Abstract
The ventricular pressure-volume relation, is an important presentation of global cardiac pump function. With every heart beat a full pressure-volume loop is described. When ventricular filling is changed, another loop starting from a different End-Diastolic Pressure and End-Diastolic Volume is described. The left top corners of the pressure-volume loops, i.e., the End-Systolic points, when interconnected, and approximated with a straight line give the End-Systolic Pressure-Volume relation, ESPVR, with its slope called E es. The Ees is independent of the (arterial) load and determined by systolic muscle properties (contractility) and wall mass. The Diastolic Pressure-Volume Relation is found by connecting the End-Diastolic Pressure and Volume points. The relation depends on diastolic muscle properties and wall thickness; the relation has considerable curvature but can be fitted with an exponential relation and its slope at end-diastole is End-Diastolic Elastance, E d. Filling changes in vivo can be obtained by partial vena cava occlusions. So-called ‘single beat’ methods have been developed to derive Ees and Ed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pressure-Volume loop
- End-Systolic Elastance
- End-Diastolic Elastance
- Frank-Starling
- Hypertrophy
- Single beat method

The ventricular pressure-volume relation, is an important presentation of global cardiac pump function. With every heart beat a full loop is described. Starting at End-Diastole, the first part of the loop is the isovolumic contraction phase (valves closed). When the (aortic) valves open, ejection begins and during this period ventricular volume decreases while pressure changes relatively little. Stroke Volume, SV, is indicated. After valve closure (End-Systole) isovolumic relaxation follows. When the mitral valves open, the filling phase starts and volume increases with a small increase in ventricular pressure until the End-Diastolic volume is reached at the start of muscle contraction. When ventricular filling is changed, another loop starting from a different End-Diastolic Pressure and End-Diastolic Volume is described. The left top corners of the pressure-volume loops, i.e., the End-Systolic points, when interconnected, and approximated with a straight line, give the End-Systolic Pressure-Volume relation, ESPVR. The slope of this line, End-Systolic Elastance, E es, is load-independent and is determined by systolic muscle properties (contractility) and wall mass. The Diastolic Pressure-Volume Relation is found by connecting the End-Diastolic Pressure and Volume points. The relation depends on diastolic muscle properties and wall thickness; it has considerable curvature leading to large errors when assumed straight. The local slope of the Diastolic Pressure-Volume relation at end-diastole is the End-Diastolic Elastance, E d . Filling changes in vivo can be obtained by partial vena cava occlusions.
1 Description
Otto Frank studied pressure-volume relations in the isolated frog heart. He found different End-Systolic Pressure-Volume Relations, ESPVR’s , for the ejecting heart and the isovolumically contracting heart. In other words, a single unique End-Systolic Pressure-Volume Relation did not appear to exist. More recent translations of his lectures given in high German (difficult for present German colleagues to understand) are being translated, so far revealing how far Frank got to the modern concept presented in this Chapter. He was certainly able to inscribe pressure-volume loops on photographic paper from mirror galvanometers connected to a pressure transducer and piston volume measuring device. These methods were inadequate, but the ideas were modern.
Measurements in the isolated blood perfused dog heart, where volume was accurately measured with a water-filled balloon, showed that the End-Systolic Pressure-Volume Relation was the same for ejecting beats and isovolumic beats. The original results suggested a linear ESPVR with an intercept with the volume axis, V d [1]. The linear relation implies that the slope of the ESPVR, the E es, with the dimension of pressure over volume (mmHg/ml), can be determined. Increased contractility, (Fig. 14.1) as obtained with epinephrine, increased the slope of the ESPVR but left the intercept volume, V d, unchanged [1]. Therefore, the E es could quantify cardiac muscle contractility. Later it turned out that both the diastolic pressure-volume relation and the ESPVR are not linear. This implies that the slope depends on the pressure and volume chosen and when approximating this locally with a straight line a virtual intercept volume is obtained, which may be positive or negative. The curvature results from failure to reach the isovolumic end-systolic pressure during ejection observed at very high systolic pressure, attributed to the longer duration of systole and limitation to maintain the active state long enough. The ESPVR, and its slope Ees, were shown to be only little dependent on the arterial load, hence the term load-independence [2]. The existence of a load-independent ESPVR is of great significance in the understanding and characterization of cardiac pump function. An extensive treatment is given in [2].
The slope (Ees) of the linearized End-Systolic Pressure-Volume Relation (ESPVR) is a measure of cardiac contractility. An increased slope implies increased contractility. (Adapted from Ref. [1] by permission)
1.1 The Varying Elastance Model
Pressure-volume loops can be analyzed by marking time points on the loop (Fig. 14.2). When different loops are obtained and the times indicated, we can connect points with the same times, and construct isochrones. The slopes of the isochrones can be determined, and the slope of an isochrone is the elastance at that moment in time. The fact that the elastance varies with time, leads to the time-varying elastance concept E(t). This means that during each cardiac cycle the elastance increases from its diastolic value to its maximal value, which is close to end-systole, E es, and then returns to its diastolic value again (Fig. 14.2).
Isochrones are lines connecting the pressures and volumes of different loops at the same moment in time. Isochrones are supposed to be straight and their slope is the instantaneous pressure-volume relation or ventricular elastance, E(t), with dimension mmHg·ml−1. The line connecting the End-Systolic corners of the loops is the End-Systolic Pressure-Volume Relation, ESPVR with slope E es. The ESPVR is not exactly a single isochrone. The slopes of the isochrones plotted as a function of time, the E(t)-curve, exemplifies the varying elastance concept (right part)
This is an oversimplification in that the ESPVR lines in Fig. 14.2 are drawn straight when they are actually curved. Nevertheless, the varying elastance model is valid as demonstrated by other methods. Cardiac muscle subjected to constant amplitude oscillations of length display increased amplitude oscillations of force during contraction, i.e., dP/dl (elastance, has increased). Intact hearts subjected to constant amplitude oscillations of volume display increase amplitude oscillations of pressure during contraction, i.e., dP/dV, elastance, has increased. Elastance is a fundamental property of the contractile mechanism.
It has been shown that the E(t) curve, when normalized with respect to its peak value and to the time of its peak, E N(t N), (see Fig. 14.3), is similar for normal and diseased human hearts [3]. Similar E N(t N) curves are found in the mouse, and dog. Thus there appears to exist a universal normalized E(t) curve in mammals including man, which is unaltered in shape in health and disease. The only differences between hearts and state of health are in the magnitude and time of peak of the E N(t N).This similarity of the varying EN(t N)-curve is very useful to construct lumped models of the heart [4, 5]. However, some doubt has been cast on the invariance of the E N(t N) curve [6].
The E N (tN) curve is the E(t) normalized in amplitude and to time to peak, and is similar in many disease states. (Adapted from Ref. [3], by permission)
The assumed mechanism is that the cardiac muscle changes its stiffness (elastance) during the cardiac cycle (Fig. 14.4) independently of its load.
1.2 Determination of End Systolic Elastance
To determine E es one needs to measure several pressure-volume loops and obtain a range of end-systolic pressure-volume points (see Figure in the Box). The determination should be done sufficiently rapidly to avoid changes in contractility due to the hormonal or nervous control systems. Both changes in arterial load and diastolic filling may, in principle, be used, but the former may illicit contractility changes by changes in coronary perfusion. Changes in filling are therefore preferred and are also easier to accomplish in practice. For instance, blowing up a balloon in the vena cava may decrease filling over a sufficiently wide range and can be carried out sufficiently rapidly to obtain a series of loops and an accurate ESPVR estimate. Measurement of both ventricular pressure and volume on a beat-to-beat basis can be carried out with the use of the so-called pressure-volume catheter [7].
Volume also can be measured using noninvasive techniques (US-Echo, X-ray, and MRI). Pressure is preferably obtained by invasively techniques. However, aortic pressure during the cardiac ejection period can be used as an acceptable approximation of left ventricular pressure in systole to determine the systolic part of the pressure-volume loop [8, 9]. Methods allowing for the calculation of ascending aortic pressure noninvasively from peripheral pressure (Chap. 27) could, if proven sufficiently accurate, allow for a completely noninvasive determination of E es.
How does one cope with the fact that most experiments show an ESPV curve that is convex to the volume axis, so that a straight line cannot be drawn? If one wants to compare contractility in an individual heart before and after an intervention, there are plenty of mathematical methods for fitting such curves and testing statistical differences between them. Alternatively, if one wants a single number for E es, one can take the maximum slope of the tangent to the curve at end systole.
1.3 Determination of End Diastolic Elastance
The diastolic pressure-volume relation is strongly nonlinear (Figure in the Box). Therefore exponential approaches have been proposed. Klotz et al. suggested P(t) = aV(t)b with some assumptions on the intercept volume, the volume at negligible transmural pressure [10]. Rain et al. and Trip et al. used P(t) = a·(eb·V(t) – 1) [10, 11]. The curve fit of the end-diastolic pressure-volume points of the different loops is used to obtain the constants a and b. The End-Diastolic Elastance, E d is the slope of the relation at end-diastolic volume, V ed, and equals E d = a·b·eb·Ved.
1.4 Derivation of End Systolic Elastance and End Diastolic Elastance from Single Beats
The slope of the end-systolic and end-diastolic Pressure-Volume relations E es and E d, are best obtained by filling changes, as for instance obtained by partial vena cava occlusion. However, this approach is often not feasible in clinical studies. Therefore so-called single beat approaches have been proposed to estimate E es and E d, see Fig. 14.5.
Single beat methods to obtain the slope of the End-Systolic Pressure-Volume Relation, E es and the End-Diastolic Pressure-Volume Relation, Ed . Left panel: The isovolumic contraction and relaxation phase of ventricular pressure (gray areas) are used to fit a (half) sine wave. The maximum of the sine-wave is assumed be isovolumic systolic pressure ‘P isovol ’. Right panel: The ‘P isovol ’, and end-systolic pressure, Pes, together with Stroke Volume is used to calculate E es = (‘Pisovol’-Pes)/SV. The diastolic pressure-volume relation can be fitted with an exponential curve using three data pressure-volume points: 0,0, begin-diastole and end-diastole. The slope at end-diastole is E d .
To derive the slope of the End-Systolic Pressure Volume Relation, E es, from a single beat Sunagawa proposed to fit a sinewave to the isovolumic contraction and relaxation periods of measured ventricular pressure [12]. The maximum of the sinewave is then assumed to be the isovolumic pressure, ‘Pisovol’ and Ees = (‘Pisovol’ – Pes)/SV. Senzaki used the generalized EN(tN) curve (Fig. 14.3) and calibrated the curve using Pes = 0.9·Pao,syst, with Pao,syst systolic aortic pressure and via tpeak, using an iterative approach the intercept pressure Vo(SB) was estimated so that Ees = 0.9·Pao,syst /(Ves – Vo(SB)) [3]. Other simulations of the isovolumic ventricular pressure, such as by polynomials, have been suggested but the results are hardly better than the use of the sine wave.
When brachial pressure is measured the systolic aortic pressure can be derived [13], see Chap. 12. Together with noninvasive volume measurement (MRI) and using the normalized E N(tN) curve (Fig. 14.3), the Ees can be derived noninvasively from a single beat [14].
To derive the End-Diastolic Pressure-Volume relation from a single beat an exponential curve between pressure and volume is assumed: P(t) = a·(eb·V(t) – 1), and fitted through the hypothetical zero pressure-zero volume point (0,0) and the pressure-volume data at beginning and end diastole as shown in Fig. 14.5 [10, 11]. The slope at end diastole then gives E d = a·b eb·Ved.
2 Physiological and Clinical Relevance
The ESPVR and E es together with the diastolic pressure-volume relation, are important characterizations of cardiac pump function and they are often used in animal research; clinical use is now increasing. The E(t) curve depends on heart size and thus on body size: Pressures are similar in different animals but volumes are not. Volumes are proportional to body mass (Chap. 32). Thus E es should be normalized with respect to ventricular lumen volume (see Chap. 11) or to heart mass or body mass to compare mammals. Since not only muscle contractility but ventricular wall thickness and lumen also contribute to E es and E d, normalization with respect to wall thickness may help to quantify changes in muscle mass and in muscle contractility to E es and E d.
2.1 The Frank-Starling Law
The varying elastance concept contains both Frank’s and Starling’s original experimental results, as shown in Fig. 14.6. Frank studied the frog heart in both isovolumic and ejecting beats, but we show here how isovolumic contractions behave in the pressure-volume plane when diastolic volume is increased. Starling also changed diastolic filling but studied an ejecting heart that was loaded with a Starling resistor. This meant that in his experiments the aortic pressure and ventricular pressure in systole were kept constant. The increase in filling resulted in an increase in Stroke Volume and thus in Cardiac Output.
Frank (left, isovolumic contractions) and Starling (right, ejections against constant ejection pressure) experiments to show the effect of ventricular filling in terms of pressure-volume relations. Increased filling causes increased isovolumic pressure (Frank) or increased Stroke Volume and Cardiac Output (Starling)
2.2 Systolic and Diastolic Dysfunction
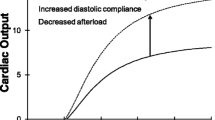
It is important to realize that both diastolic and systolic properties play an important role in cardiac function (Fig. 14.7). This can be illustrated with the following example. Systolic dysfunction results in a decreased Stroke Volume, and Cardiac Output at constant Heart Rate when not compensated by increased diastolic filling. Diastolic dysfunction, with a stiffer ventricle in diastole causes decreased filling and higher filling pressure and pulmonary venous pressure, the latter leading to shortness of breath. The smaller diastolic volume results in a decreased Stroke Volume and Cardiac Output.
Systolic and diastolic dysfunction are shown here by the red lines, and normal function by the blue lines. Left panel: In systolic dysfunction of failure the ESPVR is decreased and Stroke Volume, SV and Ejection Fraction, EF = SV/EDV, decrease also. Right panel: In diastolic dysfunction or failure with increased diastolic stiffness filling is decreased although filling pressure may be higher, End-Diastolic Volume and Stroke Volume are both decreased, but EF = SV/EDV may be similar: Heart Failure with Preserved Ejection Fraction, HFPEF
Ejection Fraction , EF, defined as the ratio of Stroke Volume , SV, and End-Diastolic Volume, EDV , is decreased in systolic dysfunction but may be unaltered in diastolic dysfunction (Fig. 14.7). This is the case when SV and EDV decrease in the same proportion. This situation is called diastolic dysfunction or Heart Failure with Preserved Ejection Fraction, HFPEF [15], and it shows that EF is not in all situations a measure of changed cardiac pump function. Breathing difficulty is a more common feature in diastolic dysfunction because systolic dysfunction usually results in a smaller increase in filling pressure and smaller increase in pressure in the lung veins.
Since EF depends on the cardiac pump and the arterial load, the EF should be considered as a ventriculo-arterial coupling factor rather than a characterization of heart function alone (Chap. 18).
2.3 Concentric and Eccentric Hypertrophy
Concentric and eccentric hypertrophy are interesting examples in the context of the varying elastance concept and the pressure-volume relation (Fig. 14.8).
Concentric hypertrophy implies an increased wall thickness with similar lumen volume. This means a stiffer ventricle both in diastole and in systole, i.e., both E es and E d are increased. The increase in Ees does not necessarily imply increased contractility of the contractile apparatus of the muscle but is mainly a result of more sarcomeres in parallel, i.e., a thicker fiber and therefore increased wall thickness. Concentric hypertrophy, with its increased diastolic stiffness results in increased diastolic pressure but smaller volume in end-diastole, and higher pressure but smaller volume in systole, but similar or slightly decreased Stroke Volume. It has been suggested that the intracellular molecular events in hypertrophy may be detrimental to cardiac muscle function [16].
In eccentric hypertrophy the ventricular lumen volume is greatly increased, more sarcomeres in series, and longer cells, while the wall thickness may be unchanged or somewhat increased. The shift of the entire pressure-volume relation to larger volumes in eccentric hypertrophy implies, by virtue of the law of Laplace (Chap. 9), that wall forces are increased. It is not clear why the cardiac muscle does not respond by hypertrophy when diastolic wall stress is increased. The increased V d in eccentric hypertrophy emphasizes that the slope of the ESPVR cannot be determined from a single pressure and volume measurement, assuming that the Vd is negligible (see below).
2.4 Modeling on the Basis of the Varying Elastance Concept
The finding that the normalized E(t) curve appears to be quite independent of the cardiac condition (Fig. 14.3), and that it is similar in mammals (Chap. 32) allows quantitative modeling of the circulation [4, 17]. The nonlinearity of the isochrones (see below) appears of little consequence in this type of modeling [4].
2.5 Limitations
It should be emphasized that the time varying elastance concept pertains to the ventricle as a whole. It allows no distinction between underlying cardiac pathologies. For instance, asynchronous contraction, local ischemia or infarction etc., all decrease the slope of the End-Systolic Pressure-Volume Relation. Also the E es depends on ventricular lumen, wall size and muscle contractility. In acute experiments cardiac contractility changes can be studied, but in long range studies as hypertrophy where muscle mass is increased both muscle mass and muscle contractility changes contribute to the changes in Ees. Normalization with respect to muscle mass and lumen have been suggested.
The pressure-volume relations, expressed by isochrones (Fig. 14.9), are not straight [17]. The systolic pressure-volume relations may be reasonably straight when muscle contractility is low, but become more and more convex to the pressure axis with increasing contractility. A curved relation implies that the E es depends on volume and pressure. It is customary to approximate the ESPVR’s in the working range by a straight line. Although this sometimes gives an acceptable approximation of reality, a virtual, often a negative, V d is found by linear extrapolation of the ESPVR to the volume axis Fig. 14.9, left panel). A negative Vd is physically impossible.
Left Panel: Neither the isochrones nor the end-systolic pressure-volume relation is linear. Thus the linear approximation may not always be correct and may lead to a negative calculated volume intercept, the virtual V d, which is physically impossible. The Ees in the working range may be approximated using the straight line. (Data of the mouse, adapted from Ref. [17], used by permission). Right panel: When the virtual volume intercept is assumed to be zero the slopes of the calculated end-systolic lines, Pes/Ves, depend on end-systolic pressure and end-systolic volume, and are not a load-independent characterization of the ventricle
In several studies the E es has been estimated from a single pressure-volume loop assuming the intercept volume Vd to be negligible. However the lines connecting the end-systolic pressure-volume points with Vd = 0, give slopes that differ between beats and are not load-independent characterizations of the ventricle (see Fig. 14.9, right panel).
It has been shown by a number of investigators that load changes affect the End-Systolic Pressure-Volume Relation. However, the effect is rather small and may be due to the fact that, at high loads, the duration of ejection is curtailed and may not be long enough for E es to be attained [2].
References
Suga H, Sagawa K, Shoukas A. A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and the effect of epinephrine and heart rate on the ratio. Circ Res. 1973;32:314–22.
Sagawa K, Maughan WL, Suga H, Sunagawa K. Cardiac contraction and the pressure-volume relationship. NewYork/Oxford: Oxford University Press; 1988.
Senzaki H, Chen C-H, Kass DA. Single beat estimation of end-systolic pressure-volume relation in humans: a new method with the potential for noninvasive application. Circulation. 1996;94:2497–506.
Segers P, Stergiopulos N, Westerhof N. Quantification of the contribution of cardiac and arterial remodeling to hypertension. Hypertension. 2000;36:760–5.
Lankhaar JW, Rövekamp FA, Steendijk P, Faes TJ, Westerhof BE, Kind T, et al. Modeling the instantaneous pressure-volume relation of the left ventricle: a comparison of six models. Ann Biomed Eng. 2009;37:1710–26.
van der Velde ET, Burkhoff D, Steendijk P, Karsdon J, Sagawa K, Baan J. Nonlinearity and load sensitivity of end-systolic pressure-volume relation of canine left ventricle in vivo. Circulation. 1991;83:315–27.
Baan J, van der Velde ET, de Bruin HG, Smeenk GJ, Koops J, van Dijk AD, et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70:812–23.
Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–7.
Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Phys. 2006;291:H403–12.
Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128:2016–25.
Trip P, Rain S, Handoko ML, van der Bruggen C, Bogaard HJ, Marcus JT, et al. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J. 2015;45:1603–12.
Sunagawa K, Yamada A, Senda Y, Kikuchi Y, Nakamura M, Shibahara T, et al. Estimation of the hydromotive source pressure from ejecting beats of the left ventricle. IEEE Trans Biomed Eng. 1980;27:299–305.
Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–63.
Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the European society of cardiology. Eur Heart J. 2007;20:2539–50.
Schiattarella GG, Hill TM, Hill JA. Is load-induced ventricular hypertrophy ever compensatory? Circulation. 2017;136:1273–5.
Westerhof N. Cardio-vascular interaction determines pressure and flow. In: Jaffrin MY, Caro CG, editors. Biological flows. New York: Plenum Press; 1995.
Claessens TE, Georgakopoulos D, Afanasyeva M, Vermeersch SJ, Millar HD, Stergiopulos N, et al. Nonlinear isochrones in murine left ventricular pressure-volume loops: how well does the time-varying elastance concept hold? Am J Phys. 2006;290:H1474–83.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Westerhof, N., Stergiopulos, N., Noble, M.I.M., Westerhof, B.E. (2019). The Pressure-Volume Relation. In: Snapshots of Hemodynamics. Springer, Cham. https://doi.org/10.1007/978-3-319-91932-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-91932-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91931-7
Online ISBN: 978-3-319-91932-4
eBook Packages: MedicineMedicine (R0)