Abstract
The widespread adoption of alternative binders are playing an increasing role in carbon dioxide (CO2) abatement in green construction and the repair of traditionally built structures. Natural Hydraulic Lime (NHL) has better environmental credentials than Portland Cement (PC) due in part to its lower calcination temperature and its ability to absorb CO2 during carbonation. However, NHL is more sensitive to climatic conditions during the setting and hardening processes and this is especially pronounced in high humidity climates. It is well understood that high humidity environments create favourable conditions for the development of the products of hydration, but the carbonation reaction can reduce or become dormant due to high sustained moisture contents. Anecdotal evidence suggests that the addition of crystalline CaCO3 into hydraulic lime mortar mixes can enhance the carbonation reaction due to ‘seeding’ of the crystal architecture. This research assesses the influence of CaCO3 modification of NHL mortars subject to high humidity environments and investigates the subsequent effect on early development of various physical properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Environmental conditions can significantly influence the set characteristics and performance of lime mortars. Relative humidity and temperature specifically, influence both hydration and carbonation set reactions with the formation of hydrates determining the mineralogical composition and the ultimate strength and stability of the matrix (Dotter 2010; Taylor 1997; Hewlett 2004). Hydraulic lime mortars placed in a high humidity environment are understood to exhibit lower rates of carbonation and may not readily attain their full set characteristics (Allen 2003) due to the potential for high and sustained moisture contents (Forster and Carter 2011). The wide scale adoption of non-Portland Cement alternative binders (including NHL) in emerging markets may be hindered by performance and behavioural uncertainty associated with high humidity conditions and equally so by the slow rates of initial set in such materials (Pavia 2008; Ball et al. 2009, 2011; El-Turki et al. 2007). Hydraulic lime mortars exposed to elevated temperatures and optimal moisture contents should follow similar hydration kinetics to that associated with belite (β-C2S) cements (Hewlett 2004) and exhibit accelerated precipitation of products of hydration (Forster 2004; Desai et al. 2011). The importance of the carbonation reaction in hydraulic limes cannot be underestimated. Carbonation in simplistic terms is the conversion of Ca(OH)2 into calcite via a chemical reaction with atmospheric CO2. The extent and rate (kinetic) of the carbonation process are also affected by physical parameters of the masses (porosity/permeability) and by the practical curing and exposure conditions (e.g. CO2 concentration, humidity, temperature, etc.) (Pacheco Torgal et al. 2012).
Evidence suggests that seeding of lime mortars encourages the early stage precipitation of calcite by accelerated carbonation reaction (Forsyth 2007). Lawrence (2006) concurs indicating that the seeding of lime mortars with 6% finely ground calcite has been shown to improve the rate of carbonation. The addition of calcitic aggregates into lime mortars has many historic precedents and these components are often found in many historic mortars (Hughes and Valek 2003; Gibbons 2003). The understanding of the perceived beneficial nature of these aggregates is little understood, however, their serendipitous addition could have inadvertently enhanced performance. In contemporary work, calcitic aggregate additives are used by specifiers and contractors to enhance the initial set in limes in high humidity environments. This is especially common on the West Coast of the UK, well known for high rainfall and low potential evaporation (Hall et al. 2010).
Seeding materials used in lime mortars can vary in physical form but are primarily, calcium carbonate in nature. There are three types of polymorph of CaCO3; calcite, aragonite and vaterite, of which calcite is the most stable. Seeding is not understood mechanistically, however, several potential reasons have been postulated for its performance. It is evident that an increase in mass of the mortar is noted within the carbonation process as the diffused CO2 transforms Portlandite into calcite (Dheilly et al. 2002). In addition it has been suggested that the presence of calcitic materials aids carbonation as it is typically a relatively porous particulate and therefore facilitates CO2 diffusion through the fresh mortar. Another hypothesised explanation is the establishment of crystal architecture that catalyses the precipitation of Ca2+ ions into growth of CaCO3. This concurs with work by various investigators (Hewlett 2004; Forster 2004) into the influence of temperature, and super-saturation of Ca2+ ions in the liquid phase upon hydration kinetics. The rate of hydration of calcium silicates is restricted by an impermeable hydrate layer of Ca2+ ions that surrounds the grain potentially leading to a temporary period of dormancy. The addition of calcitic material may minimise the effect of the dormancy period leading to an accelerated deterioration of the Ca2+ ion layer as they are readily utilised in the formation of calcite. This is potentially responsible for the reactivation of the hydraulic phase dissolution and precipitation activity. The development of early stage products of hydration have been previously associated with an impedance to CO2 diffusion in lime mortars (Radonjic et al. 2001), therefore inhibiting carbonation. It was later concluded by Ezziane et al. (2007) that the seeding processes can positively influence the mortar hydration and minimise disorders caused by the temperature rise.
2 Research Objectives
The aim of the research is to undertake an analysis of the influence of calcitic aggregate modifications in naturally hydraulic lime mortars and determine their subsequent effect on early strength development and other physical phenomena. This programme has ultimately been developed to aid specification of mortars in high temperature and high humidity environments such as the Tropics. The research objectives are;
-
i.
To investigate the effects of mortar seeding on mortars’ physical properties using laboratory testing techniques including tensile and compressive strength
-
ii.
To investigate the effects of mortar seeding on chemical properties i.e. composition of binder/carbonation front using laboratory testing techniques
-
iii.
To investigate through sorptivity testing if seeding additives affect the moisture handling characteristics of mortars.
3 Experimental Work
3.1 Materials and Characterisation
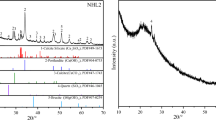
Control samples were manufactured using an NHL 3.5 (moderately hydraulic) lime binder and a well graded coarse sand. These materials were selected on the basis of being commonly used for the repair of the traditional built environment and being relatively representative of historic mortars. Material characterisation was based on sieve analysis of the aggregate in accordance with BS EN 13139 (2002), to determine the grading profile, and powder X-Ray diffraction (XRD). XRD samples were analysed on a Thermo ARL X’TRA Diffractometer with a 2Theta range of 5°–70° at a rate of 1°/min to determine mineral composition of the anhydrous binder.
3.2 Aggregate
Well graded silicaceous sand (Cloddach sand, Perthshire) was selected as it is representative of materials commonly used for the repair of traditional masonry structures in Scotland. This aggregate ensured isolation of variables due to an absence of calcitic mineral components. The grading curve was ascertained in accordance with BS EN 13139 (2002). The bulk density of the aggregate was determined to be 1630 g/l. Three types of calcite were procured for use as seeding materials, namely; (i) precipitated calcium carbonate (ii) oyster shells and (iii) limestone chippings. Precipitated calcium carbonate (99.99% pure) was used as point of reference to ensure consistency. Oyster shells composed approximately 98% of CaCO3, although the exact percentage would vary based on several factors such as the species, molecular impurities in the shell, and the number and frequency of proteins embedded in the shell. The 10 mm limestone chips are sedimentary in nature; composed largely of mineral forms of calcite and aragonite and were derived from Lancashire. The calcitic seeding materials were mechanically crushed, using a multi-frequency Siebtechnik Gyro Mill, fitted with a Tungsten Carbide pot and rings and sieved to attain a particle size in the range of 250–300 μm.
3.3 Binder
St. Astier natural hydraulic lime (NHL) (compliant with European norm BS EN 459-1 (2010) was selected for sample manufacture as it is one of the most commonly used binders in the UK and is therefore considered as reflective of site practice. NHL 3.5 (moderately hydraulic) was used for the control samples prior to seeding additives. The anhydrous NHL3.5 binder composition was established through technical data provided by the manufacturer and qualitative XRD analysis.
3.4 Sample Manufacture, Batching and Curing
Samples were prepared using a binder/aggregate ratio of 1:3 by volume. The mix proportion were selected based on the void ratio of the aggregate, and the requirement of the lime binder to fill the voids between aggregate grains to produce a ‘complete’ mortar. Cloddach sand has a void ratio of approximately 33%, hence the ratio of 1 part binder to 3 parts aggregate. All materials were batched by weight to ensure accuracy. 6% of the total Cloddach sand weight within the grading range of 250–300 μm was isolated and substituted with seeding materials within the same grading range (250–300 μm).
The manufacturing regime in BS 459-2 (2010) was modified to integrate the relative bulk density (RBD) calculations for the NHL 3.5 binder, resulting in an alteration of the quantity of binder from the standard 450 g established in BS EN 196-1 (2005) to 189 g. This adjustment was undertaken to ensure the 1:3 ratio (by volume) was maintained for the manufactured samples. The mortar was prepared by the ‘dry mixing’ method undertaken by (Allen et al. 2003; Ball et al. 2009). The samples were batched adopting the proportions shown in Table 1, and mixed mechanically for 4 min in a bench top paddle mixer at low speed. The sides of the mixer bowl were scrapped after 2 min to ensure all dry powder was incorporated into the mix. Water was added and mixed for 5 min at low speed, and finally for 1 min at high speed. The consistency was determined by flow table and was found to be 165 mm ± 2 mm. Mixing and casting of mortars was undertaken in accordance with BS 459-2 (2010), with the exception that the 160 × 40 × 40 mm steel 3 gang moulds were substituted for 160 × 40 × 40 mm single use polystyrene 3-gang moulds. Polystyrene moulds offer several benefits; ease of use; reduced weight in the environmental curing cabinet; and the elimination of the need for releasing agent which may pose uncertainties regarding the interaction of the two surfaces. Upon casting into moulds, an automatic vibrating table was used to compact mortar samples, removing any trapped air (BS EN 459 2010). Table 1 indicates the quantities of constituents required for the preparation of 15 l mortars.
All specimens were placed in the TAS Environmental Curing Cabinet under controlled environment of 33 ± 1 °C and 90 ± 5% Relative Humidity (RH). The samples were de-moulded 7 days, after which they were placed within the cabinet. To ensure satisfactory carbonation, a CO2 injection system was designed and installed, enabling a constant CO2 level.
3.5 Physical and Chemical Characteristic Testing
Physical and chemical characteristics of the mortars were established using a number of testing methods. Control specimens were used to provide a datum for subsequent materials tests. The control and the seeded mortars were subjected to the following testing regimes.
3.5.1 Carbonation Depth
Mortar specimens were used to assess the carbonation depth by spraying a freshly exposed surface of the mortar with 1% phenolphthalein solution (Lo and Lee 2002). The solution is a colourless acid/base indicator, which turns pink when the pH is above 9, denoting the presence of Ca(OH)2. This procedure indicates the boundary at which the progressing carbonated front meets the un-carbonated (unchanged) alkaline mortar.
3.5.2 Sorptivity
Sorptivity of the mortar specimens was evaluated using the ‘sharp front’ calculation method established by Hall and Hoff (2002). Tests were carried out on parallel piped specimens, after drying in an oven, at a temperature of 100 ± 10 °C. This ensured a constant weight. The specimens were coated with epoxy resin on the vertical faces, reducing the effect of water evaporation. Samples were placed on non-absorbent rods in a shallow tray of water to allow contact with the unsealed surface. The initial sample weight was recorded and subsequently re-recorded after being placed in the water at one, three, five, 10, 15 and 30 min; before weighing, the specimens’, excess water was removed by lightly dabbing on a cloth. Sorptivity was determined from the slope of the graph of mass against √time according to equation established by Hall and Hoff (2002).
3.5.3 Tensile and Compressive Strength
Mortar specimens were tested at specific time intervals (7, 14, 28, 42 and 56 days) to assess strength development over time. This was carried out using a Lloyds Universal Testing Machine (Model M5 K) with a maximum load cell of 5 kN.
4 Results and Discussion
4.1 Carbonation Depth
Early stage (7 days) carbonation testing reveal little information on the effects of seeding additives as samples were held within moulds for the first 7 days of curing, with only a single face exposed and therefore subject to carbonation. Mid-study measurements (28 days) show all seeding materials to have a positive impact on carbonation depth compared with the un-seeded control samples. This positive carbonation effect was most noticeable in the Limestone seeded materials. The positive effects of the oyster shells are however short lived, with control samples exceeding carbonation of oyster shell seeded samples by 42 days. Limestone appears to be the only material that has any significant, prolonged impact on carbonation depth; the increase in carbonation depth achieved with calcium carbonate seeding at 42 days is likely to be within the error of measuring. All samples achieved full carbonation by 56 days (Fig. 1).
These results highlight the variation in effect of seeding materials on carbonation. These variations may be the result of differing compositions and/or physical forms of the seeding materials. Additionally, the small cross sectional area of the samples does not allow for the evaluation of long-term effects on carbonation; identifying how the reaction proceeds beyond 42 days.
4.2 Sorptivity
The water absorption in control and seeded samples was evaluated by determining sorptivity between 7 and 56 days (Fig. 2).
Results show that the control samples exhibited the greatest sorptivity compared to all modified mortars. This could suggest that seeding materials create a segmented pore structure relative to the control samples interconnected system. As all seeded material was crushed to the same sieve fraction, and directly substituted for aggregate particles of the same size, it is more likely that pore resistance to liquid water occurred as a result of an increase in the formation of products of hydration, rather than from any direct impedance associated with the seeding grains themselves. That said, such effects would not typically be expected until after 28 days as hydration products in hydraulic limes form primarily from the hydration of slower acting β-belite rather than the faster acting alite or aluminium silicate components found in Portland Cement; sorptivity results exhibit relative stability by 28 days. Fluctuations in early stage sorptivity (7–28 days) are inconsistent with variations expected from the carbonation and hydration of typical hydraulic lime mortars. It may be the case that minor components within the seeding materials may have impacted upon the reactions in progress, however further trace element chemical analysis would be required to clarify this.
4.3 Tensile and Compressive Strength
Experimental results indicate a clear difference between the seeded specimens and the control samples with regards to the development of both tensile and compressive strength (Figs. 3 and 4).
All samples exhibited an elevated flexural strength at 7 days that subsequently decreased at 14 days. This could be potentially associated with high initial moisture contents in the samples; when the samples had subsequently dehydrated at 14 days the flexural strength decreased. The seeded materials exhibited higher flexural strength than the control mortar throughout the test period, elevating significantly at 56 days; it is unclear why the control sample displayed a decline in flexural strength at 56 days, this may be a reflection of changes (an increase) in pore structure associated with carbonation although this does not correlate with the formation of hydration productions, which would also be expected within this time period (42–56 days).
Figure 4 shows the variation of the compressive strength for the range of mortars. The control specimen exhibited a significant loss of strength between, 42 and 56 days unlike the seeded mortars which continued to gain strength. The relative increase in compressive strength in the seeded material is most likely associated with the disproportionate reliance on carbonation in strength development first month prior to the hydration of belite. Providing ‘nucleation sites’ for the carbonation of portlandite will in itself promote strength development, as well as producing a hydration-promoting environment at later stages (i.e. a solution that is not supersaturated with Ca2+ ions and therefore does not inhibit hydration).
5 Conclusions
This work has highlighted the impact that calcitic seeding materials have on NHL 3.5 lime mortars. The effects of seeding materials undoubtedly vary with type, possibly due to variations in trace element chemistry and in physical form/crystallinity. In general, calcitic materials have a positive influence on the strength development and rapidity of carbonation of the hydraulic lime mortars exposed to high humidity and temperature environments. In line with the impact on strength development, seeding materials have shown a negative impact on sorptivity characteristics. This is most pronounced in the limestone seeding materials.
These results go some way in identifying the most appropriate calcitic media for seeding lime mortars. The relationship between mortars and seeding materials is clearly complex. Further analysis on the seeding materials themselves may help to explain some of the discrepancies evident in this work. It is evident that the addition of limestone chips in the range of into moderately hydraulic lime used for high humidity environments will accelerated the set.
References
Allen, G. (2003). Hydraulic lime mortar for stone, brick and block masonry. Shaftesbury: Donhead.
Allen, G., Allen, J., Elton, N., Farey, N., Holmes, S., Livesey, P., Radonjic, M. (2003). Hydraulic lime mortar for stone, brick and block masonry. Dorset: Donhead.
Ball, R. J., El-Turki, A., & Allen, G. C. (2009). Influence of carbonation on the load dependant deformation of hydraulic lime mortars. In 11th International Conference on Non-conventional Materials and Technologies (NOCMAT 2009). Bath, UK.
Ball, R. J., El-Turki, A., & Allen, G. C. (2011). Influence of carbonation on the load dependent deformation of hydraulic lime mortars. Materials Science and Engineering A, 528(7–8), 3193–3199.
British Standards, I. (2010). BS EN 459-1: 2001 Building lime: Definitions, specification and conformity criteria. BSI Group, UK.
British Standards, I. (2010). BS EN 459-2: 2010 Building lime. Test methods. BSI Group, UK.
BS EN 196-1. (2005). Methods of testing cement: Part 1 determination of strength. BSI Group, UK.
BS EN 13139: 2002 Aggregates for mortars. BSI Group, UK.
Desai, S. N., Patel, S., & Patil, H. H. (2011). Elevated temperature curing of concrete with industrial wastes. International Journal of Advanced Engineering Technology, 2(4).
Dheilly, R. M., Tudo, J., Sebai bi, Y., & Quéneudec, M. (2002). Influence of storage conditions on the carbonation of powdered Ca(OH)2. Construction and Building Materials, 16.
Dotter, K. R. (2010). Historic lime mortars: Potential of local climate on the evolution of binder morphology and composition. In B. J. Smith, C. Robin, & M. Gomez-heras (Eds.), Limestone in the built environment: Present-day challenges for the preservation of the past. Bath: The Geological Society Publishing House.
El-Turki, A., Ball, R. J., & Allen, G. C. (2007). The influence of relative humidity on structural and chemical changes during carbonation of hydraulic lime. Cement and Concrete Research, 37(8).
Ezziane, K., Bougara, A., Kadri, A., Khelafi, H., & Kadri, E. (2007). Compressive strength of mortar containing natural pozzolan under various curing temperature. Cement & Concrete Composites, 29.
Forster, A. M. (2004). How hydraulic lime binders work: Hydraulicity for beginners and the hydraulic lime family. Edinburgh: Love Your Building Publishing.
Forster, A. M., & Carter, K. (2011). A framework for specifying lime mortars for rubble masonry construction. Structural Survey: Journal of Building Pathology & Refurbishment, 29(5), 373–396.
Forsyth, M. (2007). Understanding historic building conservation. Oxford: Blackwell Publishing.
Gibbons, P. (2003). Preparation and use of lime mortar. Edinburgh: Historic Scotland.
Hall, C., Hamilton, A., Hoff, W. D., Viles, H. A., & Eklund, J. A. (2010). Moisture dynamics in walls: Response to micro-environment and climate change. Proceedings of the Royal Society A Mathematics, Physical & Engineering Sciences.
Hall, C., & Hoff, W. D. (2002). Water transport in brick, stone and concrete (2nd ed.). London: Spoon Press.
Hewlett, P. (2004). Lea’s chemistry of cement and concrete (4th ed.). Oxford: Butterworth-Heinemann.
Hughes, J. J., & Valek, J. (2003). Mortars in historic buildings: a review of the conservation, technical and scientific literature. Edinburgh: Historic Scotland.
Lawrence, R. M. H. (2006). A study of carbonation in non-hydraulic lime mortars. Ph.D. Thesis. University of Bath.
Lo, Y., & Lee, H. M. (2002). Curing effects on carbonation of concrete using a phenolphthalein indicator and Fourier-transform infra-red spectroscopy. Building and Environment, 37(5).
Pacheco Torgal, F., Miraldo, S., Labrincha, J. A., & De Brito, J. (2012). An overview on concrete carbonation in the context of eco-efficient construction: Evaluation, use of SCMs and/or RAC’. Construction and Building Materials, 36.
Pavia, H. S. (2008). A study of the workability of natural hydraulic lime mortars and its influence on strength. Materials and Structures, 41(2).
Radonjic, M., Hallam, K. R., Allen, G. C., & Hayward, R. (2001). Mechanism of carbonation in lime-based materials. In Proceedings of the 8th Euro seminar On Microscopy Applied to Building Materials, Athens (Greece), pp. 465–75.
Taylor, H. F. W. (1997). Cement chemistry (2nd ed.). London: Thomas Telford Publishing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Forster, A.M., Razali, N., Banfill, P., Szadurski, E., Torney, C. (2019). The Influence of Calcitic Filler in Hydraulic Lime Mortars for Use in High Temperature and High Humidity Climatic Conditions: A Preliminary Investigation. In: Hughes, J., Válek, J., Groot, C. (eds) Historic Mortars. Springer, Cham. https://doi.org/10.1007/978-3-319-91606-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-91606-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91604-0
Online ISBN: 978-3-319-91606-4
eBook Packages: EngineeringEngineering (R0)