Abstract
Majority of world’s inhabitants consume rice (Oryza sativa L.) and rice products as staple food and major source of carbohydrate. Rice is a strong accumulator of inorganic arsenic (As). Arsenic enters into rice as a result of extensive use of As-contaminated ground water for irrigation of rice field and several other natural or anthropogenic factors. As is non-biodegradable and remains persistent in the soil for a long period of time, thereby enters into the food chain, exerting hazardous impacts on animal health. In comparison to other cereal crops, rice being an efficient arsenic bio-accumulator, the nutritional quality of rice is severely affected due to As toxicity. It enters into the rice system and accumulates through different root transporters – phosphate transporters for As(V), noduline 26-like intrinsic proteins (NIPs) for As (III) and membrane bound aquaporin channels. Researches have been focused to understand and mitigate the impact of arsenic toxicity on rice by evaluating various complex physio-molecular mechanisms associated with the arsenic transport. Screening of the landraces and other genetic stocks for better tolerance and/or resistance nature and incorporation in the breeding strategy, changing in agronomical and cultural practices, biotechnological approaches, etc. appear to be immensely important to understand the impact of the metalloid (like arsenic) in rice. This chapter encompasses the physiological and molecular insight of As transport, accumulation, tolerance and mitigation strategies towards the rice improvement program with a concern of health hazard.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Rice (Oryza sativa) is one of the important cereal crops, which is consumed as staple food by more than 50% of world’s inhabitants and fulfill the demand of major carbohydrates, thiamin, vitamin B6, and some other essential elements like iron (Fe), magnesium (Mg), zinc (Zn) and copper (Cu) (Roychowdhury et al. 2013; Hasanuzzaman et al. 2015). The estimated share of total calories obtained from rice is about 20.5% globally, 29.2% in low-income countries and 31.6% in Asia (Wailes 2005). But, unfortunately several rice producing and exporting countries are suffering from the arsenic contamination in the ground water and field soil (Bhattacharya et al. 2010; Rahman and Hasegawa 2011). Though rice production and cultivation needs water holding agro-ecosystem, global climate change has resulted in the enormous changes on global precipitation patterns posing a serious threat to rain-fed rice cultivation system and increased the dependence on the ground water for irrigation, In rice producing region of South East Asia like India, Bangladesh, China and Vietnam, majority of the ground water is contaminated with arsenic and its use for irrigation purpose that leads to the accumulation of significantly high amount of arsenic in rice (Neumann et al. 2011).
15.2 Sources of Arsenic in Agricultural Paddy Fields
Arsenic (As) is a highly toxic metalloid and group I carcinogen that exerts health hazards in animals and human as well (IARC Monographs 2004). Arsenic contamination occurs at both water and soil level (Phillips 1990). Arsenic contamination in groundwater and soil is due to the weathering of rocks and minerals, their subsequent leaching and surface runoff, and through some anthropogenic sources (Smedley and Kinniburgh 2002). The main sources of arsenic contamination in rice include some specific sources such as pesticides and herbicides, wood preservatives, phosphate fertilizers, industrial waste products, mining activities, coal burning and smelting (Jayasumana et al. 2015). Besides its natural presence, arsenic can enters into the well and other water reservoirs from other sources and when those are used for the irrigation purpose, drinking and cooking, leads to hazardous impacts not only on rice cultivation system but also on human and animal health (Rahman and Hasegawa 2011). Thus, arsenic contamination of rice is mainly due to (i) the irrigation with arsenic contaminated water, (ii) through contaminated soil, (iii) high efficiency of rice plant to absorb arsenic from both water and soil compared to the other crops, and (iv) cooking/processing with contaminated water (Islam et al. 2016). Rice plants are thus affected by arsenic and being the primary producers, arsenic enters into the food chain and affects also the heterotrophic consumers (cattles and human beings) who are depending on the plant products (Zhao et al. 2010).
15.3 Rice as a Dietary Source of Arsenic
The major source of arsenic (As) exposure to both humans and cattle comes through foods such as cereal grains, vegetables, food products and arsenic polluted drinking water. Rice and rice products being the major source of carbohydrate for more than 50% of global population, arsenic accumulation in rice is a major global concern. Rice is usually grown in flooded conditions, which make it easy for various toxic metals and metalloids including arsenic to get accumulated in the rice grain. This happens largely due to irrigation of paddy fields with arsenic tainted water, extensive use of pesticides and presence of the metalloid in the pre-existing anaerobic toxic soil (Khan et al. 2010; Shraim 2017). Such soil condition is very much favorable for the As accumulation, mobilization inside the plant parts and thus gets accumulated in the grain (Neumann et al. 2011). Thus, the consumption of arsenic contaminated rice and rice products exerts severe health hazards and being non-biodegradable remains persistent for exceedingly long period of time (Booth 2009). In comparison to other cereal crops, rice can accumulate ~10 times higher quantity of arsenic and its concentration varies depending upon various factors such as cultivation practices, soil dynamics, mineral content, irrigation practices, etc. (Meharg et al. 2008).
15.4 Physiological Implication of Arsenic Toxicity in Rice
In nature, arsenic exists in both organic and inorganic forms. The inorganic form of arsenic remains in four valance states viz., −III, 0, +III and +V. Among the various valance states of arsenic, the pentavalent arsenate (AsV) and trivalent arsenite (AsIII) are predominantly encountered in arsenic contaminated environments (Dhankher et al. 2011). These two states of arsenic are inter-convertible and their interconversion depends on the redox state of the soil (Tripathi et al. 2007; Zhao et al. 2010). The pentavalent arsenate mimic the phosphate group and hence suppress the ATP generation and associated oxidative phosphorylation (Tripathi et al. 2007). The pentavalent arsenate may be methylated to form various volatile pentavalent arsenic species like monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) in the soil and such conversion is largely influenced by the rhizosphere microbial activities and the physical/chemical state of the soil (Zhao et al. 2013). Rice is cultivated under two soil conditions viz., aerobic soil where concentration of arsenate is usually high and flooded or water lodged condition where arsenite is predominant (Clemens and Ma 2016). In comparison to other cereals, rice accumulates exceedingly high levels of arsenic (Nath et al. 2014). Studies have shown that rice can accumulates arsenic up to 2 mg/Kg in grains and 92 mg/Kg in the dried biomass (Williams et al. 2005). Since, rice cultivation needs water holding agro-ecosystem, it requires large irrigation inputs during the dry seasons and/or dry lands. As such the irrigation in majority of the rice growing regions of South East Asia are dependent on ground water that are reported to be largely tainted with arsenic, the metalloid gets transported into the rice plants that subsequently gets accumulated in the grain (Brammer and Ravenscroft 2009). Consumption of arsenic infested rice and related rice products increases severe hazardous impact on human health (Rahman et al. 2009; Tripathi et al. 2012). Studies have demonstrated that during axenic culture conditions, rice plants exposed to inorganic arsenic can accumulate methylated arsenic only when it is exposed to it, while in presence inorganic arsenic only there is such no accumulation of methylated arsenic species (Lomax et al. 2012). Recent investigations indicate that the soil bacteria can produce MMA and DMA through the methylation of inorganic arsenic and they are catalyzed by diverse S-adenosylmethionine-dependent AsIII methyltransferases (Zhu et al. 2014). In such conditions, the concentrations of both MMA and DMA sharply decrease (Arao et al. 2011). Arsenic induced oxidative stress via formation of reactive oxygen species (ROS) results due to the inter-conversation between penta- and trivalent forms of arsenic (Choudhury and Panda 2004; Tripathi et al. 2012). Arsenic inhibits the cellular and biochemical functions by interacting with the sulfhydryl (-SH) groups of proteins and enzymes. The arsenic mediated generated ROS can result the growth inhibition, leaf chlorosis and reduction of plant biomass in rice (Nath et al. 2014).
15.5 Bioavailability, Uptake and Accumulation of Arsenic in Rice
The cultivated rice efficiently accumulates arsenic from the rhizosphere because of the higher arsenite (AsIII) mobilization in the anaerobic condition and arsenic transport through highly efficient Si transport pathway (Zhao et al. 2010). Physiological studies showed that arsenic transport via roots in rice occurs through the aquaporin channels, but the detailed mechanism is not clearly known. It is assumed that noduline 26 – like intrinsic proteins (NIPs) are the possible players for arsenic transport (Ali et al. 2009). Three types of NIPs (AtNIP 5;1, AtNIP6;1 and AtNIP7;1) have been identified in Arabidopsis, which are involve in the arsenic transport and loss of function in any one of it (specially NIP7;1) results in arsenic tolerance in rice (Isayenkov and Maathuis 2008).
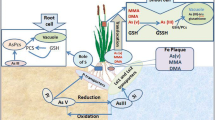
It was shown that arsenate (AsV) and other methylated forms of arsenic (MMA and DMA) in the soil are taken up by the roots due to high efficiency of AsV to enter into the plant system (Raab et al. 2007). But under the reducing condition i.e., over-flooded rice field by water, AsIII is the dominating form of arsenic as compared to AsV species. AsV has the structural analogue of phosphate (P) group and capable to mimic like a phosphate, which results in efficient uptake by the plant root system through Phosphate Transporters (PTs) like OsPht1;8 and OsPHR2 (Phosphate Starvation Response 2). In rice, 13 PT genes (OsPT1 to OsPT13) are available for AsV uptake; but OsPT1 and OsPT8 have been well investigated (Paszkowski et al. 2002). AsIII also enters directly inside the root cell through passive uptake and due to the concentration gradient. This is governed by Lsi1, a nodulin 26–like intrinsic protein (NIP) aquaglyceroporin (OsNIP2;1) (Ma et al. 2008). In rice, two types of silicon (Si) transporter genes – Oryza sativa Low Silicon 1 (OsLsi1) and 2 (OsLsi2) have been reported till date to be associated in the plasma membrane of root cells and regulate the influx (Lsi1) and efflux (Lsi2) of silicon into rice plants (Ma et al. 2007). These Si transporter (Lsi1 and Lsi2) channels also help in AsIII influx and efflux in the rhizosphere respectively. Though the Lsi1 and Lsi2 have the similar features in respect of their expression and cellular/tissue-level localization, the later one (OsLsi2) acts as As-Si efflux transporter in rice. Lsi2 is confined to the proximal side of the root exodermis and endodermis cells. Rice Lsi1 (OsLsi1) also permits the uptake of methylated arsenic species like MMA and DMA (Li et al. 2009). Immediately after the arsenate (AsV) uptake by rice plants, it converts into AsIII by the action of Arsenate Reductase (AR) enzyme, which utilizes the conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG) (Shri and Chakrabarty 2015). Then AsIII subsequently chelated with thiol ligands such as glutathione or phytochelatins (PCs) that acts as a detoxification mechanism, which is sequestered in the vacuoles from the cytosol through the ABC transporters such as OsABCC1 and OsABCC2 (Tripathi et al. 2007). Phytochelatin synthase (PC synthase or PCS) helps the production of phytochelatins from reduced glutathione (GSH) for the oxidative cellular detoxification and such process is activated by the exposure of any toxic metalloids including arsenic (Fig. 15.1). Thus, a large fraction of the toxic metalloids are bound in PC-Metalloid complex inside the cell. It was reported in rice that high amount of PC and producing enzyme PC-synthase coordinate the thiol metabolism and that gives rise the As tolerance to the plant (Tripathi et al. 2012). Along with the PCs, metallothionines (MTs) also play a major role in arsenic detoxification and mitigating oxidative damage to cells. These are the potential components that facilitate in maintaining cellular homeostasis by detoxifying the toxic metals/ metalloids. Till date, 11 number of class-I MT genes were reported in rice (Gautam et al. 2012). Based on the position and distribution of cysteine and associated controlled expression during the onset of metalloid stress, MTs are able to bind with arsenic (and/or other metalloids) and subsequently sequestered for metalloid detoxification (Singh et al. 2011). In this regard, iron (Fe) can minimize the arsenic accumulation in the plants and also controls the cellular oxidative damage in rice (Meharg 2004; Nath et al. 2014). There is another arsenic efflux transporter, Lsi2, which facilitates the transport of remaining AsIII fraction to the xylem loading. There is no transporters are identified till date which involved for the transport of MMA and DMA in the xylem. Besides these, there are also some unidentified transporters for AsIII efflux to the soil (Clemens and Ma 2016). Another transporter, OsNRAMP (Natural Resistance Associated Macrophage Protein in rice) which is present near to the Lsi2, performs the same function i.e. efflux of AsIII inside the root (Shri and Chakrabarty 2015). NRAMP is a highly conserved family of integral membrane bound protein channel, which are involved in ion transport in a wide range of organisms like humans, plants, bacteria etc. Among the planta, NRAMP (OsNRAMP1, OsNRAMP2 and OsNRAMP3) was first reported in rice (Belouchi et al. 1997).
The root histology of rice is characterized by the two casperian strips (CSs) in both exo- and endodermis region. So, just after uptake of As, it moves radially/laterally through epidermis, exodermis, cortex, endodermis and then stele (xylem and phloem). At this stage, pericycle cells will decide to translocate AsIII to the shoots. In the cells of both exo- and endodermis possess both Lsi1 (influx transporter) and Lsi2 (efflux transporter) in the opposite direction, where the former helps in arsenic influx into the cell and the later one participate in the efflux of arsenic towards the stele. So, Lsi2 is responsible for the translocation of AsIII in the xylem sap. Due to its loss-of-function mutation, the mutant planta are not able to translocate arsenic into the shoot (stem, leaves, straw and grains) and as a result, it can be found low arsenic accumulation in the rice grain (Ma et al. 2008). Through the upward translocation, AsIII is to be found in the phloem tissues at the node region of rice plant, mainly in the phloem companion cells (PCCs). From the xylem vessel, sap with arsenic enters the adjacent xylem transfer cell (XTC) and bundle sheath (BS) through an unidentified transporter (?), but later moves towards the PCC through Lsi2 of BS (Fig. 15.2). In phloem, amount of AsIII is low as compared to the xylem sap but sufficient to get stored in the grain for any human health hazards. PCC performs a filtering role by trapping as much as AsIII in the vacuole as As-PC complex and here OsABCC1 plays an important role that restricting entry of arsenic into the phloem. OsABCC1 is localized to the tonoplast of PCCs of nodal diffuse vascular bundles (DVBs) (Song et al. 2014). Thus, in the leaves, chelated PC-arsenite complex reduces the chance of arsenic translocation to the grains (Duan et al. 2011).
15.6 Arsenic Mitigation Strategies
15.6.1 Agronomic Methods for As Mitigation
In the field condition, arsenic uptake, accumulation, translocation and its impact on rice plants can be mitigated by water management and soil aeration, by creating a condition in which inorganic arsenic will form insoluble complex and precipitate in the soil itself, and through mineral nutrient management strategies where soil nutrients or externally supplied nutrient to the soil will compete with arsenic to limit its uptake by plants (Matsumoto et al. 2015).
15.6.1.1 Irrigation and Water Management
Irrigation practices have greatest impact on the iron plaque formation in the rhizosphere and arsenic uptake by rice (Somenahally et al. 2011). Sprinkler irrigation practice is a type of interval-based irrigation with non-continuous water flow, which can influence the reduction of As accumulation in plants rather than the plant those are growing in continuously irrigated (flooded) system (Moreno-Jiménez et al. 2014). So, it is very relevant for the rice agro-ecological system. In water management approach, water-saving regime has been proved as sustainable solution to decrease the arsenic content in rice, as the arsenic compound can be mobilized to the plants during water-holding conditions due to the reductive dissolution of Fe-oxyhydroxides (Arao et al. 2011). In this edaphic condition, iron-reducing microbes tend to start reduction of Fe-oxyhydroxides that leads to the formation of AsIII from AsV along with with other methylated species of arsenic (Horneman et al. 2004). Thus, the soil status with optimum saved water condition leads to change the redox state of the soil by oxidization and reducing the conversion of the most toxic soluble arsenic species i.e., AsIII from AsV to make it less bioavailable to the plant (Takahashi et al. 2004). In oxidized state, the aerated soil has the ability to form iron plaques on the rice roots and scavenge the rhizospheric arsenic (Roberts et al. 2011). Such strategies can control the mobilization and bioavailability of arsenic to the rice plants (Xu et al. 2008). In such water management practices, researchers have found low arsenic accumulation in the rice grown under aerobic system rather than the anaerobic conditions (Talukder et al. 2011).
15.6.1.2 Nutrient Management and Fertigation
Application of nutrient rich soil amendments through fertilizers, fertigation (fertilizer mixed irrigation) or by nutrient salt solutions can possibly reduce the arsenic uptake by plants and restricts the translocational accumulation of arsenic in the rice grains (Bakhat et al. 2017). External application of iron salt (Fe) efficiently forms Fe-oxide or Fe-plaque on the root surface and sequester soil soluble AsV by adsorption to reduce the arsenic bioavailability in the rhizosphere and subsequently leads to low arsenic translocation from root to shoot (Nath et al. 2014). Exogenous application of metallic iron and Fe-oxide in the paddy field significantly reduces the arsenic concentration in the grains almost by 51% and 47%, respectively (Matsumoto et al. 2015). Fe-oxide can act as a sink for arsenic, thus excess presence leads to low arsenic uptake and accumulation (Liu et al. 2015). Phosphate (P) also competes with AsV like Fe. Lee et al. (2016) suggested three important factors controlling the effect of P on the mobilization of arsenic in the soil and its uptake in rice: (1) the competition between arsenate (AsV) and P for adsorption sites on soil particles, (2) the antagonistic effect between inorganic phosphate (Pi) and arsenic during uptake by rice roots, and (3) the role of Pi in translocation of arsenic from root to shoot. Arsenic toxicity in plants depends strongly on the As/P ratio in the soil rather than the absolute arsenic concentration. In specific conditions, there is some inhibitory effect of P as it competes with AsV for the same membrane bound transporters in the roots during uptake (Abedin et al. 2002). It was reported that, it can be possible to change the status of P in shoots, the arsenic concentration in the grains can be minimized (Lu et al. 2010). In addition to this, it was reported that application of calcium (Ca) with phosphate to the arsenic contaminated soil forms Ca-P-As complex and reduce the arsenic mobilization (Neupane and Donahoe 2013). Hu et al. (2007) proposed the significant roles of sulphur (S) to mitigate the As accumulation in rice by three probable ways: (1) S mediated induction and formation of Fe-plaques on the root surface and in the rhizosphere leading to decrease the soil arsenic content; (2) sulphate (SO4) mediated enhancement for sustainable binding of arsenic with the Fe-plaques; and (3) inhibiting arsenate transport through the membrane bounded transporters using SO4. Along with these, sulfur metabolism process plays a crucial role towards detoxification As (Finnegan and Chen 2012). During high sulphur concentrations, arsenic accumulation in roots is increased due to the synthesis of thiolic ligands (GSH and PCs). It can sequester arsenic in the roots, therefore, restricting its translocation in the shoot regions (Dixit et al. 2016). It was observed during the arsenate stress in rice, the genes responsible for the sulphur uptake, transport and metabolism tend to be up-regulated (Srivastava et al. 2016). Apart from these three major mineral nutrients, higher amount of silica (Si) when present in the arsenic contaminated soil, it also competes with arsenite as both are dependent on the same transporter, nodulin-26 like intrinsic proteins (NIPs) and results in less arsenic uptake and translocation (Zhao et al. 2009). Exogenous application of Si leads to decrease the amount of arsenic in shoots, leaves and grains besides protecting the photosynthetic apparatus from arsenic induced oxidative stress (Sanglard et al. 2016). Tripathi et al. (2013) showed the controlled uptake of AsIII and upgraded antioxidant defense performance can be mediated by Si. However, the application of iron and silica contained materials like silica gel, furnace and calcium silicate slag is very common in the Southeast Asian countries and the former agent is much effective than others to control the accumulation of arsenic to the aboveground parts of the rice plant (Matsumoto et al. 2015; Bakhat et al. 2017).
Improving fertilizer use efficiency is one of the most essential means and needs to be a common practice in rice farming system. Thus, balanced fertilization practice and nutrient management in the paddy field are among the good strategies to mitigate arsenic uptake and accumulation in the rice grain (Huq et al. 2011; Matsumoto et al. 2015). Bogdan and Schenk (2009) suggested that fertilizer management such as low P fertilization along with Fe and Si application may considerably reduce the arsenic content in rice grains.
15.6.2 Biotechnological Approaches Through Transgenics
By the gene manipulation and transgenic techniques, it is now possible to upgrade arsenic mitigation and improve tolerance in rice. Such approaches include the over-expression of phytochelatin synthase gene (PCS) for the production of GSH and PC like chelators to detoxify arsenic in plants (Sharma et al. 2014). Shri et al. (2014) utilized PCS gene (CdPCS1) from Ceratophyllum demersum for its heterologous expression in rice and suggested its role to reduce the arsenic in shoot and restricts the accumulation of arsenic in the roots. Accumulation of arsenic in grains can also be controlled through the production of arsenite-thiol complex, which scavenge the arsenic in stabilized form in the root and other plant parts rather than the grains (Dhankher et al. 2011). Though, having same arsenic transporters, it was reported that transgenic plants are highly efficient to reduce the arsenic content by 30% in both underground and aerial part rather than the wild species (Duan et al. 2012). An ACR3 gene from yeast cell helps the rice transgenics to the effective efflux of AsIII by the expression of encoding arsenite efflux transporter protein; and thus minimizes arsenic accumulation in the grains. Another approach is to utilize the bacterial ArsM gene that encodes AsIII-S-adenosyl methioninemethyltransferase to make the transgenic rice. Such method is helpful as the gene product may produce MMA and DMA to bind inorganic arsenic by methylation and subsequent production of volatile trimethylarsine (TMA) (Chen et al. 2017) (Table 15.1). Apart from these molecular tailoring, modern gene editing technologies provide a new way to characterize and functionally modify the gene of interest to improve the crop production and tolerance to abiotic stresses. On the basis of bacterial cluster regularly interspaced short palindromic repeats -associated nuclease (CRISPR-CAS), gene-editing become very important using RNA guided CRISPR/CAS9 system along with the transcription activator – like effector nucleases (TALENs) and zinc-finger nucleases (ZFNs) (Chen et al. 2017).
15.6.3 Bioremediation Through Micro-organisms
Micro-organisms, present in the rhizosphere zone of the rice plant, may limit the mineral nutrient concentration in the soil. It has both beneficial as well as poses negative impact on the crop plants. But the micro-organism mediated bioremediation strategies can be utilized to detoxify the arsenic content in the soil and its mobilization inside the plant. Due to the presence of some chemical components like amino sugars, uronic acid, proteins, etc., arsenic binds with the H-bonding in the extracellular surface of the soil bacteria, e.g. Bacillus, Halobacterium, Rhodococcus (Bakhat et al. 2017). Some other microbes chelate arsenic by extracellular ferrous and ferric hydroxide production. Fungus mediated cost-effective arsenic mitigation strategy involves arbuscular mycorrhiza (AM) which represses the transcript expression of arsenic transporters (OsLsi1, OsLsi2). As a result, arsenic translocation and accumulation in the rice grain is minimized and/or stopped without affecting the cumulative yield and biomass (Chen et al. 2012).
15.7 Concluding Remarks and Future Prospects
The exposure of a major crop plant like rice to arsenic toxicity remains as a serious threat for mankind and food security. It needs well-designed strategies and fruitful actions to mitigate the arsenic contamination in grains to make it safe for human consumption. It is also important to understand the various physio-molecular mechanisms of actions in plants during arsenic stress and mechanisms related to detoxification of it. After the uptake by As-transporters present in roots, it induces oxidative damage by producing ROS, which in turn exerts several deleterious effects on cellular biomolecules with physiological and metabolic importances. Then inorganic and organic forms of arsenic tend to accumulate in rice grains. But the plant’s native ROS detoxification mechanism using phytochelatins (PCs) can minimize the arsenic concentration at a certain level. Based on the severity of stress condition, such detoxification mechanism is not adequate enough. Under such circumstances, other mitigation strategies such as agronomic practices, water and nutrient management, transgenic and biotechnological approaches appear to be useful for making arsenic free/less rice for human consumption.
Abbreviations
- AM:
-
Arbuscular mycorrhiza
- AR:
-
Arsenate reductase
- As:
-
Arsenic
- BS:
-
Bundle sheath
- CS:
-
Casperian strip
- DMA:
-
Dimethylarsinic acid
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidised glutathione
- Lsi:
-
Silicon and/or arsenic transporter
- MMA:
-
Monomethylarsonic acid
- MT:
-
Metallothionine
- NIP:
-
Noduline 26 like intrinsic protein
- NRAMP:
-
Natural resistance associated macrophage protein
- PC:
-
Phytochelatins
- PCC:
-
Phloem companion cell
- PCS:
-
Phytochelatin synthase
- PSR:
-
Phosphate starvation response
- PT:
-
Phosphate transporter
- ROS:
-
Reactive oxygen species
- TMA:
-
Trimethylarsine
- XTC:
-
Xylem transfer cell
References
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Ali W, Isayenkov SV, Zhao FJ, Maathuis FJM (2009) Arsenic transport in plants. Cell Mol Life Sci 66:2329–2339
Arao T, Kawasaki A, Baba K, Matsumoto S (2011) Effects of arsenic compound amendment on arsenic speciation in rice grain. Environ Sci Technol 45:1291–1297
Bakhat HF, Zia Z, Fahad S, Abbas S, Hammad HM, Shahzad AN, Abbas F, Alharby H, Shahid M (2017) Arsenic uptake, accumulation and toxicity in rice plants: possible remedies for its detoxification: a review. Environ Sci Pollut Res 24:9142–9158
Belouchi A, Kwan T, Gros P (1997) Cloning and characterization of the OsNramp family from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions. Plant Mol Biol 33:1085–1092
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2010) Accumulation of arsenic and its distribution in rice plant (Oryza sativa L.) in Gangetic West Bengal, India. Paddy Water Environ 8(1):63–70
Bogdan K, Schenk MK (2009) Evaluation of soil characteristics potentially affecting arsenic concentration in paddy rice (Oryza sativa L.). Environ Pollut 157:2617–2621
Booth B (2009) Cancer rates attributable to arsenic in rice vary globally. Environ Sci Technol 43(5):1243–1244
Brammer H, Ravenscroft P (2009) Arsenic in ground water: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35:647–654
Chen X, Li H, Chan WF, Wu C, Wu F, Wu S, Wong MH (2012) Arsenite transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenite stress. Chemosphere 89:1248–1254
Chen Y, Han YH, Cao Y, Zhu YG, Rathinasabapathi B, Ma LQ (2017) Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci 8:268. https://doi.org/10.3389/fpls.2017.00268
Choudhury S, Panda SK (2004) Induction of oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) both under lead and arsenic phytotoxicity. Curr Sci 87:342–348
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67:489–512
Dhankher OP, Pilon-Smits EAH, Meagher RB, Doty S (2011) Biotechnological approaches for phytoremediation. In: Altman A, Hasegawa PM (eds) Plant biotechnology and agriculture – prospect for the 21st century. Oxford Academic Press, Oxford, pp 309–328
Dixit G, Singh AP, Kumar A, Mishra S, Dwivedi S, Kumar S, Trivedi PK, Pandey V, Tripathi RD (2016) Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol Biochem 99:86–96
Duan GL, Hu Y, Lui WJ, Kneer R, Zhao FJ, Zhu YG (2011) Evidence for a role of phytochelatins in regulating arsenic accumulation in rice grains. Environ Exp Bot 71:416–421
Duan G, Kamiya T, Ishikawa S, Arao T, Fujiwara T (2012) Expressing ScACR3 in rice enhanced arsenite efflux and reduced arsenic accumulation in rice grains. Plant Cell Physiol 53:154–163
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:1–18
Gautam N, Verma PK, Verma S, Tripathi RD, Trivedi PK, Adhikari B, Chakrabarty D (2012) Genome-wide identification of rice class I metallothionein gene: tissue expression patterns and induction in response to heavy metal stress. Funct Integr Genom 12:635–647
Hasanuzzaman M, Roychowdhury R, Karmakar J, Dey N, Nahar K, Fujita M (2015) Recent advances in biotechnology and genomic approaches for abiotic stress tolerance in crop plants. In: Thangadurai D, Sangeetha J (eds) Genomics and Proteomics: concepts, technologies and applications. Apple Academic Press, Canada, pp 333–366
Horneman A, van Geen A, Kent DV, Mathe PE, Zheng Y, Dhar RK, O’Connell S, Hoque MA, Aziz Z, Shamsudduha M, Seddique AA, Ahmed KM (2004) Decoupling of As and Fe release to Bangladesh ground water under reducing conditions. Part I: evidence from sediment profiles. Geochim Cosmochim Acta 68:3459–3473
Hu ZY, Zhu YG, Li M, Zhang LG, Cao ZH, Smith FA (2007) Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ Pollut 147:387–393
Huq SMI, Sultana S, Chakraborty G, Chowdhury MTA (2011) A mitigation approach to alleviate arsenic accumulation in rice through balanced fertilization. Appl Environ Soil Sci 2011:1–18. Article ID 835627. https://doi.org/10.1155/2011/835627
IARC (2004) Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 84:1–477
Isayenkov SV, Maathuis FJM (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett 582:1625–1628
Islam S, Rahman MM, Islam MR, Naidu R (2016) Arsenic accumulation in rice: consequences of rice genotypes and management practices to reduce human health risk. Environ Int 96:139–155
Jayasumana C, Fonseka S, Fernando A, Jayalath K, Amarasinghe M, Siribaddana S, Gunatilake S, Paranagama P (2015) Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springer Plus 4:1. https://doi.org/10.1186/s40064-015-0868-z
Khan MA, Islam MR, Panaullah GM, Duxbury JM, Jahiruddin M, Loeppert RH (2010) Accumulation of arsenic in soil and rice under wetland condition in Bangladesh. Plant Soil 333(1–2):263–274
Lee CH, Wu CH, Syu CH, Jiang PY, Huang CC, Lee DY (2016) Effects of phosphorous application on arsenic toxicity to and uptake by rice seedlings in As-contaminated paddy soils. Geoderma 270:60–67
Li R, Ago Y, Liu W, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Liu C, Yu HY, Liu C, Li F, Xu X, Wang Q (2015) Arsenic availability in rice from a mining area: is amorphous iron oxide-bound arsenic a source or sink? Environ Pollut 199:95–101
Lomax C, Liu W-J, Wu L, Xue K, Xiong J, Zhou J, McGrath SP, Meharg AA, Miller AJ, Zhao FJ (2012) Methylated arsenic species in plants originate from soil microorganisms. New Phytol 193:665–672
Lu Y, Dong F, Deacon C, Hjun C, Raab A, Meharg AA (2010) Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South China. Environ Pollut 158:1536–1541
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448:209–212
Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105:9931–9935
Matsumoto S, Kasuga J, Taiki N, Makino T, Arao T (2015) Reduction of the risk of arsenic accumulation in rice by the water management and material application in relation to phosphate status. J Plant Interact 10(1):65–74
Meharg AA (2004) Arsenic in rice e understanding a new disaster for South East Asia. Trends Plant Sci 9:490–498
Meharg AA, Lombi E, Williams PN, Scheckel KG, Feldmann J, Raab A, Zhu Y, Islam R (2008) Speciation and localization of arsenic in white and brown rice grains. Environ Sci Technol 42:1051–1057
Meng XY, Qin J, Wang LH, Duan GL, Sun GX, Wu HL, Chu CC, Ling HQ, Rosen BP, Zhu YG (2011) Arsenic biotransformation and volatilization in transgenic rice. New Phytol 191(1):49–56
Moreno-Jiménez E, Meharg AA, Smolders E, Manzano R, Becerra D, Sanchez-Llerena J, Albarran A, Lopez-Pinero A (2014) Sprinkler irrigation of rice fields reduces grain arsenic but enhances cadmium. Sci Total Environ 485–486:468–473
Nath S, Panda P, Mishra S, Dey M, Choudhury S, Sahoo L, Panda SK (2014) Arsenic stress in rice: redox consequences and regulation by iron. Plant Physiol Biochem 80:203–210
Neumann RB, St Vincent AP, Roberts LC, Badruzzaman ABM, Ali MA, Harvey CF (2011) Rice field geochemistry and hydrology: an explanation for why groundwater irrigated fields in Bangladesh are net sinks of arsenic from groundwater. Environ Sci Technol 45(6):2072–2078
Neupane G, Donahoe RJ (2013) Calcium-phosphate treatment of contaminated soil for arsenic immobilization. Appl Geochem 28:145–154
Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci 99:13324–13329
Phillips DHJ (1990) Arsenic in aquatic organisms: a review, emphasizing chemical speciation. Aquat Toxicol 16:151–186
Raab A, Williams PN, Meharg A, Feldmann J (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4:197–203
Rahman MA, Hasegawa H (2011) High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci Total Environ 409(22):4645–4655
Rahman MM, Owens G, Naidu R (2009) Arsenic level in rice grain and assessment of daily dietary intake from rice in arsenic contaminated regions of Bangladesh - implications to groundwater irrigation. Environ Geochem Health 31:179–187
Roberts LC, Hug SJ, Voegelin A, Dittmar J, Kretzschmar R, Wehrli B, Saha G, Badruzzaman ABM, Ali MA (2011) Arsenic dynamics in pore water of an intermittently, irrigated paddy field in Bangladesh. Environ Sci Technol 45:971–976
Roychowdhury R, Karmakar J, Adak MK, Dey N (2013) Physio-biochemical and microsatellite based profiling of lowland rice (Oryza sativa L.) landraces for osmotic stress tolerance. Am J Plant Sci 4(12C):52–63
Sanglard LMVP, Detmann KC, Martins SCV, Teixeira RA, Pereira LF, Sanglard ML, Fernie AR, Araujo WL, DaMatta FM (2016) The role of silicon in metabolic acclimation of rice plants challenged with arsenic. Environ Exp Bot 123:22–36
Sharma S, Chatterjee S, Datta S, Mitra A, Vairale MG, Veer V, Chaurasia A, Gupta DK (2014) In vitro selection of plants for the removal of toxic metals from contaminated soil: role of genetic variation in phytoremediation. In: Gupta DK, Chattejee S (eds) Heavy metal remediation transport and accumulation in plants. Nova Science Publishers, New York, pp 155–177
Shraim AM (2017) Rice is a potential dietary source of not only arsenic but also other toxic elements like lead and chromium. Arab J Chem 10:S3434–S3443
Shri M, Chakrabarty D (2015) Arsenic in rice: a recent update. Res Rev J Bot Sci 4(2):8–11
Shri M, Dave R, Diwedi S, Shukla D, Kesari R, Tripathi RD, Trivedi PK, Chakrabarty D (2014) Heterologous expression of Ceratophyllum demersum phytochelatin synthase, CdPCS1, in rice leads to lower arsenic accumulation in grain. Sci Rep 4:5784. https://doi.org/10.1038/srep05784
Singh RK, Anandhan S, Singh S, Patade VY, Ahmed Z, Pande V (2011) Metallothionein – like gene from Cicer microphyllum is regulated by multiple abiotic stresses. Protoplasma 248:839–847
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Somenahally AC, Hollister EB, Loeppert RH, Yan W, Gentry TJ (2011) Microbial communities in rice rhizosphere altered by intermittent and continuous flooding in fields with long-term arsenic application. Soil Biol Biochem 43:1220–1228
Song W-Y, Yamaki T, Yamaji N, Ko D, Jung K-H, Fujii-Kashino M, An G, Martinoia E, Lee Y, Ma JF (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci 111:15699–15704
Srivastava S, Akkarakaran JJ, Sounderajan S, Shrivastava M, Suprasanna P (2016) Arsenic toxicity in rice (Oryza sativa L.) is influenced by sulfur supply: impact on the expression of transporters and thiol metabolism. Geoderma 270:33–42
Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K (2004) Arsenic behaviour in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38:1038–1044
Talukder AS, Meisner CA, Sarkar MA, Islam MS (2011) Effect of water management, tillage options and phosphorus status on arsenic uptake in rice. Ecotoxicol Environ Safety 74:834–839
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Tripathi P, Mishra A, Dwivedi S, Chakrabarty D, Trivedi PK, Singh RP, Tripathi RD (2012) Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol Environ Saf 79:189–198
Tripathi P, Tripathi RD, Singh RP, Dwivedi S, Goutam D, Shri M, Trivedi PK, Chakrabarty D (2013) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103
Wailes EJ (2005) Rice: global trade, proptectionist policies, and the impact of trade liberalization. In: Aksoy MA, Beghin JC (eds) Global agricultural trade and developing countries. The World Bank, Washington, DC.
Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540
Wu Z, Ren H, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157(1):498–508
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Zhao FJ, Ma JF, McGrath SP, Meharg AA (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao F-J, McGrath SP, Mehrag AA (2010) Arsenic as food chain contaminant: mechanism of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zhao F-J, Harris E, Yan J, Ma J, Wu L, Liu W, McGrath SP, Zhou J, Zhu Y-G (2013) Arsenic methylation in soils and its relationship with microbial arsM abundance and diversity, and As speciation in rice. Environ Sci Technol 47:7147–7154
Zhu Y-G, Yoshinaga M, Zhao F-J, Rosen BP (2014) Earth abides arsenic biotransformations. Annu Rev Earth Planet Sci 42:443–467
Acknowledgement
This work is the outcome of the part of Extramural Research Project vide 38(1430)/17/EMR-II supported by Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Roychowdhury, R., Khan, M.H., Choudhury, S. (2018). Arsenic in Rice: An Overview on Stress Implications, Tolerance and Mitigation Strategies. In: Hasanuzzaman, M., Nahar, K., Fujita, M. (eds) Plants Under Metal and Metalloid Stress. Springer, Singapore. https://doi.org/10.1007/978-981-13-2242-6_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-2242-6_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2241-9
Online ISBN: 978-981-13-2242-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)