window frames and cladding. Instead the flexible plasticized PVC finds use in flooring, medical products and even as a sort of synthetic leather. Because of the presence of large amount of chlorine, PVC is intrinsically fire retardant even if in some cases flame retardants can be added owing to its good compatibility with differently structured additives. All PVC-derived materials have very good electrical insulation and weatherproof properties. Over the past 15 years, it has undergone a remarkable transformation from point of view of general consideration: from being initially considered a not environment-friendly polymer to an important thermoplastic material in establishing what is called ‘a circular economy’. The PVC industry assumes a sort of role model for sustainability. This result derives from real correct technical evaluation of the concept that the sustainability of a material is achieved by inspired technology to maintain useful properties and minimizing environmental impact . To the good performances and the advantage of using as starting material chlorine derived from the kitchen salt for more than 50%, the process was made very efficient to reduce to few ppm the presence of the toxic monomer and the recycling was activated. In particular, PVC today can be recycled up to seven times without loss of performance, conserving valuable resources and raw materials. In detail, these achievements were possible as emissions of vinyl chloride monomer and dioxins during manufacturing decreased dramatically and the use of cadmium-based stabilizers was stopped. Even if plasticized PVC containing some phthalic acid derivatives is considered particularly toxic and other hazardous additives whose recycling was considered in the past a challenge and issue with particular reference to costs are involved, some interesting advances have been realized. Today the PVC is a material not only durable but fully recyclable and containing low content of carbon.
In agreement with this statement, it is of interest to the present book objective to remark that even if fully derived from fossil and invented by man PVC is not entirely derived from oil, being also produced from inorganic minerals. Indeed 57% of the polymer weight is due to chlorine, which is industrially derived from common sodium chloride and only 43% of the PVC weight is derived from hydrocarbon feedstocks. In the future, this part could be replaced with carbon derived from ethylene-originated natural resources.
3.2.7 Acrylic Polymers
This general name includes a broad number of polymeric materials obtained by polymerization of monomers containing a vinyl group to a carboxylate derivative (acrylic monomers) or a 2-propenyl group attached to the same carboxylate derivatives. Looking to the double bond type, acrylic monomers form a particular class of vinyl monomers while methacrylic monomers are vinylidenic. The corresponding polymers are usually produced by free radical polymerization and therefore the corresponding commercial homo- and copolymers are atactic and give rise to amorphous non-crystallizable materials. Some of these polymers may have Tg larger than room temperature and are used as plastics below the glass transition temperature .
For these reasons, as already discussed for PS, PC and PVC, they cannot be rigorously recognized as plastic as they lack a second transition temperature. The presence of the carbonyl functionalities attached to the double bond of these monomers generates an increased electron density on the monomer double bond that is then active for a nucleophilic attack. Typically, anions such as derivatives of metal alkyls can then start the polymerization of these monomers unless their side chain functional group does not deactivate the reaction.
The effect of molecular structure in these polymers on their thermal features can be argued by looking at the Tg values achieved when changing the monomer structure as reported in the next Table 3.2. Indeed, Tg is the property that is directly related to molecular structure more than others and the many possible molecular variations provided by human synthetic routes allow a large modulation of this property and then of the application possibility of the related polymers.
Polyacrylates and polymethacrylates differ in the structure of the side group directly bonded to the backbone. The capacity of this last group to prevent or restrict owing to steric effects the rotation of the bonds around the main chain increases with increasing steric interactions or polar groups repulsion. These restrictions, either due to steric hindrance or polarity repulsion, cause an increase of the Tg values. Thus, in polyacrylates with ester side chain, the Tg increases with the steric hindrance of the alkyl group bound to the carboxylate in the side chains, as observed when going from poly(n-butylacrylate) to poly(t-butylacrylate). If the side chain is not branched, the increase of the rotational ability of the side group with increasing chain length results in a decrease of Tg , despite the increase of Vx, as observed when comparing poly (methyl acrylate) with the polymers of ethyl, propyl and n-butyl acrylates. In other polyacrylates, the dipolar interactions between the side chains increase the energy barrier of molecular motions as it appears by the Tg values of polyacrylates with side groups having specific polarity and hydrogen bond capability (i.e. polyacrylonitrile and poly (acrylic acid)). Finally, poly(methacrylate) series, where rotational restriction is strong due to the replacement of the tertiary hydrogen atom with the methyl group, shows higher Tg than the corresponding polyacrylic analogues. The remarkable increase is around 90 °C.
The most common acrylic plastic is poly(methyl methacrylate) (PMMA) (Fig. 3.14). It has several commercial names, i.e. Plexiglas, Lucite, Perspex and Crystallite depending on the company producing it, but the molecular structure is the same. Commercial PMMA is amorphous (with at Tg values of about 100 °C) highly transparent and tough at ambient temperature. It has also a good resistance to outdoor exposition and finds applications as a convenient material for airplane windshields, automobile lights and outdoor signs. As PMMA is compatible with human tissue, it can be used in the manufacture of rigid intraocular lenses that are permanently implanted in the eye.
Soft contact lenses are often made of a related polymer (copolymers obtained by polymerizing mixtures of different acrylic monomers), where the repeating units contain hydroxyl groups thus improving the hydrophilic character of the material. Methacrylate polymers are moreover extensively employed in medical and dental applications where purity and stability are critical to performance.
It is, indeed, used for bone cement to affix implants and to remodel lost bone in orthopedic surgery. Many other applications are known for PMMA and we refer the reader to commercial information.
More appropriate with the aim of this book is rather to discuss how versatile are the polymers derived from acrylic-type monomers. PMMA is mainly used for its mechanical properties , processability and stability for applications where these characteristics are of primary importance. However, it is interesting to consider the many possibilities offered for polymer properties design by this class of momomers. Indeed, acrylic and methacrylic monomers can be easily incorporated in the same macromolecules in a random distribution by free radical polymerization processes, which is largely the most used process for industrial applications. The easy polymerizability and the possibility of selecting different functionalities as the monomer double bond substituents have been largely utilized to prepare at industrial level several functional polymers for specific applications.
Some significant examples will allow elucidating this point.
The polymer of acrylic acid, CH2=CH-COOH, can be easily prepared as a linear polymer by free radical polymerization and eventually rendered insoluble by gentle cross-linking by using in the monomers feed minor amounts of a diacrylate such as hexandiol diacrylate. The many carboxylic acid groups present as side chains can be easily salified by treatment with sodium hydroxide to give a polymeric salt very hydropylic as the salified units reach the 70% by weight of the material. When the polymer is cross-linked the polymeric salt remains solid, but it is able to absorb very large amounts of water, acting as a superabsorbent (called SAP). These materials are generally used in diapers and in sanitary napkins. Then a copolymer of an acrylic or methacrylic ester with varied amounts of acrylic acid can provide a plastic material with modulated water affinity.
On the other side, copolymers of an acrylate alkyl ester with an amino acrylate, such as 2-amino-ethyl-methacrylate can be converted into a polycation by quaternization of the side chain amino groups.
Thus, in general, the copolymerization of alkylacrylates, including MMA, with different monomers offers a flexible tool for producing a number of materials with modulated chemical and physical properties, even if, once again they have not a perfect plastic characteristic.
3.3 Bio-related Polymers from Renewable Resources
Bio-related polymers from renewable sources can have the same structure as the corresponding polymers derived from fossil or a different unique structure fully designed by nature.
The former type includes polymers with variable environmental impact and they are in general non-biodegradable, even if some are. They differ from the corresponding petrol counterpart only for the origin of the carbon and for the synthetic route adopted to produce the monomers from which the polymers are prepared. An example is the bio-polyethylene differing from the petrol-made polyethylene only for the ethylene used: the bio-ethylene is obtained by fermentation of polysaccharides (see Sect. 5.4), while the traditional ethylene is obtained by the petrol cracking process. However, the two polymers are identical and have close properties.
The latter type of bio-related polymers can be classified into two large groups based on their structure and on the synthetic procedure. One of these groups is the natural occurring polymers, the others consist mainly of biopolyesters produced by engineered microorganisms or through chemical processes capable to polymerize natural occurring monomers.
Natural occurring polymers include polysaccharides, like starches found in potatoes or wood, and proteins, such as animal-based whey or plant-derived gluten. Polysaccharides are made of glycosidic bonds, formed by reaction of hemiacetal of a saccharide and an alcohol with water loss. Proteins are made from amino acids that react to form peptide bonds, which consist of amide functional groups. An example of microorganism-made polymer is poly(hydroxybutyrate). While the most known biopolyester from renewable resources is poly(lactic acid ).
Even if then the biodegradability is not the unique desired property of bio-related polymers, it is certainly one of the most attractive from the environmental viewpoint for short life material used under conditions which do not allow collection after disposal. All biodegradable polymers should be stable and durable enough for use in their particular application, but upon disposal they should easily break down as their recycling is not possible. Polymers, specifically biodegradable polymers, should not have extremely strong carbon backbones that are difficult to break. Since the degradation begins at the surface a high surface area speed-up biodegradation and allows easy access for either the chemical, light and/or organisms able to fragment the chains. The hydrophilic character, when present, can help biodegradation as it allows the water-soluble enzyme to easily get in contact with the polymer; however, if the polymer contains hydrolizable groups as ester groups in the backbone, the material can undergo biodegradation by surface enzymatic attack even if not swollen in water. Another property of bio-related, biodegradable polymers is the non-toxicity of the degradation products, which make them suitable for biomedical application. For this last-mentioned application, it is important also the capability to preserve good mechanical integrity until the material degrades, and the control rates of degradation under use conditions. It is necessary that the material does not arouse the immune response, and obviously the products of degradation also need not be toxic. These aspects are really important: the biodegradable polymers are used for the release of drugs into the body and it is really important to control the process over time, overall when the drug cannot be issued at once; in addition, the medicine has to be stable in the bottle or blister until it is ready to be taken.
In addition to medicine, biodegradable polymers are often used to reduce the volume of waste in packaging materials. In this field, there is significant effort to replace materials derived from petrochemicals with those that can be made from biodegradable components (Sasaki et al. 2003).
Examples of bio-related polymers are described below.
3.3.1 Polyethylene from Fermentation Monomer
Natural renewable resources can be used to produce the monomer ethylene by dehydration process of ethanol obtained from various feedstocks including sugar cane, sugar beet and wheat grain (see Sect. 5.4). The corresponding renewable polyethylene is successively prepared by polymerization of this ethylene from ethanol by the various industrial processes, such high-pressure free radical and catalytic (as above discussed), invented by man. The properties of the resulting polymers are identical to those of conventional polyethylenes produced by polymerization of ethylene from oil through the same processes, respectively. This holds for the physical properties for conversion into plastics products. Then one can say that in the commercial polyethylene there are no memory and evidence of the natural origin of raw material used as starting feed. It is also versatile in terms of applications and recyclable in the same chain established for conventional PE. Therefore, the bio-derived polyethylene being chemically and physically identical to traditional polyethylene does not biodegrade in the environment and must be collected and recycled. The advantages of producing and using bio-derived polyethylene are then mainly environmental. In addition to saving not renewable resources , its use can also reduce greenhouse gas emissions. Brazilian chemical group Braskem claims that by using its method of producing polyethylene starting from sugar cane ethanol captures (removes from the environment) 2.15 tons of CO2 per ton of polyethylene produced. Annual production capacity of polyethylene from ethanol reached 200 kT in 2013. Indeed, being the process focused on the monomer, the same mature technology applied to fossil-derived ethylene can be industrially followed. Thus, high-density polyethylene (HDPE) and linear low-density polyethylene (LLDPE) materials were produced from ethylene of bio-origin. The first access to the market was directed towards products for applications in rigid and flexible packaging, closures, bags and others. In January 2014, also the production of low-density polyethylene (LDPE) was effectively performed thus allowing covering additional applications in packaging and films.
3.3.2 Thermoplastic Starch (TPS)
Starch , a polysaccharide that can be used as a thermoplastic material, is one of the most abundant renewable resources known to man as the result of the biosynthesis by numerous plants. The molecular structure of starch is a polymeric carbohydrate consisting of a hydro glucose unit linked together primarily through α-d-(1 → 4) glucosidic bonds (see Sect. 5.2). The natural product is indicated as native starch , term used to describe starch in the form in which it occurs in plants such as potatoes, wheat, cassava, rice and maize. Thermoplastic starch (TPS) is the name of the commercial products mainly made of starch that exhibit thermoplastic behaviour after various man-made treatments of the native material.
Native starch , in plants, consists of granules superstructure composed of amylose and amylopectin (see Sect. 5.2). Granular starch is partially crystalline and when it is heated thermally degrades before the crystalline melting point is reached. As a result, starch cannot be melt-processed in its native form. Addition of plasticizers is necessary in order starch can exhibit thermoplastic properties when heated at elevated temperatures under shear (see Sect. 5.5) to give gelatinized starch which is then mixed with other thermoplastic additives. Granular starch can be also plasticized by blending or grafting with a soft biodegradable polymer as polycaprolactone. This allows reducing the processing temperature while maintaining the biodegradability. Materials with glycerol as the plasticizer exhibited less rigidity. Plasticized TPS has engineering properties close to those of several plastic polymers, and it can be processed as traditional oil-derived thermoplastic polymers such as by kneading, extrusion , injection moulding , compression moulding, blow moulding or heating and casting. It can also be used as matrix for composites including nanocomposites in particular with cellulose fibres and nanofibres.
3.3.3 Poly(Lactic Acid) (PLA)
Poly (lactic acid ) is derived from the monomer lactic acid that can be industrially obtained either from natural renewable resources through bacterial fermentation of sugars or from fossil resources by chemical synthesis, where acetaldehyde is the starting molecule (Jamshidian et al. 2010). Today lactic acid is produced predominantly, or almost totally, by the former microbial fermentation process. The racemic mixture of the d and l antipodes, or mixtures where the antipode l-lactic acid predominates up to 99.9%, can be industrially produced depending on the enzymatic system used in the fermentation process. At present, the industrial-scale production of d-lactic acid by fermentation is possible, but not used to a significant level.
The polymerization of lactic acid gives PLA, at present the most popular polymer derived from renewable resources . The macromolecules of PLA contain head-to-tail units derived from the formal condensation of lactic acid as indicated in Fig. 3.15.
The same repeating unit shown in Fig. 3.15 can be formally and effectively obtained by removing water before polymerization. Actually, this reaction involves the condensation of two lactic acid molecules to yield the cyclic lactide dimer. As the condensation has been performed before polymerization to obtain the lactide, the last can undergo a chain polymerization by ring opening activated with various metal catalysts in solution, in the melt or as a suspension. l-lactide, d-lactide and meso (l,d) lactide are produced from l-lactic acid and d-lactic acid . The polymerization of each of the lactide enantiomers or mixtures leads to PLAs differing in the stereochemical features. In case of these polymers, the term poly(lactide) is more appropriate and can be used instead of PLA. This last name should be used for polymers obtained by the direct condensation of lactic acid monomers. In this case, the process is carried out at less than 200 °C to avoid the formation of the lactide during the polymerization. One equivalent of water is formed for every monomer molecule added to the growing chain by condensation. The generated water causes chain hydrolysis as side reaction so that only low molecular weight chains are obtained. To overcome this drawback, the process of polycondensation is carried out in two successive steps. The first step is the above-mentioned one giving lactic acid oligomers. In the following step, the oligomers are combined to give high molecular weight polymer chain by efficient removal of the water that is formed during the condensation so to avoid chain hydrolyses and promoting transesterification. The above polymerization routes including lactide formation are schematically represented in Fig. 3.16.
Polymer properties are dependent on monomer stereochemistry, which is on the content and distribution along the chain of l- and d-repeating units. The polymerization of the racemic mixture of l- and d-lactides may lead in the absence of any streocontrol to the random copolymer of the two antipodes, poly-dl-lactide (PDLLA), which lacks chain stereoregularity (atactic), cannot crystallize and thus is amorphous. Interestingly, isotactic crystallizable products can be obtained even from the racemic dl-lactide in case the two enantiomers polymerize in separate chains, under steric control by the catalyst or by the growing chain. Under one of the two controlled conditions, an equimolar mixture of poly-l- and poly-d-lactic acid , both isotactic, is formed instead of the random atactic copolymer. The crystallinity is then dependent on the degree of isotactic stereoregularity, and is depending on the D/L ratio between the monomers in the feed and by the stereoselective capacity control during the polymerization reaction. When the prevalence of one antipode in the polymer units is 85–90% the polymer is semicrystalline. On the contrary, it is amorphous under this enantiomeric prevalence. The crystalline domains can assume distinct crystalline modifications, the α-, β- or γ-forms, depending on the crystallization conditions. The α-form is the most frequent and is characterized by two antiparallel chains, which in case of the l-units assume a left-handed helix conformation. These helices are packed in a nearly hexagonal arrangement in an orthorhombic (or pseudo-orthorhombic) unit cell.
The isotactic poly(l-lactic acid ) derived from the pure L antipode has about 37% of crystallinity with a melting temperature of about 173–178 °C and Tg in the range 60–65 °C. Indeed, it shows a real plastic behaviour. This kind of PLA can withstand temperatures of 110 °C, is insoluble in water being substantially hydrophobic and soluble in chlorinated solvents, hot benzene, tetrahydrofuran and dioxane.
Processing of PLA allows obtaining various items of less or more complex geometry by extrusion, injection moulding, film and sheet casting. PLA is also used as a feedstock material for 3D printers. It is widely used in packaging applications, a practice that is considered environmentally useful thanks to the biodegradability of many PLA-derived items such as many films, wrappings and containers (including bottles and cups) (Masutani et al. 2015). In 2002, FDA ruled that PLA was safe to use in all food-contact packaging materials. It has also medical applications as bio-re-absorbable material able to break down inside the body within 6 months to 2 years (as absorbable suture thread).
3.3.4 Polyhydroxyalkanoates (PHAs)
Some bacteria store carbon and energy (see Sect. 5.3) by producing linear polyesters corresponding to general structure of polyhydroxyalkanoates (PHAs) in the form of homopolymers or copolymers (Fig. 3.18). Up to 150 different monomers can be combined within this family to give materials with extremely different properties. The main known polymers belonging to this class are poly-3-hydroxybutyrate [P(3HB)], poly-3-hydroxyvalerate [P(3HV)] and their random copolymer P(3HB-co-3HV) whose structures are represented in Fig. 3.17.
Poly[(R)-3-hydroxybutyrate] (P3HB) has a perfectly isotactic structure with monomer units having only the (R)-configuration, and has 55–80% of crystallinity. Two crystalline modifications are observed. The α-form produced under the most common conditions is characterized by two antiparallel chains in the left-handed 21 helical conformation. These helices are packed in an orthorhombic unit cell with axes a = 0.576 nm, b = 1.320 nm and c (fibre axis) = 0.596 nm, according to a space group of P212121 (Fig. 3.18).

(Reprinted from Yokouchi et al. 1973, by permission from Elsevier)
Crystal structure of α-form P(3HB) proposed by Yokouchi et al.
The bacteria produced P(3HB) has high molecular weight with a polydispersity of around two. It is a regular plastic material with Tg at around 4 °C and Tm near 180 °C. The densities of crystalline and amorphous P(3HB) are 1.26 and 1.18 g/cm3, respectively, larger than that of polyolefins and in the range of synthetic polyesters. On the other side, Young’s modulus (3.5 GPa) and the tensile strength (43 MPa) are close to those of isotactic PP even if P(3HB) is a stiffer and more brittle plastic material than PP. Indeed, the extension to break (5%) is markedly lower than that of polypropylene (400%).
Different from other bio-related polymers and similarly to synthetic polymers, PHAs class offers many structural possibilities and then a broad variety of properties related to distinct applications. The large number of molecular structures available by feeding various bacteria families with organic chemicals of different type even if preferably of natural origin provides a first level of differentiation in structure and then in properties providing really plastic polymers. So one can expect that these materials are suitable to replace several fossil produced polymers providing non-renewable resource saving and minor environmental impact thanks to the biodegradability and the low-carbon footprint. In spite of a large number of possible polymer compositions only about 5–10 different polymers at present are of interest for industrial production. Despite many years of research, the volume of PHAs produced at industrial level is still limited. Few examples of industrial products on the market are Mirel® and Mvera® series by Metabolic, TephaFLEX® series by Thepha and EnMat® series by TianAn Biopolymer .
3.4 Structure to Fundamental Properties
This final section of the chapter is devoted to summarize and compare the relationships between structure and main properties of plastics having different molecular features, independently of their origin. In other words, this can be regarded as an attempt to evaluate how far the molecular structure of the repeating units of the macromolecule can affect the final properties and in addition how is important monomer availability and selection. In these two last aspects, the fossil way and the nature approach present distinct characteristics. For sake of clarity in this chapter, only homopolymers and the main industrially used copolymers were considered, while a very important contribution to properties versatility is certainly offered by the blending of different polymers and other products (composites ).
The logical approach was then providing real examples to design a polymeric material with defined properties starting from the molecular structure either from fossil or renewable resources . In this connection, the comparison is referred to show the many different molecular structures designed by man that have assumed a practical interest to solve different needs of the everyday life. This approach was certainly the most efficient for technology results but has clear limitations for the environment.
The nature-related polymers fit in this general scheme in the applications range for which their molecular structure allows, taking in mind that the material properties start from the molecular structure, but acquire the ultimate value thanks also to the consequent organization. Moreover, one should keep in mind how the same properties can be reached with different structures (Tables 3.3, 3.4, 3.5 and 3.6).
The thermal and the mechanical features of a polymer (or copolymer) are strictly interconnected and mainly depend on the chemical structure of monomeric units (presence of heteroatoms, multiple bonds, aromatic moieties), order’s degree in linking the monomeric units (head–tail, tail–tail bonds, type of co-monomer sequences), molecular weights and their dispersity. These main key aspects are obviously independent of the nature of monomeric units from fossil- or bio-derived sources. Most of the mechanical performances of a polymer and in particular the interval of temperatures for its use and processing are strictly related to its crystallinity, ranging from 0 to 80%, and Tg values. These values define the classes of thermoplastic polymers as the ones whose properties are numerically reported in Tables 3.3, 3.4, 3.5, 3.6 and in the case of the amorphous (co)polymers the crystallinity is 0 (Tables 3.4 and 3.5).
The general temperature behaviour of thermoplastic polymers, assessing the state of applications and state of processing is summarized in Fig. 3.19 and in Fig. 3.20.
A polymer can crystallize only if it has linear and regular macromolecules, even if limited crystallization can take place if a small number of branches are present (like in the case of side groups derived from vinyl monomers). Crystallization requires an orderly arrangement along the polymer chain providing the structure a high degree of symmetry.
Linear polyethylene, for example the HDPE in Table 3.3, can, in a relatively easy way, reach high levels of crystallinity up to 80% even always a certain amount of the long chain can be packed in the crystal three-dimensional order. This is possible by the planar zig-zag structure easily assumed by the polyethylene macromolecules having only hydrogen atom substituents out of the carbon atom on the backbone (see Chap. 2 and Sect. 3.2). The macromolecules therefore can assume for long section a linear ribbon form giving a high degree of order and can easily be packed into dense crystals. The small substituent volume practically cancels the steric hindrance and then the polyethylene outside of the crystals can rotate freely being extremely flexible. The rotation around carbon–carbon bonds allows the chain to assume a large number of disordered conformations with high gain in entropy with respect to the unique ordered planar zig-zag conformation. For this last reason, polyethylene is characterized by a low value of Tg thus generating a wide temperature range of use for this really important plastic commodity.
Polypropylene (PP) and polystyrene (PS) can crystallize only if the macromolecules are stereoregular: that occurs if they assume an isotactic or syndiotactic structure (see Chap. 2 and Sect. 3.2). Isotactic polypropylene (iPP, Table 3.3) is the commercially interesting materials. Its configurationally ordered primary structure allows the macromolecules to assume a regular conformation in the form of left-handed and right-handed 3/1 helices as the planar zig-zag arrangement is hindered due to steric interactions of the alternating methyl group. This helical conformation can assume an ordered supramolecular arrangement in crystal, but the packing is less dense than for polyethylene. However, the larger chain rigidity due to steric limitation to the rotations around the main chain or backbone limits the entropy variation from order to disorder thus increasing both Tm and Tg . iPP has then a plastic behaviour range narrower than that of PE, but is shifted towards higher temperatures covering an interval that PE cannot reach. Conversely, atactic PS is the only industrially produced. This polymer is un-crystallizable since the polymer chain lacks any regularity. The amorphous atactic PS has a high Tg as the dipole interactions and packing of aromatic rings reduce the chain flexibility leading to high rigidity. This polymer can then be used for various applications as solid material below the Tg . The situation is similar in other atactic homopolymers and random copolymers which all do not crystallize (Tables 3.4 and 3.5) for lack of structural order and their application is as glassy phase till close to the Tg value (Fig. 3.20).
In the hydrocarbon polymers discussed before the crystallinity is related to steric interactions which may hinder ordered structures and determine the lowest energy conformations found in the corresponding crystals. In polymers having heteroatoms and functional groups, polar interactions and hydrogen bonding favour the crystallinity because the packing in crystals brings the macromolecules closer to each other and makes possible to maximize dipole–dipole and hydrogen bonding intermacromolecular forces.
The presence of ester linkages along the backbone of polyesters provides polar groups which certainly increases the strength of interchain interactions, but the effect on material properties is markedly affected by the type of hydrocarbon residue between two successive ester groups, that is on the whole molecular structure of the repeating unit. Accordingly, polycaprolactone where the connecting group consists of five methylene groups is finally flexible and has a lower melting with respect to PET (Table 3.3) to PLLA, and linear PHB (Table 3.6) which contains polar ester groups connected by more rigid aromatic or branched aliphatic short segments. In these polyesters, the dipole–dipole forces between the polar groups create strong interactions among the macromolecules in the crystals contributing to increased melting point and to high modulus values. PHB properties are rather close to those of iPP, and better than those of HDPE. Relaxation of the molecular rigidity can be achieved with longer alkyl groups as in PHV and with copolymers PHBV. Thus, PHV exhibit Tg values lower than PHB and below the room temperature explaining their rubbery state particularly by increasing the HV content, thus allowing plastic behaviour in lower temperature environments. PLLA having Tg higher than room temperature needs some plasticization to expand its plasticity range at the most popular room temperatures. The effect of intramolecular interactions is even more evident in the polyamides where the presence of the polar amide groups in the backbone chain originates a network of hydrogen bonds connecting different chains (see Nylon 6,6 or PA6 in Table 3.3). This strong binding among polyamide macromolecules is maximized in the crystals thus contributing to a remarkable extent to the melting enthalpy, which measures the energy necessary to remove macromolecules out the interaction distance. Once separated, the polyamide chains remain more rigid than purely hydrocarbon polymers with similar structure as the amide bond with its partial conjugation hinders free rotation around backbone, thus reducing the value of the entropy of melting. Both effects raise the melting point and the glass transition temperature with respect to polymers without strong intermolecular interactions, like polyethylene.
In addition to the above general effects, it is of interest to notice that polymers with small side chains crystallize more easily than polymers with large, pendant groups. An example is provided by poly(vinyl alcohol) (PVA) made by the hydrolysis of poly(vinyl acetate) (PVAc). Being the –OH groups rather small in volume PVA can stand in the planar zig-zag conformation independent of the configurational order of the substituted main chain carbon atoms, and therefore its crystallinity is independent on stereoregularity : this means that both atactic and stereoregular (iso- or syndiotactic) PVA can crystallize. The –OH groups in PVA also form strong interchain hydrogen bonds, increasing the melting up to 200 °C. By increasing the size of the side groups polymer encounters difficulties in folding itself along the crystal growth direction (like in the case of PVAc and PMMA, see Table 3.4). Thus, when the side groups are larger than –OH stereoregularity is needed for crystallization. Bulky side groups and branching reduce the ability and the possibility of a polymer to crystallize. For example, branched polyethylenes (LLDPE and LDPE) have a low degree of crystallinity and lower melting point than linear HDPE, because the side segment has less freedom to move and the incorporation of defects in the crystalline lattice spreads defect to the three-dimensional organization.
The crystalline polymers have an effective incapacity to form perfect crystals as low molecular weight compounds. A crystalline basic structure is characterized by lamellae that consist of layers of folded chains. Assembling of these layers give rise to more complex larger structures named crystallites or spherulites; these are generated by lamellae growing radially in all directions and thus, generally the resulting crystalline regions are spherical (just the spherulites), unless particular thermal gradient or geometrical constraints are applied.
The thickness of a typical crystallite is in the range of 10–20 nm. For the reasons described above, polymers are arranged in partially crystalline structures, as they are semicrystalline. In between the ordered crystalline lamellae, there are disordered regions where the chains segments have assumed different spatial arrangements. The disordered regions form the amorphous phase. As the length of macromolecules in high molecular weight polymers is larger than the dimensions of the phases in a semicrystalline material, a single polymer chain may accommodate partly in a crystalline lamella, and partly in the amorphous state. Depending on the length, some polymer chains even start in one lamella and then reach another lamella, after having gone across the amorphous region (tie molecules in the scheme of Fig. 3.21).
Crystallinity depends on the average chain length (Fig. 3.22). Indeed, the long chains tend to be more tangled and the free energy is minimized by participating in both amorphous and crystalline phases. The resulting crystallinity is significant and the behaviour of these materials implies improved strength typical of plastics (hard/soft depending on crystallinity and thus on the chain folding/packing capability). Low molecular weight may even result in improved crystallization but the polymer material is usually weaker in strength as it is more difficult that their chains act as binders of different phase domains. Under external mechanical stimuli (stress), the polymer chains can slide by each other and cause a break in the material. Therefore, an optimized balance between crystallinity and molecular weight is important for obtaining the desired mechanical response by the material.
Crystallinity has various effects, but these are interrelated with other parameters.
Crystallinity makes a material stronger (higher the tensile modulus (see Chap. 2, Fig. 2.9), but also more brittle (with low elongation at break). Indeed, a completely crystalline polymer would be too brittle and the presence of amorphous regions is necessary for toughness, that is, the ability to bend without breaking, and then what we call the plastic response in a certain range of temperature, the temperature of use (Figs. 3.22 and 2.9).
In crystallizable polymers, the crystallinity is usually induced by cooling a melt or a dilute solution below its melting point or by stretching to orient macromolecules in the stretching direction.
In the industrial production, the polymers often are cooled rapidly from the melt (see Chap. 4), and the crystallization extent is then controlled by kinetics rather than thermodynamics. These chains are entangled in the melt and may have not enough time to crystallize a high percentage; crystallinity is higher for materials cooled slowly from the melt. A typical behaviour is observed in polyesters where amorphous material from the melt can be ‘frozen into’ the solid without crystallization thanks to the kinetics control allowed by the intrinsic slow crystallization occurring in these materials. This molecular characteristic clearly holds for polyester from fossil origin and for biopolyester (especially for PLA and PHB). This behaviour is not suitable with widespread use in industrial applications, as it reduces the temperature use to that of amorphous plastics (Fig. 3.20) that is below Tg . Of consequence, prototypical polymer processing techniques such as moulding and extrusion are not possible.
Specific technological devices/designed procedures, structure modification, compounding or adding nucleating agents are therefore necessary for the conversion into valuable commercial products.
References
Billmeyer, F. W., Jr. (1984). Textbook of polymer science (3rd ed.). New York: Wiley-Interscience.
Callister, W. D., Jr. (2007). Material science and engineering, an introduction (7th ed.). Wiley & Sons, Inc. 2007; ISBN-13: 978-0-471-73696-7; ISBN-10: 0-471-73696-1.
Cowie, J. M. G., & Arrighi, V. (2007, July 27). Polymers: Chemistry and physics of modern materials (3rd ed., 520 pp.). CRC Press Textbook—268 B/W Illustrations ISBN9780849398131 - CAT# 9813.
Gandini, A. (2008). Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules, 41, 9491.
Jamshidian, M., et al. (2010). Poly-lactic acid: production, applications, nanocomposites, and release studies. Comprehensive Reviews in Food Science and Food Safety, 9, 552–571.
Klein, R. (2011). Material properties of plastics, chapter 1. In Laser welding of plastics (1st ed.). Wiley-VCH Verlag GmbH & Co. KGaA.
Masutani, K., & Kimura, Y. (2015). PLA Synthesis. From the monomer to the polymer, chapter 1. In Poly(lactic acid) science and technology: Processing, properties, additives and applications. The Royal Society of Chemistry.
Natta, G., & Corradini, P. (1959). Conformation of linear chains and their mode of packing in the crystal state. Journal of Polymer Science, 39, 29–46.
Olsson, P. A. T., et al. (2017). Ab initio and classical atomistic modelling of structure and defects in crystalline orthorhombic polyethylene: Twin boundaries, slip interfaces, and nature of barriers. Polymer, 121, 234–246.
Pan, P., & Inoue, P. Y. (2009). Polymorphism and isomorphism in biodegradable polyesters. Progress in Polymer Science, 34(7), 605.
Sasaki, S., & Asakura, S. T. (2003). Helix distortion and crystal structure of the A-form of poly(L-lactide). Macromolecules, 36(22), 8385.
Yokouchi, M., Chatani, Y., Tadokoro, H., Teranishi, K., & Tani, H. (1973). Structural studies of polyesters: 5. Molecular and crystal structures of optically active and racemic poly (B-hydroxybutyrate). Polymer, 14(6), 267.
Zhang, Y., Rempel, C., & Liu, Q. (2014). Thermoplastic starch processing and characteristics-A review. Critical Reviews in Food Science and Nutrition, 54(10), 1353.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ciardelli, F., Bertoldo, M., Bronco, S., Passaglia, E. (2019). Molecular Structure Requirements. In: Polymers from Fossil and Renewable Resources. Springer, Cham. https://doi.org/10.1007/978-3-319-94434-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-94434-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94432-6
Online ISBN: 978-3-319-94434-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)