Abstract
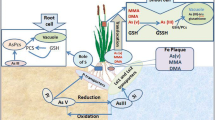
Arsenic (As) has been found to be allied part of various minerals like iron, oxides/hydroxides of aluminum, manganese, and sulfides in the earth crust. The toxicity caused by As in soil can affect paddy crop because the associated risk of As human health is mainly owed to the bioavailable species of As. Rice (Oryza sativa L.) is a most common staple food in Asia and worldwide which uptakes and accumulates As in it. The understanding of arsenic (As) biogeochemical cycle in paddy soils is very important which is related with the mobility, solubility, and bioaccessibility of this heavy metal. Rhizosphere of paddy soil becomes favorable for the oxidation of to AsIII to AsV due to the release of oxygen from roots of rice plants, development of iron plaque, and oxidation activities of rhizobacteria. Reductive dissolution of arsenopyrite discharges As(V) into groundwater, whereas dissolution of claudetite produces As(III). The article aimed to discuss the As uptake and its metabolism by rice plants when encountered with As contamination.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Arsenic Pollution
The outermost layer of the earth comprises of primary igneous olivine rocks, sedimentary sandstone, and metamorphic limestone in which arsenic (As) is also present in high concentrations. In igneous rocks its range is 0.2–10 mg kg−1, while sedimentary rocks contain approximately 0.6 mg kg−1 of As (Zhenli et al. 2005). Arsenic (As) has been found to be allied part of various minerals like iron (Fe), oxides/hydroxides of aluminum (Al), manganese (Mn), and sulfides, and it was also reported that sea salt sprigs and volcanic upsurges were among its other sources (Fitz and Wenzel 2002). Soil contains various forms of As complexes of chlorides, oxides, hydroxide, and sulfides chiefly enargite (Cu3AsA4), cobaltite (CoAsS), and skutterudite (CoAsS4) (Moreno-Jiménez et al. 2012). According to published reports, the prevalence of As in soils is thought to be caused by natural and anthropogenic sources. According to some reports, high As in soil was attributed to the extensive use of As-containing pesticides during the Green Revolution in the 1970s (Adriano 2001; Ng et al. 2003; Chopra et al. 2007). The associated risk of As human health is mainly owed to the bioavailable species of As (Rodriguez et al. 2003). Total As concentration does not indicate its bioavailability, and even no direct methods could measure the bioavailable As of soils; thus the assessment of risk is cumbersome. Hot acid extraction has been highlighted as the sole method to characterize As in soils and other media.
Quaghebeur and Rengel (2005) reported that As occurs in various chemical forms in environment. Generally, inorganic As is more toxic than organic ones; moreover, As in the trivalent oxidation state is more toxic than those in the pentavalent oxidation state. They differ from each other in physical, chemical properties, toxicity, mobility, and bioavailability (Quaghebeur and Rengel 2005). According to Gonzaga et al. (2006), As is not essential for plant growth and is highly phytotoxic in inorganic forms.
As-contaminated drinking water is serious menace for human health in the Southeast Asia and the Bengal Delta (Sharma 2006). Malik et al. (2009) reviewed As contamination and its possible remedies and reported that various aquifers and tube wells contained As above the USEPA’s recommended level in Pakistan. Smith et al. (2000, 2002) reported that among 1.4 million global As-contaminated sites, 41% were located in the USA, while the USEPA documented that As concentration was even higher in Australia (>10,000 mg kg−1). Arsenic present at high amounts (10,000–20,000 mg kg−1) in soils may pose serious health risk to human when enters food chain (Davis et al. 2001). It is also reported that high As content was associated with soil and plant samples collected near industrial estates such as Ghari Rahimabad, Pakha Ghulam, Hattar Industrial Estates, Gujranwala Industrial Estate, and Peshawar Industrial Estate of Pakistan (Rehman et al. 2008).
Human health may be seriously affected due to high As exposure and intake. Rathinasabapathi et al. (2006) reported that prolonged As contact may result in various carcinomas of the skin and internal organs, impaired neural dysfunction, and kidney and liver failures. In 1993, the WHO (1993) lowered the guideline value for As in drinking water from 50 μg L−1 to 10 μg L−1. On the other hand many developing countries still have 50 μg L−1 as MCL (Sharma 2006). As-contaminated groundwater has been reported in various parts of world, such as Vietnam, Massachusetts States, Carolina, Canada, and Bangladesh with 0.305, 30, 2460, 6590, and 0.3990 mg kg−1 As (Roychowdhury et al. 2003; Salido et al. 2003; Das et al. 2004; Bonney et al. 2007). Groundwater contamination of As was suggested as the most common consequence of high As concentrations in soil. High dependence of nearly one third of the world’s population on groundwater (Erakhrumen 2007) can be a reason of As toxicity in affected regions. As toxicity was considered as the biggest calamity mainly due to the dependence on groundwater as drinking source in Bangladesh (Chakraborti et al. 2009).
14.2 Paddy Pollution Due to Arsenic
Plants require an adequate supply of all nutrients for their normal physiological and biological functions (Gupta et al. 2003). Deficiency of specific nutrient occurs when plant cannot obtain sufficient amount as required, whereas excessive supply of the same, through contaminated soil, results in toxicity in plants. Recommended soil application by the USEPA for As is 41 mg kg−1. The understanding of arsenic (As) biogeochemical cycle in paddy soils is very important which is related with the mobility, solubility, and bioaccessibility of this heavy metal (Lim et al. 2014). The health risks associated with food chain become higher due to the high concentration of soluble and bioavailable As to living organisms (Abdul et al. 2015). Study revealed that the bioavailability of As may depend on the presence of Fe, Al, or Mn complexes of arsenic and bacterial community of pore water which reduces the As oxides to reductive liquefaction (Yang et al. 2016; Rinklebe et al. 2016). Arsenic release depends on the physicochemical and biological composition of the soil (Wang et al. 2014). In anaerobic paddy soils, sulfur and the sulfur-reducing bacteria can play an important role in the As methylation and biogeochemical cycling of As contamination by decreasing its mobility and bioaccessibility to rice plants (Jia et al. 2015). Biogeochemical cycle of As-contaminated paddy soils show that the nitrate addition reduces the As mobilization and bioavailability due to the actions of anaerobic As (III) oxidizing rhizobacteria (Zhang et al. 2017).
Rhizosphere of paddy soil becomes favorable for the oxidation of AsIII to AsV due to the release of oxygen from roots of rice plants, development of iron plaque, and oxidation activities of rhizobacteria (Jia et al. 2014). It has been analyzed that there are many As-resistant varieties of rice which accumulate 20–30 times less As than others. So As accumulation and uptake in rice grain can be controlled by the selection of As-resistant rice varieties for cultivation (Syu et al. 2015; Zhang et al. 2016). Such specific As-resistant varieties have As-responsive quantitative trait loci which control the uptake, transportation, and accumulation of As in rice grains and prevent food chain relating As toxicity (Zhang et al. 2017; Norton et al. 2014).
To overcome the toxicity of As on metabolic, biochemical, and molecular activities of cells, many plants develop phosphate and hexose carriers, enzymatic and nonenzymatic antioxidants, and synthesis of vacuolar As phytochelatin complexes (Finnegan and Chen 2012; Chen et al. 2017). Many studies revealed that under anaerobic conditions, ferric hydroxide has more affinity for adsorption and desorption of As (III) than As (V) because of possessing variable surface complexes (Ackermann et al. 2010; Postma et al. 2010; Herbel and Fendorf 2006). Anaerobic conditions not only promote dissolution of ferric hydroxide by Fe-reducing bacteria but also produce secondary minerals like magnetite, ferrihydrite, goethite, and zero-valent iron [ZVI] which may enhance sorption capacity of As rather than solubility of As (Tokoro et al. 2009; Wang et al. 2017).
14.3 Dissolution of Arsenic Minerals
Arsenic has various chemical species, but the most commonly studied are as follows: arsenopyrite (FeAsS), arsenian pyrite Fe(AsS)2, orpiment As2S3, claudetite As2O3, gersdorffite NiAsS, realgar AsS/As4S4, and arsenolite (Malik et al. 2009). Dissolution and mobility of arsenic in soils occur through the following steps: (1) reductive dissolution, (2) oxidative dissolution (3), ligand exchange, and (4) ligand-enhanced dissolution.
Reductive dissolution of arsenopyrite discharges As (V) into groundwater, whereas dissolution of claudetite produces As(III) (Foley and Ayuso 2008). Study revealed that acidic conditions and availability O2 quantity are necessary for the dissolution of arsenopyrite As2O3 (Neil et al. 2014). Oxidative dissolution comprises on three main steps: (1) As dissolution and leachability from minerals through oxidation of arsenopyrites (Yunmei et al. 2004), (2) oxidation of arsenian pyrites (Brown and Calas 2012), and (3) carbonation of arsenosulfides (Lim et al. 2009).
Oxidative and reductive dissolution of As from minerals depends on the oxygen availability and pH values of soil and water. At neutral pH some minerals of AS produce secondary minerals like orpiment, realgar, and gersdorffite which on dissolution readily changes into arsenite and thioarsenite (Drahota and Filippi 2009; Wang et al. 2015). Arsenolite is the primary mineral of As which readily dissolved and liberate As directly into water (Haffert et al. 2010). Scorodite is a type of primary mineral that naturally coexists with ferric oxyhydroxide (FO) phase at pH ranges between 2.5 and 3, but at neutral pH it produces arsenate through aqueous dissolution or through weathering of arsenic-containing minerals bedrocks (Langmuir et al. 2006).
Ligand exchange dissolution is the mechanism which is related with the exchange of anion attached on any mineral with another anion like sulfate, phosphate, oxalate, citrate, and malate. For example, exchange As(V) by phosphate from arsenopyrites:
Ligand-enhanced dissolution is the type of As dissolution in which the cations of As mineral are replaced by oxalate, malate, and citrate and release As(V) and resulted in the synthesis of complex structures of As salts.
The rate of this reaction with organic ligands, such as oxalate, malate, and citrate, varies substantially with mineral phase. The reaction rates decrease in the following trend (Fig. 14.1).
14.4 As Uptake by Rice
Rice (Oryza sativa L.) is a most common staple food in Asia and worldwide which uptake and accumulate As in it (Roychowdhury et al. 2002; Khush 2005). As is toxic heavy metal which stands first by the Agency for Toxic Substances and Disease Registry in a list of 20 hazardous substances (Goering et al. 1999). Two most predominantly occurring forms of arsenic (As) in plants are As III and As V, but most of the plants reduce As V to As III which resulted in plants death by disturbing the cellular activities of plants body (Abedin et al. 2002). It has been observed that the uptake of inorganic As species is commonly higher by rice than organic methylated As species, but after uptake methylated As species are efficiently transported to the grains and resulted in spikelet sterility syndrome which lowers down the crop yield (Zhao et al. 2013).
Several research studies have also found high concentrations of arsenic in vegetables and rice in areas where concentrations of arsenic in soil and water are also high. Higher concentrations of arsenic have been reported in rice plants (boro rice in Bangladesh) in the following orders: rice roots > rice stem > rice leaf > rice grain > rice husk (Chakma et al. 2012; Haq et al. 2012; Rai et al. 2010). Arsenic toxicity disturbs the physiological actions of plants by damaging cellular membranes of plants which ultimately cause leakage of plant electrolytes (Singh et al. 2006).
Chemical species of organic arsenic are translocated by specific aquaporin canals comprising on nodulin 26-like intrinsic (NIP) and by the phosphate transporters, and arsenite and organic As species through the nodulin 26-like intrinsic (NIP) aquaporin channels (Zhao et al. 2010). After entrance in cytoplasm, As species strives with phosphate which produced ADP arsenate by substituting a phosphate group of ATP and disturb the energy flows in cells (Meharg and Hartley-Whitaker 2002).
14.5 Rice Growth and Physiology Under As Stress
Due to irrigation of As-contaminated water to rice paddies, it accumulated in the topsoil in the form of inorganic As(V), arsenite As(III), the organic As(V), dimethyl arsinic acid (DMA), and monomethylarsonic acid (MMA) and becomes available to the next cultivated varieties of rice (Huq et al. 2006, 2008, 2011; Meharg and Rahman 2003; Williams et al. 2005). All forms of As are highly toxic, which affects the yield of rice grains resulting in straight head condition due to improper grains filling in the panicles (Yan et al. 2014). Mechanism of As detoxification in plant cell occurs by the formation of complexes with PCs, which help in the translocation of metal inside vacuole and finally its reduction from a high toxic form, i.e., As(V), to less toxic form As(III) (Rai et al. 2010). It has also been studied that in some plants, PvACR3 accumulates AsIII in the vacuole after sequestration (Indriolo et al. 2010). As toxicity disturbs the metabolism of plants which inhibits not only plants growth but also reduces the biomass, fertility, and yield (Garg and Singla 2011). Recently it has been explored that rice plants uptake AsV which rapidly reduce to AsIII by specific arsenate reductases, namely, HAC1 (High Arsenic Content 1) (Shi et al. 2016). Phytotoxicity due to As contamination of soil and water has shown the following symptoms in rice like stunt growth, reduction in roots elongation, necrosis, and decrease in size of photosynthetic pigments, which hinder the germination of seed which ultimately reduce the fruit and grains yield (Zhao et al. 2009).
14.6 Transcriptomic Study of Rice Under As Stress
Advancement in the field of sequencing technology, genomic exploration, and transcriptomic studies become helpful in understanding the effects of stress conditions in eukaryotes. In this regard the use of RNA-Seq technology is supporting the transcriptional reporting and various genes expression against stress (Wang et al. 2009). Transcriptomics has widely been utilized for the exploration of plant responsive genes under various biotic and abiotic stresses (Zeng et al. 2014; Yamamoto et al. 2015; Shaheen et al. 2017; Chaires et al. 2017). Isayenkov and Maathuis (2008) described that the AtNIP7;1 protein may contribute in the transportation of As in A. thaliana. It was highlighted that the As toxicity boosts a number of genes in rice related with metal transportation, metal-binding proteins, and antioxidant responding (Rai et al. 2010).
The entrance of arsenic species into the rice roots is possible by silicon pathway which has been elaborated by the identification of silicon transporter genes OsNIP2;1 or Lsi1 in rice plant (Ma et al. 2008). It has been studied that the silicon transporter Lsi1 in rice (Oryza sativa) also uptakes the methylated As species, i.e., monomethylarsinic acid (MMA) and dimethylarsinic acid (DMA) from paddy soil (Li et al. 2009). Previously the transcriptomic study of Arabidopsis plant showed the AsIII accumulation and translocation by the expression of heavy metals stress-responsive genes NRAMP (natural resistance-associated macrophage protein) transporter. The expression of the same protein OsNRAMP1 in rice shows that these genes may involve for the translocation and accumulation AsIII in rice. So in rice OsNRAMP1 genes may confine the epidermis and pericycle which cause the uptake of AsIII into root xylem to shoot xylem (Tiwari et al. 2014).
Other arsenate As(V) transporters in plants roots are phosphate transportation (Pi) pathways (Zhao et al. 2009). A research was conducted on comparative analysis of rice plants with phosphate (Pi) transporter OsPT8 with a rice mutant defective phosphate (Pi) transporter OsPHF1 and observed that rice having OsPT8 had higher capacities of Pi and arsenate uptake and translocation than rice with OsPHF1 (Wu et al. 2011). The As-resistant varieties of rice may be developed for the overexpression of OsABCC1 because its overexpression in wild and mutant varieties was differential upon exposures of various As concentrations. In mutant rice OsABCC1 genes were expressed equally even against low concentrations of As due to the biosynthesis of thiol complexes in the epidermis and pericycle of plant, while these genes were not expressed at low As concentration in wild type of rice (Song et al. 2014).
14.7 Remedial Measures
Arsenic remediation options in suffering countries could be possible by taking following check and balances:
-
1.
In As-contaminated sites, the wells and tube wells should be dig deeper not shallow.
-
2.
Rain water should be harvested.
-
3.
Phytoremediation by growing As hyperaccumulating plants species (duckweed) can also improve the conditions of polluted soil and water bodies (Ng et al. 2017).
-
4.
As filters should be available to community at low cost.
-
5.
Safe water supply should be made possible.
-
6.
As tolerant and hyper tolerant varieties of rice should only be referred for contaminated areas.
-
7.
Removal of As from contaminated water by using iron-coated sand is a very useful technique (Chang et al. 2012).
-
8.
Treatment of As-contaminated water with the exposure of gaseous chlorine, permanganate, hydrogen peroxide, Fenton’s reagent, and ultraviolet (UV) radiation is a useful technique for purification (Litter et al. 2010).
-
9.
Awareness programs should also be launched for education on As pollution.
-
10.
Fertilization practices can also be helpful as As mitigation strategy (Barbafieri et al. 2017).
-
11.
Bioavailability of As in the soil can also be helpful for its phytoremediation of paddy soil by the addition of phosphate-containing fertilizers (Lewinska and Karczewska 2013; Niazi et al. 2017).
-
12.
Bioadsorption is also a useful technology for the adsorption of As(III) and As(V) by a biomass or biofilm of living or dead organisms such as algae, bacteria, macrophytes or microphytes, and biopolymers (Dickinson et al. 2009).
-
13.
Adaptation of proper irrigation system can control the As contamination in rice. A research work clearly demonstrated the impact of sprinkler irrigation over flood irrigation, and the results have shown that total concentration of As in rice kernels under sprinkler irrigation was 50 times less than the constant flooding irrigation (Spanu et al. 2012).
-
14.
Biochar addition has also been studied as a best remediation for As release from contaminated sites (Li et al. 2016; Choppala et al. 2016; Yin et al. 2016).
References
Abdul KSM, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PMC (2015) Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40:828–846
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Ackermann J, Doris V, Thomas K, Klaus K, Reinhold J (2010) Minerals controlling arsenic distribution in floodplain soils. Eur J Soil Sci 61:588–598
Adriano DC (2001) Trace elements in terrestrial environments: bio geochemistry, bioavailability, and risk of metals, 2nd edn. Springer, New York. https://doi.org/10.1007/978-0-387-21510-5
Barbafieri M, Pedron F, Petruzzelli G, Rosellini I, Franchi E, Bagatin R, Vocciante M (2017) Assisted phytoremediation of a multi-contaminated soil: investigation on arsenic and lead combined mobilization and removal. J Environ Manag 203:316–329
Bonney RJA, Tyson JF, Lanza GR (2007) Phytoextraction of arsenic from soil by Leersia oryzoides. Int J Phytoremed 9:31–40
Brown GE, Calas G (2012) Section 15. Sorption processes at mineral-water interfaces in complex natural environmental systems. Geochem Perspect 1(4–5):628–649
Chaires M, Gupta D, Joshee N, Cooper KK, Basu C (2017) RNA-seq analysis of the salt stress-induced transcripts in fast-growing bioenergy tree, Paulownia elongata. J Plant Interact 12(1):128–136
Chakma S, Rahman MM, Islam P, Awal MA, Roy UK, Haq MR (2012) Arsenic in rice and rice straw. Bangla Vet 29(1):1–6
Chakraborti D, Das B, Rahman MM, Chowdhury UK, Biswas B, Goswami AB, Nayak B, Pal A, Sengupta MK, Ahamed S, Hossain A, Basu G, Roychowdhury T, Das D (2009) Status of groundwater arsenic contamination in the state of West Bengal, India: a 20-year study report. Mol Nutr Food Res 53:542–551. https://doi.org/10.1002/mnfr.200700517
Chang Y-Y, Song K-H, Yu M-R, Yang J-K (2012) Removal of arsenic from aqueous solution by iron-coated sand and manganese-coated sand having different mineral types. Water Sci Technol 65(4):683–688
Chen Y, Han YH, Cao Y, Zhu YG, Rathinasabapathi B, Ma LQ (2017) Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci 8:268
Choppala G, Bolan N, Kunhikrishnan A, Bush R (2016) Differential effect of biochar upon reduction-induced mobility and bioavailability of arsenate and chromate. Chemosphere 144:374–381
Chopra BK, Bhat S, Mikheenko IP, Xu Z, Yang Y, Luo X, Chen H, Zwieten L, McLilley R, Zhang R (2007) The characteristics of rhizosphere microbes associated with plants arsenic contaminated soils from cattle dip sites. Sci Total Environ 378:331–342
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30:383–387
Davis A, Sherwin D, Ditmars R, Hoenke KA (2001) An analysis of soil arsenic records of decision. Environ Sci Technol 35:2401–2406. https://doi.org/10.1021/es001411i
Dickinson NM, Baker AJM, Doronila A, Laidlaw S, Reeves RD (2009) Phytoremediation of inorganics: realism and synergies. Int J Phytoremed 11:97–114
Drahota P, Filippi M (2009) Secondary arsenic minerals in the environment: a review. Environ Int 35(8):1243–1255
Erakhrumen AA (2007) Phytoremediation: an environmentally sound technology for pollution prevention, control and remediation. An introductory guide for decision makers. Freshwater management series no. 2. Educ Res Rev 2(7):151–156
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil, rhizosphere, plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278
Foley NK, Ayuso RA (2008) Mineral sources and transport pathways for arsenic release in a coastal watershed, USA. Geochem Explor Environ Anal 8(1):59–75
Garg N, Singla P (2011) Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett 9:303–321. https://doi.org/10.1007/s10311-011-0313-7
Goering PL, Aposhia HV, Mass MJ, Cebrian M, Beck BD, Waalkes MP (1999) The enigma of arsenic carcinogenesis: role of metabolism. Toxicol Sci 49:5–14. https://doi.org/10.1093/toxsci/49.1.5
Gonzaga MIS, Santos JAG, Ma LQ (2006) Arsenic phytoextraction and hyperaccumulation by fern species (Arsenic and Fern Species). Sci Agric (Piracicaba, Braz.) 63:90–101. https://doi.org/10.1590/S0103-90162006000100015
Gupta M, Anil K, Mohammad Y, Pandey KP (2003) Bioremediation: ecotechnology for the present century. Environ Newsl 9(2). http://isebindia.com/01_04/03-04-2.html. Accessed 10 Mar 2018
Haffert L, Sander SG, Hunter KA, Craw D (2010) Evidence for arsenic-driven redox chemistry in a wetland system: a field voltammetric study. Environ Chem 7(4):386–397. https://doi.org/10.1071/EN10019
Haq MR, Rahman MM, Islam P, Awal MA, Chakma S, Roy UK (2012) Detection of arsenic in animal feed chain: broken rice and water hyacinth. Bangla J Vet Med 10:111–116. https://doi.org/10.3329/bjvm.v10i1-2.15656
Herbel MJ, Fendorf S (2006) Biogeochemical processes controlling the speciation and transport of arsenic within iron coated sands. Chem Geol 228:16–32. https://doi.org/10.1016/j.chemgeo.2005.11.016
Huq SMI, Correl R, Naidu R (2006) Arsenic accumulation in food sources in Bangladesh. Chapter 15. In: Naidu et al. (ed) Managing arsenic in the environment. From soil to human health. CSIRO, Clayton, VIC, pp 283–293
Huq SMI, Joardar JC, Manzurul-Hoque AFM (2008) Seasonal effect on the load in soil and subsequent transfer of arsenic to rice. Land Contam Reclam 16:357–363. https://doi.org/10.2462/09670513.908
Huq SMI, Sultana S, Chakraborty G, Chowdhury MTA (2011) A mitigation approach to alleviate arsenic accumulation in rice through balanced fertilization. Appl Environ Soil Sci 2011:1–8. https://doi.org/10.1155/2011/835627
Indriolo E, Na G, Ellis D, Salt DE, Banks JA (2010) A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 22:2045–2057. https://doi.org/10.1105/tpc.109.069773
Isayenkov SV, Maathuis FJM (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett 582:1625–1628. https://doi.org/10.1016/j.febslet.2008.04.022
Jia Y, Huang H, Chen Z, Zhu YG (2014) Arsenic uptake by rice is influenced by microbe-mediated arsenic redox changes in the rhizosphere. Environ Sci Technol 48:1001–1007. https://doi.org/10.1021/es403877s
Jia Y, Bao P, Zhu YG (2015) Arsenic bioavailability to rice plant in paddy soil: influence of microbial sulfate reduction. J Soils Sediments 15(9):1960–1967. https://doi.org/10.1007/s11368-015-1133-3
Khush GS (2005) What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol 59:1–6. https://doi.org/10.1007/s11103-005-2159-5
Langmuir D, Mahoney J, Rowson J (2006) Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings. Geochim Cosmochim Acta 70(12):2942–2956
Lewinska K, Karczewska A (2013) Influence of soil properties and phosphate addition on arsenic uptake from polluted soils by velvetgrass (Holcus lanatus). Int J Phytoremed 15:91–104. https://doi.org/10.1080/15226514.2012.683205
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150(4):2071–2080. https://doi.org/10.1104/pp.109.140350
Li H, Liu Y, Chen Y, Wang S, Wang M, Xie T, Wang G (2016) Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci Rep 6:31616. https://doi.org/10.1038/srep31616
Lim M, Han GC, Ahn JW, You KS, Kim HS (2009) Leachability of arsenic and heavy metals from mine tailings of abandoned metal mines. Int J Environ Res Pub Health 6(11):2865–2879. https://doi.org/10.3390/ijerph6112865
Lim KT, Shukor MY, Wasoh H (2014) Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/503784
Litter MI, Morgada ME, Bundschuh J (2010) Possible treatments for arsenic removal in Latin American waters for human consumption. Environ Pollut 158(5):1105–1118. https://doi.org/10.1016/j.envpol.2010.01.028
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105(29):9931–9935. https://doi.org/10.1073/pnas.0802361105
Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168:1–12. https://doi.org/10.1016/j.jhazmat.2009.02.031
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154(1):29–43. https://doi.org/10.1046/j.1469-8137.2002.00363.x
Meharg AA, Rahman MM (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37(2):229–234. https://doi.org/10.1021/es0259842
Moreno-Jiménez E, Esteban E, Peñalosa JM (2012) The fate of arsenic in soil-plant systems. Rev Environ Contam Toxicol 215:1–37. https://doi.org/10.1007/978-1-4614-1463-6_1
Neil CW, Yang YJ, Schupp D, Jun YS (2014) Water chemistry impacts on arsenic mobilization from arsenopyrite dissolution and secondary mineral precipitation: implications for managed aquifer recharge. Environ Sci Technol 48(8):4395–4405. https://doi.org/10.1021/es405119q
Ng JC, Wang JP, Shraim A (2003) A global health problem caused by arsenic from natural sources. Chemosphere 52:1353–1359. https://doi.org/10.1016/S0045-6535(03)00470-3
Ng CA, Wong LY, Lo PK, Bashir MJK, Chin SJ, Tan SP, Yong LK (2017) Performance of duckweed and effective microbes in reducing arsenic in paddy and paddy soil. AIP Conference Proceedings 1828, 020031. https://doi.org/10.1063/1.4979402
Niazi NK, Bibi I, Fatimah A, Shahid M, Javed MT, Wang H, Ok YS, Bashir S, Murtaza B, Saqib ZA, Shakoor MB (2017) Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: morphological and physiological response. Int J Phytoremed 19:670–678. https://doi.org/10.1080/15226514.2016.1278427
Norton GJ, Douglas A, Lahner B, Yakubova E, Gueirnot ML, Pinson SRM et al (2014) Genome wide association mapping of grain arsenic, copper, molybdenum and zinc in rice (Oryza sativa L.) grown at four international field sites. PLoS One 9:e89685. https://doi.org/10.1371/journal.pone.0089685
Postma D, Jessen S, Nguyen TMH, Mai TD, Koch CB, Pham HV, Pham QN, Larsen F (2010) Mobilization of arsenic and iron from Red River floodplain sediments, Vietnam. Geochim Cosmochim Acta 74:3367–3338. https://doi.org/10.1016/j.gca.2010.03.024
Quaghebeur M, Rengel Z (2005) Review: Arsenic speciation governs arsenic uptake and transport in terrestrial plants. Microchim Acta 151:141–152. https://doi.org/10.1007/s00604-005-0394-8
Rai A, Tripathi P, Dwivedi S, Dubey S, Shri M, Kumar S, Tripathi PK, Dave R, Kumar A, Singh R, Adhikari B, Bag M, Tripathi RD, Trivedi PK, Chakrabarty D, Tuli R (2010) Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere 82:1–10. https://doi.org/10.1016/j.chemosphere.2010.10.070
Rathinasabapathi B, Ma LQ, Srivastava M (2006) Arsenic hyperaccumulating ferns and their application to phytoremediation of arsenic contaminated sites. Floriculture, ornamental and plant biotechnology, Vol III. ©2006 Global Science Books, London
Rehman W, Zeb A, Noor N, Nawaz M (2008) Heavy metal pollution assessment in various industries of Pakistan. Environ Geol 55:353–358. https://doi.org/10.1007/s00254-007-0980-7
Rinklebe J, Shaheen SM, Yu K (2016) Release of As, Ba, Cd, Cu, Pb, and Sr under pre-definite redox conditions in different rice paddy soils originating from the USA and Asia. Geoderma 270:21–32. https://doi.org/10.1016/j.geoderma.2015.10.011
Rodriguez RR, Basta NT, Casteel SW, Armstrong FP, Ward DC (2003) Chemical extraction methods to assess bioavailable arsenic in soil and solid media. J Environ Qual 32:876–884. https://doi.org/10.2134/jeq2003.8760
Roychowdhury T, Uchino T, Tokunaga H, Ando M (2002) Arsenic and other heavy metals in soils from an arsenic-affected area of West Bengal, India. Chemosphere 49:605–618. https://doi.org/10.1016/S0045-6535(02)00309-0
Roychowdhury T, Tokunaga H, Ando M (2003) Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic affected area of West Bengal, India. Sci Total Environ 308:15–35
Salido AL, Hasty KL, Lim J, Butcher DJ (2003) Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian Mustard (Brassica juncea). Int J Phytotoremed 5:89–103. https://doi.org/10.1080/713610173
Shaheen S, Ahmad R, Jin W, Mahmood Q, Iqbal A, Pervez A, Shah MM (2017) Transcriptomic responses of selected genes against chromium stress in Arundo donax L. Toxicol Environ Chem 99(5–6):900–912. https://doi.org/10.1080/02772248.2017.1280269
Sharma AK (2006) Arsenic removal from water using naturally occurring iron, and the associated benefits on health in affected regions. Ph.D. Thesis, Institute of Environment & Resources, Technical University of Denmark
Shi S, Wang T, Chen Z, Tang Z, Wu Z, Salt DE et al (2016) OsHAC1;1 and OsHAC1;2 function as arsenate reductases and regulate arsenic accumulation. Plant Physiol 172:1708–1719. https://doi.org/10.1104/pp.16.01332
Singh NS, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L. Plant Sci 170(2):274–282. https://doi.org/10.1016/j.plantsci.2005.08.013
Smith AH, Lingas IO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Heal Org 78(9):1093–1103
Smith E, Naidu R, Alston AM (2002) Chemistry of arsenic in soils. J Environ Qual 31:557–563. https://doi.org/10.2134/jeq2002.5570
Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M et al (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci USA 111:15699–15704 https://doi.org/10.1073/pnas.1414968111
Spanu A, Daga L, Orlandoni AM, Sanna G (2012) The role of irrigation techniques in arsenic bioaccumulation in rice (Oryza sativa L.). Environ Sci Technol 46(15):8333–8340. https://doi.org/10.1021/es300636d
Syu C-H, Huang C-C, Jiang P-Y, Lee C-H, Lee D-Y (2015) Arsenic accumulation and speciation in rice grains influenced by arsenic phytotoxicity and rice genotypes grown in arsenic-elevated paddy soils. J Hazard Mater 286:179–186. https://doi.org/10.1016/j.jhazmat.2014.12.052
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ 37:140–152. https://doi.org/10.1111/pce.12138
Tokoro C, Yatsugi Y, Koga H, Owada S (2009) Sorption mechanisms of arsenate during coprecipitation with ferrihydrite in aqueous solution. Environ Sci Technol 44(2):638–643. https://doi.org/10.1021/es902284c
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10(1):57–63. https://doi.org/10.1038/nrg2484
Wang P, Sun G, Jia Y, Meharg AA, Zhu Y (2014) A review on completing arsenic biogeochemical cycle: microbial volatilization of arsines in environment. J Environ Sci 26(2):371–381. https://doi.org/10.1016/S1001-0742(13)60432-5
Wang Y, Xu LY, Wang SF, Xiao F, Jia YF (2015) XAS analysis upon dissolved species of orpiment in anoxic environment. Huanjing ke xue= Huanjing kexue 36(9):3298–3303
Wang S, Gao B, Li Y, Creamer AE, He F (2017) Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: batch and continuous flow tests. J Hazard Mater 322:172–181. https://doi.org/10.1016/j.jhazmat.2016.01.052
WHO (1993) Guidelines for drinking water quality. World Health Organisation, Geneva, P-41
Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39(15):5531–5554. https://doi.org/10.1021/es0502324
Wu Z, Ren H, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157(1):498–508. https://doi.org/10.1104/pp.111.178921
Yamamoto N, Takano T, Tanaka K, Ishige T, Terashima S, Endo C, Kurusu T, Yajima S, Yano K, Tada Y (2015) Comprehensive analysis of transcriptome response to salinity stress in the halophytic turf grass Sporobolus virginicus. Front Plant Sci 6:241. https://doi.org/10.3389/fpls.2015.00241
Yan W, Moldenhauer K, Zhou W, Xiong H, Huang B (2014) Rice straighthead disease–prevention, germplasm, gene mapping and DNA markers for breeding. In: Yan W (ed) Rice-germplasm, genetics and improvement. InTech. https://doi.org/10.5772/56829
Yang K, Jeong S, Jho EH, Nam K (2016) Effect of biogeochemical interactions on bioaccessibility of arsenic in soils of a former smelter site in Republic of Korea. Environ Geochem Health 38(6):1347–1354. https://doi.org/10.1007/s10653-016-9800-x
Yin DX, Wang X, Chen C, Peng B, Tan CY, Li HL (2016) Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 152:196–206. https://doi.org/10.1016/j.chemosphere.2016.01.044
Yunmei Y, Yongxuan Z, Williams-Jones AE, Zhenmin G, Dexian L (2004) A kinetic study of the oxidation of arsenopyrite in acidic solutions: implications for the environment. Appl Geochem 19(3):435–444. https://doi.org/10.1016/S0883-2927(03)00133-1
Zeng J, He X, Wu D, Zhu B, Cai S, Nadira UA, Jabeen Z, Zhang G (2014) Comparative transcriptome profiling of two Tibetan wild barley genotypes in responses to low potassium. PLoS One 9(6):e100567. https://doi.org/10.1371/journal.pone.0100567
Zhang X, Wu S, Ren B, Chen B (2016) Water management, rice varieties and mycorrhizal inoculation influence arsenic concentration and speciation in rice grains. Mycorrhiza 26:299–309. https://doi.org/10.1007/s00572-015-0669-9
Zhang J, Zhao S, Xu Y, Zhou W, Huang K, Tang Z, Zhao FJ (2017) Nitrate stimulates anaerobic microbial arsenite oxidation in paddy soils. Environ Sci Technol 51(8):4377–4386. https://doi.org/10.1021/acs.est.6b06255
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181(4):777–794. https://doi.org/10.1111/j.1469-8137.2008.02716.x
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559. https://doi.org/10.1146/annurev-arplant-042809-112152
Zhao FJ, Zhu YG, Meharg AA (2013) Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ Sci Technol 47(9):3957–3966. https://doi.org/10.1021/es304295n
Zhenli H, Yang X-E, Stophella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140. https://doi.org/10.1016/j.jtemb.2005.02.010
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Shaheen, S., Mahmood, Q. (2018). Tolerance Mechanisms of Rice to Arsenic Stress. In: Hashmi, M., Varma, A. (eds) Environmental Pollution of Paddy Soils. Soil Biology, vol 53. Springer, Cham. https://doi.org/10.1007/978-3-319-93671-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-93671-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93670-3
Online ISBN: 978-3-319-93671-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)