Abstract
The edible and non-edible varieties of mushroom can be used as a green adsorbent and can be used in modified and natural form for the adsorption of dyes, pollutants and heavy metals. However, the use of edible mushroom varieties in the adsorption of pollutants is not judicious because edible mushrooms have good nutritive and medicinal properties and can be used for consumption. Recent research is thus focused on the utilization of spent mushrooms. Spent mushroom substrate, generated as waste by mushroom industries after the harvesting of mushroom, and hence, is the source of immobilized mushroom mycelium. The species of mushroom Agaricus, Pleurotus, Lentinus, Calocybe are efficient adsorbents with 70–90% removal of pollutants in laboratory conditions. Spent mushroom substrates can also remove pollutants such as dyes, heavy metals, pesticides and fungicides in laboratory conditions with comparable efficiency as mushroom. Chemisorption and physisorption processes are involved in the adsorption. The data of adsorption are well fitted to Langmuir isotherm, revealing the involvement of monolayer adsorption irrespective of the use of mushroom fruit bodies or spent mushroom substrate. Fourier-transform infrared spectroscopic analysis reveal the presence of carboxyl, hydroxyl, amino group in the adsorption of pollutants, dyes and heavy metals.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Globally, the production and per capita consumption of mushroom has increased at a rapid rate for last five decades. According to the United Nations Food and Agriculture Organization statistics, the average annual growth rate of edible fungi is 5.6% worldwide . During 1997–2012, annual per capita consumption of mushrooms increased from about 1 kg to over 4 kg. The main producer and consumer of mushroom is China (Royse 2014). In India, more than 40,600 tons of mushrooms produced annually (Pandey et al. 2014). Mushroom cultivation is one of the environmental friendly ways to recycle agricultural and agro-industrial wastes for the production of mushroom fruit bodies or mycelium with good nutritive and medicinal properties. In 2016, market value of cultivated edible mushroom species is about 30–34 billion dollars and medicinal mushroom species is 10–12 billion dollars. Therefore, it is billion dollar agribusiness which cannot be shut down.
The problem related to mushroom cultivation industries is the disposal of waste generated after the harvesting of mushrooms. The mushroom substrate, left over after harvesting of mushroom fruit bodies, is called as spent mushroom substrate. This spent mushroom substrate creates the problem of disposal in environment-friendly way. In Iran, there is an increase in the mushroom production industries which are generating more than 50,000 tons of spent mushroom substrate annually. The generation of spent mushroom substrate was, generally two times higher than the mushroom harvested. According to Oei (1991), two tons of spent mushroom substrate remains from each ton of mushroom harvested. Now, efforts have been made to increase the mushroom production like the use of different substrate combinations and optimization of processes, which not only increased the biological efficiency and production capacity of mushroom but also reduced the generation of spent mushroom substrate. According to Pandey et al. (2014), amount of spent mushroom substrate generated from each ton of mushroom is approximately equal to the amount of generated mushroom.
Besides nutritional and medicinal properties, mushrooms are also known for their potential as adsorbent for the adsorption of pollutants from the industrial effluent and soil (Table 9.1). However, there are critics in using nutritive and medicinal species of mushroom in the adsorption of pollutants. To solve this issue, either non-edible varieties of mushroom or spent mushroom substrate of edible mushroom varieties have been used.
Spent mushroom substrate possesses leftover mycelium of the mushroom which can be utilized as a source of immobilized mushroom mycelium. Burning and landfill are the most adopted techniques for the disposal of spent mushroom substrate but are not environment friendly. Many researchers have analyzed the efficiency of mushrooms (Damodaran et al. 2014; Kan et al. 2015; Kariuki et al. 2017) and spent mushroom substrate (Kamarudzaman et al. 2015; Siasar and Sargazi 2015; Md-Desa et al. 2016) as adsorbent of pollutants, dyes and heavy metals along with evaluating the environmental impacts. This chapter describes the potential of mushroom and spent mushroom substrate in the adsorption of pollutants.
9.2 Mushroom As Green Adsorbent
Mushrooms can be served as green adsorbent which can accumulate pollutants from the surroundings and reduce their concentration (Udochukwu et al. 2014). The role of mushroom in adsorption of pollutants, dyes and heavy metals has been assessed by many researchers (Table 9.1). The mushroom mycelium can be used in the live or dead form. Live form of mycelium requires the maintenance of appropriate condition in order to maintain the viability. However, dead biomass can be used in a variety of conditions like high temperature, low temperature, acidic solution basic solution as it is not affected by pH, temperature, salinity (Kulshreshtha et al. 2014). Mushroom possesses chitin, a negative charge compound in the cell wall, which provides negative charge to the surface of mushroom mycelium. Heavy metals and pollutants possess positive charge and therefore, these can adsorb on the surface of spent mushroom substrate due to ionic interaction. Thus, spent mushroom substrate can be a good candidate for adsorption of pollutants and heavy metals based on ionic interaction.
9.2.1 Preparation of Mushroom and Its Mycelium As Adsorbent
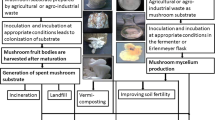
Mushroom and its mycelium are required in bulk amount for the adsorption purpose. Therefore, mushrooms can be collected in bulk amount from the natural environment or can be cultured in the laboratory. In the laboratory, mushroom can be cultured by solid state fermentation process and submerged fermentation process in the fermenter or Erlenmeyer flask. In solid state fermentation, substrate is prepared from agricultural or agro-industrial wastes which lead to the formation of fruit bodies of mushroom. In contrast, mushroom culture in Erlenmeyer flask or fermenter leads to the production of mycelial biomass of mushroom (Fig. 9.1).
Mycelium of mushroom can be cultured in an Erlenmeyer flask containing malt extract medium, and incubated at 25 °C, 125 rpm in an incubator shaker for 21 days. For harvesting the mycelium, broth was separated by filtration technique and discarded. Adsorbent can be prepared from the harvested mycelium or collected mushrooms by autoclaving for 15 min at 121 °C, 18 psi pressure, which is followed by oven drying for overnight at 60 °C. It can be ground into fine particles and then sieved to get particles of uniform size, i.e. lesser than or equal to 150 μm. This adsorbent can be used immediately, or preserved for a longer time in silica filled desiccators (Abdul-Talib et al. 2013).
9.2.2 Modified Mushroom for Adsorption
Recently, Xu et al. (2016) produced an adsorbent by using Pleurotus cornucopiae and further, used for the adsorption of hexavalent chromium ions from the aqueous solution . To prepare adsorbent from the Pleurotus cornucopiae, it was washed with deionized water for several times, which was followed by drying at 50 °C for 3 d using air-blower-driven drying closet. This was followed by grinding in a pulverized mill and sieve through 40-mesh, 60-mesh, and 100-mesh in order to get uniform particles of specific sizes. The surface of this dried mycelium was modified by chemical treatment to increase its adsorption capacity. To modify the surface for adsorption, 30 g of Pleurotus cornucopiae was agitated in 500 ml of the dodecyl dimethyl benzyl ammonium bromide solution for 24 h. Further, these particles were filtered and washed with double distilled water. Washing was done repeatedly till the complete removal of bromide ions. The washed mushroom biomass was dried at 30 °C for 24 h for self-assembling of mushroom mycelium powder. The surface scanning of modified Pleurotus cornucopiae revealed the presence of uneven surface and polyporous structure due to self-assembling of the material. Fourier-transform infrared spectroscopy analysis revealed that amine, hydroxyl, and carboxyl groups provide binding sites for adsorbate. These groups played an important role in adsorption when modified Pleurotus cornucopiae was used for the adsorption of hexavalent chromium ion. The increased dosage of this adsorbent increased the efficiency of adsorption of chromium ions. Under the optimum controllable factors like pH, temperature; hexavalent chromium removal efficiency of 75.91% was achieved. This adsorption was best fit to the Freundlich isotherm model. This is a successful approach to remove hexavalent chromium ions from the solution (Xu et al. 2016). A detailed study at pilot scale and industrial scale plant is required to assess the potential of modified Pleurotus cornucopiae biomass in the adsorption of chromium ions so that technology can be implemented for industrial effluents.
In another experiment conducted by Xie et al. (2015), Lentinula edodes was treated with a mixture of sodium hydroxide, ethanol, and magnesium chloride. The effect of this treatment was assessed on adsorption capacity. For this purpose, 20 g of powdered raw biomass of L. edodes was mixed with 400 ml of mixed solution containing 200 ml of ethanol, 100 ml of sodium hydroxide and 100 ml of magnesium chloride. The biomass of L. edodes was left in solution at 25 °C and 150 rpm for 24 h. This treated biomass was filtered and supernatant was discarded. This was followed by washing with deionized water several times till the pH of L. edodes biomass became neutral. This biomass was dried in oven at 40 °C for 24 h. After drying, biomass was ground into fine particles and filtered through 100 or 200-mesh to obtain uniform sized particles. In this study, initial concentration of heavy metals was 50 mg/L; adsorbent dosage was 5 g/L. This magnesium chloride modified Lentinula edodes was used to adsorb cadmium and copper ions from the aqueous solution with high adsorption capacities of 51.64 ± 0.65 and 59.03 ± 0.64 mg/g, respectively. This capacity of adsorption of magnesium chloride modified Lentinula edodes was reported to be greater than one order of magnitude higher than that of raw biomass. A huge number of binding sites were exposed after the treatment of L. edodes with magnesium chloride which helps in the adsorption of metals. In the adsorption of copper and cadmium ions, both physisorption and chemisorption were reported to involve. These processes are based on electrostatic interaction, ion exchange and complex formation. The study of thermodynamic parameters revealed that the process was endothermic and spontaneous. The data were fitted well to pseudo-second order kinetic model which revealed the involvement of chemisorption process. This study also focused on the recovery of heavy metals from real industrial effluent. The adsorption and heavy metal recovery efficiency was 90% and 80%, respectively (Xie et al. 2015). Therefore, magnesium chloride treated spent mushroom substrate was found to be better than raw culture of Lentinula edodes in effective adsorption and desorption of heavy metals from the real industrial effluent.
9.2.3 Limitations of Using Mushroom in Adsorption
Harvesting the mushroom mycelium for removal of pollutants is not a feasible option, especially in developing and undeveloped countries because the primary focus of mushroom cultivation is to provide protein rich food to the people. Secondly, mushrooms also have anti-mutagenic, anti-inflammatory, anti-carcinogenic, antioxidant properties and can be used for medicinal purposes. Therefore, mushroom cultivation for bioremediation of pollutants, heavy metals and dyes by adsorption is not encouraged in these countries.
When mushroom biomass or fruit bodies are used for the adsorption of pollutants, heavy metals and dyes, proper disposal practices are required if metals and pollutants are not recovered. On dumping in the environment, the adsorbed metals and pollutants can leach out and cause soil and water pollution (Fig. 9.1). Adsorption by mushroom biomass or fruit bodies is worthy to remove pollutants from the environment; nevertheless there is a need of safe disposal practices.
Another problem related to mushroom cultivation is the generation of spent mushroom substrate. Mushroom cultivation requires a substrate composed of agricultural waste fibers including wheat straw, rice straw, corn cobs, cottonseed hulls, sawdust; manures like horse manure, poultry manure, cottonseed meal; and a calcium source like calcium carbonate or gypsum. This substrate is incubated at suitable temperature and humidity condition, depending upon the species of mushroom. In the appropriate conditions, substrate supports the growth of mycelium and fruit bodies. These fruit bodies are harvested on maturation. After the harvesting of mushroom fruit bodies, mushroom substrate is discarded as solid waste which is called as spent mushroom substrate, mushroom soil, recycled mushroom compost or spent mushroom compost (Fig. 9.1). This substrate is a byproduct of the mushroom industry, which give rise to the most significant environmental issues, and therefore need a strategy for its proper disposal.
9.3 Spent Mushroom Substrate As Adsorbent
In the earlier attempts, spent mushroom substrate was disposed by landfills or by burning in open fields. In the case of landfill, spent mushroom substrate is piled up on the land; however release of leachates from the pile due to the weathering of spent mushroom substrate may adversely affect the ground water quality (Kamarudzaman et al. 2014a) and adjacent land. Later, spent mushroom substrate was used for a variety of purposes like animal feed, production of enzymes, vermicomposting and soil quality improvement (Fig. 9.2).
The use of spent mushroom substrate in laccase, xylanase, lignin peroxidase, cellulase and other enzymes production is suggested by Phan and Sabaratnam (2012). Spent mushroom substrate contains 14% protein and lots of vitamins and microelements like iron, calcium, magnesium and zinc, and essential amino acids which are generally not present in animal feed. Therefore, spent mushroom substrate can be a good feedstuff for animals (Foluke et al. 2014).
The spent mushroom substrate was also used for improving the soil quality and crop yield. Ribas et al. (2009) reported the use of spent mushroom substrate as a growth supplement for lettuce, which not only promoted the growth of lettuce by improving microbial diversity of soil and related enzymatic activities, but also degraded biocides. Fidanza et al. (2010) reported that the supplementation of spent mushroom substrate to the soil improved the structure of clay soil, reduced the surface compaction and crusting, along with promoting the microbial growth and enzymatic activity in the soil. Improvement in soil quality led to turf establishment, improved turf density and colour, increased rooting, which further reduced the need of fertilizer and irrigation (Fidanza et al. 2010). Sendi et al. (2013) replaced the peat moss with spent mushroom substrate in the cultivation media of Brassica oleracea i.e. Kai-lan and reported the higher yield of Brassica oleracea. Lopes et al. (2015) reported that the use of spent mushroom substrate as compost provided higher yield of tomatoes as compared to green, organic and chemical fertilizers. Spent mushroom substrate is considered as a source of nutrient for the soil and hence, widely used as compost (Siasar and Sargazi 2015). Besides, spent mushroom substrate possesses high adsorption capacity which can be further utilized for the removal of heavy metals , dyes, pollutants from the soil. This ability was used by Tuhy et al. (2015) for converting spent mushroom substrate into biofertilizer by adsorbing some metals like zinc, manganese and copper for fulfilling the mineral requirement of the plant. In this way, spent mushroom substrate showed promising results towards improving the soil quality and crop productivity and greening of a shattered area or desert. In contrast to this, it is also reported in the literature that the high salt and nutrient content of spent mushroom substrate disturbs the nutrient balance of soil and therefore, limits their application in improving the soil quality. Any failure in utilization of spent mushroom substrate will lead to its accumulation in the environment.
To solve the aforesaid problems of waste accumulation, the spent mushroom substrate has also been used for adsorbing the toxic metals, dyes and pollutants from the environment. The spent mushroom substrate can act as a green adsorbent due to having immobilized mycelium and excellent abilities to adsorb or degrade organic compounds and pollutants, dyes present in soil and water (Ahlawat et al. 2010). They can play an important role in environmental cleanup. Further, they have the potential to adsorb metals which can be recovered on changing certain physiological conditions. These can be used for metal recovery from industrial effluent and soil. Ribas et al. (2009) reported the use of fresh spent mushroom substrate of L. edodes for soil remediation . The spent mushroom substrate also has potential to minimize the bioavailability of heavy metals (García-Delgado et al. 2013).
Application of spent mushroom substrate in the soil and water for the removal of dyes, pollutants and heavy metals may be a viable and acceptable option as it is a waste byproduct of mushroom industry. It is available in bulk amount at a lower price or sometimes free of cost; and efficient to use for adsorbing heavy metals and pollutants from the effluent and soil and thus, can improve the quality of environment.
The effective implementation of the adsorbent in environmental cleanup depends on its properties like cost-effectiveness, potential to adsorb pollutants. Besides, adsorbent should be amenable to any physical and chemical alterations which are required to enhance their adsorption properties (Ayangbenro and Babalola 2017). The most important aspect of using spent mushroom substrate as adsorbent is its cost and availability. The spent mushroom substrate is generated in high amount as the waste of mushroom production industries (Singh et al. 2003) and generally, discarded by burning in open field and disposed in municipal solid waste landfill. Hence, spent mushroom substrate can be available in bulk amount at a low price or free of cost for using as adsorbent. The use of spent mushroom substrate for adsorbing metals and pollutants will not only solve the problem of accumulation of spent mushroom substrate but also heavy metals and pollutants. Therefore, application of spent mushroom substrate will be a feasible technology to implement as far as availability of raw material is concerned.
Another parameter for choosing spent mushroom substrate is its properties. The lignocellulosic component of spent mushroom substrate possesses negative charged groups like hydroxyl, carboxylic acid and phenolic groups, which provide them ability to bind with metals. García-Delgado et al. (2013) compared the efficiency of spent mushroom substrate of Pleurotus and Agaricus, and reported that spent mushroom substrate of Agaricus has undergone an intense composting process due to which it produces higher amounts of fatty acid and humic acid and further, acquires the higher amount of alkyl and carboxyl group compare to spent mushroom substrate of Pleurotus. Therefore, the spent mushroom substrate of Agaricus can be a better substrate than Pleurotus spent mushroom substrate for adsorbing the positive charged metals and pollutants from the environment.
For practical application considerations, it is also important that adsorbent have the potential to adsorb heavy metals and further, helps in their recovery by desorption process (Javanbakht et al. 2014). In this way, spent mushroom substrate is generated as waste and can be revenue for the industries if utilized for the adsorption of heavy metals . This recovery based approach can be implemented in future due to having financial benefits along with environmental benefits.
9.3.1 Principle of Adsorption by Spent Mushroom Substrate
Adsorption is the ability of adsorbate to adhere or attach to the adsorbent. In case of spent mushroom substrate, the adsorption occurs mainly by two processes; coulombic interaction and intrinsic adsorption. Coulombic interaction is related to the electrostatic energy of interaction between ions. When surface of adsorbate and adsorbent possess opposite charge, they are attracted by coulombic interaction. Generally, spent mushroom substrate based adsorption phenomenon relies on the fact that surface of spent mushroom substrate possesses negative charge due to the presence of anions such hydroxyl, carboxyl, amide groups; and adsorbate like heavy metals or pollutants possesses positive charge ions. The active functional groups and charge on the spent mushroom substrate can be analyzed by Fourier-transform infrared spectroscopy analysis. This analysis revealed that the carboxyl, hydroxyl, phosphate, amide group of lignocelluloses and chitin, glucans, mannans of mushroom are involved in adsorption process (Tay et al. 2012). The ability of spent mushroom substrate to bind with the pollutants, dyes and metal ions depend upon the structural component of fungi and lignocellulosic agricultural residues. Chitin is an important component of spent mushroom substrate for the adsorption of heavy metals and radionuclides. Chitosan is a more effective alternative compared to chitin, to bind and form strong complexes with pollutants and metals. However, it cannot be used due to unfavourable economics, which need high cost to implement. Moreover, it is extracted by typical extraction procedure, which results in generation of toxic wastes (Gadd 2009). The intensity of interaction is based on charge of adsorbate and adsorbent; and strength of ions (Igwe and Abia 2006).
Intrinsic adsorption is associated with the surface area of adsorbent which can be observed by the effect of different sizes of adsorbent particle on the capacity of adsorption (Igwe and Abia 2006). This process is based on the Van der Waals attractions between the adsorbent and adsorbate.
9.3.2 Mechanism of Adsorption
Generally, binding of metals, pollutants and dyes to the mushrooms and spent mushroom substrate depends on the four mechanisms: (i) adsorption (ii) ion exchange (iii) complexation and (iv) precipitation. Physical adsorption is based on the electrostatic forces and van der Waals forces as mentioned in aforesaid section. Transport of cations across the microbial cell wall depends upon metabolism of mushroom and cell membrane permeability. This is required to maintain the ionic balance of metabolically important ions like sodium, potassium and magnesium. The metabolically independent transport mechanism involves transportation through cation transport systems. Occasionally, this system transports the metal ions bearing the same charge and ionic radius along with the other required ions. It has been reported that mushroom biomass develop mechanisms to resist the heavy metals through the secretion of chelating substances that are able to bind with metal ions. Further, metal ions accumulation is reduced due to the alterations in the metal transport system. Another mechanism to develop resistance includes the binding of metal ion to intracellular molecule such as metallothionein or accumulates in intracellular organelles like vacuole or mitochondria (Ayangbenro and Babalola 2017).
The principal mechanism of heavy metal binding is related to physical binding and chemical binding of metal ions to the spent mushroom substrate. However, ion exchange can be observed as an important phenomenon in adsorption. Occasionally, bivalent metal ions are exchanged with counter ions of polysaccharides.
As mentioned earlier, complexation plays an important role in the adsorption process. It is based on the surface charge of spent mushroom substrate and mushroom. Both possess negative charge due to the presence of carboxyl, amino, thiol, amide, imine, thioether, phosphate, which provide them ability to make complexes during metal-ligand and adsorbate-adsorbent interaction (Javanbakht et al. 2014).
Precipitation, a metabolically independent process, is a chemical reaction between the metal and cell surface of mushroom and spent mushroom substrate. This leads to the deposition of heavy metals into solution and on the surface of mushroom mycelium. Zhou et al. (2005) reported that lead ions form complex with the cellulose and precipitate the product of lead complex generated by hydrolysis. The precipitation of lead complex in the form of hydroxides has also increased with an increase in the pH.
In the living mushroom mycelium, adsorption process depends upon the metabolic processes while these are independent of metabolic processes if dead biomass of mushroom is used. Dead biomass of mushroom passively binds metal ions by various physicochemical mechanisms. Nevertheless, complete knowledge of metabolism dependent processes is required in order to optimize and maintain the adsorption in the living system. In the living biomass, metabolic activities are affected by rate of respiration, the products formed during metabolism and nutrient uptake which further affects adsorption, ion exchange, complexation and precipitation.
In the case of organic pollutants, adsorption is based on chemical structure such as molecular size, charge, solubility, hydrophobicity and reactivity. In addition to adsorption and complexation, permeation of spent mushroom substrate biomass may contribute to the adsorption process.
Hydrocarbons are hydrophobic compounds, insoluble in water, however, can be associated with non-polar environment. They can be adsorbed on the surface of organic substances and spent mushroom substrate biomass. Lipophilic, hydrophobic compounds can pass through cell membranes and adsorb into organic matrix of spent mushroom substrate (Javanbakht et al. 2014). In the case of dye adsorption, chitosan, extracted derivative of chitin, is better than naturally occurring mushroom chitin. Dyes adsorbed on the surface of chitosan by various mechanisms that include surface adsorption, chemisorption, film diffusion and pore diffusion; and chemical reactions like adsorption-complexation and ion exchange. The main group involve in the adsorption of dyes is amine group; however, hydroxyl group may also contribute in the process (Javanbakht et al. 2014). It is pertinent to note that effectiveness of the substrate in adsorbing the pollutants is more important than the mechanism involved in the adsorption.
9.3.3 Selection and Preparation of Spent Mushroom Substrate As Adsorbent
During the mushroom cultivation, cellulosic, lignocellulosic and hemicellulosic fibres are utilized by the mushroom for the growth. This leads to the gradual degradation of the substrate and pore formation. The pore size is increased with increase in time due to the continued substrate degradation process, which further increase adsorption properties of spent mushroom substrate.
The choice of spent mushroom substrate may vary as per availability in the local area (García-Delgado et al. 2013). Selection of appropriate spent mushroom substrate and its application in adsorption of pollutants seems to be a sustainable technology that will not only remove the pollutants, but also solve the problem of waste disposal (Prasad et al. 2013). The most popular mushroom species around the world is Agaricus species and Pleurotus species and therefore, cultivation of these species generates huge amount of waste which accumulates in the environment and causes ill effects. Therefore, spent mushroom substrate needs proper disposal practices (García-Delgado et al. 2013). The spent mushroom substrate of these mushrooms is readily available and inexpensive biomaterial with high capacity of pollutant adsorption.
Spent mushroom substrate for adsorption of pollutants, dyes and heavy metals have been prepared by different methods and analyzed. In one method, Siasar and Sargazi (2015) mixed 2 g of spent mushroom substrate in 20 ml of water and filtered the resultant solution through filter paper AK-01 blue. Spent mushroom substrate was retained by the filter which was collected and dried; and further, used in the adsorption experiment. In the method developed by Tay et al. (2011) the sample of spent mushroom substrate was prepared at 121 °C, 18 psi for 15 min and then dried in an oven at 60 °C. This was followed by grinding and sieving to obtain particle size lesser than 710 μm. This adsorbent was stored in dry cabinet. In another method, all conditions of preparing spent mushroom substrate adsorbent from Pleurotus species were kept same, except particle size which was lesser than 150 μm. This adsorbent was stored in silica filled desiccators and used in performing the experiment for lead and cadmium adsorption (Tay et al. 2011; Abdul-Talib et al. 2013).
A different preparation of spent mushroom substrate was also used by Gill (2014) for adsorbing an organic chemical spill. In this preparation, spent mushroom substrate was mixed with dry plant material, partially composted plant material, spent mushroom substrate, dry plant material, a petroleum hydrocarbon, ammonium nitrate, iron sulfate, mixture of iron sulfate and ammonium nitrate, and a clump preventing material like sawdust, vermiculite, diatomaceous earth. This mixture was supplemented with nutrient source such as raw sugar or sugar beet residue. When this substrate mixed with the spilled chemical and water, it can neutralize the hydrocarbon spill (Gill 2014). These studies revealed that all preparations of spent mushroom substrate are effective in removing the pollutants from the environment.
9.3.4 Characterization of Spent Mushroom Substrate Adsorbent
The adsorbent must be sufficiently porous to adsorb pollutants from the surrounding. The spent mushroom substrate is composed of different types of polymers such as lignin, cellulose and hemicellulose which are degraded by mushroom mycelia during its growth and utilized as carbon and energy sources. This degradation results in numerous pores in the spent mushroom substrate that makes it a suitable substrate for adsorption (Yan and Wang 2013). These pores can be classified as micropores if pore diameter is lesser than 2 nm, mesoporous if pore diameter is 2–50 nm and macropores if pore diameter is greater than 50 nm. The macroporous material has a great potential for adsorbing large sized adsorbates as compared to mesoporous or microporous adsorbent material. The structure of cell wall, micropores, mesopores and macropores must be evaluated in order to analyze the potential of adsorbent. The characterization of spent mushroom substrate for using it as adsorbent can be done by the following methods:
9.3.4.1 Brunauer Emmett Teller Analysis
The specific surface area and total pore volume of spent mushroom substrate can be measured by surface area analyzer, a fully automated analyzer which evaluates the material by nitrogen multilayer adsorption measured as a function of relative pressure. Nitrogen does not react chemically with the substrate and therefore, used in the analyzer. This technique determines the surface area and pore area and helps in acquiring important information for performing adsorption studies. Further, adsorbent categories can be defined on the basis of Brunauer Emmett Teller analysis, such as dispersed, nonporous, macroporous materials. Macroporous material have pore diameter greater than 50 nm is well-fitted to type II isotherm . Mesoporous materials have pore diameter between 2 nm and 50 nm is well fitted to type IV isotherm. Microporous material have diameter lesser than 2 nm and well fitted to type I isotherm.
9.3.4.2 Scanning Electron Microscopy/Energy Dispersive X-ray Spectroscopy Analysis
It is an analytical technique used for the elemental analysis or chemical characterization of spent mushroom substrate. It is based on the interaction of X-ray and spent mushroom substrate. When X-rays are focused on the spent mushroom substrate, unique set of peaks can be observed on its electromagnetic emission spectrum according to the atomic structure of materials present in spent mushroom substrate.
9.3.4.3 Fourier-Transform Infrared Spectroscopy Analysis
This analysis provides an insight of adsorption process. The functional groups, involved in the adsorption process, can be determined by analyzing the peaks obtained by Fourier-transform infrared spectroscopy. Differences in the peaks of spent mushroom substrate before and after adsorption can be investigated by Fourier-transform infrared spectroscopy. These peaks reveal the important groups involved in the adsorption process (Kamarudzaman et al. 2014a).
9.3.4.4 Solid-State Cross-Polarization Magic Angle Spinning Carbon-13 Nuclear Magnetic Resonance
Solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance is used to investigate the structural changes and interactions of cellulose (Larsson et al. 1999) with the pollutant during adsorption. It is also used to detect the presence of β-D-glucan and trace compounds present in dried powder of mushroom samples. For this purpose, the high- resolution solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance spectra can be recorded at the resonance frequency of approximately 100 MHz with the use of 4 mm rotors and frequency of 12 kHz and pulse duration of 1.9 μs. A high-power proton-decoupling field of 92 kHz can be applied during data acquisition. The spectra can be obtained at room temperature averaging over 5000–33000 scans. Hydration of mushroom polysaccharide give rise to conformational stabilization, which is reflected in spectra by narrowing and splitting of resonance line (Fričová and Koval’aková 2013).
To evaluate the strategies for potential implementation, adsorption isotherms, adsorption kinetics, intra-particle diffusion ability can be used to explain the feasibility of adsorption process.
9.3.5 Adsorption of Heavy Metals by Spent Mushroom Substrate
Heavy metals are noxious products discharged by a number of industries after the completion of industrial processes and are major pollutants in the soil and water. These are discharged by electroplating and metal finishing and metallurgical industries, leather tanning, textile dyeing and printing based industries, fertilizer industries, acid mine drainages and landfill leachates. Besides, agricultural and domestic activities also discharge heavy metals in the environment.
These are recalcitrant and hence, accumulate in the environment and enter the food chain (Igwe and Abia 2006). After intake, heavy metals accumulate in the living tissue and disrupt biological processes due to their toxicity. Conventional methods for removing metal ions include a number of processes such as filtration, chemical precipitation and electrochemical treatment, ion-exchange, membrane technologies and activated carbon . Many of these processes are ineffective, especially when metal ion concentration in aqueous solution is 1–100 mg/L. Moreover, they produce huge amounts of toxic sludge which is difficult to dispose. Further, these cannot be implemented at large scale due to financial constraints. Nowadays, microbial adsorption based approaches are gaining much attention in removing the metals from the environment. The most popular microbial option for adsorbing metal is mushroom mycelium which can be obtained from mushroom industry or mushroom cultivation units. The spent mushroom substrate, as green adsorbent , presented as an alternative to traditional physicochemical means for removing heavy metals from soil and water. Spent mushroom substrate makes complex with heavy metals and increases its stability, however reduces its bioavailability, which in turn reduces the toxic effect of metal on living beings.
According to Wang and Chen (2009), there are two methods for the removal of heavy metals. The first method is related to the usage of living biomass of mushroom for metal adsorption and removal; while the other methods, rely on the immobilization technology and modification in the adsorbent for making a type of ion exchange resin to use in regeneration or reuse (Wang and Chen 2009). The use of spent mushroom substrate as green adsorbent is explained in the subsequent sections.
9.3.5.1 Spent Mushroom Substrate of Lentinus edodes
The spent mushroom substrate of Lentinus edodes is used as adsorbent for adsorbing cadmium, lead and chromium from solution. The effect of different parameters such as adsorbent concentration, initial pH, contact time and heavy metal concentration was evaluated by Tay et al. (2012). As the initial pH increase, active sites of spent mushroom substrate are deprotonated resulting in the increased adsorption of copper. In acidic condition, copper uptake is poor due to the protonation of the active sites of adsorbent. However, in basic condition, copper ions are precipitated as insoluble hydroxides resulting in decrease adsorption of copper. On increasing the adsorbent dosage, adsorption of heavy metals increased. Fourier-transform infrared spectroscopy analysis revealed that the carboxyl, phosphoryl, and phenolic groups are involved in adsorption (Tay et al. 2012). This study shows the potential of Lentinula edodes in removing heavy metals.
9.3.5.2 Spent Mushroom Substrate of Pleurotus ostreatus
Tay et al. (2012) used Pleurotus ostreatus and spent mushroom substrate for adsorbing heavy metals. The copper adsorption experiment, conducted by him, was based on the assessment of half saturation constant of adsorption so that results can be obtained with minimum adsorbent dosage and in shorter time. The findings revealed that optimum adsorption parameters were initial pH 5, contact time 10 min and initial copper concentration 50 mg/L. The adsorption co-efficient for Pleurotus mushroom and Pleurotus spent mushroom substrate was found 0.9598 and 0.9920, respectively. These values showed that both mushroom and spent mushroom substrate has same adsorption capacity. Fourier-transform infrared spectroscopy analysis revealed that carboxyl, hydroxyl and amide groups of lignocelluloses, chitin, and proteins were involved in the adsorption study. The kinetics of the study showed that chemisorption, complexation and ion exchange are the mechanisms involved in the adsorption of copper on spent mushroom substrate and mushroom fruit body.
Adsorption of Nickel
Nickel, a toxic metal, discharged with industrial effluent from mineral processing, electroplating and battery manufacturing industries. It is an essential micronutrient for some plant and microbes in minute amount, nevertheless toxic in high amount. It accumulates but not magnified along the food chain (Tay et al. 2011). Long term exposure to nickel causes serious health issues. Therefore, spent mushroom substrate was used to remove this metal from the environment.
Tay et al. (2011) studied the potential of Pleurotus ostreatus spent mushroom substrate as a green adsorbent for nickel adsorption. For this purpose, analytical grade of nickel nitrate hexahydrate salt was used to prepare nickel solution with ultrapure water. Pleurotus ostreatus spent mushroom substrate adsorbent was prepared using the protocol as mentioned by Tay et al. (2011) and particle size lesser than 710 μm was chosen for nickel adsorption study. Nickel adsorption was carried out in batch process. Erlenmeyer flasks were filled with 50 ml nickel solution and placed on incubator shaker at 125 rpm and 25 °C. The effect of different parameters such as adsorbent concentration, initial pH, and contact time and nickel concentration was evaluated. After withdrawal, samples were filtered and supernatants were analyzed by inductively coupled plasma optical emission spectrometry. Further, feasibility of technique in terms of effectiveness and cost was also compared. This was done by performing the same experiment with commercially available synthetic adsorbent, i.e. Amberlite IRC-86. Results of the study revealed that 50% of nickel adsorption was obtained at half saturation constant of 0.7 g adsorbent concentration, initial pH in range of 4–8, contact time of 10 min., 50 ml solution of nickel with initial nickel concentration 50 mg/L. In this way, maximum nickel adsorption was 3.04 mg/g and data was fitted to Langmuir isotherm model showing the occurrence of monolayer adsorption on the surface of adsorbent. Fourier-transform infrared spectroscopy analysis determined the involvement of alkyl, hydroxyl, amino, aliphatic, alcohol and carbonyl functional groups in the adsorption process. The mechanism of adsorption involves physisorption and chemisorption ion exchange process. Characterization of adsorbent indicates that Pleurotus spent mushroom substrate is weak ion exchanger and therefore, its ion exchange performance was evaluated against Amberlite IRC-86. It is reported that ion exchange capacity of spent mushroom substrate is comparable to Amberlite IRC-86 to remove nickel. Nevertheless, Pleurotus spent mushroom substrate was found to be six times cheaper than Amberlite IRC-86 in terms of cost per unit of metal removed (Tay et al. 2011). Therefore, a costly option Amberlite IRC-86 can be replaced with an economic option spent mushroom substrate.
Tay et al. (2016) also conducted the study on adsorption of bi-metal solution, containing copper and nickel by Pleurotus spent mushroom substrate. The maximum uptakes of copper and nickel were 3.54 mg/g and 1.85 mg/g, respectively. However, Pleurotus spent mushroom substrate has higher selectivity for nickel as compared to copper. To find out the possible mechanism involved in the adsorption, results were evaluated through isotherm , kinetic and thermodynamic studies. Data of adsorption study were in agreement with Langmuir and Freundlich isotherms. This confirms the involvement of chemisorption process. Further, the adsorption reaction is spontaneous and exothermic is elucidated by thermodynamic parameters. Inductively coupled plasma optical emission spectrometry technique of measuring metal concentration and differences in pH depicted the involvement of ion exchange mechanism.
The above mentioned findings indicate the effectiveness of Pleurotus spent mushroom substrate in adsorbing nickel from the effluents. After studying the detailed insight of the process and mechanism , in the future, this spent mushroom substrate will be a promising green adsorbent for removing the metals in environmental friendly manner.
Adsorption of Lead
Pleurotus spent mushroom substrate was assessed for its potential in adsorbing lead ions. In a study, conducted by Liew et al. (2010) the suitability of Pleurotus spent mushroom substrate for removal of lead was determined. The effect of pH of lead containing solution, contact time, initial lead concentration was analyzed. The percentage removal of lead from solution using different amount of Pleurotus spent mushroom substrate by keeping the constant contact time i.e. 60 mins, was also assessed. Results of the study revealed that Pb removal increased with an increase in the amount of Pleurotus spent mushroom substrate. However, a maximum of 88% lead removal was achieved by 0.5 g of Pleurotus spent mushroom substrate (Liew et al. 2010). The removal percentage of lead increased at the initial stage with increasing the amount of adsorbent which is possible due to the increase in binding sites in adsorbent. At saturation stage, the lead binding sites decreased and resulted in the reduced lead adsorption. This is possibly due to screening effect which can reduce the accessibility of binding sites (Liew et al. 2010) by previously bind lead ions. The study showed that Pleurotus spent mushroom substrate is an efficient adsorbent for lead ions.
Adsorption of Iron
Iron is a toxic heavy metal that is commonly found in various industrial effluents. The exposure of human causes respiratory diseases, heart attacks, tissue damage. Therefore, it is necessary to remove iron from the industrial effluents. Spent mushroom substrate has been used for adsorbing the iron from the industrial effluents.
Kamarudzaman et al. (2013) performed an experiment to adsorb iron from the solution by using spent mushroom substrate as adsorbent. Standard solution of iron i.e. 1000 mg/L was prepared by dissolving ferric nitrate in ultrapure water. It was further diluted to obtain different concentrations. The pH was adjusted by using 1 M hydrochloric acid or sodium hydroxide solutions. Scanning electron microscopy for the analysis of surface morphology of Pleurotus ostreatus spent mushroom substrate before adsorption, revealed the rough and porous surface of spent mushroom substrate, which was efficient enough to support the adsorption process due to large surface area. The optimum adsorption of iron by Pleurotus spent mushroom substrate was achieved at an initial pH ranging from 4 to 5, contact time 10 min. and initial iron concentration of 50 mg/L using half-saturation constant of 0.4 g adsorbent dosages. After adsorption, SEM analysis showed that the spent mushroom substrate surface appears to be fully covered with iron molecules due to the deposition of iron molecules in the pores. Further, energy-dispersive X-ray spectroscopic analysis confirmed the adsorption of iron molecule by rise in peaks of iron. Fourier-transform infrared spectroscopy spectra revealed the involvement of carboxyl, hydroxyl and amide functional groups. Physical and chemical characterization of spent mushroom substrate supports the use for the adsorption of iron (Kamarudzaman et al. 2014a).
Later, the potential of Pleurotus spent mushroom substrate for adsorbing iron in the fixed bed column reactor was also investigated to find out the influence of flow rate, bed depth, and initial concentration of iron. Results show that decrease in flow rate and initial iron concentration leads to increase in breakthrough time and exhaustion time. In contrast to this, the breakthrough and exhaustion time decreased with decrease in bed depth (Kamarudzaman et al. 2014b). The study of physical and chemical characterization revealed the suitability of spent mushroom substrate for the adsorption of iron.
Adsorption of Copper
Copper is used as anti-corrosive agent and also used to make a decorative coating on the metal alloys (Siasar and Sargazi 2015). It is used in electrical and electroplating industries. It reaches the water bodies with the industrial effluents. In natural environment, metal persist for a longer time due to their non-degradable nature. The most serious threat to the environment and living beings is the toxicity and bioaccumulation potential of heavy metals . Adsorption based approaches are the most promising option for removing copper from the industrial effluents.
Pleurotus ostreatus spent mushroom substrate was used to remove copper from the effluent. The copper adsorption on spent mushroom substrate was carried out in the batch experiment. The maximum adsorption of copper was reported to be 3.54 mg/g. To find out the mechanism of metal adsorption, isotherm , kinetic and thermodynamic studies were conducted. Copper adsorption can be explained by Langmuir and Freundich isotherms. The mean energy indicated the involvement of chemisorption process for copper adsorption.
The adsorption data fitted well to the pseudo-second order kinetic model which revealed the involvement of chemisorption process in the adsorption of copper ions on spent mushroom substrate. Another mechanism involved in copper adsorption was ion exchange mechanism which was confirmed by inductively coupled plasma optical emission spectrometry. Copper forms weaker and reversible bond after adsorption, revealing the involvement of ion-exchange mechanism in the copper adsorption (Tay et al. 2016). This study showed that copper can be adsorbed by using spent mushroom substrate, which is an economic and feasible option.
According to Sarioglu et al. (2009), copper removal efficiency has been affected by the formation of insoluble complexes between copper ions and anionic charges which is present on the surface of adsorbent such as hydroxide, carbonate and sulfate.
Adsorption of Manganese
Manganese in industry is used for rust and corrosion on steel. Ionized manganese provides different colours depending on the oxidation state of ions. Manganese is also used as cathode in zinc-carbon and alkaline batteries. Manganese compounds are less toxic than the other heavy metals like nickel and copper, however exposure to high doses even for short period produces toxic effects. Therefore, it is important to treat manganese containing effluent prior to discharge in water bodies.
Pleurotus spent mushroom substrate was selected as adsorbent to remove manganese ions in a batch experiment and effect of pH, contact time and initial manganese concentration were investigated by Kamarudzaman et al. (2015). The optimum adsorption of manganese ions was achieved at pH 6, contact time 20 min., 10 mg/L using 1 g dosage of Pleurotus spent mushroom substrate. The data of this study were fitted well to Langmuir isotherm model and pseudo-second order kinetic model. Again, this revealed the involvement of chemisorption process in manganese adsorption by spent mushroom substrate. This experiment indicates the suitability of spent mushroom substrate for adsorption of manganese (Kamarudzaman et al. 2015).
Thereafter, Kamarudzaman et al. (2015) conducted an experiment in a fixed-bed column for the removal of manganese by Pleurotus spent mushroom substrate. The effect of various parameters such as flow rate, bed height and initial concentration of manganese in the solution was studied. The results of breakthrough time, exhaustion time revealed the influence of flow rate, bed height and initial manganese concentration. The breakthrough time and exhaustion time increased with decreasing the flow rate and initial manganese concentration. In contrast to this, breakthrough time and exhaustion time increased with increasing the bed height. The process involved in the removal of manganese is confirmed as an adsorption process by energy-dispersive X-ray spectroscopic analysis. Fourier-transform infrared spectroscopy analysis revealed the involvement of carboxyl, hydroxyl and amide functional groups as the active binding sites for manganese ion adsorption (Kamarudzaman et al. 2015).
Adsorption of Zinc
The concentration of Zinc above the critical toxicity level, i.e. 200 mg per Kg is reported to be toxic for cotton crops. Excessive amount of zinc in the soil severely affects Gossypium hirsutum i.e. cotton plant and results in stunted growth. This toxicity can be reduced by increasing the pH of soil because this pH helps in binding the zinc to the soil organic matter and thus, reducing the bioavailability of zinc to the plant (Williams 1980). Therefore, increase in pH of soil results in eliminating the problem of zinc toxicity which is not practically feasible option.
Another method of ameliorating the zinc toxicity is the application of higher organic matter in the soil (Romney et al. 1977). Shuman and Li (1997) performed a study to find out the effect of lime and mushroom substrate in zinc adsorption from the soil in order to reduce the effect of zinc on cotton plant. A pot experiment was done by using spent mushroom substrate as adsorbent and zinc contaminated soil and results were compared with non-polluted soil. Lime was used as a supplement. In the pot, spent mushroom substrate alone and in combination with lime was reported to reduce the zinc toxicity. There was a steady decline in the zinc toxicity with increasing the lime supplementation. This experiment proved that lime and spent mushroom substrate supplementation is effective in reducing zinc toxicity from the soil where zinc concentration is very high. However, lime supplementation alone is sufficient to remove zinc, when it is present at low concentration.
9.3.5.3 Spent Mushroom Substrate of Agaricus
Agaricus species are widely cultivated mushroom on composted and non-composted autoclaved substrate. After harvesting mushroom fruit bodies, a huge amount of spent mushroom substrate is generated which discards as waste. Spent mushroom substrate has also been used as adsorbent in the adsorption of dyes, heavy metals and pollutants.
Adsorption of Lead
Agaricus spent mushroom substrate was used for removing the lead from aqueous solution by Huang et al. (2009) and further, lead removal conditions were optimized by Tagushi method (Taguchi and Konishi 1987). This was performed with four control factors, i.e. pH, contact time, adsorbent mass and initial lead concentration. Huang et al. (2009) investigated the adsorption capacity of spent mushroom substrate and percent removal of lead. The optimum adsorption capacity was reported to be at pH 5, time 5 h, adsorbent biomass 10 mg, initial lead concentration 50 mg/L. However, the optimum condition for removal of lead was reported to be pH 4, time 4 h, sorbent mass 0.010 g, initial lead concentration 50 mg/L. In these conditions, optimum lead adsorption capacity and percent removal was 60.76 mg/g and 80.50%, respectively.
Adsorption of Copper
Agaricus spent mushroom substrate was also used for the adsorption of copper. Agaricus spent mushroom substrate was mixed in 20 ml water and then filtered through filter paper. After filtration, mushroom mycelium was collected on filter paper. Prior to use in the adsorption studies, collected mycelium was dried. For adsorption studies, copper solution of 1 g/L, (w/v) was prepared by using copper sulphate penta-hydrate (Siasar and Sargazi 2015). Adsorption, at initial stage was faster compared to that occurred at later stage. The slow rate of adsorption at later stage is possibly due to the low number of available sites for binding with adsorbate or due to the shielding effect (Bishnoi et al. 2007).
The adsorption of copper is related to increase in pH of the solution from 2 to 6 due to the presence of negative charged ions on the surface of biomass. However, adsorption of copper decreases dramatically between pH 6–8 which is possibly due to the formation of anionic hydroxide complexes of the metal ions and their competition with the active sites. Adsorption of copper increases the density of positive charge on the surface of adsorbent at low pH values, which further leads to reduced rate of adsorption due to the repulsive forces. The maximum adsorption capacity of copper ions was reported to be present at pH 6. In this experiment, Langmuir model explained this as non-linear adsorption and suggested that uptake occurs on a homogenous surface by monolayer adsorption without interaction between adsorbed molecules. This experiment sheds a light on the fact that spent mushroom substrate has good efficiency of adsorbing copper ions from the aqueous solutions, especially with low metal concentrations.
9.3.5.4 Modified Spent Mushroom Substrate
The adsorption capacity of spent mushroom substrate can be modified by preparing activated carbon . Tay et al. (2015) prepared the spent mushroom substrate activated carbon by using central composite rotatable design-response surface methodology. In pre-carbonization stage, spent mushroom substrate was soaked in an activating agent i.e. potassium hydroxide. This was followed by drying at 80 °C for 24 h and further, carbonized in muffle furnace. Optimization of activated carbon percent yield can be described by central composite rotatable design-response surface methodology. This method helps in minimizing the use of chemicals and energy in the preparation of activated carbon , which is utmost important to implement the technology for achieving healthy environment in a sustainable way.
Adsorption of Nickel
The spent mushroom substrate was modified in the form of activated carbon by using Box-Nehnken design of response surface methodology. Md-Desa et al. (2016) prepared the activated carbon by spent mushroom substrate using potassium hydroxide as an activating agent. Potassium hydroxide forms the pores in activated carbon due to intercalation of metallic potassium ion in the carbon structure and increases the porosity of activated carbon. For the preparation of activated carbon, the spent mushroom substrate was soaked in 3 M potassium hydroxide for 24 h which was followed by drying at 80 °C for 24 h. This material was carbonized in the furnace at 500 °C with heating rate of 10 °C/min. The produced char was washed with hydrochloric acid and distilled water to remove residual potassium hydroxide and again, dried at 80 °C. The prepared activated carbon was sieved to 150 μm and kept at drying cabinet prior to use. A software generated experimental design was used to assess the effect of preparation parameters of impregnation ratio, and activation time. The potential of activated carbon derived from Pleurotus ostreatus spent mushroom substrate was assessed for the removal of nickel. The batch adsorption studies by varying adsorbent dosage, pH, contact time, metal concentration and temperature were determined. The samples were shaken at 125 rpm, filtered through filter paper and analyzed using inductively coupled plasma optical emission spectrometry. In this study, 50% nickel was adsorbed at 0.63 g adsorbent, pH 5–6, contact time 60 min, nickel concentration 50 mg/L and temperature 25 °C. Further, these results were also compared with Amberlite IRC86 resin and zeolite NK3. The result indicated that spent mushroom substrate derived activated carbon have a great potential (51.35%) to adsorb nickel from the water as compared to Amberlite IRC86 resin (39.31%) and zeolite NK3 (34.35%) (Md-Desa et al. 2016). The spent mushroom substrate is an economic alternative to adsorb nickel from waste water.
9.3.5.5 Super Adsorbent Preparation from Spent Mushroom Substrate
A super adsorbent was prepared by Ding and Gong (2013) using spent mushroom substrate. Spent mushroom substrate from the mushroom bed is, generally, obtained in the thick- slurried form with 80% water content. For the preparation of super-adsorbent, spent mushroom substrate was allowed to air-dry to reduce its moisture content up to 12%. This was followed by crushing into fine powder and passed through 100-mesh sieve in order to get particles of uniform size. Now, spent mushroom substrate along with ammonium persulfate, acrylic acid, N,N-methylene-bisacrylamide, water and solution of 40% sodium hydroxide were added into heat resistant bottles. This formed a suspension of 200 g in the bottle. After this, bottles were placed in a microwave oven. The whole mixture was heated for 8 min at low-grade-firepower till the porous hydrogel was formed. The gel was spread on an aluminum plate and dried in a vacuum oven to reduce the water content up to 9%. Again, the resin was passed through a 20-mesh screen. This material acted as a good quality adsorbent and further, adsorption strength and gel strength increased with an increase in the spent mushroom substrate ratio. The speed of adsorption was not much affected by ammonium persulphate dosage unlike spent mushroom substrate ratio. Scanning electron microscopy revealed the presence of multilayered and loose particles in the prepared super adsorbent because spent mushroom substrate particles interfere in the continuity of composite resin. The spent mushroom substrate particles distribute uniformly in the acrylic resin and form sub-microscopically homogenous composites (Ding and Gong 2013). This super adsorbent has been produced successfully, however, not used in pollutant adsorption studies to till date.
9.3.6 Adsorption of Dyes
Dyes are considered as one of the toxic pollutant, released from various industries. During the process of dyeing, about 10–20% of dyes is not utilized which releases with the effluent and reaches the water bodies. These discharges increase the biological oxygen demand and chemical oxygen demand of the water bodies and make them unsuitable for use. The dyes are complex molecules and therefore, not efficiently degraded by bacteria (Singh et al. 2013). The partial degradation of dyes leads to the production of toxic and mutagenic compounds like aromatic amines (Lade et al. 2015). Fungi possess a variety of enzymes that helps them to grow in a variety of environmental conditions . Moreover, live or dead biomass of fungi can adsorb a variety of pollutants. Consider this fact, fungal culture bearing spent mushroom substrate is used as adsorbent for dyes by many researchers (Toptas et al. 2014; Yan et al. 2015).
9.3.6.1 Spent Mushroom Substrate of Agaricus bisporous
The spent mushroom substrate of Agaricus bisporus was used for the dye adsorption. Toptas et al. (2014) developed the procedure to use spent mushroom substrate of Agaricus for adsorption of dyes. The collected Agaricus spent mushroom substrate was washed with water and then dried in an oven at 60 °C overnight. This was followed by grinding of spent mushroom substrate in fine particles of 0.2 mm or lesser. The characterization of spent mushroom substrate revealed the presence of chitin in the substrate (Toptas et al. 2014). This adsorbent was used in decolorization studies of Acid Red 111 and Basic Red 18 and Levafix Braun E-RN dyes in batch process. The dyes solutions were prepared in flasks and pH of the dye solution was adjusted with dilute hydrochloric acid and sodium hydroxide using pH meter. The spent mushroom substrate was added to the dye solution and then left at room temperature. After the fixed time intervals, samples were withdrawn and filtered to separate adsorbent and analyzed for absorbance at 418 nm for Levafix Braun E-RN and 504 nm for Acid red 111 or Basic red 18. The conditions were optimized for pH, contact time and amount of adsorbent. pH has an important role in adsorption of dyes. The effect of pH on the Acid red 111 dye adsorption by spent mushroom substrate was tested for a pH range from 2 to 6 and for different contact times and maximum uptake of Acid red 111 was reported at pH 3. The equilibrium was reached after 6 h depending on pH of the solution. pH of the solution affects both surface charges of spent mushroom substrate and dyes; and solubility of dyes and their colour in solution. Further, pH influences the ionic interaction between adsorbent and adsorbate due to its effect on the charge. At lower pH, the amino groups of chitin in spent mushroom substrate possess negative charge due to which excess hydronium ions are present on the surface of spent mushroom substrate. These negatively charged ions of spent mushroom substrate can bind with the cations of dyes. Hence, the adsorption of dyes on the surface of spent mushroom substrate occurs due to ionic interaction. The dye binding ability of chitin is not affected by changing the pH in the range of 2–7 because chitin remains insoluble and unaffected by the presence of protonated amines. At pH 3, contact time of 2 h was required to achieve equilibrium. Depending on pH, maximum time required to achieve the equilibrium is 6 h. With increasing the amount of spent mushroom substrate from 0.05 to 1.0 g to fixed dye concentration, i.e. 100 mg/L, rate of adsorption increased and 0.2 g was found to be the optimum amount of spent mushroom substrate. Initially, dyes adsorption efficiency was reported to increase with an increase in the amount of adsorbent up to 0.2 g due to increase in the availability of adsorption sites. However, the efficiency of adsorption did not increase beyond this amount (Toptas et al. 2014).
In the case of adsorption of Levafix Braun E-RN (LB) dye using Agaricus spent mushroom substrate, the pH range 2–5 was assessed in dye adsorption studies. Results of the study showed that the adsorption capacity decreased with increasing pH. The optimum adsorption was found at pH 2 (Toptas et al. 2014).
In case of reactive dyes, a covalent bond is formed between reactive groups of the dye molecule and nucleophilic group within the fibers of spent mushroom substrate. The hydroxyl group of cellulosic spent mushroom substrate, the amino and hydroxyl groups of proteins, and amino groups of polyamides contribute in the dye adsorption process (Toptas et al. 2014).
It is reported in the literature that the equilibrium data fitted well with the Langmuir and Freundlich isotherms in case of acidic and basic dyes. In contrast, equilibrium data fitted well with the Langmuir isotherm model in case of reactive dyes (Toptas et al. 2014). Thermodynamic parameters depicted that adsorption of dyes on spent mushroom substrate is spontaneous and exothermic. Fourier-transform infrared spectroscopy analysis revealed the involvement of hydroxyl, amine and carboxyl groups in the dye adsorption process. Therefore, dye adsorption depends on different parameters such as dye concentration, substrate concentration, pH and temperature.
9.3.6.2 Modified Spent Mushroom Substrate As Adsorbent
Oxalic Acid Treated Spent Mushroom Substrate
The spent mushroom substrate can also be used in modified form in order to increase the adsorption capacity. To enhance its adsorption capacity, spent mushroom substrate can be treated with chemicals. In a study, conducted by Yan et al. (2015) spent mushroom substrate was collected after the harvesting of mushroom fruit bodies and washed thoroughly with running tap water which was followed by rinsing with distilled water several times to remove impurities. The washed spent mushroom substrate was dried in an oven at 85 °C for 6 h. The dried spent mushroom substrate was ground into fine particles and sieved to 60–80 mesh particle size. The dried spent mushroom substrate was treated with 0.6 mol/L oxalic acid solution at 60 °C for 2 h. Then, the oxalic acid was removed by filtration. The filtrate was discarded and the oxalic acid treated spent mushroom substrate was dried initially at 50 °C for 24 h and then by increasing temperature at 120 °C for 3 h. After drying, oxalic acid treated spent mushroom substrate was stored in airtight container. The oxalic acid treated sms was used as adsorbent in the methylene blue (80 mg/L) adsorption experiments with 30 to 300 mg doses at original solution pH. The decolorization of dye solution was assessed at different temperature ranges 303–333 K for 12 h with continuous stirring. Adsorption kinetic experiments were performed by using 100 mg of oxalic acid treated spent mushroom substrate. Then, the flasks were taken out at some intervals for sample withdrawal. The adsorbent was removed by filtration through a 400 mesh nylon screen. The dye concentration of supernatant was measured by UV-visible spectrophotometer. Characterization of untreated and oxalic acid treated substrate by Fourier-transform infrared spectroscopy analysis revealed the stretches and new peaks in the region of 2800–2900 and 1670–1760 cm−1 which correspond to C-H stretching vibrations of carbonyl group in the other constituents or in the carboxylic acid, respectively. This shows the modification of surface of spent mushroom substrate in terms of increased number of carboxyl group (Yan et al. 2015). Oxalic acid treatment helps in increasing the number of carboxyl group on spent mushroom substrate which further increases the adsorption capacity.
In the adsorption experiment, conducted by Yan et al. (2015) methylene blue adsorption capacity of oxalic acid treated spent mushroom substrate was analyzed in the pH range of 2–10. The methylene blue adsorption capacity was reported to increase in pH range 2–4. Further, increase in pH did not improve the adsorption capacity. Methylene blue is basic dye and possesses positive charge in aqueous solution while in the same condition; spent mushroom substrate is surrounded by hydronium ions. Hence, methylene blue competes with the hydronium ions for binding sites on spent mushroom substrate and electrostatic repulsion occurs, which prevents the adsorption of methylene blue dye. Carboxylic groups are not deprotonated and ionize at pH below 4 which results in decreased adsorption. Oxalic acid treated spent mushroom substrate possesses negatively charged carboxylic groups that have ability to deprotonate at higher pH. Therefore, at higher pH 6, carboxylic groups are easily deprotonated and carried out methylene blue adsorption process due to electrostatic attraction between negative charged carboxylic group and positive charged dye. The oxalic acid treated spent mushroom substrate can also interact with methylene blue dye due to hydrogen bonds formation (Yan et al. 2015).
The effect of contact time and initial concentration revealed the maximum adsorption within the first 60 min., equilibrium was achieved in 120 min at dye concentration of 100 mg/L and thereafter, no adsorption or little adsorption occurred. Similar types of results were found at other concentrations. Again, this reveals the presence of a huge number of adsorption sites on oxalic acid treated spent mushroom substrate. When time is increased, the available site for adsorption decreased. With the increase in initial methylene blue concentration ranging from 40 to 100 mg/L, the driving force for the mass transfer between adsorbent spent mushroom substrate and dye solution increased which resulted in the increased adsorption capacity. It was also reported that equilibrium adsorption capacity decreases from 128.2 to 19.7 mg/g with increasing the amount of oxalic acid treated spent mushroom substrate from 25 to 200 mg. To study the effect of temperature on adsorption, equilibrium adsorption capacity was reported to increase from 89.3 to 97.2%. The lower temperature was found to favour the adsorption process. Boyd’s film-diffusion method elucidated the mechanism of methylene blue adsorption by external mass transfer. The thermodynamic parameters indicated that the methylene blue adsorption process by oxalic acid treated spent mushroom substrate is exothermic, feasible and spontaneous. This study sheds the light on the fact that oxalic acid treated adsorbent can be used successfully for the adsorption of dyes and therefore, can be used for the treatment of effluent possess methylene blue dye (Yan et al. 2015).
Recently, spent mushroom substrate co-pyrolyzed with Saccharina japonica i.e. kelp seaweed biomass and used for adsorption studies of crystal violet dye by Sewu et al. (2017). The potential of this co-pyrolyzed biochar was compared with biochars having different compositions: (i) biochar from spent mushroom substrate alone (ii) biochar prepared by adding 10% kelp extract to spent mushroom substrate (iii) biochar from kelp extract added spent mushroom substrate. The results of this study showed that biochar from kelp extract containing spent mushroom substrate had the highest dye adsorption capacity with highest fixed carbon content (70.60%) and biochar yield (31.6%). Spent mushroom substrate co-pyrolyzed with 10% kelp extract was also reported to be a good adsorbent with 2.2 times higher adsorption capacity i.e. 610.1 mg/g than spent mushroom substrate used alone i.e. 282.9 mg/g. Spent mushroom substrate co-pyrolyzed with 10% kelp extract had high ash content, abundant functional groups as revealed by Fourier-transformed infrared spectroscopy and coarse surface morphology. Langmuir model of adsorption is well fixed to the adsorption data (Sewu et al. 2017). This study shows that the adsorption potential of spent mushroom substrate can be increased by using kelp seaweed extract.
9.3.7 Adsorption of Pollutants
Industrial and agro-industrial activities lead to the release of effluent in the water bodies. This effluent is loaded with a variety of chemicals, dyes or pollutants which poses adverse effect on the environment and living beings. The natural remediation process is slow and depends on the presence of microbes in soil. The supplementation of soil and water with spent mushroom substrate can be useful in the remediation of pollutants.
9.3.7.1 Polycyclic Aromatic Hydrocarbon Degradation by Spent Mushroom Substrate of Agaricus
Polycyclic aromatic hydrocarbon is a contaminant, discharged by many industries, in the environment. Many polycyclic aromatic hydrocarbons are toxic, mutagenic and carcinogenic. These are lipid soluble and readily absorbed by gastrointestinal tract of mammals and remains in body fat (Abdel-Shafy and Mansourb 2016). Hence, it needs to be removed from the environment. Recently, García-Delgado et al. (2015) reported the use of spent mushroom substrate of Agaricus bisporous in polycyclic aromatic hydrocarbons remediation from soil. To analyze the effect of Agaricus spent mushroom substrate in terms of type or density of microbial population on polycyclic aromatic hydrocarbons removal, it was amended in autoclaved soil and un-autoclaved soil. First set was prepared in the autoclaved soil in order to assess the effect of indigenous microbial population on the spent mushroom substrate supplemented soil. This data provided the comparison of impact of indigenous soil microflora on the bioavailability of polycyclic aromatic hydrocarbons; and the remediation potential of spent mushroom substrate and indigenous microbes. Results indicated that naturally occurring indigenous microbial population is ineffective in removing polycyclic aromatic hydrocarbons. Therefore, soil was amended in two ways by using spent mushroom substrate. Firstly, spent mushroom substrate was used directly without treatment. Secondly, spent mushroom substrate was applied after sterilization and bioaugmented with A. bisporus in order to find out the use of spent mushroom substrate as a microbial carrier. Polycyclic aromatic hydrocarbon removal efficiency of spent mushroom substrate was 17%. No significant difference was reported to be present in terms of number of rings degradation. The bioaugmented Agaricus bisporus to sterilized spent mushroom substrate showed the most efficient degradation rate i.e. 29% as compared to spent mushroom compost alone i.e. 21%. Results showed that spent mushroom substrate bioaugmented with fresh Agaricus bisporous has great potential in removing the polycyclic aromatic hydrocarbons and it can be used as carrier molecule (García-Delgado et al. 2015).
This experiment showed that spent mushroom substrate has the highest potential in removing polycyclic aromatic hydrocarbons and therefore, can be used as a potent adsorption tool.
9.3.7.2 Pesticide/Fungicide Adsorption by Spent Mushroom Substrate
Pesticides are toxic chemicals, however, used to control the pest in the agricultural field. When pesticides used in an uncontrolled and unrestricted way to control the pest, they accumulate in the environment. They produce adverse effects in the living beings like toxicological effects, neurological disorder, cancer and hormonal disorder, fetal development. Hence, the use of pesticides must be controlled and strategies are required to remove them from the environment.
Recently, Álvarez-Martín et al. (2016a) performed the experiment on the adsorption of polar and non-polar pesticides/fungicides by spent mushroom substrate. In this study, cymoxanil and pirimicab were used as polar pesticides and tebuconazole and triadimenol were used as non-polar pesticides. The spent mushroom substrate was applied to the soil in different amounts that varies between 2 and 75%. The spent mushroom substrate amended soil showed 90% adsorption of non-polar pesticides. However, adsorption was only 56.3% for polar pesticides. Adsorption efficiency for non-polar pesticides was reported to increase with increasing the amount of spent mushroom substrate in soil. In contrast, such type of increment was not observed in the case of polar pesticides. The possible reason of this was indicated by increase in organic carbon content of the soil by spent mushroom substrate supplementation. Therefore, spent mushroom substrate can be used as a tool to adsorb non-polar pesticides from the soil and help in removing them.
The spent mushroom substrate can be used to immobilize pesticides when it is applied to the soil. The spent mushroom substrate is rich in organic matter and therefore, it can adsorb pesticides and reduces their bioavailability. In a study, conducted by Herrero-Hernández et al. (2011) for tebuconazole distribution in soil was assessed in the soil depth upto 50 cm after 124, 209 and 355 days of application of spent mushroom substrate. The study revealed the higher level of fungicide in the deep layer of amended soil compared to non-amended soil. This was possible due to the effect of organic matter of spent mushroom substrate on the soil. The spent mushroom substrate was reported to bind with the pesticide to immobilize it and further, prevents its spreading in the soil. Application of spent mushroom substrate and fungi together in the field requires validation of data by comparing the field data and laboratory data in order to prevent water and soil contamination (Herrero-Hernández et al. 2011). This also prevents the leaching of pesticides in soil and ground water. Thus, the spent mushroom substrate is an important tool not only to adsorb the pesticides, but also to degrade them by producing a variety of enzymes.
Further, the efficiency of spent mushroom substrate in the adsorption of linuron, diazinon and myclobutanil was also compared with sewage sludge and grape marc by Rodríguez-Cruz et al. (2012). The spent mushroom substrate was found to be more effective for myclobutanil pesticide adsorption while grape marc was reported to be effective for linuron and diazinon (Rodríguez-Cruz et al. 2012). Therefore, spent mushroom substrate can be used for adsorbing pesticides from the soil, however, it depends on a variety of factors which affects the adsorption of one pesticide not the other. These factors need to be investigated in detail and considered in order to apply spent mushroom substrate in the field.
The efficiency of spent mushroom substrate as an adsorbent of fungicides was evaluated by Sánchez-Martín and Rodríguez-Cruz (2012). In this study, different types of spent mushroom substrate were used with eight different fungicides. In fungicide adsorption studies by spent mushroom substrate, non-linear sorption isotherms were observed. Pre-adsorbed and post-adsorbed spent mushroom substrate was analyzed by Fourier-transform infrared spectroscopy and changes in peaks and bands revealed the active groups involved in the adsorption process. The spent mushroom substrate also showed very low potential to desorb the fungicides (Sánchez-Martín and Rodríguez-Cruz 2012). In a laboratory experiment, the efficiency of Agaricus spent mushroom substrate was assessed for carbendazim and moncozeb pesticides. The spent mushroom substrate was sterilized and used as carrier after loading with Trichoderma sp. and Aspergillus sp. (set 1). Another set (set 2) was loaded with Trichoderma sp., Aspergillus sp. and a bacterial isolate. The highest degradation of mancozeb was achieved with set 2 while the highest degradation of carbendazim was achieved with set 1 in 15 days of incubation at 30 ± 2 °C. The possible reason for this is the production of lignocellulolytic enzymes produced by fungi to degrade carbendazim. In spent mushroom substrate-amended soil, adsorption of fungicides results in decrease bioavailability of fungicides (Álvarez-Martín et al. 2016b).
Herrero-Hernández et al. (2015) reported the effect of spent mushroom substrate supplementation on the removal of azoxystrobin fungicide in laboratory conditions and natural field conditions with and without spent mushroom substrate supplementation. The spent mushroom substrate supplementation helps in more adsorption of fungicide from the soil compared to non-supplemented soil. Bioavailability of fungicide and its immobilization depends on the supplementation of soil with spent mushroom substrate. The fungicide showed a stimulatory effect on the dehydrogenase activity of soil. The spent mushroom substrate supplementation or non-supplementation directs the adsorption of azoxystrobin fungicide. There are significant differences in the behavior of fungicide in two conditions i.e. laboratory conditions and field condition. In laboratory conditions, the dissipation of fungicide much slower than that in field condition. Therefore, it is suggested to perform the experiment in field condition to derive any conclusion regarding the application of spent mushroom substrate.
The fungicide adsorption depends on rate of spent mushroom substrate amendment, use of composted and/or fresh spent mushroom substrate, incubation time. The effect of the addition of fresh and composted spent mushroom substrate to the soil was analyzed for penconazole and metalaxyl pesticides. The spent mushroom substrate amendment also leads to the increase in immobilization of metalaxyl (water soluble pesticide) and retention of penconazole (highly hydrophobic). Therefore, spent mushroom substrate supplementation immobilized metalaxyl and considered as good tool for removing metalaxyl from the soil (Marín-Benito et al. 2009). The spent mushroom substrate is a useful tool to increase the organic content and immobilize the pesticides. The spent mushroom substrate amended soil was found to adsorb more pesticide than the non-amended soil. Further, in the next experiment, the effect of composted or fresh spent mushroom substrate was assessed for iprovalicarb, penconazole, metalaxyl and pyrimethanil fungicides. The degradation rate of all fungicides decreased in the composted spent mushroom substrate amended soil. However, it decreased only for iprovalicarb and penconazole in fresh spent mushroom substrate amended soil. The highest mineralization of fungicides was obtained in non-amended soil for metaxyl and penconazole fungicide. However, the fresh spent mushroom substrate amended soil showed the formation of non-extractable residues with metalaxyl fungicide. This study shows the differences in the rate of degradation of fungicide with the fresh and composted spent mushroom substrate supplementation and the characteristics of spent mushroom substrate affecting the degradation process. Fresh and composted spent mushroom substrate has different abilities to adsorb fungicides and therefore, a detailed investigation is required for their application (Marín-Benito et al. 2012).
9.3.7.3 Adsorption of Antibiotics by Spent Mushroom Substrate
Sulphonamides are very popular antibiotics use to treat various diseases of human beings and animals. The treated patients or animals secret these antibiotic with excreta. These antibiotics reach the water bodies with run-off water; and agricultural field due to the application of animal manure. In China, spent mushroom substrate was used for the treatment of sulfa antibiotics containing water. Recently, experiment was performed in laboratory conditions by Zhou et al. (2016). For this purpose, spent mushroom substrate was collected and soaked in distilled water for 24 h, then washed, filtered and dried in an oven at 35 °C for 4 h. The dried material were sieved to the desired mesh size i.e. 100–300 μm and stored in airtight container. Experiments were conducted by varying contact time, initial concentration range 0.5–10 mg/L and pH 1–11. The result of this study indicated that the data best fit to pseudo second order kinetic model. Intra-particle diffusion study showed that film diffusion occurred during the adsorption of sulfadrugs on to spent mushroom substrate. Therefore, spent mushroom substrate is a good adsorbent to adsorb the traces of sulfa drugs from waste water (Zhou et al. 2016).
9.3.7.4 Modified Spent Mushroom Substrate for Pollutants Adsorption
Aluminum Hydroxide Coated Biochar for Fluoride Adsorption
Chen et al. (2016) suggested the use of aluminum hydroxide coated spent mushroom substrate biochar for the adsorption of fluoride. Spent mushroom substrate was modified to produce carbonized spent mushroom substrate biochar, which was further coated by aluminum hydroxide. The preparation of aluminum hydroxide-coated biochar was done by using 4 g spent mushroom substrate biochar and 40 ml aluminum sulfate solution. Both were added to the beaker and stirred vigorously at 60 °C. The pH was then adjusted to 5 by using sodium hydroxide and hydrochloric acid solution. This solution was filtered by vacuum filtration and put in the hot air oven at 110 °C for 3 h to coat the spent mushroom substrate biochar with aluminum hydroxide. The coated biochar was washed several times with distilled water in order to remove sodium and sulfate salts and then dried in hot air oven at 100 °C for 5 h. The coated spent mushroom substrate biochar was ground into powder. To keep uniform size particles, biochar was filtered through 6-mesh and then, placed in an airtight container. The coated and uncoated biochar were characterized for adsorption properties. The specific surface area of coated and coated spent mushroom substrate biochar was determined by BET method using the automatic surface area analyzer. A scanning surface image of the coated and uncoated spent mushroom substrate biochar was obtained by SEM technique. To analyze the elemental composition of coated and uncoated spent mushroom substrate biochar, energy-dispersive spectrometer was used. An X-ray powder diffractometer was used to analyze the structures and phases of uncoated and coated spent mushroom substrate biochar surface.
This adsorbent was used in the adsorption studies and impact of adsorbent dosage, initial fluoride concentration, contact time, pH, and coexisting fluoride ion adsorption. The adsorbent dosage between 0.4 and 8 g/L, initial fluoride concentration between 5–100 mg/L, contact time of 1–960 min was evaluated in 50 mL centrifuge tubes in a batch experiment. These tubes were kept at shaker which was set at the speed of 300 rpm for 180 min at room temperature i.e. 25 ± 2 °C. The solution was separated by filtration. Fluoride ion concentration was measured by an electrode based method using fluoride ion selective electrode (Chen et al. 2016). In this way, aluminum hydroxide coated spent mushroom substrate biochar was reported to be more effective in removing the fluoride as compared to uncoated spent mushroom substrate biochar. Langmuir isotherm is the best fit isotherm model to show fluoride adsorption. The maximum fluoride adsorption capacity by aluminium hydroxide coated spent mushroom substrate biochar was 36.5 mg/g and due to its application, fluoride concentration was reduced to 1 mg/L from its initial concentration i.e. 10 mg/L (Chen et al. 2016).
The coated spent mushroom substrate biochar was reported to be having fluoride ion adsorption capacity higher than that of other biomass-based adsorbents. Besides this, coated spent mushroom substrate biochar can be used over a wide pH range. It provides great operational advantages which are helpful in transferring this technology to the field. The spent mushroom substrate is generated as waste and has the capacity to remove fluoride; this technique is economic to remove fluoride from the water and hence is a very feasible method to implement.
9.4 Factors Affecting the Process of Adsorption
A number of factors influence the adsorption of metals from the effluents such as pH, temperature, concentration of adsorbent and adsorbate, and bioavailability of pollutants. The bioavailability of metal depends on various factors like buffering capacity, mineral content of the substrate, organic content of the substrate and cation exchange capacity. Besides, the use of living and dead biomass require different requirement for the removal of heavy metals and pollutants. Living biomass requires the optimum conditions for growth like nutrient requirement, pH and temperature, which is a dispensable factor for dead biomass (Gadd 2009).
Keeping all the factors in mind, an efficient method can be developed for the adsorption of heavy metals and pollutants to reduce the risk of exposure of living beings to heavy metals and pollutants (Ayangbenro and Babalola 2017). The factors affecting the adsorption process are discussed here.
9.4.1 pH
The pH is an important factor as it affects the selectivity of spent mushroom substrate. Therefore, it is necessary to maintain desired pH in order to induce the binding of spent mushroom substrate to a variety of pollutants and metals. The selectivity of spent mushroom substrate depends on the functional groups, which may vary according to pH. The binding properties of metal in the solution are also dependent on pH (Dursun 2006) as it also affects the solubility of metal ions in the solution. In order to demonstrate the effect of pH on adsorption capacity, several experiments were conducted with different metals and spent mushroom substrate (Tay et al. 2012; Siasar and Sargazi 2015; Chen et al. 2016).
In a study, the effect of pH was demonstrated on the uptake of copper ions by using different pH ranges 2–8 and found that copper ions adsorption increases with increase in pH values up to 6. The increased pH up to 6 corresponds to the increase number of negatively charged active sites which further facilitates the interaction with positively charged metal ions. At pH 2, the surface of biomass is protonated which exerts repulsive force on metal ions. With an increase in the pH up to 6, pollutants and metals bind to spent mushroom substrate due to electrostatic interaction and saturate the active sites of spent mushroom substrate which exert a repulsive force, resulting in decreased rate of adsorption after attaining a limit. In contrast, at higher pH values, the anionic hydroxides are formed. Siasar and Sargazi (2015) reported the formation of anionic hydroxide at pH 6. In contrast to this, Tay et al. (2012) reported the formation of insoluble hydroxide at pH 8. Generally, at highly basic condition i.e. pH about 8, anionic hydroxide complexes are formed which competes for active sites and results in the decrease adsorption due to competition between anionic hydroxide and metals for the active binding sites (Vimala and Das 2009; Siasar and Sargazi 2015; Ayangbenro and Babalola 2017).
Similar type of observation was observed for nickel adsorption by spent mushroom substrate in aqueous solution. At low pH, the surface of spent mushroom substrate is negatively charged due to which it attracts protons and surface of spent mushroom substrate becomes protonated. When initial pH rises, the nickel adsorption increases due to deprotonation of binding sites. After saturation of binding sites of spent mushroom substrate with nickel (II), adsorption decreases. The optimum pH value for nickel adsorption is 4.5 which is also the initial pH of the aqueous solution of nickel and therefore, there is no need of adjusting pH (Tay et al. 2011). Similar type of effect of pH on adsorption was observed for lead and cadmium by spent mushroom substrate of Pleurotus platypus, A. bisporus and C. indica, respectively (Vimala and Das 2009) and lead uptake by P. osteatus (Liew et al. 2010). Hence, adsorption of cationic metals reduced at very low pH i.e. between 2 and 4 and increases with increase in pH up to 6. Again with increase in pH, adsorption decreases due to insoluble metal hydroxide formation.
In contrast to above, it is also reported that the anionic metal species like platinum chloride chromium oxide, gold cyanide adsorption increases with decrease in pH. These studies proved that the solubility and bioavailability of heavy metals can be affected by the pH values of the solution. Occasionally, adsorption process is not dependent on pH like adsorption of silver ions, mercury ions and gold chloride due to the formation of covalent complexes with nitrogen and sulphur containing ligands (Gadd 2009).
9.4.2 Bioavailability of Pollutants in Natural Condition
In natural condition, bioavailability of pollutant is considered as an important factor in planning the adsorption strategy. For e.g., soil characteristics play an important role in the adsorption of pollutants from the soil by using spent mushroom substrate. Soil properties are affected by the presence of organic matter because organic matter has a strong impact on the cation exchange capacity, buffer capacity, and on the retention and bioavailability of heavy metals. Soil with low organic matter usually found to have a high content of heavy metals which is bioavailable to plants and microbes. In contrast to this, organic content of the soil binds with the heavy metals and make them less mobile and less bioavailable to microbes and plants (Ayangbenro and Babalola 2017). Therefore, idea of using spent mushroom substrate is related to increase the organic content of the soil, which further immobilize metals in the soil due their adsorption and make them unavailable to plants. Therefore, spent mushroom substrate based amendments help in reducing the toxicity of heavy metals by reducing their bioavailability to the plants.
9.4.3 Temperature
Temperature also plays an important role in adsorption of heavy metals and pollutants. The raise of temperature increases the fluidity of liquid due to decrease in viscosity and hence, increases the adsorption. Another reason of high adsorption due to increase in temperature is related to increase rate of diffusion of adsorbate particles into adsorbent. Temperature also affects the stability of metal ions present in the solution (Ayangbenro and Babalola 2017). Therefore, increase in temperature increases the metal adsorption capacity.
9.4.4 Contact Time
Another important factor in the study of metal removal and pollutants removal by spent mushroom substrate is contact time. It is the time to which spent mushroom substrate is exposed to the metal or pollutant. The adsorption is a process in which attaining equilibrium is an important criterion. Adsorption of heavy metals , dyes and pollutants occurs initially at faster speed, however on increasing the contact time the process gradually decreases. After applying spent mushroom substrate to the solution, adsorption of heavy metals or pollutants gradually decreases with increase in the contact time. Initially, a large number of binding sites are available on the spent mushroom substrate to which metal or pollutant binds. However, on increasing the contact time, spent mushroom substrate covered with adsorbate and reduced vacant sites. The remaining vacant sites cannot be occupied further, due to repulsive forces between the molecules of adsorbate and pollutant or metal covered adsorbent (Siasar and Sargazi 2015).
9.4.5 Characteristics of Biosorbent
The most important characteristic of biosorbent includes its porosity. The biosorbent must be porous in order to allow the uptake of metals and pollutants. Secondly, it must possess active functional groups bearing negative charge like alkyl, hydroxyl, or amino compound, aliphatic alcohol, carboxyl and carbonyl groups. The spent mushroom substrate is rich in calcium because calcium salts are used in the preparation of mushroom substrate. These calcium ions can be replaced by the metal ions by ion exchange process during adsorption. This replacement of calcium ions with metal ions can be observed by changing peaks in the Fourier-transform infrared spectroscopy analysis (Tay et al. 2011).
The binding of cations on the negatively-charged surface of spent mushroom substrate may increase adsorption of anions. Occasionally, the binding of cations to spent mushroom substrate may enhance the adsorption of another cation due the pH based buffering effects. This can be understood by the example of adsorption of zinc on the calcium-rich spent mushroom substrate. In the preparation of mushroom substrate, calcium carbonate is used which saturate the spent mushroom substrate with the calcium ions. It is reported that this calcium containing spent mushroom substrate have better efficiency for zinc adsorption (Fourest et al. 1994) because calcium ions are replaced by zinc during adsorption process. The adsorption process also depends on the characteristic and charge of spent mushroom substrate and interference of metals ions. For e.g. some negative charge ions on spent mushroom substrate can increase the adsorption of metals. In contrast to this, presence of carbonate, chlorides and phosphate interferes in the adsorption process due to the formation of insoluble precipitates with metals. For e.g., chloride ions may form complex with cadmium like cadmium chloride, which may influence the adsorption process (Trevors et al. 1986).
9.4.6 Status of Fungal Biomass on Spent Mushroom Substrate
As mentioned earlier, the spent mushroom substrate is an immobilized source of mushroom mycelium. During mushroom cultivation, mushroom mycelium is immobilized on agricultural or agro-industrial wastes. During the mushroom cultivation, ambient conditions are provided to cultivate mushrooms. Therefore, these substrates possess living biomass. This biomass remains viable in spent mushroom substrate even after the harvesting of fruiting bodies. However, mycelium loses its viability when stored for a longer time. After long-term storage, mushroom mycelium can be used as source dead fungi. Besides long-term preservation of spent mushroom substrate, the viability of mycelium is also affected by its modification in any form. Initially, when it is applied immediately after collection, there is need to maintain the conditions in order to maintain its viability. After drying, chemical treatment, or using old/preserved spent mushroom substrate, better adsorption can be achieved without the need of maintaining the conditions like pH, temperature during adsorption process. Live mycelium bearing spent mushroom substrate, usually, adsorbs metal and pollutants on the basis of metabolism-dependent process. However, dead mycelium bearing spent mushroom substrate, treated spent mushroom substrate adsorbs the metals by the metabolism-independent process. This type of adsorption is the result of interaction between pollutant or metal ions and surface of spent mushroom substrate. The mushroom mycelium is composed of chitin and other complex macromolecules. These surface molecules possess negative charge due to the presence of carboxyl, sulfate, phosphate, carbonyl, amino groups. The presence of these groups remains unaffected by living or dead status of biomass. Therefore, the spent mushroom substrate can bind with positive charge metals and involved in metal chelation. In this way, spent mushroom substrate adsorbs metals which are present in the solution (Javanbakht et al. 2014).
9.4.7 Particle Size of Biosorbent
The particle size of biosorbent is an important property to consider for adsorption process. The fine particles have more surface area as compared to coarse particles. Therefore, fine particles can adsorb more compare to coarse particles. The adsorption is not a rate controlling process. It depends on intra-particle mass transfer, which controls the rate of adsorption and therefore, considered as a constraint for the adsorption process (Javanbakht et al. 2014).
9.4.8 Influence of Initial Metal Concentration
The initial concentration of pollutants, dyes, or metals poses a great influence on the adsorption process. Initially, adsorption increases with increase in the concentration of pollutant or metals due to their availability in high amount. There is interference of mass transfer resistance in the adsorption process. However, higher initial concentration of pollutant exerts high driving force to overwhelm the mass transfer resistance between pollutant/metal ions and adsorbents (Gadd 2009). This type of phenomenon for copper ions adsorption was reported by Tay et al. (2012).
9.5 Advantages of Spent Mushroom Substrate As Biosorbent
The spent mushroom substrate is waste from mushroom industry and therefore, it is beneficial to use for adsorption of metals and pollutants. Moreover, it is economic technique to implement in the field. A few studies conducted in the field shed the light on the suitability of spent mushroom substrate for adsorption of pollutants (Herrero-Hernández et al. 2011).
The spent mushroom substrate can be reused, recycled for the adsorption of pollutants (Javanbakht et al. 2014). The adsorption capacity of spent mushroom substrate may be increased by using different chemical treatments and by modifying it in activated carbon form (Tay et al. 2015; Md-Desa et al. 2016). There is also possibility of metal recovery after adsorption of metals on spent mushroom substrate. A detailed study on the metal recovery options will make the use and disposal of spent mushroom substrate, a safer option. Proper utilization of spent mushroom substrate will also reduce the generation of waste from mushroom industry. The spent mushroom substrate can be composted and stored for a longer time with maintaining its sorption capacity. It will not release any toxin which adversely affects the growth of soil microbes and living beings.
9.6 Limitations of Spent Mushroom Substrate As Biosorbent
As mentioned earlier, spent mushroom substrate is generated as waste from mushroom production units. Therefore, it is economic option, if used in natural form, to adsorb the pollutants from the environment. The cost is slightly higher in the cases where a special treatment is given to spent mushroom substrate to modify its properties. The major limitation with spent mushroom substrate is its disposal after adsorption. There is scarcity of reports on the fate of spent mushroom substrate after adsorption. As the spent mushroom substrate is loaded with high amount of pollutants and heavy metals , it need to be treated and disposed in a proper way in order to prevent the spread of its toxicity in the environment. The current options of disposing spent mushroom substrate like incineration and landfill are not feasible option (Javanbakht et al. 2014). Heavy metals loaded spent mushroom substrate may be used for the recovery of metals. The detailed methodology is required to be developed for the extraction of metals and reuse of spent mushroom substrate (Siasar and Sargazi 2015). Another limitation of using spent mushroom substrate for adsorption lies in the differences in the adsorption capacity of different species of mushroom. As mentioned in the aforesaid paragraph, a single species of mushroom do not possess the capacity to adsorb all types of pollutants from the environment. Moreover, there is scarcity of reports on the field scale studies. A laboratory based experiments do not provide complete information about the adsorption of pollutants from the environment because standard conditions cannot be maintained in the environment and interference of indigenous population cannot be avoided. Therefore, a detailed investigation is required for the adsorption of pollutant by spent mushroom substrate in field conditions. There is need of many efforts to improve biosorption process like optimization of process at pilot scale to implement technology.
9.7 Conclusion
In this chapter, the possibility of using mushroom and spent mushroom substrate as green adsorbent have been discussed along with their pros and cons. A detailed review of the adsorption of pollutants using spent mushroom substrate and mushroom revealed their suitability in the adsorption of pollutants. Mushroom and spent mushroom substrate can be modified for enhancing their adsorption capacities. This technology provides a good option for adsorption of pollutants. However, the main problem of using mushroom or spent mushroom substrate is the generation of toxic sludge after adsorption. It is necessary to address and solve the problem of toxic sludge generation and utilization by doing research in this area. The utilization of spent mushroom substrate has high potential to be developed into a sustainable technology. It is an environmental friendly approach for adsorbing pollutants from industrial effluents and soil. Moreover, the utilization of spent mushroom substrate will not only reduce the waste of mushroom farm, but also remove pollutants from the effluent and soil. Therefore, efforts need to be done to implement the technology in the field.
References
Abdel-Shafy HI, Mansourb MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123. https://doi.org/10.1016/j.ejpe.2015.03.011
Abdul-Talib S, Tay CC, Abdullah-Suhaimi A, Liew HH (2013) Fungal Pleurotus ostreatus Biosorbent for cadmium (II) removal in industrial wastewater. J Life Sci Technol 1:65–68. https://doi.org/10.12720/jolst.1.1.65-68
Ahlawat OP, Gupta P, Kumar S, Sharma DK, Ahlawat K (2010) Bioremediation of fungicides by spent mushroom substrate and its associated microflora. Indian J Microbiol 50:390–395. https://doi.org/10.1007/s12088-011-0067-8
Álvarez-Martín A, Sánchez-Martín MJ, Pose-Juan E, Rodríguez-Cruz MS (2016a) Effect of different rates of spent mushroom substrate on the dissipation and bioavailability of cymoxanil and tebuconazole in an agricultural soil. Sci Total Environ 550:495–503. https://doi.org/10.1016/j.scitotenv.2016.01.151
Álvarez-Martín A, Rodríguez-Cruz MS, Andrades MS, Sánchez-Martín MJ (2016b) Application of a biosorbent to soil: a potential method for controlling water pollution by pesticides. Environ Sci Pollut Res Int 23:9192–9203. https://doi.org/10.1007/s11356-016-6132-4
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94. https://doi.org/10.3390/ijerph14010094
Bishnoi NR, Kumar R, Kumar S, Rani S (2007) Biosorption of Cr(III) from aqueous solution using algal biomass Spirogyra spp. J Hazard Mater 135:142–147
Bressa G, Cima L, Costa P (1988) Bioaccumulation of Hg in the mushroom Pleurotus ostreatus. Ecotoxicol Environ Saf 16:85–89
Chen Z, Deng H, Chen C, Yang Y, Xu H (2014) Biosorption of malachite green from aqueous solutions by Pleurotus ostreatus using Taguchi method. J Environ Health Sci Eng 12:63. https://doi.org/10.1186/2052-336X-12-63
Chen G, Peng C, Fang J, Dong YY, Zhu X, Cai H (2016) Biosorption of fluoride from drinking water using spent mushroom compost biochar coated with aluminum hydroxide. Desalin Water Treat 57:12385–12395. https://doi.org/10.1080/19443994.2015.1049959
Damodaran D, Raj Mohan B, Shetty VK (2013) The uptake mechanism of Cd(II), Cr(VI), Cu(II), Pb(II), and Zn(II) by mycelia and fruiting bodies of Galerina vittiformis. Biomed Res Int 2013:149120. https://doi.org/10.1155/2013/149120
Damodaran D, Vidya Shetty K, Raj Mohan B (2014) Uptake of certain heavy metals from contaminated soil by mushroom-Galerina vittiformis. Ecotoxicol Environ Saf 104:414–422. https://doi.org/10.1016/j.ecoenv.2013.10.033
Ding R, Gong K (2013) Super-absorbent resin preparation utilizing spent mushroom substrate. J Appl Polym Sci. https://doi.org/101002/app39285
Dursun AY (2006) Acomparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. Biochem Eng J 28:187–195
Emuh FN (2010) Mushroom as a purifier of crude oil polluted soil. Int J Sci Nat 1(2):127–132
Falandysz J, Danisiewicz D (1995) Bioconcentration factors (BCF) of silver in wild Agaricus campestris. Bull Environ Contam Toxicol 55:122–129
Falandysz J, Bona H, Danisiewicz D (1994) Silver uptake by Agaricus bisporus from an artificially enriched substrate. Z Lebensm-Unters Forsch 199:225–228
Falandysz J, Danisiewicz D, Galecka K (1995) Mercury in mushrooms and underlying soil in the city of Gdansk and in the adjacent area. Bromatol Chem Toksykol 28:155–159
Fidanza MA, Sanford DL, Beyer DM, Aurentz DJ (2010) Analysis of fresh mushroom compost. Hortitechnology 20:449–453
Foluke A, Olutayo A, Olufemi A (2014) Assessing spent mushroom substrate as a replacement to wheat bran in the diet of broilers. Am Int J Contemp Res 4:178–183
Fourest E, Canal C, Roux JC (1994) Improvement of heavy metal biosorption by mycelial dead biomasses (Rhizopus arrhizus, Mucor miehei and Penicillium chrysogenum): pH control and cationic activation. FEMS Microbiol Rev 14:325–332
Fričová O, Koval’aková M (2013) Solid-State 13C CP MAS NMR Spectroscopy as a tool for detection of (1→3, 1→6)-β-D-glucan in products prepared from Pleurotus ostreatus. ISRN Anal Chem. Article ID 248164. https://doi.org/10.1155/2013/248164
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28. https://doi.org/10.1002/jctb.1999
García-Delgado C, Jiménez-Ayuso N, Frutos I, Gárate A, Eymar E (2013) Cadmium and lead bioavailability and their effects on polycyclic aromatic hydrocarbons biodegradation by spent mushroom substrate. Environ Sci Pollut Res 20:8690–8699. https://doi.org/10.1007/s11356-013-1829-0
García-Delgado C, D’Annibale A, Pesciaroli L, Yunta F, Crognale S, Petruccioli M, Eymar E (2015) Implications of polluted soil biostimulation and bioaugmentation with spent mushroom substrate (Agaricus bisporus) on the microbial community and polycyclic aromatic hydrocarbons biodegradation. Sci Total Environ 508:20–28. https://doi.org/10.1016/j.scitotenv.2014.11.046
Gill P (2014) Spill cleanup material and pet litter, and methods of making and using same US 8739734 B2. Patent US8739734 B2
Herrero-Hernández E, Andrades MS, Marín-Benito JM, Sánchez-Martín MJ, Rodríguez-Cruz MS (2011) Field-scale dissipation of tebuconazole in a vineyard soil amended with spent mushroom substrate and its potential environmental impact. Ecotoxicol Environ Saf 74:1480–1488. https://doi.org/10.1016/j.ecoenv.2011.04.023
Herrero-Hernández E, Marín-Benito JM, Andrades MS, Sánchez-Martín MJ, Rodríguez-Cruz MS (2015) Field versus laboratory experiments to evaluate the fate of azoxystrobin in an amended vineyard soil. J Environ Manag 163:78–86
Huang H, Cheng G, Chen L, Zhu X, Xu H (2009) Lead (II) removal from aqueous solution by spent Agaricus bisporus: determination of optimum process condition using Taguchi method. Water Air Soil Pollut 203:53–63. https://doi.org/10.1007/s11270-009-9991-1
Huo CL, Shang YY, Zheng JJ, He RX, He XS, Zhu LM (2011) 2011 International Symposium on Water Resource and Environmental Protection (ISWREP) 3:2317–2320
Igwe JC, Abia AA (2006) A bioseparation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol 5:1167–1179. https://doi.org/10.4314/ajb.v5i11.43005
Iqbal M, Edyvean RG (2005) Loofa sponge immobilized fungal biosorbent: a robust system for cadmium and other dissolved metal removal from aqueous solution. Chemosphere 61:510–518
Jarzynska G, Falandysz J (2011) The determination of mercury in mushrooms by CV-AAS and ICP-AES techniques. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:569–573. https://doi.org/10.1080/10934529.2011.562816
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanispent mushroom substrate of heavy metal removal using microorganispent mushroom substrate as biosorbent. Water Sci Technol 69:1775–1787. https://doi.org/10.2166/wst.2013.718
Jibran AK, Milsee Mol JP (2011) Pleurotus sajor-caju Protein: a potential biosorptive agent. Adv Bio Tech 11:25–27
Kamarudzaman AN, Tay CC, Jalil MFA, Abdul-Talib S (2013) Biosorption of iron (III) from aqueous solution using Pleurotus ostreatus spent mushroom compost as biosorbent. Adv Mater Res 781-784:636–642. https://doi.org/10.4028/www.scientific.net/AMR.781-784.636
Kamarudzaman AN, Tay CC, Amnorzahira A, Liew HH, Abdul-Talib S (2014a) Characterization of Pleurotus spent mushroom compost as a potential biosorbent for Fe(III) ions removal. Adv Environ Biol 8:1–6
Kamarudzaman AN, Tay CC, Amnorzahira A, Liew HH, Abdul-Talib S (2014b) Study of Fe(II) biosorption using pleurotus spent mushroom compost in a fixed-bed column. In Mechatronics and Mechanical Engineering I (vol 664, pp 392–396). (Appl Mechanics Mater; vol 664). Trans Tech Publications Ltd. https://doi.org/10.4028/www.scientific.net/AMM.664.392
Kamarudzaman AN, Tay CC, Amir A, Abdul-Talib S (2015) Mn(II) ions biosorption from aqueous solution using Pleurotus spent mushroom compost under batch experiment. Appl Mech Mater 773–774:1101–1105
Kan SH, Sun BY, Xu F, Song QX, Zhang SF (2015) Biosorption of aquatic copper (II) by mushroom biomass Pleurotus eryngii: kinetic and isotherm studies. Water Sci Technol 71:283–288. https://doi.org/10.2166/wst.2014.511
Kariuki Z, Kiptoo J, Onyancha D (2017) Biosorption studies of lead and copper using Rogers mushroom biomass ‘Lepiota hystrix’. S Afr J Sci 23:62–70. https://doi.org/10.1016/j.sajce.2017.02.00
Kulshreshtha S, Mathur N, Bhatnagar P (2014) Mushroom as a product and their role in mycoremediation. AMB Express 4:1–7. https://doi.org/10.1186/s13568-014-0029-8
Lade H, Kadam A, Paul D, Govindwar S (2015) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J 14:158–174. https://doi.org/10.17179/excli2014-642 eCollection 2015
Larsson PT, Hult EL, Wickholm K, Pettersson E, Iversen T (1999) CP/MAS 13C-NMR spectroscopy applied to structure and interaction studies on cellulose I. Solid State Nucl Magn Reson 15(1):31–40
Liew HH, Tay CC, Yong SK, Surif S, Abdul-Talib S (2010) Biosorption characteristics of lead [Pb(II)] by Pleurotus ostreatus biomass. In: International conference on Science and Social Research (CSSR 2010), December 5–7, 2010. https://doi.org/10.1109/CSSR.2010.5773766 2010
Lopes RX, Zied DC, Martos ET, de Souza RJ, da Silva R, Dias ES (2015) Application of spent Agaricus subrufescens compost in integrated production of seedlings and plants of tomato. Int J Recycl Org Waste Agricult 4:211–218. https://doi.org/10.1007/s40093-015-0101-7
Marín-Benito JM, Sánchez-Martín MJ, Andrades MS, Pérez-Clavijo M, Rodríguez-Cruz MS (2009) Effect of spent mushroom substrate amendment of vineyard soils on the behavior of fungicides: 1. Adsorption-desorption of penconazole and metalaxyl by soils and subsoils. J Agric Food Chem 57
Marín-Benito JM, Rodríguez-Crua MS, Andrades MS, Sánchez-Martín MJ (2012) Assessment of spent mushroom substrate as sorbent of fungicides: influence of sorbent and sorbate properties. J Environ Qual 41:814–822. https://doi.org/10.2134/jeq2011.0437
Mathialagan T, Viraraghavan T, Cullimore DR (2003) Adsorption of cadmium from aqueous solutions by edible mushrooms (Agaricus bisporus and Lentinus edodes). Water Qual Res J Can 38:499–514. https://doi.org/10.1081/SS-120016698
Md-Desa NS, Ab-Ghani Z, Abdul-Talib S, Tay CC (2016) Performance of spent mushroom farming waste (SMFW) activated carbon for Ni (II) removal. IOP Conf Ser: Mater Sci Eng 136:012059
Melgar MJ, Alonso J, García MA (2007) Removal of toxic metals from aqueous solutions by fungal biomass of Agaricus macrosporus. Sci Total Environ 85:12–19
Muraleedharan TR, Iyengar L, Venkobachar C (1995) Screening of tropical wood-rotting mushrooms for copper biosorption. Appl Environ Microbiol 61:3507–3508
Oei P (1991) Some aspects of mushroom cultivation in developing countries. In: Mahe M J (ed) Proceeding of the 13th international congress on the science and cultivation of fungi, vol 2. Rotterdam, Netherlands XIII, pp 777–780
Oyetayo VO, Adebayo AO, Ibileye A (2012) Assessment of the biosorption potential of heavy metals by Pleurotus tuber-regium. Int J Adv Biol Res 2:293–297
Pan R, Cao L, Huang H, Zhang R, Mo Y (2010) Biosorption of Cd, Cu, Pb, and Zn from aqueous solutions by the fruiting bodies of jelly fungi (Tremella fuciformis and Auricularia polytricha). Appl Microbiol Biotechnol 88:997–1005
Pandey M, Senthil Kumaran G, Vasudeo G (2014) Making mushroom production process a zero waste enterprise. Int J Environ Sci 5:236–242. https://doi.org/10.6088/ijes.2014050100019
Phan CW, Sabaratnam V (2012) Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl Microbiol Biotechnol 96(4):863–873. https://doi.org/10.1007/s00253-012-4446-9
Prasad ASA, Varatharaju G, Anushri C, Dhivyasree S (2013) Biosorption of lead by Pleurotus florida and Trichoderma viride. Br Biotechnol J 3:66–78
Ribas LCC, de Mendonça MM, Camelini CM, Soares CHL (2009) Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: growth promotion and soil bioremediation. Bioresour Technol 100:4750–4757. https://doi.org/10.1016/j.biortech.2008.10.059
Rodríguez-Cruz MS, Herrero-Hernández E, Ordax JM, Marín-Benito JM, Draoui K, Sánchez-Martín MJ (2012) Adsorption of pesticides by sewage sludge, grape marc, spent mushroom substrate and by amended soils. Int J Environ Anal Chem 92:933–948. https://doi.org/10.1080/03067319.2011.609933
Romney EM, Wallace A, Wood R, El-Gazzer AM, Childress JD, Alesander GV (1977) Role of soil organic matter in a desert soil on plant responses to silver, tungsten, cobalt and lead. Commun Soil Sci Plant Anal 8:719–725
Royse DJ (2014) A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In: Proceedings of 8th international conference on Mushroom Biology and Mushroom Products (ICMBMP8), vol I & II 2014, New Delhi, India, 19–22 November 2014, pp 1–6
Sánchez-Martín MJ, Rodríguez-Cruz MS (2012) Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J Agric Food Chem 60:6936–6945. https://doi.org/10.1021/jf301322h
Sarı A, Tuzen M (2009) Biosorption of as(III) and as(V) from aqueous solution by macrofungus (Inonotus hispidus) biomass: equilibrium and kinetic studies. J Hazard Mater 164:1372–1378
Sarioglu M, Gülera UA, Beyazit N (2009) Removal of copper from aqueous solutions using biosolids. Desalination 239:167–174
Sendi H, Mohamed MTM, Anwar MP, Saud HM (2013) Spent mushroom waste as a media replacement for peat moss in Kai-Lan (Brassica oleracea var. Alboglabra) production. Sci World J 2013:258562. https://doi.org/10.1155/2013/258562
Sewu DD, Boakye P, Jung H, Woo SH (2017) Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresour Technol 244:1142–1149. https://doi.org/10.1016/j.biortech.2017.08.103
Shuman LM, Li Z (1997) Amelioration of zinc toxicity in cotton using lime or mushroom compost. J Soil Contam 6:425–438. https://doi.org/10.1080/15320389709383576
Siasar H, Sargazi F (2015) Biosorption of copper from aqueous solution using the water of leaching spent mushroom compost. Int J Rev Life Sci 5:925–929
Singh AD, Noorlidah A, Vikineswary S (2003) Optimization of extraction of bulk enzymes from spent mushroom compost. J Chem Technol Biotechnol 78:743–752. https://doi.org/10.1002/jctb.852
Singh MP, Vishwakarma SK, Srivastava AK (2013) Bioremediation of direct blue 14 and extracellular ligninolytic enzyme production by white rot fungi: Pleurotus spp. Biomed Res Int 2013:180156. https://doi.org/10.1155/2013/180156
Širić I, Humar M, Kasap A, Kos I, Mioč B, Pohleven F (2016) Heavy metal bioaccumulation by wild edible saprophytic and ectomycorrhizal mushrooms. Environ Sci Pollut Res 23:18239–18252. https://doi.org/10.1007/s11356-016-7027-0
Skariyachan S, Prasanna A, Manjunath SP, Karanth SS, Nazre A (2016) Environmental assessment of the degradation potential of mushroom fruit bodies of Pleurotus ostreatus (Jacq.: Fr.) P. Kumm. towards synthetic azo dyes and contaminating effluents collected from textile industries in Karnataka, India. Environ Monit Assess 188:121. https://doi.org/10.1007/s10661-016-5125-6
Srinivasan A, Viraraghavan T (2010) Oil removal from water by fungal biomass: a factorial design analysis. J Hazard Mater 175:695–702. https://doi.org/10.1016/j.jhazmat.2009.10.065
Taguchi G, Konishi S (1987) Taguchi methods, orthogonal arrays and linear graphs, tools for quality American supplier institute. American Supplier Institute, Dearborn, pp 8–35
Tay CC, Liew HH, Redzwan G, Yong SK, Surif S, Abdul-Talib S (2011) Pleurotus ostreatus spent mushroom compost as green biosorbent for nickel (II) biosorption. Water Sci Technol 64(12):2425–2432. https://doi.org/10.2166/wst.2011.805
Tay CC, Liew HH, Yong SK, Surif S, Redzwan G, Abdul-Talib S (2012) Cu(II) removal onto fungal derived biosorbents: biosorption performance and the half saturation constant concentration approach. Int J Res Chem Environ 2:138–143
Tay CC, Khoshar-Khan MIA, Md-Desa NS, Ab-Ghani Z, Abdul-Talib S (2015) Sustainable optimization of spent mushroom compost activated carbon preparation method using central composite rotatable design response surface methodology. J Eng Sci Technol, Special Issue on ACEE 2015 Conference August (2015):40–51
Tay CC, Liew HH, Abdul-Talib S, Redzwan G (2016) Bi-metal biosorption using Pleurotus ostreatus spent mushroom substrate (PSMS) as a biosorbent: isotherm, kinetic, thermodynamic studies and mechanism. Desalin Water Treat 57:9325–9331. https://doi.org/10.1080/19443994.2015.1027957
Tian X, Li C, Yang H, Ye Z, Xu H (2011) Spent mushroom: a new low-cost adsorbent for removal of Congo red from aqueous solutions. Desalin Water Treat 27:319–326. https://doi.org/10.5004/dwt.2011.2152
Toptas A, Demierege S, Ayan EM, Yanik J (2014) Spent mushroom compost as biosorbent for dye biosorption. Clean (Weinh) 42:1721–1728. https://doi.org/10.1002/clen.201300657
Trevors JT, Stratton GW, Gadd GM (1986) Cadmium transport,resistance and toxicity in algae, bacteria and fungi. Can J Microbiol 32:447–464
Tuhy L, Samoraj M, Witkowska Z, Wilk R, Chojnacka K (2015) Using spent mushroom substrate as the base for organic –mineral micronutrient fertilizer-field tests on maize. Bioresources 10:5709–5719
Udochukwu U, Nekpen BO, Udinyiwe OC, Omeje FI (2014) Bioaccumulation of heavy metals and pollutants by edible mushroom collected from Iselu market Benin-city. Int J Curr Microbiol App Sci 3:52–57
Vimala R, Das N (2009) Biosorption of cadmium(II) and lead(II) from aqueous solutions using mushrooms: a comparative study. J Hazard Mater 168:376–382. https://doi.org/10.1016/j.jhazmat.2009.02.062
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002
Williams JH (1980) Effect of soil pH on thc losicil! of zinc and nickel to vegetable crops. In: Inorganic pollution and agriculture. Proceedings of a conference organizcd by the Agricultural Development and Advisory Service, April, 1977, London, pp 21–218
Xie H, Zhao Q, Zhou Z, Wu Y, Wang H, Xu H (2015) Efficient removal of Cd(II) and Cu(II) from aqueous solution by magnesium chloride-modified Lentinula edodes. RSC Adv 5:33478–33488. https://doi.org/10.1039/C4RA17272H
Xu F, Liu X, Chen Y, Zhang K, Xu H (2016) Self-assembly modified-mushroom nanocomposite for rapid removal of hexavalent chromium from aqueous solution with bubbling fluidized bed. Sci Rep 6:26201. https://doi.org/10.1038/srep26201
Yan T, Wang L (2013) Adsorption removal of methylene blue from aqueous solution by spent mushroom substrate: equilibrium, kinetics and thermodynamics. Bioresources 8:4722–4734
Yan T, Wang P, Wang L (2015) Utilization of oxalic acid modified spent mushroom substrate for removal of methylene blue from aqueous solution. Desalin Water Treat 55:1007–1017. https://doi.org/10.1080/19443994.2014.922440
Yang X, Guo M, Wu Y, Wu Q, Zhang R (2014) Removal of emulsified oil from water by fruiting bodies of macro-fungus (Auricularia polytricha). PLoS One 9:e95162. https://doi.org/10.1371/journal.pone.0095162 eCollection 2014
Zhou D, Zhang L, Guo S (2005) Mechanisms of lead biosorption on cellulose/chitin beads. Water Res 39:3755–3762. https://doi.org/10.1016/j.watres.2005.06.033
Zhou A, Zhang Y, Li R, Su X, Zhang L (2016) Adsorptive removal of sulfa antibiotics from water using spent mushroom substrate, an agricultural waste. Desalin Water Treat 57:388–397. https://doi.org/10.1080/19443994.2014.979239
Zulfadhly Z, Mashitah MD, Bhatia S (2001) Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ Pollut 112:463–470
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kulshreshtha, S. (2018). Mushroom Biomass and Spent Mushroom Substrate As Adsorbent to Remove Pollutants. In: Crini, G., Lichtfouse, E. (eds) Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-92162-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-92162-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92161-7
Online ISBN: 978-3-319-92162-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)