Abstract
Tertiary hyperparathyroidism is hypercalcemia after kidney transplantation. A near-total parathyroidectomy is curative for tertiary hyperparathyroidism and should be considered within the first year of kidney transplantation if hypercalcemia does not resolve. Cure rates of hypercalcemia after a parathyroidectomy for tertiary hyperparathyroidism are >95%.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
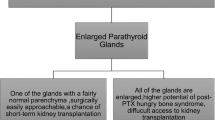
Tertiary hyperparathyroidism (3HPT) is described as persistent hyperparathyroidism with hypercalcemia despite correction of secondary hyperparathyroidism (from chronic renal failure) with renal transplantation. The reported incidence of post renal transplant hyperparathyroidism ranges from 25–50% at 1 year after transplant and about 17% at 4 years after transplant [1, 2]. The average time for parathyroid levels to return to normal post transplant varies between three to 6 months [3]. Calcium levels post renal transplant are also highly variable. The natural history of post transplant calcium levels can be categorized into three distinct groups: eucalcemia, hypercalcemia, and fluctuating calcium levels. These varying calcium levels present an obstacle to identifying patients with true tertiary hyperparathyroidism as opposed to patients who are still undergoing gland involution. Resolution of hypercalcemia >1 year post-transplant is highly unlikely. The incidence of “true” 3HPT with hypercalcemia post renal transplant ranges f rom 0.5% to 5.6% [4,5,6,7,8,9] (Table 18.1).
Indications for Parathyroidectomy in Tertiary Hyperparathyroidism
Tertiary hyperparathyroidism leads to decreased bone mineral density, changes in mental status, myopathies, and; importantly, volume depletion and calcification of the renal allograft, which may affect allograft function [10,11,12]. Parathyroidectomy (PTX) is the only definitive treatment option for 3HPT. Current indications for PTX include highly increased and persistent hypercalcemia, symptomatic hypercalcemia, increased parathyroid hormone levels, hypophosphatemia, decreased bone mineral density, or kidney function decline associated with hyperparathyroidism; however, it is unclear as to what calcium or parathyroid hormone level and at what time point after renal transplantation one should refer a patient for PTX [5]. At our institution and many others, patients are referred for PTX by the transplant nephrology department, and their criteria for referral are based on the level and persistence (greater than 6 months) of serum calcium and increases in PTH. Pharmaceutical therapy for hypercalcemia management is often initiated in these patients with a varied response. The role and timing of implementation of calcimimetics is controversial and it can be associated with worsening allograft function with a paucity of long term outcomes data. The decision to initiate calcimimetic therapy or to proceed with parathyroidectomy is often individual and institution dependent with a paucity of consensus derived guidelines. Studies have shown similar renal graft survival between patients who undergo PTX and control groups even though some studies report a decrease in GFR in patients who have undergone PTX. Benefits of PTX may include improved patient survival, improved bone mineral density, and alleviation of symptoms [13,14,15,16,17].
The optimal timing of PTX post-kidney transplant in patients with 3HPT is also difficult and the decision to proceed with PTX is based upon expectation of resolution of hypercalcemia, risks of surgery, cure rates and improvement in symptomology. Near-total or total PTX with autograft are both effective in curing 3HPT with numerous series documenting a cure rate >95% when resolution of hypercalcemia is used as a marker for cure [18]. However, up to 20% of patients will have elevated PTH levels and this is likely due to vitamin D deficiency, fluctuations in GFR and remaining hyperplastic tissue [19, 20]. Although, the rate of reoperations is low; patients with persistent marked elevations in PTH should be followed closely for hypercalcemia and some require a reoperation. The risks of parathyroidectomy are related to anesthesia and neck surgery. Permanent voice alteration from recurrent laryngeal nerve injury rates vary between 0–8% and are relatively uncommon in the hands of experienced endocrine surgeons [16]. Transient and permanent hypoparathyroidism is a known complication of any parathyroidectomy and the rates are low after PTX for 3HPT. Bone hunger and vitamin D deficiency should be assessed during the postoperative course and invariably patients will require calcium supplementation and vitamin D repletion. Permanent hypoparathyroidism is an extremely rare and avoidable complication. Careful assessment of the remnant parathyroid and its vascular viability should be routine in the operation and an immediate autograft of parathyroid fragments should be performed if the remnant is ischemic. Cryopreservation of parathyroid fragments should be performed in all patients with storage of fragments for at least 3–6 months. Intraoperative parathyroid hormone (IOPTH) monitoring can not only help assess the adequacy and completeness of a PTX, and IOPTH also helps assess parathyroid remnant function.
Effect on allograft function from 3HPT and PTX is an area of controversy with conflicting data on outcomes [6, 12]. Hypercalcemia and hyperparathyroidism can cause vasoconstriction, renal arterial calcification and tubulointerstitial calcification which can lead to decrease allograft function and lead to graft failure [20]. There are direct effects of PTH on vascular contractility and vascular calcifications which can lead to decrease allograft blood flow [21, 22]. Increasing GFR without evidence of rejection can serve as a marker for consideration of PTX. There are conflicting reports on improvement in GFR and a decrease in rejection episodes after PTX in 3HPT. In case-controlled series an increase in GFR was noted in patients undergoing PTX when compared to patients with medical management, however, this group also had a higher rate of eventual graft failure.
Uncontrolled 3HPT is associated with increased mortality, myocardial disease, vascular calcification, worsening bone disease, calciphylaxis, neuropsychiatric symptoms, generalized malaise and fatigue [4,5,6,7,8,9]. Improvements in all of these are well documented in literature from PTX and calcimimetic therapy. In the past few years calcimimetic therapy is more readily available and therefore more likely to be utilized. However, PTX results in immediate and the most profound control of hypercalcemia and symptoms and it is also cost-effective.
Early intervention may improve outcomes in patients with 3HPT. Kidney transplantation results in normal urinary excretion of phosphorus and restores calcium and vitamin D homeostasis, which in >95% patients is curative for hyperparathyroidism. However up to 5% of patients will develop 3HPT. In patients undergoing PTX for 3HPT there are pre-transplant and post-transplant factors associated with an increased risk for 3HPT [23]. Pre-transplant factors with increased risk of the development of 3HPT were elevated serum calcium, very high PTH levels (>1000 pg/mL), longer vintage of dialysis, failed parathyroidectomy and use of calcimimetics (Table 18.2). Hypercalcemia immediately after kidney tra nsplantation is observed in many patients with resolution seen in the majority of patients. However, when patients are hypercalcemic at 1 or 3 months they are 11 and 15 times more likely to require PTX, respectively. When PTH and serum calcium are used concurrently at 1 month post-kidney transplant they are strong predictors of the development of 3HPT. We believe that an early PTX (within the first year of transplant) is cost-effective and likely to offer a greater benefit to the patient and further waiting is unlikely to be curative for 3HPT.
Techniques of Parathyroidectomy in Tertiary Hyperparathyroidism
The technique described here is as a near-total parathyroidectomy is a more precise variant of subtotal parathyroidectomy. In 3HPT parathyroid glands are hyperplastic, three and three fourths are removed, leaving a vascularized remnant of one gland which approximates the size of 2 normal parathyroids; and the fragment which are equivalent of 8–10 normal glands are cryopreserved. The in-situ gland remnant may be marked with a permanent suture for future identification (Fig. 18.1). Alternately, all parathyroids may be removed from the neck, and the equivalent of 1–2 normal parathyroids may be minced into 1–2 mm fragments and autografted into either the sterno-cleidomastoid or nondominant forearm muscle. Autografted fragments should not be prepared from nodular parathyroids but from the more uniform parathyroids. In this instance, additional fragments should be cryopreserved as a safeguard in case the autografted tissue is insufficient. Total parathyroidectomy and autotransplantation has the advantage that recurrent parathyroid hyperfunction may be treated by partial autograft excision under local anesthesia. However, some autografts fail to function. In these patients a vascularized parathyroid remnant often functions better, and patients may be discharged sooner. Furthermore, in our experience, vascularized remnant regrowth is distinctly uncommon if the size of the remnant is small (the equivalent of 2 normal parathyroids in mass, approximately 80 mg). In some institutions, a small group of patients undergo a less than subtotal or near-total parathyroidectomy due to variation in appearance, size and vascularity of the observed parathyroid glands. Outcomes of this type of “limited” parathyroidectomy in 3HPT have been good in some series by experienced surgeons. Although this practice may be efficacious for some patients, we believe there are pitfalls in such an approach and this has led to referrals to many endocrine surgeons for reoperations in patients for persistent 3HPT. Also the pathophysiology of 3HPT is of renal insufficiency triggered hyperplasia leading to abnormality in all glands. This may play role in recurrence of hyperparathyroidism for patients after a kidney transplant since a significant number of patients develop chronic renal insufficiency with decrease graft function and up to 20% of patients also progress to failure of the transplanted kidney and resumption of dialysis. Leaving as small an amount of parathyroid tissue as possible without leading to hypoparathyroidism with cryopreservation of parathyroid fragments is the ideal approach.
References
Apaydin S, Sariyar M, Erek E, Ataman R, Yiğitbaş R, Hamzaoğlu I, Serdengeçti K, Ulkü U. Hypercalcemia and hyperparathyroidism after renal transplantation. Nephron. 1999;81(3):364–5.
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant. 2004;19(5):1281–7.
Triponez F, Clark OH, Vanrenthergem Y, Evenepoel P. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248(1):18–30. https://doi.org/10.1097/SLA.0b013e3181728a2d.
Gwinner W, Suppa S, Mengel M, Hoy L, Kreipe HH, Haller H, Schwarz A. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant. 2005;5(8):1934–41.
D’Alessandro AM, Melzer JS, Pirsch JD, Sollinger HW, Kalayoglu M, Vernon WB, Belzer FO, Starling JR. Tertiary hyperparathyroidism after renal transplantation: operative indications. Surgery. 1989;106(6):1049–55. discussion 1055–6.
Kandil E, Florman S, Alabbas H, Abdullah O, McGee J, Noureldine S, Slakey D, Zhang R. Exploring the effect of parathyroidectomy for tertiary hyperparathyroidism after kidney transplantation. Am J Med Sci. 2010;339(5):420–4. https://doi.org/10.1097/MAJ.0b013e3181d8b6ff.
Kinnaert P, Nagy N, Decoster-Gervy C, De Pauw L, Salmon I, Vereerstraeten P. Persistent hyperparathyroidism requiring surgical treatment after kidney transplantation. World J Surg. 2000;24(11):1391–5.
Kerby JD, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg. 1998;227(6):878–86.
Evenepoel P, Claes K, Kuypers DR, Debruyne F, Vanrenterghem Y. Parathyroidectomy after successful kidney transplantation: a single Centre study. Nephrol Dial Transplant. 2007;22(6):1730–7.
Rix M, Lewin E, Olgaard K. Posttransplant bone disease. Transplant Rev. 2003;17:176–86.
Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89(5):1227–39. https://doi.org/10.1016/j.suc.2009.06.011.
Schwarz A, Rustien G, Merkel S, et al. Decreased renal transplant function after parathyroidectomy. Nephrol Dial Transplant. 2007;22:584–91.
Madorin C, Owen RP, Fraser WD, Pellitteri PK, Radbill B, Rinaldo A, Seethala RR, Shaha AR, Silver CE, Suh MY, Weinstein B, Ferlito A. The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol. 2012;269(6):1565–76. https://doi.org/10.1007/s00405-011-1833-2.
Sharma J, Raggi P, Kutner N, Bailey J, Zhang R, Huang Y, Herzog CA, Weber C. Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg. 2012;214(4):400–7 discussion 407–8. https://doi.org/10.1016/j.jamcollsurg.2011.12.046.
Heyliger A, Tangpricha V, Weber C, Sharma J. Parathyroidectomy decreases systolic and diastolic blood pressure in hypertensive patients with primary hyperparathyroidism. Surgery. 2009;146(6):1042–7. https://doi.org/10.1016/j.surg.2009.09.024.
Milas M, Weber CJ. Near-total parathyroidectomy is beneficial for patients with secondary and tertiary hyperparathyroidism. Surgery. 2004;136(6):1252–60.
Daniel WT, Weber C, Bailey JA, Raggi P, Sharma J. Prospective analysis of coronary calcium in patients on dialysis undergoing a near-total parathyroidectomy. Surgery. 2013;154(6):1315–21 discussion 1321–2. https://doi.org/10.1016/j.surg.2013.06.030.
McPhaul JJ, McIntosh DA, Hammond WS, Park OK. Autonomous secondary (renal) parathyroid hyperplasia. N Engl J Med. 1964;271:1342–5.
Evenepoel P, Van Den Bergh B, Naesens M, De Jonge H, Bammens B, Claes K, Kuypers D, Vanrenterghem Y. Calcium metabolism in the early posttransplantation period. Clin J Am Soc Nephrol. 2009;4(3):665–72. https://doi.org/10.2215/CJN.03920808.
Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. J Am Soc Nephrol. 2002;13(2):551–8.
Sutliff RL, Weber CS, Qian J, Miller ML, Clemens TL, Paul RJ. Vasorelaxant properties of parathyroid hormone-related protein in the mouse: evidence for endothelium involvement independent of nitric oxide formation. Endocrinology. 1999;140(5):2077–83.
Massfelder T, Parekh N, Endlich K, Saussine C, Steinhausen M, Helwig JJ. Effect of intrarenally infused parathyroid hormone-related protein on renal blood flow and glomerular filtration rate in the anaesthetized rate. Br J Pharmacol. 1996;118(8):1995–2000.
Dewberry L, Tata S, Graves S, Weber C, Sharma J. Predictors of tertiary hyperparathyroidism: who will benefit from parathyroidectomy? Surgery. 2014;156:1631–7.
Somnay Y, Weinlander E, Alfhedi A, Schneider D, Sippel R, Chen H. Radioguided parathyroidectomy for tertiary hyperparathyroidism. J Surg Res. 2015;195:406–11.
Hsieh TM, Sun CK, Chen YT, Chou FF. Total parathyroidectomy versus subtotal parathyroidectomy in the treatment of tertiary hyperparathyroidism. Am Surg. 2012;78:600–6.
Park JH, Kang S-W, Jeong JJ, Nam K-H, Chang HS, Chung WY, et al. Surgical treatment of tertiary hyperparathyroidism after renal transplantation: a 31-year experience in a single institution. Endocr J. 2011;58:827–33.
Sadideen HM, Taylor JD, Goldsmith DJ. Total parathyroidectomy without autotransplantation after renal transplantation for tertiary hyperparathyroidism: long-term follow-up. Int Urol Nephrol. 2012;44:275–81.
Schlosser K, Endres N, Celik I, Fendrich V, Rothmund M, Fernández ED. Surgical treatment of tertiary hyperparathyroidism: the choice of procedure matters! World J Surg. 2007;31:1947–53.
Triponez F, Kebebew E, Dosseh D, Duh QY, Hazzan M, Noel C, et al. Less-than-subtotal parathyroidectomy increases the risk of persistent/recurrent hyperparathyroidism after parathyroidectomy in tertiary hyperparathyroidism after renal transplantation. Surgery. 2006;140:990–7.
Yang RL, Freeman K, Reinke CE, Fraker DL, Karakousis GC, Kelz RR, Doyle AM. Tertiary hyperparathyroidism in kidney transplant recipients: characteristics of patients selected for different treatment strategies. Transplantation. 2012;15(94):70–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Sharma, J., Weber, C. (2018). Early Versus Late Parathyroidectomy for Tertiary (Posttransplant) Hyperparathyroidism. In: Angelos, P., Grogan, R. (eds) Difficult Decisions in Endocrine Surgery. Difficult Decisions in Surgery: An Evidence-Based Approach. Springer, Cham. https://doi.org/10.1007/978-3-319-92860-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-92860-9_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92858-6
Online ISBN: 978-3-319-92860-9
eBook Packages: MedicineMedicine (R0)