Abstract
Peat has been used as an alternative, low-cost and efficient material capable of retaining metals. Most studies of adsorption have tended to focus on the characterization and adsorption mechanisms of temperate peats rather than tropical ones, therefore there is insufficient data about their characteristics and subsequent use in contaminated areas. The purpose of this study is to assess the chemical characteristics of tropical peats from the Mogi-Guaçu river Basin (Brazil), to evaluate their ability to capture potentially toxic metals in a contaminated mine area in Brazil. The peats were classified as H5–H6 on the Von Post scale of humification and had 48% ash content. The \( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \), ∆pH and the point of zero salt effect (PZSE) for peat 1 was 5.1, −1.0 and 3.6, while for peat 2, the values were 5.9, −2.4 and 3.1, respectively. These data showed materials with low acidity characteristic and a predominance of negative charges, which allows great cation retention. The cation exchange capacity (CEC) was considered high (91.0 and 116.0 cmolc kg−1), especially when considering the organic matter content (520.43 and 510.06 g kg−1). The removal of lead (Pb II) ions from the aqueous solution, investigated under different experimental conditions, revealed a satisfactory efficiency of 1/50, peat/solution ratio. Metals were removed in the descending order Pb > Zn > Cd, and both peats showed similar efficiency of lead sorption in high concentrations. The results show that the tropical peats have good characteristics to be used as alternative adsorbent materials in abandoned and contaminated mining areas.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Peat has become important as an organic low cost and efficient material for ion metallic adsorption (Gisi et al. 2016). Peatlands are soils formed by the humification of plant residues in acidic, moist and environments with low oxygen such as wetlands, river floodplains, coastal plains and lacustrine regions (Oliveira et al. 2014; Rezanezhad et al. 2016). Tropical peats are defined as organic soils between latitudes of 35°N and 35°S, including the whole of Brazil and Uruguay where there are large areas of peatlands (Andriesse 1988). The composition and texture of tropical peats differ from humid temperate peat deposits (Yonebayashi et al. 1992). In tropical areas, trees are more prevalent in peatland composition than in temperate regions, which are mainly composed by sphagnum moss (Andriesse 1988). The differences in characteristics may also affect adsorption efficiency. Peat is a material with a large surface area (>200 m2 g−1), high porosity and functional groups responsible for the polar character of this material. These characteristics make peat an efficient agent in the adsorption process (Tripathi and Ranjan 2015).
Adsorption has emerged as an alternative over other processes due to its effectiveness, safety, flexibility and economical treatment (Tripathi and Ranjan 2015). It is an efficient technique often used for soil, groundwater and wastewater treatment (Kocasoy and Guvener 2009). Adsorption is a process that is adaptable for both treatment techniques and containment of pollutants, meaning the adsorbents could be used not only as impermeable barriers (sealing soil) but also for permeable reactive barriers (treatment), controlling the amount of contaminants transported in a porous medium (Zuquette et al. 2008).
Numerous studies have attempted to explain the mechanisms of adsorption, controlled by the properties of temperate peats (Kalmykova et al. 2008). However, there is limited information available on the adsorption and desorption of metals of tropical peats, especially their chemical and physical characterizations for metal retention (Oliveira et al. 2014). Tropical peats are rarely used in the treatment of contaminated mining areas due to a lack of knowledge about the material. This paper will investigate the chemical characteristics of two tropical peats from the Mogi-Guaçu river Basin (Brazil), including their adsorption pre-test efficiency, in order to assess peats’ ability to be used as future alternative adsorbent material in an abandoned and contaminated mining area in southeastern Brazil (soil and water with high metal concentrations).
2 Materials and Methods
Peat 1 was collected in the Mogi-Guaçu river Basin, in city of Cravinhos (the state of São Paulo), Brazil. The area is a commercial peat extraction zone. Peat 2 was also extracted from the Mogi-Guaçu river Basin, but at Km 40 of the SP-255 road in Luis Antonio city, Brazil. Both peats were from a warm tropical climate region, characterized by a dry fall/winter and a rainy summer/spring. As a commercial product, Peat 1 was originally air-dried and homogenized, while peat 2 was air-dried, homogenized in a porcelain mortar and sieved through a 2 mm mesh sieve, after extraction. Both were stored at room temperature prior to analysis. Analysis of the pH in water, the pH in KCl, the degree of decomposition, the cation exchange capacity (CEC), the point of zero salt effect (PZSE), ash and organic matter content were carried out for peat characterization, besides adsorption pre-tests.
2.1 \( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \), pHKCl and ∆pH
The pH of the peats was potentiometrically measured in the supernatant suspension of a 1/2.5 peat(g)/liquid(mL) ratio according to Brazilian Public Agricultural Research Corporation (2011), in triplicate and using a digital pH meter (Digimed DH 21). The liquid was either distilled water (\( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \)) or a 1 M KCl solution (pHKCl). The ∆pH index was used to estimate the sign of the net charge and it was calculated from the difference between pHKCl and \( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \) values (Mekaru and Uehara 1972).
2.2 Ash Content, Organic Matter and Degree of Decomposition
The ash and organic matter content was determined through ignition in a muffle furnace at 550 °C. The degree of decomposition was determined using the Von Post Scale (Grover and Baldock 2013), which provides a qualitative classification system of humification ranging from H1 (insignificant or very slightly decomposed peat) to H10 (highly humified).
2.3 Determining of Point of Zero Salt Effect (PZSE)
The peats’ points of zero salt effect were determined using potentiometric titration (Sposito 1984). For each peat, 24 samples of 4 g each were divided into two groups of 12 samples, which distributed in Erlenmeyers containing: 20 mL of KCl 0.1 M, KCl 0.01 M and KCl 0.001 M. Samples from each of these salt concentration groups received 0.002, 0.004, 0.008 and 0.016 M of HCl (a total of 12 acid solutions) and 0.002, 0.004, 0.008 and 0.016 M of NaOH (12 basic solutions). For peat 1, two more concentrations were added to the salt concentration: 0.0032 and 0.064 of HCl (a total of 18 acid solutions) and 0.0032 and 0.064 of NaOH (18 basic solutions). The samples were agitated every 30 min for 24 h. The titration curves were obtained by plotting the pH values determined at the four points of each electrolyte concentration (KCl) as a function of adsorption of OH− or H+, according to the amounts of acid/base (HCl/NaOH) added. The common point of intersection of the three curves was defined as the pH value equivalent to the PZSE.
2.4 The Cation Exchange Capacity (CEC)
The CEC in peats were based on the occupation of the exchange sites with hydrogen- ions from a diluted solution of hydrochloric acid, the elimination of excess acid, the displacement of adsorbed hydrogen ions with calcium acetate solution and then the titration of the acetic acid formed with NaOH. The procedure was in accordance with the Brazilian Ministry of Agriculture, Livestock, and Supply (2013) and it is recommended for organic materials.
2.5 Adsorption Test
The BET analysis test at room temperature was used in adsorption tests. First, as a preliminary step for adsorption, peat 1 was tested in order to verify the favorable condition of peat/solution ratio for the BET equilibrium test (Roy et al. 1992). Five different ratios were tested: 1/5, 1/10, 1/50, 1/80, 1/100. For this first situation, Pb was used at 100 mg L−1 because it is a concentration above the highest value of Pb leaching (77 mg L−1 according to Raimondi (Raimondi 2014) from mine waste) in the region intended for use of the peats as adsorbent materials. Secondly, an adsorption test was carried out for Pb, Zn and Cd at 150 mg L−1 using the favorable ratio from step 1 for both peats. The metal contents (Pb, Zn and Cd) were determined using atomic absorption in PerkinElmer PinAAcle 900F equipment.
3 Results and Discussion
Analytical data for the peat samples are given in Table 1. According to the Von Post scale of humification degree, the samples of both peats were between two categories: moderately and moderately highly decomposed peats (H5 and H6). It’s important to emphasize the large amount of recognizable plant structure (fibres) resistant to decomposition present in both peats, an unusual characteristic for moderately decomposed temperate peats. However, the fibrous feature is commonly found in tropical peat soils (Andriesse 1988). Brazilian peats from the Southeast region (including São Paulo state) can be classified as fibric, being composed by fibers and filaments immersed in a gelatinous and dark color matrix (Kiehl 1985), in accordance with the results obtained for Mogi-Guaçu peats. The high fiber content may interfere in adsorption efficiency.
Regarding the organic matter and ash content (Table 1), there were no significant differences between both peats. For organic matter, Peat 1 showed an average of 520.43 ± 2.08 g kg−1 as well as peat 2, which was 510.06 ± 8.26 g kg−1. For ash content peat 1 and peat 2 were 47.9 ± 0.21% and 48.9 ± 0.82%, respectively. In terms of ash content, samples had comparatively higher amounts than the average values found in other studies (Kiehl 1985; Huat et al. 2011). And consequently, lower levels of organic matter (Kiehl 1985; Huat et al. 2011), probably due to the degree of degradation of the peat. For (Huat et al. 2011), peats usually have at least 65% organic matter or less than 35% mineral content. However, in the state of São Paulo (Brazil) high levels of ash is an ordinary characteristic for peats formed near rivers to have, as a consequence of periodic flooding (Cabral Junior et al. 2001). For (Wust et al. 2003) attempted to classify tropical peats in Malaysia based on ash content to divide the peats into a new system. For the authors, most peat classification schemes are developed in boreal and humid temperate regions, therefore they fail to adequately characterize tropical deposits. Both peats analyzed from the Mogi-Guaçu basin were better adjusted to this new classification system, being classified as very high ash content peats (40–55%).
The cation exchangeable capacity (CEC) was considered favorable for future use of the materials in the adsorption of potentially toxic metals ions (peat 1: 91.0 cmolc kg−1 and peat 2: 116.0 cmolc kg−1—Table 1), despite the organic matter content. To compare results, peats from others Brazilian states (Santa Catarina and Rio de Janeiro) had CECs of 140 and 67 cmolc kg−1 for their respective organic matter contents: 75 and 32.5% (Petroni et al. 2004; Crescêncio Junior 2008). The organic matter present in soils and peats contributes significantly to the cation exchangeable capacity values (Sparks 1995; Alleoni et al. 2009) and the main exchangeable sites are the functional acid groups (humic acids) (Huat et al. 2011). So, the CEC found indicates both materials are favorable for adsorption of metal in mine contaminated areas.
As the pH of peat increases with increasing humification, CEC is also pH-dependent and generally raises with an increase in the degree of decomposition (Delicato 1996). At pH 7.0, less decomposed peats have CEC values around 100 cmolc kg−1. On the other hand, highly decomposed peats have CEC values around 200 cmolc kg−1 (Andriesse 1988).
Peats can be classified into four broad categories based on pH (American Society for Testing and Materials 2013) (Table 2).
Peats usually have low pH, caused by reactions to acid decomposition (Kiehl 1985). Both peats can be classified as slightly acidic according to ASTM D4427—13 (Table 2), with pH values of 5.1 for peat 1 (from Cravinhos city) and 5.9 for peat 2 (from Luis Antônio city) (Table 1). For (Dick et al. 2009) at 4 and 5 pH values, over 90% of the carboxylic functional groups of organic matter (COOH of pKa-3) are already dissociated. And the carboxylic groups are the major contributors to CEC under natural conditions, since phenolic hydroxyl groups dissociate at higher pH (pH 8 to 9), limiting their contribution to CEC.
As shown in Table 1, in relation to the pH in water, the pH of peat suspension is lower when adding an electrolyte to it like KCl (pHKCl for peat 1is 4.1 ± 0.0 and for peat 2 is 3.5 ± 0.1). These results are important for providing information about whether peats’ colloids possess net negative or net positive charges (Mekaru and Uehara 1972). The ΔpH, calculated by the difference between pHKCl and \( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \) is an efficient index used for estimating the signal and the magnitude of the net surface charge of tropical soils (Alves and Lavorenti 2005).
The ΔpH determined are −1.0 ± 0.1 for peat 1 and −2.4 ± 0.1 for peat 2 (Table 1). These results (ΔpH < 0) indicate the predominance of negative charges, demonstrating the peat´s ability to retain more cations than anions—a favorable feature for ion metallic adsorption.
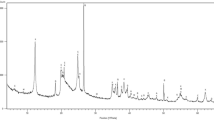
With respect to the point of zero salt effect (PZSE), Fig. 1 shows the titration curves for the two peats. The PZSE for peat 1 and for peat 2 were 3.6 and 3.1, respectively. PZSE is one distinct point of zero charge (Sposito 1984), meaning it is the pH value in which the ionic concentration of the solution does not influence the magnitude of the variable surface charges (Alves and Lavorenti 2005). The pH values in water for peats (5.1 ± 0.1 and 5.9 ± 0.1) were greater than the value of PZSE. These results indicated that the net surface charge of the materials is negative, therefore they have the ability to retain cations from the environment. The results were consistent with the ΔpH values of Table 1 (−1.0 ± 0.1 and −2.4 ± 0.1). PZSE values generally present a positive correlation with ΔpH, since as ΔpH approaches zero or becomes more positive, there is a corresponding increase in PZSE (Silva et al. 1996). Humic substances of organic soil matter are a major factor in pH-dependent charges in soils (Sparks 1995) and the functional groups existing could gain or lose protons, being the cause of the variation in the superficial liquid charge. At values above 3.6 for peat 1 and 3.1 for peat 2 (\( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \) > PZSE), the hydroxyl groups of the phenolic and carboxylic groups on the surface of the peat undergo deprotonation, becoming negatively charged. For the values below 3.6 and 3.1 (\( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \) < PZSE), the inverse occurs. A low PZSE value for any soil (or for peat) indicates that the material has a negative charge over a wide pH range and consequently a wide pH action to adsorb cations (Sparks 1995).
Regarding the favorable condition of peat/solution ratio for the BET equilibrium test, Fig. 2 provides the results from the preliminary adsorption test. From the data, it’s possible to observe the increase in the percentage of Pb removal up until the ratio 1/50, which is close to the maximum removal (being that 99% of removal is at ratio 1/50). For the following ratios (1/10 and 1/5), the efficiency reaches 100%. Thus, the 1/50 ratio was considered satisfactory and feasible for subsequent adsorption tests (Table 3).
For the adsorption of Pb, Zn and Cd at initial concentration of 150 mg L−1, the ratio of 1/50 was used. During the test there was no significant variation of pH or electrical conductivity in any of the samples. Lead ion removal from solutions was high for both peats (peat 1: 93.2%, peat 2: 93.6%—Table 3). While the removal of zinc and cadmium were lower (peat 1: Zn-48.2% and Cd-37.8%; peat 2: Zn-39.8% and Cd-23.6%). Peat 1 showed to be more adsorbent for Zn and Cd. Metal ions were removed in a descending order, Pb > Zn > Cd, for both peats, showing the affinity of the sorbent surface for lead. For (Kalmykova et al. 2008), the preference for Pb2+ could be explained through the binding of specific chelating groups in peats. In their studies, (Kalmykova et al. 2008) showed the metal ions were removed in the following order: Pb > Cd > Zn.
4 Conclusions
Both peats from the Mogi-Guaçu River Basin in São Paulo State (Brazil) have organic matter content considered relatively low, compared with the average content in other studies about peats. Still, other results like the CEC, the natural condition of pH (\( {\text{pH}}_{{{\text{H}}_{{\text{2}}} {\text{O}}}} \)), the ΔpH and the point of zero salt effect showed a predominance of negative charges in the material and characteristics favorable for potentially toxic metal ion adsorption.
The peats were classified as moderately and moderately highly decomposed peats (H5 and H6), according the Von Post scale of humification, but with high levels of fiber. They also had a high ash content. Those characteristics are considered typical for tropical peats, especially for peats from São Paulo state. However, they are characteristics that may influence the adsorption efficiency.
Adsorption pre-tests demonstrated that the maximum efficiency of lead adsorption is reached (or very close to maximum) at a ratio of 1/50 (peat/solution). The metals were removed in the descending order of Pb > Zn > Cd, showing the affinity of both materials for lead. Pb was 93% removed from the solution at an initial concentration of 150 mg L−1 and a ratio of 1/50.
The results of this study indicated that both peats are promising materials to be used in adsorption techniques for contaminated mining areas. However, only effective adsorption tests can prove this efficiency and reveal their potential for future use as protective barriers against contamination.
References
Alleoni, L.R., Mello, J.M.V., Rocha, W.S.D.: Electrochemistry, adsorption and ion exchange in soil. In: Mello, J.W., Alleoni, L.R. (eds.) Chemistry and Mineralogy of Soil, chap. XII, vol. 2, pp. 69–130. Brazilian Society of Soil Science, Viçosa (2009)
Alves, M.E., Lavorenti, A.: Point of zero salt effect relationships with clay mineralogy of representative soils of the São Paulo State Brazil. Pedosphere 5(15), 545–553 (2005)
American Society for Testing and Materials—ASTM. D4427: Standard classification of peat samples by laboratory testing (2013)
Andriesse, J.P.: Nature and Management of Tropical Peat Soils, vol. 59. FAO Bulletin—Food and Agriculture Organization of the United Nations, Rome (1988)
Brazilian Ministry of Agriculture, Livestock, and Supply: MAPA: Manual of Official Analytical Methods for Fertilizers and Correctives, 1st edn. MAPA, Brasilia (2013). in Portuguese
Brazilian Public Agricultural Research Corporation: EMBRAPA: Manual of Methods of Soil Analysis, 2nd edn. EMBRAPA, Rio de Janeiro (2011). in Portuguese
Cabral Junior, M., Motta, J.F.M., Mello, I.S.C., Tanno, L.C., Sintoni, A., Salvador, E.D., Chieregatti, L.A.: Mineral resources of the fenerozoic in São Paulo State. Geociências 20(1), 105–159 (2001). (in Portuguese)
Crescêncio Junior, F.: Study in laboratory for peats as a reactive barrier in aquifers remediation. PhD thesis at Universidade Federal do Rio de Janeiro. Rio de Janeiro (2008) (in Portuguese)
Delicato, D.M.S.: Physical-chemical properties and sorption characteristics of peat. PhD thesis Dublin City University, Dublin (1996)
Dick, D.P., Novotny, E.H., Dieckow, J., Bayer, C.: Chemistry of soil organic matter. In: Mello, J.W., Alleoni, L.R. (eds.) Chemistry and Mineralogy of Soil, chap. XII, vol. 2, pp. 69–130. Brazilian Society of Soil Science, Viçosa (2009)
Gisi, S.D., Lofrano, G., Grassi, M., Notarnicola, M.: Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain. Mater. Technol. 9, 10–40 (2016)
Grover, S.P.P., Baldock, J.A.: The link between peat hydrology and decomposition: Beyond von Post. J. Hydrol. 479, 130–138 (2013)
Huat, B.B.K., Kazemian, S., Prasad, A., Barghchi, M.: State of an art review of peat: general perspective. Int. J. Phys. Sci. 6(8), 1988–1996 (2011)
Kalmykova, Y., Strömvall, A.N., Steenari, B.M.: Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J. Hazard. Mater. 152(2), 885–891 (2008)
Kiehl, E.J.: Organic Fertilizers. Ceres Agronomic Publisher, Piracicaba (1985). (in Portuguese)
Kocasoy, G., Guvener, Z.: Efficiency of compost in the removal of heavy metals from the industrial wastewater. Environ. Geol. 57, 291–296 (2009)
Mekaru, T., Uehara, G.: Anion adsorption in ferruginous tropical soils. Soil Sci. Soc. Am. Proc. 36(2), 296–300 (1972)
Oliveira, L.K., Melo, C.A., Goveia, D., Lobo, F.A., Armienta Hernández, M.A., Fraceto, L.F., Rosa, A.H.: Adsorption/desorption of arsenic by tropical peat: influence of organic matter, iron and aluminium. Environ. Technol. 36(1–4), 149–159 (2014)
Petroni, S.L.G., Pires, M.A.F., Munita, C.S.: Use of radiotracer in adsorption studies of copper on peat. J. Radioanal. Nucl. Chem. 259(2), 239–243 (2004)
Raimondi, I.M.: Geological and geotechnical characterization of mining tailings—Adrianópolis (PR). Master thesis at São Paulo University, São Carlos (2014) (in Portuguese)
Rezanezhad, F., Price, J.S., Quinton, W.L., Lennartz, B., Milojevic, T., Van Cappellen, P.: Structure of peat soils and implications for water storage, flow and solute transport: a review update for geochemists. Chem. Geol. 429, 75–84 (2016)
Roy, W.R., Krapac, I.G., Chou, S.F.J., Griffin, R.A.: Batch Type Procedures for Estimating Soil Adsorption of Chemicals. Technical resource document. EPA/530-SW-87-006-F, Cincinnati, EUA (1992)
Silva, M.L.N., Curi, N., Marques, J.J.G.S.M., Guilherme, L.R.G., Lima, J.M.: Point of zero salt effect and its relations with mineralogical and chemical properties of Brazilian latosols. Pesquisa agropecuária brasileira 31(9), 663–671 (1996). (in Portuguese)
Sparks, D.L.: Environmental Soil Chemistry. Academic Press, San Diego, California (1995)
Sposito, G.: The Surface Chemistry of Soils. Oxford University Press, New York (1984)
Tripathi, A., Ranjan, M.R.: Heavy metal removal from wastewater using low cost adsorbents. Bioremediat. Biodegradation 6(6), 1–5 (2015)
Wust, R.A.J., Bustin, R.M., Lavkulich, L.M.: New classification systems for tropical organic-rich deposits based on studies of the Tasek Bera Basin, Malaysia. Catena 53, 133–163 (2003)
Yonebayashi, K., Okazaki, M., Pechayapisit, J.: Woody fragments in tropical peat soils. In: Kyuma, K., Vijarnsorn, P., Zakaria, A. (eds.) Coastal Lowland Ecosystems in Southern Thailand and Malaysia. Showado-Printing, Kyoto (1992)
Zuquette, L.Z., Silva Júnior, E.M., Garcia, A.: Sorption aspects for the unconsolidated materials of the region of São Carlos (SP), Brazil (In Portuguese). Revista Escola de Minas 61(2), 219–230 (2008)
Acknowledgements
The authors are grateful for the National Council for
Scientific and Technological Development (CNPq) for financial support (305096/2015-0) and for their PhD fellowship (54134/2016-3). The authors also thank the São Paulo Research foundation (FAPESP) for a research fellowship (2014/07180-7) and for a scholarship (2015/02529-4).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Raimondi, I.M., Lima, J.Z., Rodrigues, V.G.S. (2019). The Characterization of Tropical Peats for Potentially Toxic Metals Adsorption Purposes in an Abandoned Mine Area. In: Shakoor, A., Cato, K. (eds) IAEG/AEG Annual Meeting Proceedings, San Francisco, California, 2018 - Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-319-93127-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-93127-2_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93126-5
Online ISBN: 978-3-319-93127-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)