Abstract
Among all the vector-borne diseases occurring worldwide, mosquito-borne diseases prevail by far resulting in approximately 700,000 deaths from clinical complications annually. Although malaria still accounts for the highest disease burden, currently emerging and resurging mosquito-borne viral diseases like dengue, West Nile, chikungunya, Rift Valley and even yellow fever viruses became either epidemic or pandemic, affecting many regions in the world. In February 2016, the World Health Organization declared the Zika virus public health emergency of international concern following large outbreaks with rapid geographical spread in the Pacific and Southern America, while Mayaro, Wesselsbron, Usutu and St. Louis encephalitis viruses have been identified as new disease agents showing pandemic potential.
The transmission of mosquito-borne disease agents can be readily interrupted by prevention from potentially infective mosquito bites. Therefore, personal protective measures against bites of hematophagous vectors constitute the first line of defence against mosquito-borne diseases. Besides the use of skin repellents and bite-proof textiles, long-lasting insecticide-impregnated bed nets and clothing have been developed during recent years which synergistically contribute to optimized personal protection. The aim of this study is to give an overview on the most current and widely used textile impregnation techniques, their efficacy in public health protection as well as their mosquito bionomic-specific use against daytime-, night-time-, indoor- and/or outdoor-biting vector mosquitoes. We strongly recommend the use of long-lasting permethrin-impregnated clothing for the prevention of mosquito-borne diseases transmitted by daytime- and night-time-active-, indoor- and outdoor-biting mosquitoes, including chikungunya, dengue and Zika fevers combined with the extensive use of long-lasting insecticide-treated bed nets preventing primarily from nocturnal, anthropophilic, indoor-biting mosquitoes vectoring, e.g. malaria, lymphatic filariosis and West Nile fever.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Permethrin-impregnated clothing
- Insecticide-treated bed nets
- Malaria prevention
- Long-lasting activity
- Personal protection
- Mosquito bite protection
- Public health implications
12.1 Introduction
Currently, 11 major global vector-borne human diseases have been identified by the World Health Organization (WHO) which results in approximately 17% of the estimated burden of communicable diseases, thus accounting for more than 700,000 deaths associated with these diseases (WHO 2017a). Among these diseases defined, approximately two thirds, namely, malaria, dengue, lymphatic filariosis, chikungunya, Zika virus disease, yellow fever and Japanese encephalitis, are mosquito-borne (GBD 2015; WHO 2017a). When compared, malaria still accounts for more than 60% of the estimated or reported number of annual cases of mosquito-borne diseases as well as for almost 96% of disease-related deaths which are estimated to sum up to 447,860 fatal cases per year (WHO 2017a). According to this analysis, malaria is still the most frequent mosquito-borne disease worldwide. Especially for the sub-Saharan Africa region, anthropophilic and endophagic anopheline vector mosquitoes currently are of biggest concern (WHO 2014).
Nevertheless, mosquito-borne viruses like West Nile, dengue, chikungunya and Zika viruses are emerging quickly and globally resulting in extreme outbreaks during recent years in known endemic as well as in formerly nonendemic areas followed by introduction either of the disease agent with transmission to indigenous or newly introduced vector mosquito species. The risk of human infection for these viral diseases is primarily associated with vector mosquito-specific behavioural aspects, bionomics and preferred environment. West Nile, dengue, chikungunya and Zika diseases are transmitted either by daytime-biting endophageous Aedes or nocturnal indoor-biting Culex mosquitoes, especially confined to, and prevailing, in an urban environment (WHO 2014). The majority of the globally emerging mosquito-borne viral diseases like dengue, chikungunya and Zika fevers are Aedes-borne, primarily and effectively transmitted by the yellow fever mosquito, Aedes (Stegomyia) aegypti, as well as the Asian tiger mosquito, Ae. albopictus (Stegomyia albopicta) (GBD 2015; WHO 2017b, c, d). Unlike sylvatic Aedes species, these two species are weak flyers and favour anthroponotic environments and urban areas.
Since 2014, major outbreaks of dengue, malaria, chikungunya and yellow fever have been reported in many countries worldwide afflicting billions of people while claiming lives and overwhelming the public health systems in many countries. Since the first detection of autochthonous transmission of chikungunya fever in Saint Martin in December 2013, more than 2.9 million suspected and confirmed cases including 296 deaths have been reported in the Americas until late July 2016 (Yactayo et al. 2016). Following the fulminant rise and spread of chikungunya virus in the new world, Zika virus—primarily transmitted by the very same mosquito vector, Aedes aegypti—raged in South America, the Caribbean and beyond since its first occurrence in Brazil in late 2014 (WHO 2017b, d). Since 2015, hundreds of thousands of cases occur each year in the Americas, linked with clinical—especially neurological—complications like microcephaly and Guillain-Barré syndrome (WHO 2017d). Following its first introduction, the Zika virus’ incredibly high average speed of spread within Brazil was estimated to be 42 km/day or 15,367 km/year (Zinszer et al. 2017).
While the WHO (2017c) calculated 3.2 million cases of disease of which 500,000 are severe leading to 12,500 deaths annually, Bhatt et al. (2013) estimated 390 million new dengue virus infections occurring annually worldwide. According to this data, dengue currently represents the by far most frequent mosquito-borne viral disease in the world thus increasing the public health impact of Aedes mosquitoes (Stanaway et al. 2016). While mosquito-borne viral diseases are currently quickly emerging globally, the number of annual malaria cases and associated deaths reported worldwide are steadily decreasing during recent years with 212 million annual cases and 429,000 deaths from malaria estimated for 2015 (WHO 2016). New studies reveal that rather rare and yet neglected mosquito-borne viruses show a high potential for emerging into the human population on a larger scale, among them Mayaro virus (Hotez and Murray 2017) as well as the Wesselsbron, Usutu and St. Louis encephalitis flaviviruses (Smith 2017).
When analysing the increasing number of vector-borne diseases of public health impact which are currently emerging or resurging worldwide, only few are effectively vaccine-preventable. Chemoprophylactic drugs are available only as a means of secondary prevention for malaria, but multidrug resistance is on the increase and spreading, especially in southeastern Asia and Africa. For this reason, personal protective measures against hematophagous vectors constitute an effective primary prevention method against arthropod-borne diseases in endemic areas (Faulde et al. 2006).
Since decades, the synergistic use of a safe and appropriate skin repellent formulation combined with long-lasting insecticide-treated fabrics, including clothing, tents and netting, is highly recommended for the protection of at-risk personnel exposed to vector arthropod-infested areas (WHO 2001a, b; Faulde and Uedelhoven 2006; Pennetier et al. 2010; Banks et al. 2014). Until recently, combined personal protective measures have been primarily employed individually during occupational-, travel- or leisure time-related exposure in the field. This philosophy changed markedly during the current Zika virus epidemics still occurring in the Americas when the extensive use of N,N-diethyl-meta-toluamide (DEET) skin repellent in combination with clothing, impregnated with the synthetic pyrethroid permethrin, has been strongly recommended as a public health core strategy to mitigate the spread of this disease within the affected human population (Wylie et al. 2016). Following detailed toxicological analyses, this method has been highly recommended by the United States Centers for Disease Control for Zika virus-exposed women, especially during pregnancy, in order to prevent from the devastating neuropathological consequences for foetuses (Wylie et al. 2016).
The aim of this study is to (a) give an overview on current knowledge, growing impact, principles of action and efficacy and (b) to analyse and discuss optimization options for use and development, shortcomings and future research fields of insecticide-treated textiles designed for mosquito bite protection in the public health sector.
12.2 Insecticide-Impregnated Clothing
The use of insecticides for fabric impregnation aiming at disease prevention is not new. Since Napoleonic times, it is well known that soldiers are highly vulnerable to infestations with body lice and, consequently, to epidemics of louse-borne diseases (Faulde 2006; Pagès et al. 2010). First attempts were made during World War II to treat combat uniforms with the newly developed organochlorine-insecticide dichlorodiphenyltrichloroethane (DDT) by using the dipping technique or simply by dusting individuals directly. Primarily, the aim was to control body louse infestations in order to prevent from potentially infected lice bites and mitigate further spread of louse-borne diseases (Faulde 2006). During the 1940s, it has been quickly realized that insecticide-treated clothing also prevent from mosquito, flea and other arthropod vector bites because military populations are frequently deployed to active vector-borne disease foci while experiencing an increased risk of contracting vector-borne diseases (Pagès et al. 2010). During the 1970s, DDT has been banned by many, especially industrial nations which made DDT impregnation of clothing obsolete. Fortunately, the first residual synthetic pyrethroids like permethrin have been developed at that time which had the potential to replace DDT for clothing impregnation purposes until today (Faulde et al. 2003). Because of its highly advantageous properties which include excito-repellency, hot-feet, knockdown, kill, high residual activity, laundering resistance and user safety, the synthetic residual pyrethroid permethrin has been widely used until today as an effective arthropod contact repellent for fabric impregnation (US Armed Forces Pest Management Board 2009; Vaughn et al. 2014; Faulde et al. 2016). Currently, permethrin is the only recommended and used synthetic insecticide for the impregnation of clothing.
To date, different methods for fabric impregnation with permethrin have been developed, all specifically affecting essential parameters like protective efficacy, residual activity, laundering resistance and bioavailability as well as homogeneity of distribution of permethrin molecules. Known and widely used methods are:
-
The absorption method, wherein fabrics are individually treated by dipping or spraying ready-to-use permethrin solutions (Evans et al. 1990; Carnevale and Mouchet 1997).
-
The incorporation method, known also as “Eulanisierung ”, which uses heat and salt gradients to bind permethrin into wool or silk fibres and, most frequently, carpets (Zimmermann and Höcker 1988).
-
The polymer-coating or foularding method, achieved by embedding the permethrin into single or multiple plastic layers by polymerization onto the fabrics prior to the tailoring process (Faulde et al. 2003).
-
The treatment of fabrics by dipping or spraying methods with various types of micro- or nanocapsules containing permethrin (Yao et al. 2015; Forgearini et al. 2016).
-
The permethrin micro-/nanocapsule-enhanced polymer-coating (or foularding) method combining the specific advantages of micro-/nanocapsule and polymer-coating impregnation systems allowing to extremely increase the long-lasting efficacy (Faulde unpublished).
The last three methods have been developed only recently in order to (a) avoid toxicological and logistical shortcomings of self-impregnation while saving the environment from contamination of residues stemming from the manual impregnation process; (b) increase user safety by reducing the migration rate of permethrin, which is embedded in plastic layers and/or micro-/nanocapsules, into the human body; (c) increase long-lasting efficacy by enhancing residual bioactivity and laundering resistance; and (d) avoid inhomogeneity of permethrin surface concentrations which could reduce the protective efficacy. It has to be considered that each impregnation method as well as any changes regarding treatment parameters may directly and substantially affect the delicate balance between the insecticide’s bioactivity, bioavailability, migration rate, surface homogeneity, laundering resistance and residual activity as well as dermal exposure rates, transdermal migration, physiological incorporation and, therefore, user safety and bite protection efficacy (Faulde et al. 2003, 2016).
To date, most studies on impregnated fabrics efficacy have been conducted under laboratory conditions prior to and after laundering or under experimental conditions. The protective effect of impregnated clothes against mosquito bites has, however, been demonstrated in field or near-field conditions in two recent studies (Londono-Renteria et al. 2015; Osborne et al. 2016). Two additional studies have attempted to investigate the protective effect of permethrin-impregnated clothing against mosquito-borne diseases under field conditions in disease-endemic areas (Soto et al. 1995; Most et al. 2017). In a randomized, double-blind study published by Soto et al. (1995), Colombian soldiers wore permethrin-impregnated battle dress uniforms under noncombat conditions during a 4–6 weeks’ period resulting in an incidence reduction rate of 80% against malaria and 78% against cutaneous leishmaniasis. Combat uniforms have been impregnated by a spraying technique which was commercially available on the US American market. Unfortunately, permethrin concentrations, residual activity, bioactivity, laundering and environmental effects were not monitored during this relatively short field study. A more recent investigation performed by Most et al. (2017) analysed the preventive effect of factory-treated long-lasting polymer-coated permethrin-impregnated clothing against malaria infection after military worst-case exposure to high-level disease transmission sites in the rain forest of French Guiana. Between August 2011 and June 2012, 25 personnel wearing impregnated clothing and exposed for 9.5 person-months in hyperendemic foci contracted no cases of malaria, whereas 125 persons wearing untreated uniforms only, exposed for 30.5 person-months, contracted 11 cases of malaria. The use of impregnated clothing significantly protected against malaria by reducing the malaria incidence rate more than 3.4-fold. Impregnated uniforms were laundered up to 218 times. 4.3% of blouses and 13% of trousers showed insufficient protective efficacy when tested according to the standardized licencing algorithm TL 8305-0331 due to permethrin loss associated with frequent laundering, mechanical abrasive processes and weathering. Although the polymer-coating technique allows high-residual long-lasting binding of permethrin onto the fabric, experiences made after long-term worst-case use in hyperendemic disease foci in rain forests revealed the need for an optimized impregnation method allowing an even higher residual activity as well as increased stability against laundering, abrasion and weathering processes (Most et al. 2017). Preliminary data of another recent study among migrant rubber tappers wearing permethrin-impregnated clothing in malarious areas in Myanmar showed a high acceptability among users as well as reduced mosquito biting rates (Crawshaw et al. 2017). No information is yet available concerning the preventive effect against malaria infections under the given study conditions and locations. Furthermore, no data is yet available on the protective efficacy of long-lasting permethrin-impregnated clothing against Ae. aegypti- and Ae. albopictus-borne viral diseases like dengue, chikungunya and Zika fevers during exposure in hyperendemic areas or during epidemics in the field.
12.3 Mode of Action of Insecticides and Repellents Used for Textile Impregnation
Until today, five chemical classes of synthetic insecticides are used to control adult mosquito vectors within the public health sector: organochlorines, organophosphates, carbamates, pyrethroids and neonicotinoids. All of them are acting as acute neurotoxins by either targeting the acetylcholinesterase (organophosphates, carbamates), the nicotinic acetylcholine receptor (neonicotinoids) or the voltage-gated sodium channels (pyrethroids, organochlorides) within the neuronal system. Insecticides usually affect—to a higher or lesser extent—the neurologic system of both target animals like insects and other arthropods and vertebrate nontarget animals including man. The higher the toxic effect of an insecticide to target animals and the lower its toxic impact to humans and other nontarget organisms, the higher its selectivity and, consequently, safety for the user and the environment. Generally, organochlorides, organophosphates and carbamates show a lower selectivity rate whereas pyrethroids and neonicotinoids are characterized by a higher selectivity (=safety) rate. These characteristics principally make pyrethroids and neonicotinoids excellent candidates for the safe use of impregnated textiles worn close to or directly on the human body. Unlike the “old” pyrethroids which have been developed in the 1970s, the newest class of insecticides, the neonicotinoids, are currently discussed controversially due to their accumulating toxic effects in the environment. It has been proven that neonicotinoid use is strongly linked to the currently widely experienced honeybee population devastation while simultaneously affecting many other pollinators (Lundin et al. 2015). It is, therefore, unclear whether licencing of neonicotinoids will be extended in the future and whether they will remain commercially available for textile impregnation purposes on the European and/or American market.
Consequently, ester-, non-ester and α-cyano pyrethroids are used most widely for the impregnation of textiles, especially long-lasting insecticide-impregnated bed nets (Faulde et al. 2012). Among them, permethrin, etofenprox, deltamethrin, cyfluthrin, λ-cyhalothrin and α-cypermethrin are recommended by the WHO for the treatment of mosquito nets at substance-specific application concentrations (WHO 2013), and to date, permethrin remains the only recommended pyrethroid for clothing impregnation including mattresses (US Armed Forces Pest Management Board 2009; Vaughn et al. 2014; Faulde et al. 2016).
Usually, arthropods try to actively avoid direct exposure to contact pyrethroids by showing a so-called “hot-feet” effect including an enhanced motility reaction (Hoffmann 1995). The resulting excito-repellency effect is finally leading to reduced landing, probing and biting behaviour by vector mosquitoes on pyrethroid-treated clothing when using the arm-in-cage test (Faulde et al. 2012; DeRaedt Banks et al. 2015). However, when blood feeding is easily possible under laboratory conditions, e.g. bites through pyrethroid-impregnated bed net fabric characterized by a smaller or larger mesh size which has been wrapped around the forearm in order to expose the skin, mosquitoes readily feed on commercially available LLITNs despite direct contact to and intoxication with insecticides as well as excito-repellency effects (Faulde et al. 2012). For example, Fig. 12.1a shows Ae. aegypti mosquitoes biting through newly purchased, unlaundered PermaNet 2.0® bed net fabric treated with 55 mg permethrin/m2, whereas Fig. 12.1b depicts this effect against the Conmanet® impregnated with 25 mg deltamethrin/m2, and Fig. 12.1c shows an own polymer-coated research net containing 2000 mg etofenprox/m2, all tested against the negative control (Fig. 12.1d) using the arm-in-cage test. Although excitatory behaviour of the test mosquitoes was documented on insecticide-treated netting, the time of exposure to the insecticide during the complete feeding process, which takes between 150 and 329 s (mean, 240 s) (Gillett 1967), was long enough to allow >95% mortality of all fed mosquitoes within the following 24 h when using the etofenprox-treated fabric. Although containing tenfold of the WHO-recommended etofenprox concentration of 200 mg/m2 landing, probing and biting count of test mosquitoes did not differ from that of the negative control (Fig. 12.1c, d) (Faulde et al. 2012). Obviously, direct access to the skin as well as successful probing and blood feeding overcame the excito-repellency effect of insecticides when topically exposed in a test cage which finally lead to an increased exposure to contact biocides together with increased mortality in test mosquitoes.
Arm-in-cage tests showing landing, probing and biting/feeding behaviour of Aedes aegypti mosquitoes exposed to two brands of commercially available LLITNs and one type of research textile vs. negative control after 2 min of exposure: (a) PermaNet 2.0®; (b) Conmanet®; (c) research fabric treated with 2000 mg etofenprox/m2; (d) untreated fabric (negative control)
New innovative insecticide combinations and treatment methods are necessary in order to overcome the growing lack of protective effect of long-lasting insecticide-treated bed nets (LLITNs) in geographic areas where mosquito resistance mechanisms are prevalent. Insecticide class-specific resistance mechanisms against pyrethroids include knockdown resistance mechanisms, caused by kdr and ace-1R gene mutations, as well as increased production of detoxification enzymes (Marius et al. 2017). In order to neutralize resistance mechanisms, three different main approaches are currently favoured which are (a) fabric impregnation with insecticide classes, e.g. carbamates, organochlorides or neonicotinoids, which overcome pyrethroid-specific resistances (Guillet et al. 2001), (b) the simultaneous use of the pyrethroid synergist piperonyl butoxide (PBO) (Marius et al. 2017), and (c) the combined use of pyrethroid contact insecticides together with skin repellents, like N,N-diethyl-m-toluamide (DEET) or insect repellent 3535 (IR3535) (Faulde and Nehring 2012).
Like residual pyrethroids, carbamates act as contact insecticides due to their low volatility. Furthermore, carbamates are known to be extremely effective in areas where highly pyrethroid-(kdr-) resistant vector mosquitoes are endemic (Djénontin et al. 2009). Carbamates used on bed nets or tarpaulins were carbosulfan at a concentration of 300 mg/m2 as well as bendiocarb at 100 and 200 mg/m2 (Guillet et al. 2001; Djénontin et al. 2009). Although highly effective against pyrethroid-resistant mosquitoes, carbamates show—when compared to pyrethroids—on average a markedly higher acute human toxicity and a considerably lower selectivity rate. Both parameters directly affect the user safety. Consequently, about 20% of sleepers in that study reported potential toxicological side effects, like headache and sneezing, when using a carbosulfan-treated bed net (Guillet et al. 2001). Due to the toxicological impact for human health combined with a widely occurring ace-1R mutation associated with carbamate and organophosphate resistance, long-lasting carbamate-impregnated bed nets are currently neither available commercially nor recommended for use.
Another new attempt is to impregnate fabrics with a PBO-pyrethroid combination in order to increase the effectiveness of LLITNs in areas characterized by simultaneously occurring kdr- and ace-1R mosquito resistance mechanisms as well as detoxification enzyme upscaling. PBO is a well-known synergist which is widely used in combination with pyrethroids for insect pest control. The primary mode of action of the methylenedioxyphenyl compound, PBO, is to inhibit P-450 monooxygenase enzymes, also known as the mixed-function oxidase system (MFO) . The MFO system is the main route of detoxification in insects and causes the oxidative breakdown of insecticides such as natural pyrethrins as well as chemically altered synthetic pyrethroids. Consequently, when PBO is added to synthetic pyrethroids, higher insecticide levels remain in the insect body which enhance their lethal effect (Moores et al. 2009). Different brands of new generation LLITNs, coated with pyrethroids and PBO, are now available on the market. According to a recent study, Olyset Plus®, coated with 2% (w/w) permethrin and 1% (w/w) PBO, and PermaNet 3.0® treated with 2.8 g/kg ± 25% deltamethrin plus 4 g/kg ± 25% PBO both showed better protective efficacy when compared to conventional LLITNs in malarious areas with a high vector mosquito resistance level in Benin (Marius et al. 2017).
In order to increase the protective effect of LLITNs in geographic areas where mosquito resistance mechanisms are widely occurring, the additional use of effective skin repellents is considered essential and is strongly recommended in order to ameliorate bite protection (WHO 2001a; Norris and Coats 2017). Primarily, licenced synthetic and safe skin repellent compounds like DEET, IR3535, KBR 3023/Picaridin/Icaridin/Saltidin or para-menthane-3,8-diol (PMD) are widely in use, known for their high repellent activity against mosquitoes, user friendly and are considered toxicologically safe (WHO 2001a, b; Faulde 2010). Many efforts have been undertaken in the past to treat clothing, bed nets and tents with skin repellents, especially with DEET, by using the spraying or dipping technique (Faulde 2010). Because molecules of skin repellents are usually characterized by a high natural vapour pressure, the active compound evaporates freely into the atmosphere where the chemicals can be smelled and avoided by mosquitoes. Therefore, skin repellents are characterized by a spatial activity (Faulde 2010; Norris and Coats 2017). Consequently, even when used at higher concentrations, the average bite protection time does not exceed 6–8 h when applied onto the skin or fabric due to complete evaporation of the molecules into the atmosphere (Fei and Xin 2007; Faulde et al. 2010). Durability of the repellent effect could be enhanced by employing microencapsulated DEET on cotton fabric. During graft copolymerization of butyl acrylate onto chitosan in an aqueous solution, resulting DEET microcapsules revealed 100% repellency for 8 h, including a partially preserved repellent activity for up to 48 h (Fei and Xin 2007). Another method to produce LLRTNs has been developed by binding DEET, or IR3535, onto fibres of bed net fabric using the polymer-coating technique (Faulde et al. 2010). By polymerizing multilayers onto the fibres in which the repellent molecules have been embedded within the plastic coating, extremely high DEET and IR3535 concentrations >10 g/m2 could be obtained. One hundred per cent repellency, measured by complete landing, probing and biting protection using the arm-in-case test, could be achieved at DEET concentrations >3.7 g/m2 (Fig. 12.2a) as well as for IR3535 contents >10 g/m2 (Abb. 2b) (Faulde et al. 2010). One hundred per cent landing, probing and biting protection could be achieved with DEET-impregnated fabrics for 29 weeks at an initial concentration of 4.66 g/m2, 54 weeks at 8.8 g/m2, 58 weeks at 9.96 g/m2 and 61 weeks at 10.48 g/m2 as well as for 23 weeks using IR3535-coated fabric at a concentration of 10.02 g/m2 (Faulde et al. 2010). In spite of the highly promising long-term protective efficacy of DEET and IR3535 polymer-coated onto bed net fabrics, laundering stability was, unfortunately, extremely low, resulting in a loss of the 100% repellency in treated clothing after the first washing process according to EN ISO 6330:2000 (International Organization for Standardization 2012), thus making its use for cloths obsolete in case frequent launderings are necessary (Faulde unpublished).
Arm-in-cage tests showing long-lasting complete (100%) landing, probing and biting/feeding protection against Aedes aegypti mosquitoes after 5 min of exposure using skin repellents DEET and IR3535 together with permethrin and etofenprox, all polymer-coated onto textiles: (a) textile containing 3590 mg/m2 DEET plus 1208 mg/m2 permethrin; (b) bed net fabric containing 5930 mg/m2 IR3535 plus 2139 mg/m2 etofenprox. Notice landing and probing behaviour on the bite-protecting glove, serving as host-seeking control
Besides behavioural aspects including spatial repellency, insecticidal effects of the skin repellents DEET, IR3535 and KBR 3023 have been detected when tested against Ae. aegypti (Licciardi et al. 2006; Pridgeon et al. 2009; Faulde et al. 2010). Obviously, these skin repellents do not behave like a homogeneous class of compounds expressing a single mode of toxic action. DEET, for example, exhibits more complex insecticidal properties when compared with the other chemicals. Although their detailed molecular and physiological mechanisms of action remained unknown, toxicological studies revealed that the mode of action of skin repellents differs markedly from that of pyrethroids and other insecticide classes (Licciardi et al. 2006; Faulde et al. 2010). Figure 12.3a shows the knockdown and kill effects of fabric impregnated with 0.5 g DEET/m2 after 120 min of exposure against Ae. aegypti mosquitoes following continuous contact exposure (Fig. 12.3b) using the cone test. Synergistic insecticidal and repellent effects have been documented when testing combined pyrethroid and repellent-impregnated fabrics impregnated by the long-lasting polymer-coating multilayer technique (Faulde and Nehring 2012). In this study, the ester pyrethroid permethrin and the non-ester pyrethroid etofenprox have been cotreated with either DEET or IR3535 at different concentrations which depended on the number of heterogenic, substance-embedding binding layers polymerized. When using the arm-in-cage test against Ae. aegypti, 100% probing and biting protection was preserved for 83 weeks with the 5930 mg DEET/m2 combined with 2139 mg etofenprox/m2 fabric, for 72 weeks with the 5002 mg DEET and 2349 mg etofenprox/m2 material, for 63 weeks with the 3590 mg DEET/m2 and 1208 mg permethrin/m2 tissue and for 61 weeks with the 4711 mg DEET/m2 and 702 mg etofenprox/m2 fabric (Faulde and Nehring 2012). Simultaneously, an up to 75% quicker contact toxicity of repellent pyrethroid fabrics was documented when compared to the corresponding pyrethroid-specific contact toxicity alone using the WHO cone test (WHO 2013) including continuous forced contact of test mosquitoes (Faulde and Nehring 2012; Marius et al. 2017). Obviously, this novel method represents a highly promising approach because (1) insecticide-repellent combinations on fabric are shown to be highly effective against kdr and ace-1R mutation-containing mosquitoes (Pennetier et al. 2010) and (2) host-seeking mosquitoes come into close contact with the evaporated spatial skin repellent molecules, evidently leading to an acute intoxication due to the insecticidal properties of the skin repellents DEET and IR3535 (Pennetier et al. 2010; Faulde and Nehring 2012). More research work is needed in this area in order to further analyse the effectiveness against mosquito-borne diseases, entomological aspects regarding behavioural, physiological and genetic resistances in vector mosquitoes, epidemiological aspects as well as user safety. Although considered as being very promising, long-lasting insecticide-repellent-treated fabrics, including bed nets and clothing, are not yet commercially available.
12.4 Entomological, Behavioural and Epidemiological Aspects of Insecticide-Treated Textiles
In order to optimize the protective efficacy of insecticide-treated textiles and for the development of synergistic combinations of available personal protective measures against mosquito bites, it is essential to precisely know the species-specific bionomics as well as host finding and feeding behaviour of the relevant mosquito vectors abundant in a given region or ecotope. Since only anthropophilic (species primarily biting man) and anthropozoophilic (species biting humans as well as vertebrate and other animals) mosquito vectors are of special public health concern and importance, less relevant zoophilic disease agent-carrying mosquitoes have been neglected in the context of this study. Furthermore, it is essential to differentiate between indoor (endophilic) biters and those species, which exclusively bite outdoors (exophilic) while never or very rarely entering human dwellings. Additionally, it has to be taken into special consideration whether the vector species of interest are daytime or night-time (nocturnal) biters or follow another circadian rhythm, e.g. biting activities exclusively during dusk, dawn or high noon. In general, Aedes mosquitoes are daytime biters, whereas Culex and Anopheles mosquitoes are primarily feeding during the night-time. Consequently, LLITN use aims at preventing from bites of nocturnal, indoor-biting mosquitoes of the genera Culex and Anopheles particularly and is, therefore, a key element in roll-back programmes, e.g. against malaria or lymphatic filariosis. Vice versa, LLITN use (a) prevents sleeping healthy humans from infectious bite of nocturnal indoor biters in human dwellings and (b) protects noninfected mosquitoes from indoor infection during blood feeding on sick as well as bedridden persons who contracted a mosquito-borne disease. Unlike LLITNs, insecticide-impregnated clothing protects from mosquito bite at any time at any place and is, therefore, a highly effective means of prevention whenever and wherever the wearer is active outside human dwellings in any urban, rural or sylvatic environment or in buildings while not sleeping under a LLITN.
Like LLITNs, permethrin-impregnated clothing may protect from mosquito bite and can, as well, reduce transmission potential and speed in case asymptomatic viremic human disease reservoirs are exposed to susceptible but not yet infected mosquito vector species. This effect may contribute to a disruption of the transmission chain and is discussed as an option for public health protection (Wylie et al. 2016; Most et al. 2017). Due to a differing genus- and species-specific susceptibility of mosquitoes to insecticides, concentration-dependent permethrin-related excito-repellency, knockdown and kill effects were highest in Aedes mosquitoes, followed by Anopheles and, finally, Culex species (Faulde et al. 2016). Consequently, the protective effect of permethrin-impregnated clothing should be as follows: Aedes-borne diseases > Anopheles-borne diseases ≫ Culex-borne diseases (Faulde et al. 2016).
Interestingly, long-term insecticide exposure may also trigger behavioural changes in mosquitoes which may include change of bionomics, circadian rhythm, sensing of toxic compounds as well as avoiding insecticide-contaminated or insecticide-treated surfaces. In the past, behavioural changes affecting circadian activity and host-finding mechanisms as well as adaption to an altered environment have been well described in anopheline malaria vectors. For example, An. gambiae sensu stricto (s.s), one of the most effective African chief vectors of malaria, became more exophilic after introduction of LLITNs in Kenya, whereas it was previously known to be exclusively endophagic (Githeko et al. 1996). Despite its new exophilic behavioural shift, An. gambiae s.s. remained highly anthropophilic which increased the overall malaria transmission rate in this area. Following introduction of LLITNs, the most commonly observed effect in An. funestus has been a markedly increased exophilic behaviour including a trophic deviation to cattle (Russell et al. 2011). While An. funestus populations disappeared in some African regions following introduction of LLITNs (Sokhna et al. 2013), this species developed an entirely new strategy, e.g. in Benin (Moiroux et al. 2012). The formerly nocturnal An. funestus strains became more and more daytime biters, as >26% were caught during daylight between 6 a.m. and 9 a.m. (Moiroux et al. 2012). In a more recent study, LLITN use induced a significant behavioural change in anopheline mosquitoes after the third year of use resulting in an activity pattern and host-seeking shift to earlier hours of the evening (Thomsen et al. 2017). Earlier mosquito biting activity during the evening, which happened outside the regular human sleeping period, was linked to an elevated anopheline biting rate outdoors and indoors. As a consequence, LLITN users as well as non-bed net users experienced an increased level of malaria transmission when compared with the conditions prior to the LLITN intervention (Thomsen et al. 2017). In this case, the simultaneous use of permethrin-impregnated clothing could have contributed to enhanced personal protection effectiveness from mosquito bites under the given environmental and epidemiological conditions.
Besides insecticide exposure, anthropogenic environmental changes may also lead to a remarkable chance in mosquito circadian activity and host-seeking behaviour. In India, the usually daytime-active yellow fever mosquito, Ae. aegypti, is currently becoming more and more nocturnal while expanding its activity period late into the evening and night-times (ProMED 2017). Obviously, ubiquitous ambient light sources together with higher urban environment-specific night-time temperatures have altered Ae. aegypti’s biting habits. Nowadays, dengue, chikungunya and other viral fevers are more frequently transmitted during the night-time outdoors and indoors in this area, making LLITN use more and more effective against Aedes-borne diseases (ProMED 2017).
LLITNs, either impregnated with PBO-synergized permethrin or deltamethrin or treated with these pyrethroids alone, do not reduce entry rates of anopheline vector mosquitoes. These results indicate that both synergized and non-synergized pyrethroid-treated textiles do not show a spatial repellent effect due to a missing vapour pressure of this class of contact insecticides (Spitzen et al. 2017). Nevertheless, sublethal toxic effects of pyrethroid-treated bed nets to mosquitoes reduced the number of mosquitoes re-entering the house. Although this insecticide exposure-avoiding mosquito behaviour may be advantageous for LLITN users, malaria transmission may more frequently affect neighbouring, unprotected houses (Spitzen et al. 2017).
12.5 Standardized Efficacy Testing and User Safety
Historically, only military fabrics were impregnated with insecticides by dipping or spraying, but the residual activity was short. In recent years, many armies have developed their own long-lasting impregnated battle dress uniforms using different types of impregnation methods on various kinds of fabric. Although excellent testing guidelines exist for LLITNs (WHO 2013), no World Health Organization Pesticides Evaluation Scheme (WHOPES) or other national or public health guidelines exist for the standardized testing and licencing of insecticide-treated clothing. When taking into account that permethrin-impregnated clothing is widely commercially available in the civilian market since more than a decade, this capability gap strongly deserves consideration in the near future (Faulde et al. 2016).
In order to guarantee that wearing the permethrin-impregnated fabric is protective and safe, both the initial concentration and release rate of permethrin should be monitored through an appropriate quality assurance procedure during the production process, as recommended by the German Federal Institute for Risk Assessments (GFIFRA) (Appel et al. 2008). This is why different internal testing and licencing specifications have been developed among national forces and agencies. In order to ensure that manufacturers fully comply with minimum quality requirements, especially those concerning protective efficacy and user safety, the German Armed Forces (Bundeswehr) implemented the standardized testing and licencing algorithm TL 8305-0331 (WIWeB 2016). Launched in 2002, this standard has been revised to accord with increased technical and scientific knowledge or specific force health protection needs during military deployments. Currently, a first attempt for national standardization of impregnated clothing has been undertaken by the Dutch standardization office by setting up the NEN 8333 “protective clothing—clothing that supports the protection against ticks and is industrially treated with permethrin” (NEN 2017). The national implementation of this norm is planned for early 2018.
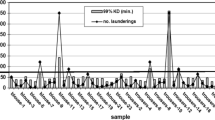
In order to analyse the brand-specific characteristics and possible heterogeneity of commercially available permethrin-impregnated clothing on the international market, a selection of widely used products needs to be investigated and compared in detail. In a comparison of the residual bioactivities and laundering resistances of five commercially available, factory-treated permethrin-impregnated fabrics designed for the prevention of mosquito-borne diseases, an extremely high variability in initial permethrin concentrations, residual bioactivity and permethrin loss during laundering was observed (Faulde et al. 2016). The resulting data indicate that only one of the examined products completely met all the necessary efficacy and safety requirements defined by TL 8305-0331 (Faulde et al. 2016). Because of the lack of mandatory international testing and licencing procedures, industrial producers of impregnated clothing generally do not inform on specific impregnation techniques employed, initial insecticide concentrations, arthropod toxicity, residual activity, laundering resistance or durability of the insecticidal ingredient. It was, therefore, interesting to observe that two products (40%) exceeded the initial maximum permethrin concentration of 1300 ± 300 mg/m2 recommended according to the current risk assessments for human safety (Appel et al. 2008), showing an extremely high permethrin concentration ≥4000 mg/m2. One product exhibited a residual permethrin concentration of 1800 mg/m2 which exceeded the maximally recommended concentration even after 100 standardized machine launderings according to EN ISO6330:2000. When compared with the initial concentrations, the percentage permethrin loss, following 100 launderings was extremely diverse and ranged from 58.14 to 98.46% (Faulde et al. 2016). Chiefly, the higher the binding capacity, or fixation rate, of permethrin fixed onto the fibres was, the lower was the permethrin concentration-dependent bioactivity when investigated against arthropods. Experiences made in this study indicate that a certain impregnation method-related optimal equilibrium has to be identified in order to maximize bioactivity, bioavailability, insecticide stability and protective efficacy of a permethrin-impregnated fabric. Furthermore, the residual permethrin concentration found after 100 launderings was considerably low in two products which showed less than 40 mg/m2. Too low and potentially sublethal permethrin concentrations on clothing of ≤200 mg/m2 should be avoided because (a) the corresponding bioactivity falls short of the minimum protective efficacy needed, (b) undesired behavioural changes in arthropod vectors may occur including stimulation or acceleration of attachment of ticks and (c) genetic, physiological or behavioural resistance mechanisms can be triggered (Most et al. 2017).
Depending on the information needed, three main laboratory test systems have been reported for efficacy testing of insecticide-treated textiles: the tunnel test for bed net testing, the cone test and the arm-in-cage test (WHO 2013; DeRaedt Banks et al. 2015; Marius et al. 2017; Most et al. 2017). The use of the tunnel test is recommended by WHO in order to test LLITNs for mortality and blood-feeding success of host-seeking mosquitoes (WHO 2013). As blood host, an animal as bait (usually a guinea pig or a rabbit) is exposed against test mosquitoes in a choice box simulating human bait.
The cone test (Fig. 12.4) measures acute toxicological aspects of test animals exposed for a defined time frame, or continuously, to a contaminated surface. When analysing toxic effects of contact insecticides like pyrethroids, carbamates or organochlorides, direct exposure of test animals is essential. In contrast, substances like organophosphate insecticides as well as arthropod repellents—characterized by a more or less pronounced vapour pressure—affect the health of test animals not only by direct body contact to the treated surface but additionally due to the atmosphere-borne incorporation of molecules which evaporated into the aerosphere. Consequently, forced contact to insecticide-contaminated test surfaces is requisite especially when examined against flying test arthropods like mosquitoes which can actively avoid contact with chemicals (Fig. 12.3b). In case direct exposure of test animals to contaminated surfaces is not carried out continuously, it is absolutely necessary to exactly define the sum of time of exposure required in order to detect the desired insect-specific toxicological actions in detail, like hot-feed, knockdown, excito-repellency or kill effects. As an example, an exact contact exposure time of 3 min is given for test mosquitoes employed in the WHO cone test bioassay set up for standardized LLITN testing (WHO 2013). When compared with Anopheles and Culex vector mosquito species recommended for testing purposes, the yellow fever mosquito, Aedes aegypti, has been identified as the most suitable biosensor for testing the toxicological effects of pyrethroids on fabrics, especially when monitoring for low doses or cut-off values of less residual impregnation methods (Faulde et al. 2016; Osborne et al. 2016).
The arm-in-cage test has been designed to analyse and monitor the spatial effects of skin repellents (Masetti and Maini 2006). Non-blood-fed test mosquitoes, reared in a cage, were directly exposed to treated (positive control) vs. untreated (negative control) skin of human or animal bait. This system has been also used to measure the spatial repellent effects of textiles impregnated with skin repellents (Faulde et al. 2010). Besides spatial repellency testing, the arm-in-cage test can be modified in order to measure excito-repellency effects, including landing counts, probing activity and blood feeding of pyrethroid-treated textiles (Fig. 12.5) wrapped around the forearm of a human volunteer or around test animals (Faulde et al. 2012; DeRaedt Banks et al. 2015). This test system can also be employed for investigations aiming at further determination whether a clothing fabric is mosquito bite-proof due to mechanical characteristics, including special knitting techniques, internal semipermeable membrane layers or just thickness of the fabric exceeding the length of the mosquito proboscis. For example, in Fig. 12.6, bite-proof combat gloves are quality-tested against Ae. aegypti using the arm-in-cage test.
In order to guarantee user safety, permethrin concentration on clothing is limited to 1250 mg/m2 according to the US Environmental Protection Agency (Young and Evans 1998; US Armed Forces Pest Management Board 2009) or to 1300 ± 300 mg/m2 by the GFIFRA (Appel et al. 2008), respectively. GFIFRA performed a toxicological risk assessment following human biomonitoring investigations among users of the German Federal Armed Forces battle dress uniforms (BDUs) as well as on the BDU itself including toxicological studies regarding permethrin concentration, migration rates and exposure criteria (Appel et al. 2008). It has been concluded that normal use of BDUs, impregnated by a defined polymer-coating technique at a concentration of 1300 mg permethrin/m2 with a 25:75 cis/trans ratio, would not affect human health while ensuring sufficient protection against bites of arthropod vectors (Appel et al. 2008). Corresponding data are not yet available when permethrin treatment has been carried out by spraying or dipping techniques. Additionally, GFIFRA recommended monitoring the maximum permethrin concentration as well as its binding method-specific release rate through an appropriate quality assurance procedure during the production process in order to assure that wearing impregnated clothing is both protective and safe (Appel et al. 2008). Human biomonitoring studies using Bundeswehr BDUs revealed that permethrin metabolites increased approximately 200-fold during an 8-h workday when compared with the negative control group. When analysed quantitatively, the permethrin content migrated into the human corresponded to roughly 20% of the acceptable daily intake (ADI) of 0.05 mg/kg body weight per day (Appel et al. 2008). During military deployment when wearing impregnated BDU on average 16 h per day, the quantitatively measured permethrin concentration reached approximately 40% of the ADI (Zimmer and Faulde 2009). Consequently, continuous use of newly impregnated BDUs under worst-case scenarios would theoretically lead to a maximum incorporation rate of 60% of the ADI and is, therefore, considered safe. It is yet unclear in how far markedly varying impregnation technique-dependent migration and release rates of permethrin may affect the ADI value during worst-case use, because the permethrin release rate of dipping or spraying methods doubled when compared to the polymer-coating technique and the cross-contamination rate during storage increased 3.5- to 10-fold, respectively (Faulde et al. 2006).
Concurrent with the ongoing Zika virus epidemics in the Americas which showed devastating consequences for foetuses and newborns during prenatal virus infection, the urgent need to optimize personal protection of pregnant women against infected mosquito bite has been identified. Since the synergistic combination of skin repellents together with permethrin-impregnated clothing has been considered as most suitable option, the safety and toxicity of DEET and permethrin have been re-evaluated, especially for its combined use during pregnancy (Wylie et al. 2016). Results obtained led to the strong recommendation that pregnant women should treat their clothing with registered products containing the excito-repellent permethrin which should not be applied directly to the skin (Wylie et al. 2016). Furthermore, the WHO considers permethrin use as being compatible with breastfeeding (WHO 2002). Additionally, a new study published by GFIFRA documented that allergies, linked to sensitization of users by permethrin which has been impregnated on textiles, are highly unlikely (BfR 2017). It has been, furthermore, determined that the systemic cancerogenic effect of permethrin-treated textile use is negligible (BfR 2017).
12.6 Conclusions
Besides chemoprophylactic regimens, personal protective measures against mosquito bites, especially the use of long-lasting factory-treated permethrin-impregnated clothing, may prevent both individual infection as well as the further spread of mosquito-borne diseases of public health concern. Furthermore, it is highly recommended to employ long-lasting impregnation methods exclusively, while aiming at initial maximum permethrin concentrations ≤1300 ± 300 mg/m2 and remaining minimum residual permethrin concentrations ≥200 mg permethrin/m2 during field use in order to prevent from possible adverse health effects for users and to ensure sufficient protective efficacy while simultaneously avoiding undesired behavioural side effects and/or pyrethroid resistance in arthropod disease vectors. New and improved strategies for personal protection from bites of hematophagous vector mosquitoes are urgently needed, especially in the light of increasing insecticide resistances including genetic (kdr- and ace-1R), physiological and behavioural resistance mechanisms. One new attempt, the long-lasting textiles containing a combination of spatial skin repellents, characterized by spatial insecticidal properties, together with excito-repellent and safe contact insecticides, like the pyrethroid permethrin, definitely deserve special consideration and future research work.
In concordance with the EU Biocides Regulation 528/2012, it is strongly recommended that manufacturers of impregnated clothing provide data on concentrations, migration rates, homogeneity on impregnated fabrics, protective efficacy and laundering resistance of the insecticide used for their products. This information is critical to designing safe and effective personal protection products which especially ensures secure use for children and during pregnancy. Although the polymer-coating method has been shown to provide excellent laundering resistance, residual stability and long-term efficacy, current experiences made point out the need for further improvements in residual permethrin-binding techniques and bioavailability (speed of toxic action depending on molecule diffusion processes), especially when garments are intended for long-term worst-case field use.
Long-lasting factory-based polymer-coated permethrin-impregnated clothing provided excellent protection against bites of infectious anopheline mosquitoes, thereby reducing malaria incidence rates significantly in high-transmission foci. Due to a documented higher susceptibility of Aedes mosquitoes to permethrin, it can be further concluded that the protection rates against Ae. aegypti- or Ae. albopictus-borne diseases are equivalent or even higher. In the light of available research data, long-lasting permethrin-impregnated clothing is highly recommended for personal protection against mosquito-borne diseases of public health importance, including chikungunya, dengue, West Nile and Zika fevers, all of which are currently resurging globally.
References
Marius A, Gnanguenon V, Yovogan B, Akinro B, Anagonou R, Agossa F, Houtoukpe A, Padonou GG, Akogbeto M (2017) WHO cone bio-assays of classical and new-generation long-lasting insecticidal nets call for innovative insecticides targeting the knock-down resistance mechanism in Benin. Malar J 16:77
Appel KE, Gundert-Remy U, Fischer H, Faulde M, Mross KG, Letzel S, Rossbach B (2008) Risk assessment of Bundeswehr (German Federal Armed Forces) permethrin-impregnated battle dress uniforms (BDU). Int J Hyg Environ Health 211:88–104
Banks SD, Murray N, Wilder-Smith A, Logan JG (2014) Insecticide-treated clothes for the control of vector-borne diseases: a review on effectiveness and safety. Med Vet Entomol 28(S1):14–25
BfR - Bundesinstitut für Risikobewertung (2017) Allergien: Sensibilisierung durch Permethrin in Textilien ist unwahrscheinlich. https://doi.org/10.17590/20170510-135,840. http://www.bfr.bund.de/cm/343/allergien-sensibilisierung-durch-permethrin-in-textilien-ist-unwahrscheinlich.pdf
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496:504–507
Carnevale P, Mouchet J (1997) La protection individuelle contre les insectes vecteurs. Med Trop 57:505–510
Crawshaw AF, Maung TM, Shafique M, Sint N, Nicholas S, Li MS, Roca-Feltrer A, Hii J (2017) Acceptability of insecticide-treated clothing for malaria prevention among migrant rubber tappers in Myanmar: a cluster-randomized non-inferiority crossover trial. Malar J 16(1):92
DeRaedt Banks S, Orsborne J, Gezan SA, Kaur H, Wilder-Smith A, Lindsey SW, Logan JG (2015) Permethrin-treated clothing as protection against the dengue vector, Aedes aegypti: extent and duration of protection. PLoS Negl Trop Dis 9(19):e0004109
Djènontin A, Chabi J, Baldet T, Irish S, Pennetier C, Hougard JM, Corbel V, Akogbéto M, Chandre F (2009) Managing insecticide resistance in malaria vectors by combining carbamate-treated plastic wall sheeting and pyrethroid-treated bed nets. Malar J 8:233
Evans SR, Korch GW Jr, Lawson MA (1990) Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol 27:829–834
Faulde MK, Uedelhoven WM, Robbins RG (2003) Contact toxicity and residual activity of different permethrin-based fabric impregnation methods for Aedes aegypti (Diptera: Culicidae), Ixodes ricinus (Acari: Ixodidae), and Lepisma saccharina (Thysanura: Lepismatidae). J Med Entomol 40:935–941
Faulde M, Uedelhoven W (2006) A new clothing impregnation method for personal protection against ticks and biting insects. Int J Med Microbiol 296(Suppl 1):225–229
Faulde MK, Uedelhoven WM, Malerius M, Robbins RG (2006) Factory-based permethrin impregnation of uniforms: residual activity against Aedes aegypti and Ixodes ricinus in battle dress uniforms worn under field conditions, and cross contamination during the laundering and storage process. Mil Med 171(6):472–477
Faulde M (2006) Emergence of vector-borne diseases during war and conflict. Med Corps Int 26(1):4–14
Faulde M (2010) Insektizide, Akarizide und Repellenzien. In: Aspöck H (ed) Krankheiten durch Arthropoden. Denisia-Verlag, Wien. Denisia 30:109–122.
Faulde MK, Albiez G, Nehring O (2010) Insecticidal, acaricidal and repellent effects of DEET- and IR3535-impregnated bed nets using a novel long-lasting polymer-coating technique. Parasitol Res 106(4):957–965
Faulde MK, Nehring O (2012) Synergistic insecticidal and repellent effects of combined pyrethroid and repellent-impregnated bed nets using a novel long-lasting polymer-coating multi-layer technique. Parasitol Res 111(2):755–765
Faulde M, Albiez G, Nehring O (2012) Novel long-lasting impregnation technique transferred from clothing to bed nets: extended efficacy and residual activity of different pyrethroids against Aedes aegypti as shown by EN ISO 6330-standardized machine laundering. Parasitol Res 110(6):2341–2350
Faulde MK, Pagès F, Uedelhoven W (2016) Bioactivity and laundering resistance of five commercially available, factory-treated permethrin-impregnated fabrics for the prevention of mosquito-borne diseases: the need for a standardized testing and licensing procedure. Parasitol Res 115(4):1573–1582
Fei B, Xin JH (2007) N,N-diethyl-m-toluamide-containing microcapsules for bio-cloth finishing. Am J Trop Med Hyg 77:52–57
Forgearini JC, Michalowski CB, Assumpção E, Pohlmann AR, Guterres SS (2016) Development of an insect repellent spray for textile based on permethrin-loaded lipid-core nanocapsules. J Nanosci Nanotechnol 16(2):1301–1309
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602
Gillett JD (1967) Natural selection and feeding speed in a blood-sucking insect. Proc R Soc Lond B Biol Sci 167(1008):316–329
Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA (1996) Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol 82(3):306–315
Guillet P, N’Guessan R, Darriet F, Traore-Lamizana M, Chandre F, Carnevale P (2001) Combined pyrethroid and carbamate ‘two-in-one’ treated mosquito nets: field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med Vet Entomol 15(1):105–112
Hoffmann G (1995) Wirkung, Einsatzgebiete und Erfordernis von Pyrethroiden im nicht-agrarischen Bereich. Bundesgesundheitsblatt 38:294–303
Hotez PJ, Murray KO (2017) Dengue, West Nile virus, chikungunya, Zika-and now Mayaro? PLoS Negl Trop Dis 11(8):e0005462
International Organization for Standardization (ISO) (2012) Domestic washing and drying procedures for textile testing. EN ISO 6330:2012. www.iso.org
Licciardi S, Herve JP, Darriet F, Hougard J-M, Corbel V (2006) Lethal and behavioural effects of three synthetic repellents (DEET, IR3535 and KBR 3023) on Aedes aegypti mosquitoes in laboratory assays. Med Vet Entomol 20:288–293
Londono-Renteria B, Patel JC, Vaughn M, Funkhauser S, Ponnusamy L, Grippin C, Jameson SB, Apperson C, Mores CN, Wesson DM, Colpitts TM, Meshnick SR (2015) Long-lasting permethrin-impregnated clothing protects against mosquito bites in outdoor workers. Am J Trop Med Hyg 93:869–874
Lundin O, Rundlöf M, Smith HG, Fries I, Bommarco R (2015) Neonicotinoid insecticides and their impacts on bees: a systematic review of research approaches and identification of knowledge gaps. PLoS One 10(8):e0136928
Masetti A, Maini S (2006) Arm in cage tests to compare skin repellents against bites of Aedes albopictus. Bull Insectiol 59:157–160
Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, Djègbé I, Guis H, Corbel V (2012) Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 206:1622–1629
Moores GD, Philippou D, Borzatta V, Trincia P, Jewess P, Gunning R, Bingham G (2009) An analogue of piperonyl butoxide facilitates the characterisation of metabolic resistance. Pest Manag Sci 65(2):150–154
Most B, Pommier de Santi V, Pagès F, Mura M, Uedelhoven WM, Faulde MK (2017) Long-lasting permethrin-impregnated clothing: protective efficacy against malaria in hyperendemic foci, and laundering, wearing, and weathering effects on residual bioactivity after worst-case use in the rain forests of French Guiana. Parasitol Res 116:677–684
NEN (2017) NEN 8333: Beschermende kleding – Kleding die helpt te beschermen tegen teken en fabrieksmatig behandeld is met permethrine. https://www.nen.nl/NEN-Shop-2/Standard/NEN-83332017-Ontw.-nl.htm
Norris EJ, Coats JR (2017) Current and future repellent technologies: the potential of spatial repellents and their place in mosquito-borne disease control. Int J Environ Res Public Health 14:124
Osborne J, DeRaedt-Banks S, Hendy A, Gezan SA, Kaur H, Wilder-Smith A, Lindsay SW, Logan JG (2016) Personal protection of permethrin-treated clothing against Aedes aegypti, the vector of dengue and Zika virus, in the laboratory. PLoS One 11(5):e0152805
Pagès F, Faulde M, Orlandi-Pradines E, Parola P (2010) The past and present threat of vector-borne diseases in deployed troops. Clin Microbiol Infect 16:209–224
Pennetier C, Chabi J, Martin T, Chandre F, Rogier C, Hougard JM, Pagès F (2010) New protective battle-dress impregnated against mosquito vector bites. Parasit Vectors 3:81
Pridgeon JW, Bernier UR, Becnel JJ (2009) Toxicity comparison of eight repellents against four species of female mosquitoes. J Am Mosq Control Assoc 25:168–173
ProMED (2017) Dengue/DHF update (16). Archive Number: 20171028.5410616 dated 28 Oct 2017. http://www.promedmail.org/
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF (2011) Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J 10:80–90
Smith DR (2017) Waiting in the wings: the potential of mosquito transmitted flaviviruses to emerge. Crit Rev Microbiol 43(4):405–422
Sokhna C, Ndiath MO, Rogier C (2013) The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin Microbiol Infect 19(10):902–907
Soto J, Medina F, Dember N, Berman J (1995) Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis 21(3):599–602
Spitzen J, Koelewijn T, Mukabana WR, Takken W (2017) Effect of insecticide-treated bed nets on house-entry by malaria mosquitoes: the flight response recorded in a semi-field study in Kenya. Acta Trop 172:180–185
Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castañeda-Orjuela CA, Chuang TW, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL (2016) The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 16(6):712–723
Thomsen EK, Koimbu G, Pulford J, Jamea-Maiasa S, Ura Y, Keven JB, Siba PM, Mueller I, Hetzel MW, Reimer LJ (2017) Mosquito behavior change after distribution of bednets results in decreased protection against malaria exposure. J Infect Dis 215:790–797
US Armed Forces Pest Management Board (2009) Technical Guide No. 36: personal protective measures against insects and other arthropods of military significanc. http://www.afpmb.org/sites/default/files/pubs/techguides/tg36.pdf
Vaughn MF, Funkhouser SW, Lin F-C, Fine J, Juliano JJ, Apperson CS, Meshnick SR (2014) Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med 46:573–580
WIWeB (2016) Bedingungen für die Zulassung der Vektorenschutzausrüstung gemäß TL 8305–0331. http://www.lhbw.de/fileadmin/user_upload/Ze_2016-01-26___8305-0331___Vektorenschutzausruestung_fuer_textile_Flaechengebilde___Herstellerzulassun_P000294636_.PDF
World Health Organization (2001a) Vectors of diseases: hazards and risks for travellers—part I. WER. 25:189–194
World Health Organization (2001b) Vectors of diseases: hazards and risks for travellers—part II. WER. 26:201–203
WHO (2002) Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. World Health Organization, Geneva. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf
WHO (2013) Guidelines for laboratory and field-testing of long-lasting insecticidal nets. World Health Organization, Geneva, document WHO/HTM/NTD/WHOPES/20131
WHO (2014) A global brief on vector-borne diseases. http://apps.who.int/iris/bitstream/10665/111008/1/WHO_DCO_WHD_2014.1_eng.pdf
WHO (2016) World malaria report 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/
WHO (2017a) Global vector control response 2017–2030. http://www.who.int/malaria/areas/vector_control/Draft-WHO-GVCR-2017-2030.pdf
WHO (2017b) Chikungunya fact sheet. http://www.who.int/mediacentre/factsheets/fs327/en/
WHO (2017c) Fact sheet: dengue and severe dengue. Updated April 2017. http://www.who.int/mediacentre/factsheets/fs117/en/
WHO (2017d) Zika situation report: Zika virus, microcephaly and Guillain–Barré syndrome. http://who.int/emergencies/zika-virus/situation-report/2-february-2017/en/
Wylie BJ, Hauptmann M, Woolf AD, Goldman RH (2016) Insect repellants during pregnancy in the era of the Zika virus. Obstet Gynecol 128(5):1111–1115
Yactayo S, Staples JE, Millot V, Cibrelus L, Ramon-Pardo P (2016) Epidemiology of chikungunya in the Americas. JID 214(S5):S441–S445
Yao TT, Wang LK, Cheng JL, Hu YZ, Zhao JH, Zhu GN (2015) Optimization of pyrethroid and repellent on fabrics against Stegomyia albopicta (=Aedes albopictus) using a microencapsulation technique. Med Vet Entomol 29:37–43
Young D, Evans S (1998) Safety and efficacy of DEET and permethrin in the prevention of arthropod attack. Mil Med 163(5):1–7
Zimmer J, Faulde M (2009) Biomonitoring in the German armed forces: focusing on risk assessments during deployments. Clin Chem Lab Med 47:A42–A43
Zimmermann M, Höcker H (1988) Untersuchungen zur Mottenschutzausrüstung von Wolle mit Permethrin. Melliand Textilber 69:909–915
Zinszer K, Morrison K, Brownstein JS, Marinho F, Santos AF, Nsoesie EO (2017) Reconstruction of Zika virus introduction in Brazil. Emerg Infect Dis 23(1):92–94
Conflict of Interest Statement
The author declares that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Faulde, M.K. (2018). Long-Lasting Insecticide-Treated Textiles Preventing from Mosquito Bite and Mosquito-Borne Diseases. In: Benelli, G., Mehlhorn, H. (eds) Mosquito-borne Diseases. Parasitology Research Monographs, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-319-94075-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-94075-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94074-8
Online ISBN: 978-3-319-94075-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)