Abstract
Acute encephalitis is a rapidly progressive encephalopathy due to brain inflammation. Historically, the most frequently identified causes of acute encephalitis have been infectious, though recently an increasing number of autoimmune encephalitides have been described. While autoimmune encephalitis can occur in the setting of a tumor, infections such as herpes simplex encephalitis can also serve as a trigger. The clinical presentation, along with neuroimaging and cerebrospinal fluid studies, are vital in making the diagnosis of autoimmune encephalitis and in distinguishing it from infectious encephalitis and from other causes of encephalopathy. First line treatments include corticosteroids, intravenous immunoglobulin, and plasma exchange, while second line agents include targeted or broad spectrum immunosuppressive agents. Long-term outcomes following autoimmune encephalitis are poorly characterized and persistent neurocognitive symptoms are likely underrecognized.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Acute encephalitis is a rapidly progressive encephalopathy due to brain inflammation, progressive over the course of weeks, and associated with significant morbidity as well as care burden to patients, families, and society [1, 2]. Historically, the most frequently identified causes of acute encephalitis have been infectious; however over the past decade, an increasing number of autoimmune encephalitides have been described. A subset of these autoimmune encephalitides are paraneoplastic in that they occur physically and potentially temporally remote from a tumor. Paraneoplastic autoimmune encephalitis, like other paraneoplastic neurological syndromes, is often the by-product of the immunological response directed against a cancer, and the development of a paraneoplastic syndrome can herald the detection of cancer or its recurrence by years [3,4,5]. In contrast, primary autoimmune encephalitides have been described in the absence of detected cancer at diagnosis or in longitudinal clinical care, typically characterized by immune responses directed against cell surface proteins including neurotransmitter receptors, water channels, and ion channels [6].
The diagnosis of an autoimmune encephalitis carries import for not only the immediate care for a patient presenting with a rapidly progressive encephalopathy but also the detection and monitoring for occult malignancy when appropriate [7]. The diagnosis of autoimmune encephalitis can be challenging, prompting the recent development of consensus clinical criteria for autoimmune encephalitis to help providers better identify patients and to differentiate autoimmune encephalitis from other neurological and psychiatric disorders [6]. As described below, additional challenges arise when diagnosing and treating patients with autoimmune encephalitis, including syndrome recognition, antibody testing in the commercial or research laboratory setting, the interpretation of antibody test results, the utility of various diagnostic modalities, and the acute and chronic management of the autoimmune encephalitis and its sequelae (Table 12.1).
The field of autoimmune encephalitis has matured from syndrome recognition and description to the exploration of disease mechanisms, potential relationships of infectious and autoimmune encephalitides, the evaluation of treatment approaches and pharmaceuticals, and the potential for novel treatment approaches in the paradigm of precision medicine. In this chapter we explore the diagnostic and treatment challenges that face the neurologist caring for a patient with possible autoimmune encephalitis as well as future directions in diagnosis and care.
Diagnosis
Clinical Presentation
Encephalitis is a severe, debilitating inflammatory disorder of the brain, with varied possible etiologies of a rapidly progressive encephalopathy leading to a broad differential diagnosis (Table 12.2) and potentially extensive diagnostic evaluation [1, 6, 8].
Syndrome onset and tempo play important roles in differentiating acute encephalitis from more chronic neurodegenerative and psychiatric syndromes. In general, acute encephalitis is characterized by the development and progression of brain inflammation leading to a debilitating neurological disorder in a matter of weeks, usually less than 6 weeks [1, 6]. More specifically for autoimmune encephalitis, consensus clinical criteria require subacute onset with rapid progression of less than 3 months of working memory deficits (or short-term memory loss), altered mental status, or psychiatric symptoms [6]. Altered mental status is further defined as decreased or altered level of consciousness, lethargy, or personality change [6]. These symptoms may be accompanied by other neurological symptoms or examination findings, some of which have been associated with specific autoantibodies [4, 6, 9].
The subsequent evaluation of patients presenting with signs and symptoms consistent with autoimmune encephalitis should include a conventional neurological evaluation to assess for potential alternative etiologies as well as to investigate for supportive findings by standard diagnostic tests, including magnetic resonance imaging (MRI), cerebrospinal fluid (CSF), and electroencephalography (EEG) studies. The diagnosis of autoimmune encephalitis is clinical and not dependent on the detection of an autoantibody as at times autoantibody testing is not readily accessible, the results of autoantibody testing may take weeks to return, the failure to detect an autoantibody in the serum or CSF does not exclude an autoimmune encephalitis, and false-positive antibody assay results can occur. As early immunotherapy appears to be associated with improved clinical outcome, the diagnostic evaluation is undertaken to support the diagnosis of autoimmune encephalitis while quickly clarifying the presence or absence of other etiologies, particularly infectious, to allow for rapid initiation of immunotherapy with treatment escalation as clinically indicated [10, 11].
Diagnostic Tests
As mentioned previously, the standard diagnostics used in the evaluation of patients with suspected autoimmune encephalitis include MRI of the brain, CSF assessment, and EEG [6]. The sensitivity and specificity of each of these standard diagnostics vary for autoimmune encephalitis in general and for specific autoantibody syndromes.
CSF assessment is of import in ruling out a number of infectious encephalitides, supporting a diagnosis of possible autoimmune encephalitis, and in diagnosing a specific autoantibody syndrome [6]. Routine CSF studies typically demonstrate a moderate lymphocytic predominant pleocytosis (≥5 WBC/mL), with normal glucose and potentially elevated CSF protein. The detection of intrathecal oligoclonal bands and an elevated serum to CSF immunoglobulin G (IgG) index indicate intrathecal antibody synthesis and are further supportive. It should be noted that a CSF pleocytosis may be transient, potentially only evident in the early stages of the encephalitis, as has been observed in anti-NMDA receptor (anti-NMDAR) encephalitis [6, 10, 12]. In addition, when evaluating patients for possible autoimmune encephalitis, it is recommended that autoantibody testing is sent from the CSF in addition to autoantibody testing in the serum [6]. The reasons for this are manifold. First, in some syndromes (e.g., anti-NMDAR and anti-LGI1), CSF antibody testing has been demonstrated to be more sensitive than serum testing alone [10, 13]. In addition, multiple antibodies can be detected in the serum, potentially in addition to those detected in the CSF. In such cases, CSF antibodies are more likely pathologic, with a lower rate of false-positive and false-negative results compared to serum antibody testing [6].
EEG is of variable sensitivity in autoimmune encephalitis, with the most frequent findings being non-specific slowing and disorganized cortical activity [6, 12, 14]. Consensus criteria for the diagnosis of possible autoimmune encephalitis and definite limbic encephalitis include temporal slowing (either unilateral or bilateral) [6]. Patients with autoimmune encephalitis may be found to have electrographic seizures, potentially as nonconvulsive status epilepticus [14]. There have been descriptions of rare electrographic findings in specific autoimmune encephalitis syndromes, such as extreme delta brush in anti-NMDAR encephalitis which is noted in a minority of cases [15]. Patterns commonly associated with other neurological syndromes have been noted in cases of autoimmune encephalitis, such as periodic sharp wave complexes commonly described in Creutzfeldt-Jakob disease also observed in patients with autoimmune encephalitis with autoantibodies directed against the voltage-gated potassium channel complex [16].
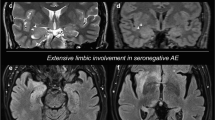
Brain MRI is of variable sensitivity, for instance, being abnormal in 33–50% of patients with anti-NMDAR encephalitis. Depending on the syndrome, there can be abnormalities of the mesial temporal lobes, gray matter, and/or white matter on T2 sequences with subtle gadolinium enhancement. Some lesions may also appear consistent with demyelinating diseases. Findings by MRI may be subtle and transient, resolving spontaneously through the course of disease or with treatment [6, 10, 12].
Though currently included in the consensus criteria for definite autoimmune limbic encephalitis [6], FDG-PET may in the future prove to play an important role in the diagnostic evaluation of patients with suspected autoimmune encephalitis. Consensus criteria include hypermetabolism of the mesial temporal lobe in lieu of T2 hyperintensities on MRI as meeting the radiographic criterion for definite autoimmune limbic encephalitis [6]. This criterion is based on primarily qualitative observations of FDG-PET studies from small series of patients with a variety of autoantibody syndromes, chiefly anti-NMDA receptor and anti-LGI1 encephalitis. In a recent retrospective series applying semiquantitative techniques, brain FDG-PET/CT was observed to often be abnormal in patients with possible autoimmune encephalitis, most commonly demonstrating hypometabolism [17, 18]. Demonstration of abnormalities by brain FDG-PET/CT was also noted to be in weak agreement with detection of abnormalities on at least two of the routine diagnostic assessments (CSF analysis, brain MRI, and/or EEG), suggesting its potential utility in addition to these routine studies in the diagnosis of possible autoimmune encephalitis [17]. Some series have also found that FDG-PET may be more sensitive than brain MRI for abnormalities in autoimmune encephalitis [18]. Finally, characteristic metabolism patterns have been noted in some autoimmune encephalitides which have been found to resolve with patient clinical improvement, such as parieto-occipital hypometabolism and relative anterior hypermetabolism in anti-NMDAR encephalitis [18,19,20]. Much work remains to prospectively assess the utility of FDG-PET in the diagnosis and clinical monitoring of autoimmune encephalitis, including its differentiation from other causes of encephalitis (e.g., infectious encephalitides) and syndromes (e.g., psychiatric, drug-induced).
Several autoantibodies directed against neuronal targets have been described in autoimmune encephalitis, with patients at times presenting with additional neurological symptoms and signs suggestive of particular autoantibody syndromes (Table 12.3). The autoantibodies themselves may play a direct role in disease pathogenesis or may be markers of systemic immunoreactivity directed against the nervous system [4, 21, 22]. It is not uncommon for multiple autoantibodies to be detected in the serum. For instance, in a review over 550 seropositive patients evaluated for a paraneoplastic neurological syndrome at a tertiary medical center, nearly a third were found to have multiple autoantibodies [23]. The pattern of autoantibodies detected was suggestive of the cancer ultimately detected and was not specific for a particular neurological syndrome [23]. In addition, autoantibodies have been detected in non-paraneoplastic, non-encephalitic syndromes, including Creutzfeldt-Jakob disease [24, 25]. Thus, in utilizing autoantibody testing in the serum alone, one runs the risk of detecting multiple autoantibodies, many of which are not involved in the pathogenesis of autoimmune encephalitis, leading to potential misdiagnosis. This issue of diminished specificity is compounded by the poorer sensitivity for serum autoantibody testing compared to autoantibody testing in the CSF [10, 13]. In light of these observations, current consensus recommendations include not only autoantibody testing in the serum but also concurrent testing in the CSF [6].
Intersection of Infection and Autoimmunity
As many as 10–25% of patients who experience an episode of herpes simplex encephalitis (HSE) will develop a relapse of neurologic symptoms weeks to months later in the absence of evidence of ongoing virus production [26]. Until recently the pathophysiology of these symptoms remained unclear and represented both a diagnostic and therapeutic challenge to clinicians. However, evidence has emerged that a number of these cases represent an autoimmune phenomenon in association with the development of antibodies to the NMDA receptor, thus representing a post-infectious autoimmune encephalitis. Indeed, such patients typically develop symptoms 4–6 weeks after HSE, have negative testing for herpes virus at the time of relapse, develop new enhancing or confluent lesions on brain MRI, demonstrate the presence of anti-NMDAR antibodies in the serum and/or CSF, and improve following the administration of immunotherapy [27, 28]. While an infection may lead to the generation of autoimmunity by a number of differing mechanisms including molecular mimicry, dysregulation of immune checkpoints, uncovering of cryptic neural epitopes, and bystander activation [28], the mechanisms by which HSE leads to the generation of anti-NMDAR antibodies remain to be discovered.
Identification of Autoantibodies
Autoantibody identification in autoimmune encephalitis is a rapidly emerging field that is typically based upon one or a combination of methodologies, including immunohistochemistry (IHC) on rodent brain sections, immunocytochemistry (ICC) of live primary neurons, and cell-based assays (CBA) where nonneural cells are transfected with an antigen of interest. Each of these methodologies has advantages and disadvantages, and together they can complement each other in the identification of autoantibodies . With ICC, for example, the tissue is typically from an adult animal and thus expresses mature (and likely relevant) antigens, various brain regions can be utilized, both cell surface and intracellular staining can be appreciated, and there is tremendous experience in interpretation of specific staining patterns [29]. Disadvantages are that the tissue is typically fixed in paraformaldehyde which even when done briefly may result in alteration of antigens and that cross-species differences between proteins may result in false negatives in some cases. ICC typically involves addition of serum or CSF to live primary rat hippocampal neurons such that the autoantibody only has access to the extracellular compartment, an advantage being specific detection of binding to extracellular epitopes. Disadvantages include the possibility that cultured hippocampal neurons may not express the range of antigens expressed in mature tissue, and that antigens expressed by other neuronal subtypes may not be found in hippocampal neurons, thus contributing to false negatives. Most CBAs utilize transfection of the antigen of interest into human embryonic kidney (HEK) cells, followed by either fixed or live staining utilizing either ICC or flow cytometry. Such methodologies theoretically allow for the precise detection of single autoantigens that serve as a target for patient autoantibodies [30, 31] and have been reported to have high sensitivity and specificity [30, 32, 33]. However, confounding factors include the potential need to express additional proteins to aid in targeting or localization of the antigen of interest to the cell surface, the potential for excitotoxicity in the setting of overexpression of ion channels, and subjectivity with scoring of ICC. Moreover, the need for a priori knowledge of the antigen of interest limits the potential for discovery of new autoantigens by CBA [29].
Treatment
Treatment of patients with autoimmune encephalitis entails a three-part approach that addresses (1) the autoimmune syndrome with immunotherapy, (2) an underlying malignancy if detected, and (3) treatment of associated sequelae of the syndrome. As autoimmune encephalitis is rare, our understanding of disease mechanisms, expert opinion, case series, and a few prospective trials guides treatment selection. Important factors in treatment consideration are the autoantibody detected, patient comorbidities and sensitivities, and the phase of illness (Table 12.4).
In the acute setting, autoimmune encephalitides associated with autoantibodies directed at cell membrane proteins tend to respond well to antibody-directed therapies such as intravenous immunoglobulin and plasmapheresis. These treatments often follow or accompany courses of intravenous corticosteroids such as intravenous methylprednisolone. Second-line therapies used in the acute phase include rituximab and cyclophosphamide. Mycophenolate and azathioprine are typically used in the maintenance phase, as are rituximab, cyclophosphamide, corticosteroids, and intravenous immunoglobulin [34,35,36].
In the case of autoimmune encephalitides associated with autoantibodies directed against intracellular antigens, immunomodulatory therapies such as plasmapheresis do not seem to be of benefit [9, 37]. Therapies directed at reducing the cell-mediated immune response, such as the cytotoxic agent cyclophosphamide and lymphocyte-specific medications such as mycophenolate, play an important role in mitigating the cytotoxic response and with hopes of minimizing the extent of consequent neuronal injury. The detection and treatment of an underlying cancer can have a dramatic clinical impact and play an important role in treatment. For instance, resection of detected ovarian teratomas has been considered as first-line treatment along with intravenous steroids, intravenous methylprednisolone, and plasmapheresis exchange in anti-NMDAR encephalitis [10]. Similarly, the chemotherapeutic medications used in the treatment of cancer have effects not only on the antigenic source, the cancer, but also immunosuppressive effects which can impact the immune response underlying the autoimmune encephalitis.
There are no guidelines of when is it appropriate to escalate from first- to second-line treatments, with administration of second-line agents typically utilized for cases of non-response or incomplete response to first-line therapies or for severe presentations of disease. In the largest series of anti-NMDAR encephalitis, the relapse rate for those treated with first-line therapy alone was 12%, while 10% of those treated with second-line therapy relapsed within the same time period [10]. There is mounting evidence for the use of rituximab as second-line immunotherapy in autoimmune encephalitis, regardless of antibody status, given reported tolerability and improved outcomes after first-line treatment [11, 38]. In addition, there is consideration for its use as a first-line agent, though prospective studies of this approach are lacking [6]. An additional therapeutic challenge revolves around duration of treatment. As with the decision to escalate treatments in autoimmune encephalitis, there are no guidelines as to how long to maintain such treatments. Goals of long-term immune treatment include cessation of neuroinflammation and attendant neurodegeneration, as well as limiting the risk of autoimmune relapse. While in many cases the ongoing neuroinflammation may subside over months, relapses can occur many years after the initial event [39]. A practical approach for patients with moderate to severe autoimmune encephalitis is to continue immunotherapy for 18–24 months with ongoing clinical and radiographic assessment of disease activity. Upon reaching a period of clinical stability, immune treatments can be gradually weaned with careful and frequent reassessment to determine whether treatment needs to be reinstituted.
Emerging Therapies
In patients who do not respond adequately to rituximab, tocilizumab, a monoclonal antibody against the interleukin-6 (IL-6) receptor, may hold promise. IL-6 is an important pro-inflammatory cytokine that has broad effects on multiple immune cells, and a number of recent efforts have focused on targeting the cytokine or its receptor to modulate inflammatory disease [40]. In a retrospective institutional cohort study of patients with autoimmune encephalitis initially treated with rituximab, tocilizumab resulted in better long-term outcomes compared to those given further rituximab or no subsequent treatment [41]. More recently, bortezomib, a proteasome inhibitor, was employed in patients with anti-NMDAR encephalitis, with the rationale that this drug can deplete plasma cells and potentially decrease levels of pathogenic autoantibodies. Four of five patients with treatment-refractory anti-NMDAR encephalitis treated with bortezomib were reported to show clinical improvement or disease remission and a corresponding fall in CSF antibody levels [42]. Another pro-inflammatory cytokine, interleukin-1 (IL-1), has also received recent attention as a potential therapeutic target, since levels of its antagonist are elevated in patients with a good outcome following encephalitis of infectious or autoimmune cause [43]. Indeed, a recent case report described the recovery of a patient with a chronic autoimmune meningoencephalitis following treatment with anakinra, an IL-1 receptor antagonist [44]. Notably, despite the growing number of potential therapeutic options, at the moment there is not enough evidence to inform a rationale treatment algorithm for those with autoimmune encephalitis refractory to conventional second-line agents.
Major Gaps
Despite the many advances described above, substantial gaps remain in our knowledge of diagnosis, treatment, and outcomes of autoimmune encephalitis. Here we discuss three such gaps: (1) arriving at an etiologic diagnosis for patients, (2) development of therapies based upon personalized medicine, and (3) achieving a more refined understanding of the sequelae of autoimmune encephalitis.
An Etiologic Diagnosis
Despite extensive testing for infectious and autoimmune conditions, up to 40% of all cases of acute encephalitis remain without an etiologic diagnosis [45, 46]. It is likely that some of these cases are accounted for by autoimmune conditions. Indeed, novel autoantibodies are being identified at a rapid clip via the methodologies mentioned above coupled with mass spectrometric identification of autoantigens [29]. However, screening techniques based upon rodent tissue may miss some human autoantigens, and thus the development of human protein-, cell-, or tissue-based platforms to identify novel autoantibodies is of importance. Protein display technologies such as phage immunoprecipitation sequencing (PhIP-Seq) can be utilized to identify binding between autoantibodies and large libraries of overlapping peptides that span most, if not all, of the human peptidome and have already been utilized to identify novel paraneoplastic autoantigens [47]. More recently, an in vitro translation platform termed parallel analysis of translated ORFs (PLATO) has been developed that enables translated proteins to remain bound to their mRNA. Thus, when autoantibody-antigen complexes are identified, the still attached mRNA allows for ready identification of the antigen of interest [48]. Notably, the approaches detailed above focus only on identification of autoantibodies, and it is becoming increasingly likely that additional novel autoantibodies will account for small proportions of disease. Disorders of cell-mediated immunity, which are not as readily identified as autoantibody-mediated disease, will likely account for a substantial proportion of undiagnosed autoimmune encephalitis cases. A combination of approaches, including careful clinical and immunophenotyping as well as immunogenetics, will be needed to elucidate these causes.
Toward Personalized Therapy
Current therapeutic paradigms for autoimmune encephalitis utilize broad strokes to impact the immune system and in so doing place patients at particular risk for opportunistic infections, malignancy, and systemic complications. Thus, a major challenge is to develop a more personalized approach to therapy based upon the specific pathogenic mechanism driving the disease process in each patient. There has been much interest in developing antigen-specific approaches that induce immune tolerance by targeting antigen-presenting cells (APCs) or T cells. For example, when an autoantigen is presented by an APC in the presence of low levels of co-stimulatory molecules and without additional activating stimuli, the T cell can be driven toward an anergic state that may at least transiently halt the autoimmune process. Engagement of additional negative signals between APCs and T cells can lead to death of T cells via clonal deletion or apoptosis, potentially resulting in longer-lived antigen-specific effects [49,50,51]. Current efforts are focused on cytokine-, cell-, and particle-based approaches as well as alternate antigen delivery methods (i.e., oral) that can cause specific reprogramming of lymphocytes either directly or through effects on APCs [49]. T cells can also be engineered to specifically detect and kill cells expressing a particular cell surface receptor [52, 53]. This technology, termed chimeric antigen receptor T cells (CAR-T), has been recently applied to a model of the autoimmune disease pemphigus vulgaris in which autoreactive antibodies target the protein desmoglien-3 (DSG3). CAR-T cells were found to selectively kill DSG3-reactive B cells, decrease autoreactive antibody titers, and prevent disease in this disorder of systemic autoimmunity [54]. It will be of interest to determine whether such approaches readily translate to disorders of CNS autoimmunity. Recent work on neuromyelitis optica (NMO), an autoimmune demyelinating disorder of the CNS, may also provide direction on novel specific therapies for autoimmune encephalitis. NMO is caused by binding of pathogenic autoantibodies to the aquaporin-4 (AQP4) water channel on astrocytes, resulting in complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. Mutation of the antibody to remove the pathogenic effector functions while maintaining tight binding to AQP4 resulted in a nonpathogenic antibody that competed with pathogenic antibodies for AQP4 binding, resulting in amelioration of lesion formation in a mouse model of disease [55]. Such approaches may be applicable to autoimmune encephalitis. Notably, methodologies that enable the identification and cloning of patient-specific autoantibodies in autoimmune encephalitis may facilitate the development of blocking antibodies as specific therapies [56].
Sequelae of Autoimmune Encephalitis
Following an episode of autoimmune encephalitis, patients experience a variety of neurocognitive sequelae and are at risk for seizures; however our understanding of the true impact of these is limited to case series and retrospective studies [57]. Not only are seizures a common initial presentation of autoimmune encephalitis, but many patients develop postencephalitis epilepsy [58, 59]. Antiepileptics are therefore commonly used both acutely and in the maintenance phase after the initial episode of encephalitis has resolved. In a subset of patients, antiepileptic medications alone were effective in controlling seizures [59], with consideration for antiepileptic selection based on patient-specific factors. Additionally, patients can experience long-term cognitive effects as a consequence of structural damage to underlying systems [60]. As such, patients may benefit from comprehensive rehabilitation services, with therapies tailored to specific patient deficits. Patients may also experience psychiatric sequelae such as psychosis and catatonia, both acutely as a part of the autoimmune encephalitis syndrome and chronically, necessitating psychiatric management. One point of caution is the use of antipsychotic medications in patients with anti-NMDAR encephalitis given observation of intolerance to these medications characterized by high temperature, mutism, coma, muscle rigidity, and rhabdomyolysis [61]. Finally, some of the treatments used may themselves have neurobehavioral side effects, such as steroid-induced encephalopathy or antiepileptic effects on concentration, memory, and mood [62, 63]. Future prospective studies of the long-term outcomes in patients with autoimmune encephalitis as well as sequelae of encephalitis and adverse effects of treatment are needed to help guide our care of patients as they recover as well as in counseling of patients and families regarding diagnosis, prognosis, and treatment selection.
References
Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–28.
Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82:443–51.
Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–54.
Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327–40.
Tarin D. Update on clinical and mechanistic aspects of paraneoplastic syndromes. Cancer Metastasis Rev. 2013;32:707–21.
Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404.
Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18:19–e13.
Frontera JA. Metabolic encephalopathies in the critical care unit. Continuum. 2012;18:611–39.
McKeon A. Paraneoplastic and other autoimmune disorders of the central nervous system. Neurohospitalist. 2013;3:53–64.
Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65.
Lee WJ, Lee ST, Byun JI, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86:1683–91.
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
Arino H, Armangue T, Petit-Pedrol M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87:759–65.
Johnson N, Henry C, Fessler AJ, Dalmau J. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75:1480–2.
Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–100.
Savard M, Irani SR, Guillemette A, et al. Creutzfeldt-Jakob disease-like periodic sharp wave complexes in voltage-gated potassium channel-complex antibodies encephalitis: a case report. J Clin Neurophysiol. 2016;33:e1–4.
Probasco JC, Solnes L, Nalluri A, et al. Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e352.
Solnes LB, Jones KM, Rowe SP, et al. Diagnostic value of 18F-FDG PET/CT versus MRI in the setting of antibody specific autoimmune encephalitis. J Nucl Med. 2017;58:1307–13.
Leypoldt F, Buchert R, Kleiter I, et al. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681–6.
Yuan J, Guan H, Zhou X, et al. Changing brain metabolism patterns in patients with ANMDARE: serial 18F-FDG PET/CT findings. Clin Nucl Med. 2016;41:366–70.
Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8.
McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies: pathology and mechanisms. Acta Neuropathol. 2011;122:381–400.
Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56:715–9.
Kim B, Yoo P, Sutherland T, et al. LGI1 antibody encephalopathy overlapping with sporadic Creutzfeldt-Jakob disease. Neurol Neuroimmunol Neuroinflamm. 2016;3:e248.
Rossi M, Mead S, Collinge J, Rudge P, Vincent A. Neuronal antibodies in patients with suspected or confirmed sporadic Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2015;86:692–4.
Sköldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol. 2006;253:163–70.
Prüss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902–11.
Venkatesan A, Benavides DR. Autoimmune encephalitis and its relation to infection. Curr Neurol Neurosci Rep. 2015;15(3):3.
van Coevorden-Hameete MH, Titulaer MJ, Schreurs MW, de Graaff E, Sillevis Smitt PA, Hoogenraad CC. Detection and characterization of autoantibodies to neuronal cell-surface antigens in the central nervous system. Front Mol Neurosci. 2016;9:37.
Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–77.
Ramberger M, Peschl P, Schanda K, et al. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-D-aspartate receptor antibodies in serum using a live cell-based assay. PLoS One. 2015;10:e0122037.
Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76.
Höftberger R, van Sonderen A, Leypoldt F, et al. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology. 2015;84:2403–12.
Venkatesan A, Geocadin RG. Diagnosis and management of acute encephalitis: a practical approach. Neurol Clin Pract. 2014;4:206–15.
Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12:1–13.
Varley J, Taylor J, Irani SR. Autoantibody-mediated diseases of the CNS: structure, dysfunction and therapy. Neuropharmacology. 2017;132:71–82.
Didelot A, Honnorat J. Paraneoplastic disorders of the central and peripheral nervous systems. Handb Clin Neurol. 2014;121:1159–79.
Byun JI, Lee ST, Jung KH, et al. Effect of immunotherapy on seizure outcome in patients with autoimmune encephalitis: a prospective observational registry study. PLoS One. 2016;11:e0146455.
Gabilondo I, Saiz A, Galán L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77:996–9.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57.
Lee W-J, Lee S-T, Moon J, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. 2016;13:824–32.
Scheibe F, Prüss H, Mengel AM, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology. 2017;88:366–70.
Michael BD, Griffiths MJ, Granerod J, et al. The Interleukin-1 balance during encephalitis is associated with clinical severity, blood-brain barrier permeability, neuroimaging changes, and disease outcome. J Infect Dis. 2016;213:1651–60.
Novroski AR, Baldwin KJ. Chronic autoimmune meningoencephalitis and periodic fever syndrome treated with Anakinra. Case Rep Neurol. 2017;9:91–7.
Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–44.
Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. 2015;84:359–66.
Larman HB, Zhao Z, Laserson U, et al. Autoantigen discovery with a synthetic human peptidome. Nat Biotechnol. 2011;29:535–41.
Zhu J, Larman HB, Gao G, et al. Protein interaction discovery using parallel analysis of translated ORFs (PLATO). Nat Biotechnol. 2013;31:331–4.
Pearson RM, Casey LM, Hughes KR, Miller SD, Shea LD. In vivo reprogramming of immune cells: technologies for induction of antigen-specific tolerance. Adv Drug Deliv Rev. 2017;114:240–55.
Luo X, Miller SD, Shea LD. Immune tolerance for autoimmune disease and cell transplantation. Annu Rev Biomed Eng. 2016;18:181–205.
Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34.
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4.
Gross G, Eshhar Z. Endowing T cells with antibody specificity using chimeric T cell receptors. FASEB J. 1992;6:3370–8.
Ellebrecht CT, Bhoj VG, Nace A, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–84.
Tradtrantip L, Zhang H, Saadoun S, et al. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol. 2012;71:314–22.
Kreye J, Wenke NK, Chayka M, et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain. 2016;139:2641–52.
Granerod J, Davies NW, Ramanuj PP, Easton A, Brown DW, Thomas SL. Increased rates of sequelae post-encephalitis in individuals attending primary care practices in the United Kingdom: a population-based retrospective cohort study. J Neurol. 2017;264:407–15.
Gaspard N, Foreman BP, Alvarez V, et al. New-onset refractory status epilepticus: Etiology, clinical features, and outcome. Neurology. 2015;85:1604–13.
Feyissa AM, Lopez Chiriboga AS, Britton JW. Antiepileptic drug therapy in patients with autoimmune epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4:e353.
Finke C, Pruss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with Leucine-rich, Glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74:50–9.
Lejuste F, Thomas L, Picard G, et al. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e280.
Sacks O, Shulman M. Steroid dementia: an overlooked diagnosis? Neurology. 2005;64:707–9.
Kowski AB, Weissinger F, Gaus V, Fidzinski P, Losch F, Holtkamp M. Specific adverse effects of antiepileptic drugs—a true-to-life monotherapy study. Epilepsy Behav. 2016;54:150–7.
Iorio R, Lennon VA. Neural antigen-specific autoimmune disorders. Immunol Rev. 2012;248:104–21.
McKeon A. Immunotherapeutics for autoimmune encephalopathies and dementias. Curr Treat Options Neurol. 2013;15:723–37.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Venkatesan, A., Probasco, J.C. (2018). Autoimmune Encephalitis. In: Hasbun, R. (eds) Meningitis and Encephalitis. Springer, Cham. https://doi.org/10.1007/978-3-319-92678-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-92678-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92677-3
Online ISBN: 978-3-319-92678-0
eBook Packages: MedicineMedicine (R0)