Abstract
Understanding the molecular mode of actions of nanoantmicrobial will be helpful in creating viable administration systems to control critical pathogenic plant diseases. Similarly, the understanding of those mechanisms may assist to avoid resistance mechanisms, which are known and used in the case of pathogenic microorganisms. The potential mechanism of toxicity has been attributed to several possible mechanisms; the disintegration or arrival of particles from the nanoparticles inspire either provocative reaction, mitochondrial brokenness, interruption of cell layer respectability, oxidative pressure, protein or DNA degradation and harm, or reactive oxygen species (ROS) age, influencing the proteins and phospholipids and eventually causing cell passing. Specific attention was given to antimicrobial agents antimicrobial instruments with center around age of reactive oxygen species (ROS) including hydrogen peroxide (H2O2), OH-(hydroxyl radicals), and O2−2 (peroxide). ROS has been a major consideration for a few systems including cell wall harm because of NPs-restricted association and improved membrane permeability.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanoantimicrobials
- Nanoparticles (NPs)
- Nanostructures
- Chitosan
- Nanocomposites
- Magnetic nanoparticles
- Bimetallic nanoparticles
11.1 Introduction
Nanotechnology, the procedure to produce, control, and release nanomaterials, represents a zone holding huge guarantee for the agricultural scenario (Baruah and Dutta 2009; Navrotsky 2000; Kuzma 2007). Nanoparticles (NPs) having at least one dimension in the order of 100 nm or less (Auffan et al. 2009), in light of the fact that it is at this scale, the properties of materials vary as for their physical, substance, and organic properties from those at a higher scale. Nanostructuring increases the value of customary materials by improving their mechanical quality, superconductivity, and capacity to join and effectively convey dynamic substances into biological systems, at low expenses and with restricted agroecosystem effect (García-Rincóna et al. 2010). From an agricultural viewpoint, nanotechnology can possibly turn into a helpful method for plant pathologists in the diagnosis and treatment of plant diseases by the utilization of nano-based kits, pathogen detection plan by the application of nanosensors, improved ability of plants for micronutrients absorption, maximized plant yield by nanoporous zeolites, and plant insect management by using nanocapsules (Chaudhry et al. 2008; Sharon et al. 2010; Rai and Ingle 2012; Prasad 2014; Prasad et al. 2014, 2017a; Ismail et al. 2017). Researchers are occupied with preparing various types of natural, inorganic, and crossover nanoparticles having physical, optical, and organic properties (Salata 2004; Rai and Ingle 2012; Prasad et al. 2016). Nanobiotechnology works at a similar level with viral infection or disease-infecting particle and in this manner holds the potential for primordial identification and suppression. It additionally holds out the likelihood that smart sensors and conveyance frameworks will enable the agricultural industry to fight viruses and other plant pathogens (Prasad et al. 2014, 2017a). Then again, nanobio-innovation can enhance our comprehension of the nanobiology of different crops and consequently can possibly improve yields or nutritional values and also create enhanced systems for monitoring agroecosystem and improving the capacity of plants to preserve micronutrients or pesticides (Fakruddin et al. 2012; Tarafdar et al. 2013).
In order to completely and accurately define the mechanisms of toxicity that nanomaterials exhibit in a cellular environment, researchers at the forefront of this field will need to take a highly interdisciplinary approach utilizing both chemical and biological techniques to substantiate their claims. The current chapter will summarize recent progress toward an understanding of the antimicrobial mechanisms of nanostructures, with a focus on studies providing evidence for oxidative stress induction, membrane disruption, and genotoxicity. With a specific end goal to totally and precisely define the mechanisms of toxicity that nanomaterials display in a cellular environment, scientists at the forefront of this field should adopt an exceedingly interdisciplinary strategy using both concoction and natural procedures to substantiate their cases. The current chapter will summarize recent progress toward an understanding of the antimicrobial mechanisms of nanostructures, with a focus on studies providing evidence for oxidative stress induction, membrane disruption, and genotoxicity.
11.2 Resistance to Conventional Antimicrobial
Antimicrobial resistance is a regularly developing issue, yet what is an antimicrobial resistance? The expression “antimicrobial resistance ” is not only a potential danger, it is a severe health problem that is quickly spreading around the world. Over the past decades, people depended on regular antibiotic and antifungal eventually this led to improve the genotype of microorganisms to become more resistant, thus made researcher become more interested to provide new solutions using nanostructures, heavy metals are known to be toxic to various pathogens. In nature, microbial resistance from most dangerous substantial metals is because of their compound detoxification and because of vitality subordinate particle efflux from the cell by layer proteins that capacity either as ATPase or as chemiosmotic cation or proton antiporters. Modification in dissolvability additionally assumes a part in microbial resistance (Liu et al. 2011b).
11.3 Nanostructures as an Antimicrobial
Morphology and surface properties of colloidal nanoparticles are imperative. So that smaller nanoparticles than larger nanoparticles have further antimicrobial activity (Chwalibog et al. 2010), polymer-based copper nanocomposites have been examined for antifungal efficacy against plant pathogenic fungi; also silver nanoparticles are used in controlling spore-producing fungal plant pathogens and also showed the highest inhibition rate for both before and after the outbreak of disease on cucumbers and pumpkins and maximum inhibition for the growth of fungal hyphae and conidial germination in vivo assay (Kim et al. 2009; Cioffi et al. 2004). Also, a portion of the nanoparticles that have entered into the arena of controlling plant diseases are nanocarbon, silica, and aluminosilicates (Prasad et al. 2014). Ag NPs have an inhibitory effect on fungal colony and on spores of F. oxysporum. Silver nanoparticles might be directly devoted to penetrate cell layer membranes to kill spores (Abkhoo and Panjehkeh 2017; Morones et al. 2005). As of late, chitosan has turned out to be viable against microorganisms, plant pathogens, and viral pathogens (Xing et al. 2015). Furthermore zinc oxide (ZnO) and magnesium oxide (MgO) nanoparticles are effective antibacterial and anti-odor agents (Shah and Towkeer 2010; Bhuyan et al. 2015), and platinum nanoparticles TiO2 (Goswami et al. 2010) were achieved using the metal ion-reducing bacterium Shewanella algae growth (Konishi et al. 2007). Likewise it is notable that graphene oxide (GO) and its composites possess antimicrobial properties and have been used as antibacterial and antifungal agents (Santos et al. 2012; De Faria et al. 2014), and alumina NPs have been evaluated on bacteria such as Escherichia coli, Pseudomonas aeruginosa, and Bacillus subtilis and algae Scenedesmus sp. and Chlorella sp., and the toxic mechanism has been suggested as the interaction of the nanoparticles with the cell surface which cause leakage in the membrane (Sadiq et al. 2011). In addition, the likelihood to conjugate the surface of the NSs or to consolidate them with different materials (e.g., polymers, characteristic and manufactured fibers, clay) enables to achieve nanoantimicrobials with tunable properties as far as productive bioactivity against the focus of microorganisms and constrained, assuming any, toxicity toward human cells.
11.4 Applied Nanostructures and Their Mechanisms
The biocidal mode of action of the biofungicides is diverse relying upon the type of microorganisms utilized, viz., rhizosphere fitness, parasitism, and antibiosis, activating metabolic changes, promoting plant growth, and so forth (Saraf et al. 2014; Shrivastava et al. 2014). At the point when nanomaterials bind electrostatically to the bacterial cell wall and membranes, prompting modification of film potential, film depolarization, and loss of integrity, thus, result in roughness of transport, hampered breath, interruption of energy transduction and/or cell lysis, and eventually cell death (Pelgrift and Friedman 2013). Subsequently, the fundamental mechanisms that have been proposed to clarify the antimicrobial activity of inorganic and metal nanoparticles were ROS, which prompt oxidative stress and liberate superoxide, free radicals, and particles that can respond with the peptide linkages in the cell wall of microscopic organisms and in this manner upset them (Makhluf et al. 2005). To explain In the mitochondria of cells, ATP is synthesized by reduction of molecular oxygen to water through a sequence of coupled proton and electron transfer reactions. During this process, a small percentage of the oxygen is not reduced completely, resulting in the formation of superoxide anion radicals, and subsequently other oxygen-containing radicals. Thus, ROS need aid impacts from claiming cell division oxidative metabolism, much of which occurs in the mitochondria. Biologically relevant ROS include superoxide anion radicals, hydroxyl radicals, singlet oxygen, and hydrogen peroxide (H2O2) (Yin et al. 2012; Prasad et al. 2017b). The burst of ROS causes, through extreme oxidative stress, harm to all the cell’s macromolecules, prompting lipid peroxidation, adjustment of proteins, interruption of enzymes, and RNA and DNA destruction. At high concentrations the ROS lead to cell death and at low concentrations cause serious DNA damage and mutations (Wang et al. 2010; Matˇeejka and Tokarsk´y 2014). The nanoparticle is unable to cross the nuclear membrane and thus accumulates in the cytoplasm, where they can gain access to the nucleus during mitosis when the nuclear membrane breaks down (Singh et al. 2009). The direct interaction of nanoparticles with the DNA and DNA-related protein may lead to physical damage in the genetic material. Interference with the structure or function of the DNA repair enzymes in the nucleus may be another reason for DNA damage. The nanoparticle cannot cross the nuclear membrane and accordingly aggregates in the cytoplasm, where they can access the core amid mitosis when the nuclear membrane separates (Singh et al. 2009). The direct interaction of nanoparticles with the DNA and DNA-related protein may lead to physical destruction of the nucleic acids. Interference with the structure or function of the DNA repair enzymes in the nucleus might be an extra reason for DNA damage (Huang et al. 2015). And the alkalin effect also there is ionic mimicry mechanism that based on the donor atom selectivity and/or speciation of metals: metal ions in general bind to some atoms of donor ligands, such as O, N and S, through strong and selective, depending on whether metal ions or metal complexes are involved. In this way, some metals can stimulate the damage of Fe-S clusters, for instance, from bacterial dehydratases that are particularly susceptible to site-specific inactivation by toxic metals interactions, exterior metal ions, or their complexes that can replace original metals present in biomolecules leading to cellular dysfunction. Metals can also replace non-catalytic metal-binding sites inhibiting enzyme activity (Lemire et al. 2013; Grass et al. 2011; Ruparelia et al. 2008; Xu and Imlay 2012).
11.4.1 Silver NPs
Silver NPs are being utilized as part of various advancements and consolidated into a wide exhibit of customer items that exploit their attractive optical, conductive, antifungal, and antibacterial properties (Aziz et al. 2016; Joshi et al. 2018). The fundamental utilization of Ag NPs is, be that as it may, as antimicrobial agents (Cioffi and Rai 2012; Prasad et al. 2012). Silver-based nanocomposites have been utilized broadly as antimicrobial agents in various areas including therapeutic, pharmaceutical, material, food safety, ecological, and agrarian applications (Kim et al. 2007). With the antimicrobial activity of AgNPs, it is encouraging to note that they are predominantly utilized for plant disease control (Jo et al. 2009; Kim et al. 2011). Although, AgNPs have been proved effective against over 650 microorganisms including bacteria (both Gram-positive and negative), fungi and viruses; however, the exact mechanism of silver action on microbes is still not known, but the possible mechanism of action of metallic silver, silver ions, and silver NPs have been suggested according to the injuries and changes, induced in microbial cells (Malarkodi et al. 2013). Several mechanisms maybe involved in the antimicrobial activity of Ag NP's most of them damage the microbial's cell structure integrity and result in leakage of intracellular compounds, and eventually cell death (Durán et al. 2016). Concerning the activity of Ag+, the subsequent actions are caused: (1) binding to negatively charged proteins and nucleic acids (particularly with functional groups like imidazole, indole, hydroxyl, phosphate, thiol) causing changes in structure. As an example, it is known that Ag + ions bind to cysteine-containing proteins on plasma membranes, causing both physiological and biochemical destructions that compromise membrane integrity. Subsequent penetration of Ag into the cytoplasm causes the inactivation of critical enzyme systems and condenses DNA which then reacts with the thiol group proteins and triggers cell death (Ocsoy et al. 2013; Aziz et al. 2014, 2015, 2016). The antifungal activity of AgNPs takes place due to the high attraction between the nanoparticles and the functional chemical groups existing in the cell membrane of fungi and other microorganisms (Jo et al. 2009). It has been suggested that AgNPs with a positive surface charge are more easily internalized through the cell membrane than particles negatively charged or neutral (Jo et al. 2015). This is caused by 1) a procedure called electromagnetism that occurs between the positively charged AgNPs and negatively charged bacterial cell membranes, due to the presence of phosphate and carboxyl groups that 2) alter the functions of the ribosome, causing an inhibition of protein synthesis and locking mechanisms of transcription and translation (Abbaszadegan et al. 2015). DNA loses its ability to duplicate when the fungal culture was treated with AgC and Ag+, which may lead to a deactivated expression of ribosomal subunit proteins and to the synthesis of disabled enzymes and cellular proteins, important for the adenosine triphosphate production (Yamanaka et al. 2005; Sang et al. 2012) and which also prevent protein expression related to ATP fabrication (Kim et al. 2009). The biogenic silver NPs bind with protein of the outer cell wall of some pathogens including bacterial, fungal, or viral bodies that disrupt the lipoproteins of the microbial cell wall. Finally the cell division was stopped and cell leads to death (Kuppusamy et al. 2016; Prasad et al. 2016) and (3) deterioration of the external cell membrane, due to the fact that the synthesis of the component proteins is affected. When the membrane is not totally functional, the protein precursors of the envelope proteins and periplasmic constituents accumulate in the cytoplasm, such as when the membrane potential is dissipated by small molecules (Lok et al. 2006). Due to the abundance of sulfur-containing structural proteins and enzymes on the bacterial cell membrane, silver NPs can interact with them and in turn reduce cell functionality and viability. Furthermore, they interact with phosphorus-containing compounds like DNA. Nanoparticles less than 20 nm in diameter may attach to the cell membrane, make pores on the cell wall, leading to more permeability, and release cytoplasmic content outside the cells, destroying enzymes and attacking the respiratory chain and cell division, which cause the death of bacteria (Morones et al. 2005). Additionally, AgNPs are recognized to (4) generate ROS and liberated radicals, which lead both mitochondrial dysfunction and DNA harm. In addition, a study improved by Rai et al. (2009) indicated that the AgNPs affect the performance of reactive oxygen species (ROS) and lead to a process of lipid peroxidation of the cell membrane, changes in cell cycle, and damage to the DNA of the microorganisms. (5) intercalate between DNA bases, silver ions (particularly Ag+) released from silver nanoparticles can interact with phosphorus moieties in DNA, resulting in inactivation of DNA replication, or can react with sulfur-containing proteins, leading to the inhibition of enzyme functions (Gupta and silver 1998; Matsumura et al. 2003). Silver NPs might prevent many oxidative enzymes, including alcohol dehydrogenase, and prohibit the uptake of succinate by the membrane vesicles. They cause oxidative DNA harm and interfere with DNA replication processes (Petrus et al. 2011; Prasad et al. 2017b). In short, the antimicrobial mode of action for AgNPs is associated with four well-defined mechanisms: (1) adherence of AgNPs onto the surface of cell wall and membrane, (2) AgNPs penetration inside the cell and damaging of intracellular structures (mitochondria, vacuoles, ribosomes) and biomolecules (protein, lipids, and DNA), (3) AgNPs induced cellular toxicity and oxidative stress caused by formation of reactive oxygen species (ROS) and free radicals, and (4) modulation of signal transduction pathways.
11.4.2 TiO2 NPs
Titanium dioxide nanoparticles (TiO2 NPs) can be utilized in different agricultural applications. The photocatalytic properties of TiO2 NPs play a major role in the management of different pathogens. There have been many new reports on the inhibitions of microorganisms in the presence of pure TiO2 nanoparticles with several crystalline phases under UV irradiation (Lin et al. 2014). When TiO2 is irradiated with near-UV light, this semiconductor exhibits robust photocatalytic chemical reaction, which is a sophisticated chemical reaction method for the removal of trace contaminants and microorganism pathogens (Hossain et al. 2014). The photocatalytic antimicrobial activity of TiO2 is attributed to lipid peroxidation that cause a rise within the membrane thinness, discontinuity of the cell structure, and production of ROS, as well as peroxide (H2O2), superoxide radical (O2•), and free radicals (•OH), upon exposure to near-UV and UVA radiation (Choi et al. 2007; Niazi and Gu 2009; Huh and Kwon 2011; Carré et al. 2014).
The generated ROS causes harm to the molecular structure of the cell, including DNA, lipid, and protein damage (Fu et al. 2014). Mathur et al. (2015) reported the effects of TiO2 NPs on intracellular levels of two major types of ROS – hydroxyl radical (•OH) and superoxide radical (O)−2. These free radicals can interact with macromolecules, such as lipids, proteins, enzymes, and nucleic acid molecules in bacteria, viruses, and different microorganisms, which can destroy cell structures through a series of chain reactions (Yu et al. 2002; Sonawane et al. 2003; Zhao et al. 2000; Prasad et al. 2016). Formation of oxidative stress (hydroxyl and superoxide radicals) was higher in bacterial cells presented to NPs and UVA light. Eventually, a significant increase in membrane permeability was noted in cells exposed to NPs and UVA light in comparison to that in dark and visible light conditions. Therefore, opinions exist that the primary mechanism of action of titania NPs is based on creation of ROS, which induce oxidative stress. TiO2 NPs also have bactericidal effects in the absence of irradiation, suggesting that they use other antimicrobial mechanisms unrelated to photocatalytic ROS production (Choi et al. 2007). Inactivation of microorganisms depends upon several factors, e.g., concentration of TiO2, type of microorganism, intensity and wavelength of light, degree of hydroxylation, pH, temperature, availability of oxygen, and ROS retention time (Markowska-Szczupak et al. 2011; Hossain et al. 2014).
The antimicrobial activity of titanium nanoparticles have been broadly examined throughout the years. TiO2 is economical, nontoxic, and insoluble food additive. Titanium dioxide NP is photocatalytic; their poisonous quality is initiated by obvious light, close UV, or UV (Pelgrift and Friedman 2013). The antialgal activity of titania nanoparticles against microalgae species have likewise been led for which a focus subordinate reduction in chlorophyll content was noticed (Sadiq et al. 2011). Later reports have demonstrated its efficiency against different viral species and pathogens (Brady-Est´evez et al. 2008; Allahverdiyev et al. 2013). The effect of TiO2 NPs on the symbiotic behaviour of symbiotic arbuscular mycorrhizal fungi (AMF) colonising rice at 0, 25, 50 and 100 mg plant-1 to the rhizosphere of mycorrhizal rice plants maintained in pots. TiO2 NPs had an inhibitory affected AMF in plant roots (Priyanka et al. 2017). The TiO2 nanoparticles could protect the wood against white- and brown-rot fungi (De Filpo et al. 2013). TiO2 nanoparticles had viable antifungal properties at the concentration of 5.14 and 5.35 g/mL for fluconazole-susceptible and fluconazole-resistant strains of Candida albicans biofilms contrasted with fluconazole medication, respectively (Haghighi et al. 2012).
11.4.3 ZnO NPs
Zinc nanoparticles have been utilized as nanofertilizers on numerous plant species and indicated positive outcomes in ideal concentration; however ZnO NPs as fungicide against various plant pathogens is less studied. ZnO NPs are extremely successful antimicrobial agents and are effective against both, bacteria, fungi, toxicogenic fungi in addition to the thermophilic and barophilic spores (Sondi and Salopek-Sondi 2004; Raghupathi et al. 2011; Sierra-Fernandez et al. 2017). ZnO could also be utilized as antimicrobial agents against microorganisms that could be causes of food-borne pathogens and plant diseases. The exact mechanism of this activity is not fully understood yet. Be that as it may, one hypothesis proposes the development of a solid oxidant, hydrogen peroxide (H2O2). The superoxides and hydroxyl radicals cannot penetrate into the membrane because of their negative charges (Xie et al. 2011). Accordingly, these species are found on the external surface of bacteria, and by differentiation, H2O2 particles can go through the bacterial cell wall, in this way prompting wounds and destroying and lastly activating cell death (Zhang et al. 2007; Sawai et al. 1996). Some other possible mechanisms include cell membrane disruption, generation of ROS on the NP surface, the influx of zinc particle in the cell, membrane dysfunction, or internalization of NPs, which could help in its antimicrobial activity (Li et al. 2012a). ZnO NPs caused liquefaction of cytoplasmic substances, making the cytoplasm less electron-dense and creating an eminent separation of the fungal cell wall (Arciniegas-Grijalba et al. 2017). A few investigations recommended that ZnO NPs may cause support changes of the microbial cell membrane, causing cytoplasm leakage and in the long run the demise of bacterial cells (Sawai and Yoshikawa 2004; Brayner et al. 2006). ZnO nanoparticles showed noteworthy antibacterial activity and exhibited a deadly impact against C. jejuni, even at low concentrations. ZnO nanoparticles prompted noteworthy morphological changes and assessable membrane leakage (Xie et al. 2011). Different researchers show that the event of ROS is the principal mode of action responsible for the killing efficiency of ZnO NPs (Bhuyan et al. 2015; Arciniegas-Grijalba et al. 2017). The formation of ROS, for example, hydrogen peroxide H2O2, hydroxyl radical *OH, and superoxide O*2 −, is the aftereffect of ZnO initiation by UV or unique light (photocatalysis).
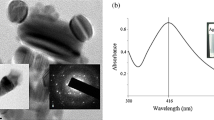
The SEM micrographs of Botrytis cinerea mycelium treated with photoactivated ZnO NPs plainly show different degenerative modifications in the conidial heads and hyphal morphology. Twisted conidial heads and withered hyphal wall were usually seen in treated mycelia compared with control. Fungistatic mode of action of ZnO activity on fungi was seen by He et al. (2011). Authors discovered control of conidial advancement by the distortion of conidiophores in Penicillium expansum, while the fungal mat of B. cinerea was disfigured after treatment with ZnO NPs (He et al. 2011). Jayaseelan et al. (2012) showed that ZnO NPs suppress the development of pathogenic microbes P. aeruginosa and Aspergillus flavus. ZnO NPs rate the germination of fungal spores of Alternaria alternata, Fusarium oxysporum, Rhizopus stolonifer, and Mucor plumbeus (Wani and Shah 2012). SEM micrographs got by Kairyte et al. (2013) showed distorted conidial heads and withered hyphal wall in B. cinerea mycelia after treatment by photoactivated ZnO NPs (Fig. 11.1).
Scanning electron microscopy of B. cinerea mycelium after treatment with 5 × 10−3 M photoactivated ZnO NPs (24 h incubation; 34.56 J/cm 2 illumination dose) (d–f) in comparison with control (a–c), non-treated ones. (Reprinted from Kairyte et al. 2013)
Distortion of the hyphal cell structure may be routed to over-the-top collection of nucleic acids and sugars, since ZnO NPs can influence cell physiology and trigger higher generation of nucleic acids. In addition, the increase in the production of nucleic acids can be considered as a stress response of fungal hyphae, and increase production of starches might be a consequence of cell self-protection from the activity of ZnO NPs (Perez Espitia et al. 2012). ZnO nanoparticles demonstrated less phytotoxicity on plants in contrast with AgNP and can be valid as a nanopesticide. There have been various investigations that determined that controlling the measure of ZnO NPs is basic for antimicrobial-related applications. As needs be, the state of the zinc oxide nanostructures can influence penetration of cell membranes; spherical nanoparticles cannot enter as effectively as rod structures (Sirelkhatim et al. 2015). Be that as it may, the most essential part in antimicrobial movement is played by molecule size and concentration of ZnO NPs (Sirelkhatim et al. 2015). In this manner the high antimicrobial activity of ZnO NPs is normally identified with a substantial surface area and a high concentration of particles. This can be clarified by methods of entry into the bacterial cell wall; a smaller-sized particle can easily penetrate bacterial membranes and injure or kill the cells. The fungal wall, in controlling cell permeability, is the part of the cell that interacts with the external environment, and thus with the ZnO NPs present in the fungal culture of interest in this work. This part of the fungal cell is made primarily out of polysaccharides and proteins. In particular, there are β-1,3-D-glucan and β-1,6-D-glucan macroproteins, chitin, proteins, and lipids, and among the polysaccharides, chitin, glucan, and mannan or galactomannan (Pontón 2008) prevail. The last factors that are accepted to affect the component of antimicrobial activity are the changes that can happen on the surface of ZnO NPs, in this way making it possibly responsive. Another approach to improve a better antimicrobial agent is to functionalize the surface of zinc oxide nanostructures. In such manner, if the surface area of the zinc oxide nanostructures is reformed, it could advance the generation of ROS and the release of ZnO, consequently expanding antimicrobial activity. Other than the previously mentioned applications, the mix of photoactive nanomaterials and microorganisms can then again be utilized as a part of different new fields later on. For instance, the good bacteria which fight off the pathogens can be designed with nanomaterials to upgrade the general antimicrobial impact.
11.4.4 MgO NPs
Magnesium oxide nanoparticles (MgO NPs) are extremely fixed, biocompatible, and exceptionally efficient as an antibacterial agent. The formation of reactive oxygen species (ROS) mechanism has been proposed to explain the antimicrobial mechanism of MgO nanoparticales, the interaction of nanoparticles with bacteria, subsequently leads to damaging the bacterial cell (Tang and Bin-Feng 2014). It has been described that the development of the surface area of MgO particles prompts a rise of the O2 − concentrations in solution and thus results in a more effective damage to the cell wall of the bacteria (Sawai et al. 2000; Yamamoto et al. 2000). It was proposed that the cell death was caused by the electrostatic contact between the bacterial cell surface and MgO nanoparticles. MgO nanoparticles showed high bactericidal effect against microbes because of the association of particles and bacteria (Peter et al. 2002; Makhluf et al. 2005). It was discovered that MgO nanoparticles could take up halogen gases because of the defective nature of their surface and its positive charge, which resulted in a strong interaction with bacteria, which are negatively charged (Stoimenov et al. 2002). Conversely, non-ROS-interceded bacterial toxicity was additionally found in MgO nanoparticles, proposing that oxidative stress would not be the mode of action for cell death (Leung et al. 2014). The alkaline effect has been considered as another primary factor in the antibacterial action of MgO nanoparticles (Sawai et al. 2001; Yamamoto et al. 2000). The possible antibacterial mode of action was the preservation of water humidity on the MgO nanoparticle surfaces, which could frame a thin water layer around the nanoparticles. The local pH of this thin water layer formed around the nanoparticles might be much higher than its equilibrium value in the solution. At the point when the nanoparticles are in contact with the bacteria, the high pH in this thin surface layer of water could harm the cell membrane, resulting in cell death (Sawai et al. 1997).
These antibacterial mechanisms of MgO NPs are dissimilar to the membrane lipid peroxidation caused by oxidative stress, based on the following three points:
-
1.
When the bacterial cell membrane is broken and surface pores are visibly clear, MgO NPs are not detected in the cell. Moreover, no extreme Mg ions are visible in energy-dispersive X-ray spectroscopy spectra. Hence, the inhibitory impact of MgO harms the cell membrane.
-
2.
Only one kind of MgO NP can identify little measures of ROS; the other two cannot.
-
3.
Lipopolysaccharide (LPS) and phosphatidylethanolamine (PE) in the cell wall are not significantly changed by MgO NP treatment, which shows that MgO does not cause lipid peroxidation in the cell membrane (Leung et al. 2014; Wang et al. 2017a).
The antifungal impact of MgO NPs on a few fungal pathogens like Alternaria alternata, Fusarium oxysporum, Rhizopus stolonifer, and Mucor plumbeus detailed the most noteworthy impact utilizing the 30 and 50 nm nanoparticle size (Wani and Shah 2012). MgO nanoparticles have a good antibacterial effect against three essential food-borne pathogens. The interaction of nanoparticles with bacterial cells causes cell membrane outflow, stimulates oxidative stress, and at last prompts cell death (He et al. 2016). MgO nanoparticles induced systemic resistance in tomato against bacterial wilt disease. The quick fabrication of O2 or phenoxyl radicals in tomato roots treated with MgO NPs may assume a related part in the plant resistance response of tomatoes against Ralstonia solanacearum (Imada et al. 2016). Several mechanisms for the action of MgO nanoparticles on bacteria are the following: (1) MgO nanoparticles continuously produce a specific level of H2O2 while in suspension, which induces oxidative stress in cells; (2) physical interaction between nanoparticles and bacterial cell surface interrupts bacterial membrane integrity and causes membrane leakage; (3) higher concentrations of nanoparticles prompt serious membrane damage, cell content release, irreversible oxidization of biomolecules (e.g., DNA, proteins, and lipids), and eventually cell death (He et al. 2016).
11.4.5 Magnetic Nanoparticles (MNPs)
Nanotechnology application in plant disease management in the early stage. Magnetic nanomaterials could be used for site-targeted delivery of systemic nanoagrochemical plant protection, for improving plant disease resistance, increasing effective nutrient consumption, and improvement of plant growth (Nair and Kumar 2013). Fe2O3 NP-coated seeds have indicated enhanced seed germination and root and shoot lengths of Solanum lycopersicum. Based on the improved Fe2+/Fe total ratio found in iron extracted from dry biomass of the plant, we affirm the take-up of Fe2O3 NPs and their internalization and/or biomineralization in the plant body (Shankramma et al. 2016). Furthermore, superparamagnetic iron oxide could be able to destroy macromolecules, including DNA, lipids, and proteins, through the Fenton reaction, leading to bacterial death. Iron increases the generation of ROS through oxidative stress and promotes the electron transport chain to produce superoxides (O−2), which destroy the iron clusters (Leuba et al. 2013). Consequently, more divalent iron participates in the oxidation in Fenton reaction, leading to the generation of more hydroxyl radicals (·OH) and stimulating the death of residual bacteria through the catabolism of the carbon source and the formation of nicotinamide adenine dinucleotide. The intake of superparamagnetic iron may also increase simultaneously because of functionalized polycarboxylate. The uncovering of magnetic fields to attract nanoparticles can change the organic movement and raise the bacteriostaticity of these nanocomposites in bacterial medium. Momentary nanomagnets may fill in as a helpful model framework to apply electromagnetic interactions of nanoparticles in agrobiology system (Sadjad et al. 2017). Fe3O4/ZnO/AgBr nanocomposites with diverse weight ratios of Fe3O4 to ZnO/AgBr were prepared using a facile microwaved-assisted technique, and their antifungal activities were investigated against Fusarium graminearum and Fusarium oxysporum, the causal agents of wheat head blight and lentil-vascular wilt diseases, respectively. Magnetic nanocomposites completely deactivated F. oxysporum after 60 min, which is a shorter time duration than for F. graminearum (Hoseinzadeh et al. 2016). The prepared magnetic nanocomposites could be used as an effective nanofungicide in plant pathology applications.
11.4.6 Ni NPs
The antimicrobial activity of nickel nanoparticles (Ni NPs) depends on the formation of ROS and release of nickel ions Ni(II). Enhanced leakage of proteins from Ni NPs treated bacterial and fungal cell membranes into culture medium is due to generation of free radicals from NiGs surface that induced membrane damage and leaked membrane and cellular contents (Choi and Hu 2008; Pandian et al. 2016). Stimulation of ROS synthesis leads to the generation of highly responsive radicals that destroy the cells by damaging cell membranes, proteins, DNA, and intracellular system (Kim et al. 2011; Jyoti et al. 2007). The Ni NPs can affect the quantity of lactate dehydrogenase, an important cytoplasmic enzyme (Pandian et al. 2016). Generally ROS generation has been suggested as a mode of action that clarifies the phytotoxicity effect of metal oxide NPs in the microbial cell. In the mitochondria of cells, ATP is produced by reduction of molecular oxygen to water through a sequence of attached proton and electron transfer reactions. Amid this procedure, the low level of oxygen is not reduced totally, bringing about the arrangement of superoxide anion radicals and therefore other oxygen-containing radicals. Hence, ROS are by-products of cellular oxidative metabolism, much of which happens in the mitochondria. Naturally released significant ROS include superoxide anion radicals, hydroxyl radicals, singlet oxygen, and hydrogen peroxide (H2O2) (Yin et al. 2012). The antifungal efficacy of Ni NPs against Fusarium wilt of tomato and lettuce was studied under in vitro and in vivo assay (Fig. 11.2). The Ni NPs suppressed the fungal growth of F. oxysporum f. sp. lactucae and F. oxysporum f. sp. lycopersici by 60.23 and 59.77%, respectively, at 100 ppm concentration compared with control (Ahmed et al. 2016). Nickel and cobalt ferrite nanoparticles (NiFe2O4 and CoFe2O4) are effectively confirmed for antifungal activity against three fungal plant pathogens: Fusarium oxysporum, Colletotrichum gloeosporioides, and Dematophora necatrix. Furthermore, it is also investigated that these ferrite nanoparticles decrease disease incidence of Fusarium wilt in pepper (Sharma et al. 2017).
Zone inhibition of Fusarium wilt pathogens. First row, Fusarium oxysporum f. sp. lycopersici (a, control; b, 50 ppm nickel nanoparticles ; c, 100 ppm nickel nanoparticles); second row, Fusarium oxysporum f. sp. lactucae (d, control; e, 50 ppm nickel nanoparticles; f, 100 ppm nickel nanoparticles). (Reprinted from Ahmed et al. 2016)
11.4.7 Carbon-Based NPs
Carbon-based nanomaterials, for instance, carbon nanotubes (CNTs) , single-walled carbon nanotubes (SWCNTs), multiwalled carbon nanotubes (MWCNTs), graphene oxide (GO) nanoparticles, and fullerenes, presented prospective antimicrobial activities (Wang et al. 2014; Dizaj et al. 2015). A specific investigation evaluated the microbial effects of carbon nanotubes and fullerenes on some pesticide uptake by agricultural plants (De La Torre-Roche et al. 2013). The CNTs’ antimicrobial mode of action is not totally clear. Former reports on CNTs divided the antimicrobial mechanism into two major kinds (Kang et al. 2007; Li et al. 2014). The first one is physical interaction, which includes membrane leakage or cell growth inhibition caused by interactions of the cell or cell membrane with the GNPs. The second category contains chemical reactive ions giving rise to the formation of reactive oxygen species (ROS). One of the most interesting, and the simplest, mechanism is the mechanical damage of bacterium cell envelope by some carbon forms. With the cell membrane damage resulting from direct contact with SWCNT, the membrane damage leads to leakage of intracellular materials (e.g., cytoplasm, ribosomes, and nucleic acids), which will eventually lead to cellular death (Kang et al. 2007; Jastrzębska et al. 2012). This mode of action will be affected significantly by some characteristics of the carbon nanomaterial, such as size, contact time, concentration, functionalization, and others. Another important factor affecting the antimicrobial efficacy of CNTs is emanated from their electronic structure.
Different investigational assays discovered that physical damage of pathogens resulted from their interaction with graphenes by two potential mechanisms: by extreme insertion and breaking of the cell membrane and by damaging extraction of phospholipids from lipid membranes (Zhou and Gao 2014). The oxidative stress mechanism has been suggested as a major cytotoxicity mechanism of graphene (Roda et al. 2014). Reactive oxygen species (ROS) are formed by GO, which would affect microorganisms sustainability. These ROS contain hydrogen peroxide, superoxide anion radicals, singlet oxygen, hydroxyl radicals, and nitric oxide. To help ensure against the dangerous impacts of ROS, oxygen-consuming life-forms and facultative anaerobic microorganisms deliver defensive cell reinforcement catalysts such as catalase, superoxide dismutase, and glutathione peroxidase. Catalases are proteins that catalyse the conversion of Hydrogen Peroxide (H2O2) to water and molecular oxygen, thereby protecting cells from the toxic effects of hydrogen peroxide Catalases are produced by all microorganisms utilized as part of this examination with the exception of S. faecalis which is microaerophilic (Roda et al. 2014). The antibacterial property of graphene does not come from ROS-initiated harm but rather through electron transfer communication from microbial membrane to graphene (Li et al. 2014). Other, more changeable mode of action for antimicrobial activity has been shown for graphene-based structures too. But previously mentioned disturbing the integrity of the cell wall (mechanical harm of the cell), these materials can (1) wrap around the microorganisms isolating them from the agrosystem, (2) produce hurtful reactive oxygen species (ROS) , (3) remove phospholipid atoms of the microorganisms by the presence of the lipophilic graphene, and (4) lower the metabolic activity of the bacterial cells (Akhavan et al. 2011; Liu et al. 2011a; Gurunathan et al. 2012; Sawangphruk et al. 2012; Tu et al. 2013; Chen et al. 2014).
There are not many examples of the antifungal mechanism of the graphene family materials in literature. According to Sawangphruk et al. (2012), inhibition of the mycelia growth results perhaps from direct interaction of rGO nanosheets with the cell wall (Aspergillus niger, Aspergillus oryzae, and Fusarium oxysporum). Researchers propose that there is a chemical reaction between oxygen (from rGO) and polysaccharides (e.g., chitin) in the wall. The six CNTs involved, SWCNTs, MWCNTs, graphene oxide (GO), reduced graphene oxide (rGO), fullerene (C60), and activated carbon (AC), were studied against two fungal pathogens: Fusarium graminearum and Fusarium poae (Wang et al. 2014). The SWCNTs had the maximum antifungal efficacy tracked by MWCNTs, GO, and rGO. C60 and AC showed no important antifungal activity. The antifungal properties of MWCNTs with diverse surface groups against F. graminearum were examined by Wang et al. (2017b). As per their discoveries, spore germination was strangely suppressed by surface-modified MWCNTs, with germination rate being 18%, threefold lower than for pristine MWCNTs.
The antifungal mode of action was assumed to target the spores in three steps: (1) depositing on the surface of the spores, (2) inhibiting water uptake, and (3) inducing plasmolysis. Different reports asserted no antifungal efficacy of GO toward Candida albicans and Candida tropical (Li et al. 2013b; Cui et al. 2014). The antimicrobial mode of action for fullerenes is still under open discussion. Precisely, fullerenes and their subordinates have exhibited effective antibacterial action against a wide range of microorganisms when presented to light (Chen et al. 2016). Fullerenes can integrate light and along these lines create reactive oxygen species (Kleandrova et al. 2015). Other possible mechanisms have additionally been accounted for, including impact on respiratory chain, disturbance of the cell membrane structure (Cataldo and Da Ros 2008), interaction with membrane lipids, and intercalation into them (Cataldo and Da Ros 2008; Dizaj et al. 2015). The antimicrobial property of fullerene is likewise influenced by the size and surface area of it (Dizaj et al. 2015) and the form of functional group used (Li et al. 2012a). CNTs are unable to substitute/compete with the currently used antimicrobial materials (e.g., polymers, Cu NPs, and Ag-NPs) for many reasons, for instance, their toxicity profile for human cells has not been well addressed yet (Al-Jumaili et al. 2017). Currently, most antimicrobial carbon nanomaterials are still lack research/development.
11.4.8 Cu NPs
Furthermore to the control of growth of yeasts and molds,copper nanoparticles have also found to be effective against Gram-positive and Gram-negative bacteria, the activity of copper oxide nanoparticles (100–150 nm) coated to fabric showed 100 % reduction of E. coli, S. aureus, and Aspergillus niger after 48 h of incubation (Schrand et al. 2010; Theron et al. 2008; Usha et al. 2010). Copper nanoparticles gained position as innovative antimicrobial agents due to their high antimicrobial activities against widespread microorganisms including multidrug-resistant organisms. Similarly, copper is economical and simply obtainable, therefore synthesis of copper nanoparticles is cheap. One more advantage of copper nanoparticles is that they oxidize and form copper oxide nanoparticles, which can simply mix with polymers or macromolecules to produce nanocomposites, and are relatively stable in terms of both chemical and physical properties (Cioffi et al. 2005; Usman et al. 2012). Cu nanoparticles (Cu NPs) can penetrate the cell directly through the pores present in cell membrane due to their small size, or they enter through ion channels and transporter proteins present in the plasma membrane. Nanoparticles which are introduced into the cell can have direct contact with oxidative organelles such as mitochondria. Furthermore, redox-active proteins can stimulate reactive oxygen species (ROS) production in cells, and ions (Cu2+) produced by nanoparticles can induce ROS by several chemical reactions. Also, Cu2+ ions have the ability to form chelates with biomolecules or remove the metal ions in specific metalloproteins, which may result in functional protein inhibitions. Cu2+ released by copper oxide nanoparticles increases their local concentration and disrupts cellular metal cation homeostasis resulting in cell toxicity (Chang et al. 2012). Linoleic acid capped copper nanoparticles after penetrating into the bacteria deactivate their enzymes, generating hydrogen peroxide resulting from ROS, which leads to bacterial cell death (Das et al. 2010). Schrand et al. (2010) hypothesized that copper nanoparticles act as actual antibacterial agent against the wide range of bacterial pathogens due to interactions with -SH groups, leading to protein denaturation. ROS may bind with DNA molecules and interrupt the helical structure by cross-linking within and between the nucleic acid strands and affect gene expression (Fig. 11.3). Copper ions inside bacterial cells also disrupt biochemical progressions (Kim et al. 2000; Stohs and Bagchi 1995). Still there is an absence of definite data regarding the mechanism of action; however till now, completely different activity pathways are advised for the two copper oxides (I and II), with the involvement of ROS primarily within the case of CuO NPs and therefore the specific binding of Cu(I) to macromolecule surfaces for Cu2O NPs . However, the exact mechanism behind bactericidal effect of copper nanoparticles is not known and needs to be further studied on the broader range of bacteria strains.
11.4.9 Al NPs
The marketable use of aluminum oxide nanoparticles Al2O3 extremely increased during the last decade, and, by no means, it enhances the risk of environmental pollution. Additionally, the toxic effects of Al2O3 NPs are also described on some model organisms for ecotoxicity assays, such as Bacillus subtilis, Escherichia coli, and Pseudomonas fluorescens (Jiang et al. 2009: Sadiq et al. 2009). There are chronic toxicity studies on Al2O3 nanoparticle exposure that cause neurotoxic effects on locomotion behaviors by prompting more ROS generation and interruption of ROS defense mode of actions in nematode Caenorhabditis elegans (Li et al. 2012b). The variation in cytotoxicity between micron-sized and nanosized alumina nanoparticles toward Scenedesmus sp. and Chlorella sp. was investigated. The antialgal inhibitory effect of the nanoparticle was studied against both the species, and an evident reduction in the chlorophyll content was also investigated in the cells treated with nanoparticles (Sadiq et al. 2011). Alumina nanoparticles showed a mild growth-inhibitory effect, only with very high concentration, which might be due to surface charge interactions between the particles and cells. Free-radical scavenging properties of the particles prevented cell wall disruption and drastic antimicrobial action (Sadiq et al. 2009). Alumina nanoparticles (Al2O3 NPs) showed a minor development inhibitory effect, so to speak, with extremely high focus, which may be because of surface charge naturally occurring between the particles and cells. Free-radical scrounging properties of the particles have foreseen cell wall disturbing influence and extraordinary antimicrobial action (Rupareli et al. 2008; Sadiq et al. 2009). SEM images of nanoalumina-interacted cells of E. coli indicate the changes in cell shape and agglomerated particles on the cell wall. Moreover, TEM micrographs show disruption and disorganization of cell membrane and cell wall (Ansari et al. 2013). The cell membrane was widely injured and, most probably, the intracellular content has leaked out. Al2O3 carry a positive charge on its surface, electrostatic interaction between bacteria and NPs results in the adhesion of them on the bacterial surface and expressed antimicrobial activity. Al2O3 NPs not only adhered at the surface of cell membrane, but also penetrated inside the bacterial cells, cause formation of irregular-shaped pits and perforation on their surfaces and may also interact with the cellular macromolecules causing adverse effect including cell death. However more investigations as regards to the connection of alumina nanoparticles with cells should be done before its broad use in restorative and horticultural and crop application.
11.4.10 Au NPs
The antimicrobial property of Au NPs has been confirmed in various microbes, including Gram-positive bacteria, Gram-negative bacteria, and some pathogenic fungi. The green-synthesized gold nanoparticles (Au NPs) (45–75 nm) act as an active antifungal agent against wheat stem rust caused by Puccinia graminis tritici, and other fungal pathogens including Aspergillus flavus, Aspergillus niger, and Candida albicans using standardized well diffusion technique and hence have a great prospect in the preparation of fungicides used against different plant diseases (Jayaseelan et al. 2013). This action is credited to unique properties of Au NPs in illumination centering, solid cationic attractions to the negatively charged plasma layer of organisms, or conjugation with antimicrobial agents and antibodies.
In another way, it can bind to the DNA of microorganisms and repress DNA transcription (Rai et al. 2010). Gold NPs , 4,6-diaminopyrimidine thiol as a simple of bacterial tRNA base, has potential capability to prevent the subunit of ribosome for tRNA which influence on its capacity that affect protein synthesis (Carbon and David 1968; Sayed et al. 2006; Zhao et al. 2010). Au NPs can change membrane potential and suppress ATP synthase activities to decrease the ATP level, indicating a general decrease in metabolism, and also improve chemotaxis in the early-stage reaction (Cui et al. 2012). Those properties prompt cell layer disturbance, ROS collection, hindering DNA translation, and subsequent cell demise (Huh and Kwon 2011). In the first mechanism, Au nanoparticles generate holes in the cell wall, resulting in leakage of the cell contents, formation of biofilm, and finally cell death (Chwalibog et al. 2010). A second mechanism had been suggested that the strong electrostatic attractions among Au NPs and the cell wall surface of the pathogens introduce adhesin-mediated interaction between the pathogenic cells and the substrate surfaces (Yu et al. 2016).
11.4.11 Bimetallic NPs
Bimetallic nanoparticles made out of two kinds of metal components and metallic nanoparticles can be sorted as bimetallic or trimetallic relying upon the quantity of segment metallic fixings, for example, metal oxide NPs (Cu, Mg, Zn, and Ag) (Roopan et al. 2014). A synergistic antimicrobial effect is achieved when silver nanoparticles are hybrid with other metal nanoparticles or oxides acting as a shell or a core to form bimetallic nanoparticles (Chou and Chen 2007). The superparamagnetic bimetallic Ag/Co polymeric nanocomposite was evaluated to exhibit bactericidal activity during treatment of bacteria-contaminated aqueous solutions (Alonso et al. 2011). The Fe-Ag NPs showed high antimicrobial activity against E. coli (Gram-negative bacteria). Cu-chitosan and Zn-chitosan nanocomposites (NCs) were prepared by reduction of metal precursors in the presence of chitosan in sc CO2 medium and deposition of organosol on chitosan, respectively. Inorganic bimetallic blends (BBs) in light of understood fungicide nanoscale Cu(OH)2 were acquired with the basic properties of salt hydrolysis. The BBs and Cu-chitosan demonstrated the most astounding antifungal adequacy against both R. solani anastomosis gatherings. The in vivo assessment of Cu-chitosan NC and Trichoderma hybrid with BBs indicated plant growth promotions and synergistic inhibitory impact against R. solani.
This exploration could prompt the likelihood of applying Cu-chitosan NCs, BBs and Trichoderma as nanobiofungicides at the field level. The most astounding group of BBs and NCs influenced DNA molarity and resulted in significant degradation. Copper particles discharged may likewise cooperate with DNA atoms and intercalate into nucleic corrosive strands. Cu nanoparticles degrade DNA in a single oxygen-mediated fashion even in the absence of any external agents like hydrogen peroxide or ascorbate. Low-molecular weight chitosan can enter cell dividers and interface with cell DNA of growths and microorganisms which therefore hinders mRNA interpretation and protein production (Abd-Elsalam et al. 2017). The antimicrobial mechanism of bimetallic might the ROS generation and cell wall damaged. Therefore, the combination of this metal oxide can be enhanced antimicrobial action (Liu et al. 2012; Vidic et al. 2013).
11.4.12 Chitosan NPs
Chitosan is a nontoxic, biodegradable biopolymer showing antimicrobial and plant immunity-eliciting properties. Nanochitosan has been shown to be useful in many different areas, specifically in agriculture, plant pathology, food, and biomedicine (Cota-Arriola et al. 2013; El Hadrami et al. 2010; Saharan et al. 2015; Abd-Elsalam et al. 2017). Chitosan stimulates various plant responses, including induction of disease and abiotic stress resistance, enhancement of plant growth and yield and shelf life of flowers and fruits, and activation of secondary metabolite production (Pichyangkura and Chadchawan 2015). Chitosan antimicrobial activities, mechanism, and induction of plant defense responses were reviewed and discussed (Xing et al. 2015). Chitosan demonstrated antimicrobial activities against different plant pathogens including parasites, microorganisms, and fungi and goes about as an elicitor of plant barrier systems. With a wide range of antimicrobial effects, chitosan has been used to reduce or keep the spread of pathogens (Mansilla et al. 2013) or to upgrade plant intrinsic resistance (Fondevilla and Rubiales 2012).
The correct mode of action for antimicrobial activity of chitin, chitosan, and their derivatives is as yet unclear, notwithstanding extraordinary systems that have been proposed. The positively charged amino gatherings of the glucosamine units connect with negatively charged particles on pathogen surfaces, which is named as electrostatic collaborations, and can destroy the cell structure, cause direct cell surface modifications, and increase membrane permeability and in this manner cause the demise of microscopic organisms (Helander et al. 2001; Rabea et al. 2009; Chung et al. 2004; Liu et al. 2004; Zakrzewska et al. 2005; Je and Kim 2006; Chung and Chen 2008).
The growth inhibition of F. oxysporum as a response to chitosan was accompanied by marked cellular changes, which included hyphal swelling, increased vacuolation, retraction and alteration of the plasma membrane, cytoplasm aggregation, and irregular cell wall deposition (Benhamou 1992). In electron micrographs, the outer membrane of chitosan-treated E. coli was disrupted and covered by an additional toothlike layer. In micrographs of chitosan-treated S. aureus, the membrane of dividing cells was disrupted in the constricting region with the loss of bacterial cell substances (Liu et al. 2004). Furthermore the efflux of potassium particles was recognized as an early reaction of the cell to the nearness of some cationic mixes. A quick efflux of potassium dependent on the chitosan fixation was investigated. Furthermore, there was an important inhibitory effect of chitosan on H+-ATPase activity in the plasma membrane of Rhizopus stolonifer. The decrease in the H+-ATPase’s activity could provoke the accumulation of protons inside the cell, which would result in the suppression of the chemiosmotic-driven transport that allows the H+/K+ exchange (García-Rincóna et al. 2010).
A parallel confirmation of method of activity of chitosan has been shown in view of the interactions with DNA or RNA. Chitosan with low molecular weight can penetrate cell wall and interact with cellular DNA of fungi and bacteria which consequently prevents RNA and protein synthesis (Sudarshan et al. 1992; Goy et al. 2009), destroys intracellular components from colloidal state to flocculation and degeneration, disrupts the normal physiological metabolic activity of bacteria, or directly interferes with genetic materials (Come et al. 2003; Issam et al. 2005), and then stops the reproduction of bacteria, resulting in the death of microorganisms eventually. It is presumable that chitosan could bind with DNA and inhibit synthesis of messenger RNA (mRNA) through penetration toward the nuclei of the microorganisms and interfere with the synthesis of mRNA and proteins (Sudarshan et al. 1992; Rabea et al. 2009).
A report used fluorescence visualization to determine that oligochitosan can penetrate the cell membrane of Phytophthora capsici and A. niger and that, as it is positively charged, chitosan can bind to intracellular targets, such as DNA and RNA, which are negatively charged (Li et al. 2008). Infiltrated chitosan oligomers (molecular weight = 8000 and 5000) were suggested to block the transcription from DNA to inhibit the growth of bacteria (Liu et al. 2001) and then disrupt the related protein synthesis. The phosphate group might be an extracellular target contributing to its interaction with the positively charged chitosan, ultimately resulting in damage of vital bacterial activity. There are also phosphate groups in the primary structure of nucleic acid (DNA/RNA). It is possible that the amino groups of chitosan that possess positive charges would attract the negatively charged phosphate groups of DNA/RNA. The brightness of bands weakened gradually as the concentration of chitosan nanoparticles increased, showing the aggravation of chitosan-DNA/RNA interactions. The possible reason might be that negative charges of DNA/RNA had been counteracted by chitosan so that they could not move in the electric field accordingly. The gel retardation experiment pointed out that DNA and RNA might be the intracellular targets of chitosan (Xing et al. 2009).
11.4.13 Elicitation of Plant Defense Responses by Chitosan
Many reports presented that chitosan is not only an antimicrobial agent but also an active elicitor of plant systemic induced resistance to pathogens (El Hadrami et al. 2010; Xing et al. 2015). Most investigations with respect to the utilization of chitosan on rural items center on diseases caused by parasites at preharvest and postharvest stages. The use of adjusted chitosan derivatives and the blend of chitosan with different substances have as of late been looked into somewhere else (Das et al. 2015). Management of plant diseases especially postharvest diseases by chitosan or essential oil treatments seems to happen through two various modes of action: a direct germicide effect on plant pathogens and an indirect effect by inducing defense mechanisms in plant tissue (Zhang et al. 2011; Shao et al. 2013). Plant resistance toward pathogens occurs through hypersensitive responses that result in cell death at the penetration site, structural alterations, accumulation of reactive oxygen species (ROS), synthesis of secondary metabolites and defense molecules, and activation of pathogenesis-related (PR) proteins (Van-Loon and Van-Strien 1999). Chitosan can increase pathogenesis-related (PR) quality capacity through different modes, which incorporates enactment of cell surface or layer receptors, and inner impacts on the plant DNA compliance, which can, thus, influence gene translation (Hadwiger 1999). Chitosan application has been mentioned to increase phenylalanine ammonia lyase (PAL) activity in treated fruit tissue. PAL elicitation via chitosan was established with table grapes in the vineyard sprayed with or without C. laurentii and covered with chitosan at postharvest, and then stored at 0 °C (Meng and Tian 2009; Meng et al. 2010). Chitinase and β-1,3-glucanase are two PR proteins that participate in defense against pathogens, because they can partially degrade the fungal cell wall (Van-Loon and Van-Strien 1999). Increases within the activities of chitinase and β-1,3-glucanase are evidenced as a result of chitosan application in Valencia oranges (Canale Rappussi et al. 2009). Chitosan remedy may start compliant resistance of fruit through regulation of ROS levels, inhibitor enzymes, and also the ascorbate-glutathione cycle. Changes in the content of ROS, such as H2O2 and O2−, are the earliest events that correlate plant resistance to pathogens (Wang et al. 2014), since ROS are involved in the development of disease resistance in fruit (Torres et al. 2003). This may be because of direct effects, as chitosan itself has inhibitor activity and scavenges hydroxyl group radicals (Yen et al. 2008), or to indirect effects, like chitosan causation of the plant inhibitor system. Chitosan treatment has been reported to influence inhibitor catalyst activities within the tissues of fruits and vegetables. Compared to untreated strawberries, those treated with chitosan maintained higher levels of many defense-related enzymes, such as catalase, glutathione peroxidase, guaiacol peroxidase, polyphenol oxidase, superoxide dismutase, dehydroascorbate reductase, and monodehydroascorbate reductase (Wang and Gao 2012). Chitosan significantly improved the production of polyphenol oxidase activity in rice seedlings following infestation with two rice phytobacteria (Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola) (Li et al. 2013a). Secondary metabolites are not directly involved in growth or reproduction, but they are often involved with plant defense. Elicitation is a method widely used for improving secondary metabolite yields (Xing et al. 2015). The antimicrobial efficacy of chitosan is impacted by a variety of things that represent the kind of chitosan, the degree of chitosan polymerization, relative molecular weight, solvent, pH, its charges, and solubility (Tavaria et al. 2013). The five fundamental modes of action of chitosan are electrostatic interactions, plasma membrane harm mechanism, chitosan-DNA/RNA interactions , metal chelation potential of chitosan, and deposition onto the microbial cell membrane. The mechanisms of how chitosan acted on plant immune system have now not been elucidated virtually. It is assumed that the mode of motion of chitosan is probably greatly complicated than assumed above; similarly researches need to clarify the precise mechanism.
11.4.14 Chitosan Nanocomposites
However, much work has to be done regarding the mode of action of chitosan-based nanomaterials against plant pathogens (El Hadrami et al. 2010; Abd-Elsalam et al. 2017). It seems that seed treatment with chitosan NCs ends in extra induction of plant defense mechanisms as previously demonstrated by using Saharan et al. (2013, 2015). Chitosan nanoparticles express more affinity towards pathogen’s outer membrane and thus easily enter into the pathogens’ cell (Van et al. 2013). Interactions between undoubtedly charged nanochitosan molecules and the polyanionic structures of microbial cellular membranes result in destabilization of cell membrane. This induces the leakage of intracellular contents and subsequently causes death of pathogens. Disruption of protein synthesis and membrane destabilizations is in all likelihood primary and secondary modes of antimicrobial activity of chitosan (Marquez et al. 2013). Furthermore, nanochitosan mechanism entails the penetration of low-molecular weight chitosan into the mobile, binding to DNA and subsequently inhibiting RNA and protein synthesis. Chitosan has also been shown to prompt several protection methods in plant tissues and inhibit the production of pollutants and microbial growth. Nanochitosan upregulates the plant defense mechanisms which involve enzymes such as phenylalanine ammonium lyase, polyphenol oxidase, tyrosine ammonium lyase, and antioxidative enzymes SOD, CAT, and POD (superoxide dismutase, catalase, and peroxidase) (Ma et al. 2014; Katiyar et al. 2015). It also induces the hypersensitivity-related reactions in different plant species to keep away or put off the invading pathogen from the cell (Chandra et al. 2015). Chitosan has been used to control seed-borne fungi of flowers as an elicitor as opposed to a fast-acting toxic agent because it has been stated that chitosan can activate plant defenses in infected flora (Kaur et al. 2012). Cu-chitosan nanoparticles enhanced enzyme activities involved in plant defense by chitosan participants in the reactive oxygen species (ROS) scavenging system (Saharan et al. 2013; Saharan et al. 2015). The plant microenvironment becomes acidic as a result of mycotic contamination which leads to the breakup of nanostructure and discharge of Cu particles (Brunel et al. 2013). The released Cu ions produce reactive hydroxyl radicals to prevent fungal pathogens (Borkow and Gabbay 2005). Cu-chitosan nanonetwork was evident through a higher Cu accumulation in porous areas which supported the ionic and chelating interaction mechanism to inhibit enzymes and toxins used by fungal pathogens throughout pathogenesis (Vahabi et al. 2011). Cu nanoparticles and Cu-chitosan and Zn-chitosan NCs have nearly the same mode of action as Cu-chitosan nanocomposites, for instance, the production of ROS, and membrane disruption (Xie et al. 2011; Ingle et al. 2013). Similarly, zinc is an important micronutrient for plant growth and is absorbed by plants through diffusion and specific transporters in the form of divalent ions. Another important mechanism includes penetration of the chitosan oligomer into the cells of microorganisms which inhibits the growth of microbial cells by stopping the transcription of DNA into mRNA (Hernández-Lauzardo et al. 2011). Cu-chitosan nanocomposites could penetrate cell walls of fungi and bind to DNA or mRNA. Disruption of fungal metabolism and duplication should in the long run lead to pathogen demise. The common nanometals used as antimicrobial agents collectively with their mechanisms of action are summarized in Table 11.1.
11.4.15 Nanoemulsions Mechanism
The nanoemulsion (NE) droplets with antimicrobial agents fuse with lipid containing organism thereby destroying them by numerous modes of action. The fusion between the nanodroplets is driven by the electrostatic attraction between the droplet charge and the charge on pathogens. When certain amounts of droplets fuse with the pathogens, the active ingredient from the nanodroplets is released to the lipid membrane causing lysis and death of pathogens. They observed that the antimicrobial activity depended on the target microorganism and nanoemulsions with smaller diameters showing better antimicrobial activity due to the fast delivery via the cellular membrane of the target pathogens (Donsì et al. 2011). This fusion among the emulsion and the anionic rate of the pathogen could bring about the antimicrobials’ lysis and death. Due to this unique, nonspecific mode of action, there are no possibilities for development of resistant microbial strains (Karthikeyan et al. 2011; Moghimi et al. 2016). Strong electrostatic attraction could improve the fusion, and then nanoemulsions with positive charge exhibited higher antimicrobial activity (Hamouda and Baker 2000). Strong electrostatic attraction ought to improve the fusion, after which nanoemulsions with positive charge exhibited better antimicrobial activity (Hamouda and Baker 2000). Synergistic effect between one-of-a-kind antimicrobial agents is constantly taken into consideration to improve the antimicrobial activity of nanoemulsions. The basic theory behind these studies was that NE particles were thermodynamically driven. The anionic charge on pathogen and the electrostatic appeal between the cationic charges of the emulsion complements their mixing capability. The fusion of an adequate number of nanoparticles with pathogens assists in the release of some of the energy trapped within the emulsion (http://nano.med.umich.edu/platforms/Antimicrobial-Nanoemulsion. html). It is this trapped electricity and the actively worried components that weaken the pathogen lipid membrane leading to destruction of cells and their final dying. However, NEs, to be more effective in the case of spores, extra germination enhancers are required to be integrated with emulsion. The moment germination begins, the germination spores end up vulnerable to antimicrobial activity of the NE (Fig. 11.4). One peculiar aspect of NE is that concentrations exert selective toxicity on microbes.
Action mechanism of NE against spores. (Reprinted from Kaur 2016)
11.4.16 Photocatalyst Mechanism
Other than the previously mentioned mechanisms, there are other unique antimicrobial mechanisms of nanomaterials that are available in the literature. One such unique mechanism is photocatalysis-mediated antimicrobial activity. Photocatalysis has been shown to be capable of killing an wide range of microorganisms together with bacteria, fungi, algae, and viruses (Paspaltsis et al. 2006; Foster et al. 2011). One such nanomaterial showing antimicrobial properties during photoactivation was nano-TiO2, a semiconductor (Mueller and Nowack 2008). Moreover, ZnO is also a semiconductor that, upon absorption of photons, transported its electrons between the valence and conduction band. Not all the nano-based material had photo-mediated antimicrobial capacity. Just semiconducting nano metal oxides like TiO2 and ZnO nanomaterials are found to have this sort of photocatalytic antimicrobial impact. As the component manages the arrangement of ROS and hinderd microorganisms through photocatalytic impact, broad investigations must be completed to decrease the cytotoxicity among higher living beings because of the ROS delivered by these nano products.
11.5 How to Investigate the Mode of Actions?
While composing and examining nanomaterials for their antimicrobial abilities, data is not just required to focus on nanoparticles but also on measure disseminations and shape and level of collection of the particles, making representation techniques vital to material characterization. Moreover, with a specific end goal to evaluate the antimicrobial efficacy of nanosized materials, representation of the association among microorganisms and the material is required, and the result of such connections on the feasibility of the microbial cell must be known. The nanocidal abilities of a mass material, which itself has no investigated antimicrobial effects, depend on supported oxidation systems and consequently on little amounts of the silver particle that are discharged into the fluid condition. The systems hostile to microbial efficacy should likewise be anticipated. The nanomaterial may straightforwardly harm the target cell membrane (of both prokaryotic and eukaryotic cells), or the impact might be because of compound activity or enhanced membrane permeability, bringing about spillage of cell substance or interruption of DNA replication. These effects can often be directly seen using the latest high-resolution microscopy methods. The stability and enduring efficacy of antimicrobial activity also depends largely on the properties of the nanomaterials once combined with the bulk matrix, particularly final particle size, shape, and availability (Marambio-Jones and Hoek 2010). Consequently, direct visualization of the particles incorporated in situ within the bulk matrix is also required, although this is also perhaps one of the most difficult to achieve without the introduction of significant sampling artifacts. A suite of high-resolution microscopy techniques are now readily available, and most bulk sample types, including fully hydrated samples, can now be visualized by a range of methods. However, given the wide-ranging nature of materials in which nanoparticles are now being incorporated, including polymers, powders, aerosols, and zeolites, no single technique will be able to provide all the required information. Rather, as pointed out by Samberg et al. (2011) in their investigation of the antibacterial impacts of silver nanoparticles, an examination of techniques is required, keeping in mind the end goal is to accomplish characterization of any antimicrobial activities.
A review of the literature indicates that the majority of studies published to date on the antimicrobial nature of nanomaterials rely on several methods to visualize such materials. Electron microscopy techniques such as transmission electron microscopy (TEM) are frequently coupled with a scanning probe microscopy (SPM) technique such as atomic force microscopy (AFM). Without a doubt the current has intense far-reaching accessibility and adaptable methods, for example, AFM has altered characterization of nanosized materials over the most recent two decades, and it can be influentially contended that the accessibility of AFM and related strategies are eventually in charge of the quick development of nanotechnology investigation, by and large and particularly interface and colloidal science (Butt et al. 2007). Several imaging and molecular techniques will perform to evaluate the molecular mechanisms that underlie the microorganisms response to the nanomaterials (NMs).
11.5.1 Electron Microscopy Techniques
The field of electron microscopy covers a wide assortment of systems that can be used for imaging both nanomaterials and the mass material in which the nanomaterials are consolidated. An assortment of both auxiliary and concoction data can be inferred, despite the fact that EM overwhelmingly gives subjective basic data about the examples inspected. Three fundamental electron microscopy methods are utilized for material characterization, transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM), and scanning electron microscopy (SEM). Together these three imaging techniques and their derivatives are capable of offering a numerous range of records on a selected pattern, ranging from sub-nanometer resolution as in the case of TEM to structural information on bulk materials many centimeters in measurement which can be achieved through scanning electron microscopes with specially designed chambers. The disadvantages of EM techniques include the fact that EM can only deliver statistics in dimensions and lacks distinct statistics on 3-D morphology of samples without specialized software. However, the principle negatively looks at the end result from the destructive nature of the sample-guided techniques required for EM and the tough imaging situations essential for a postive microscopy.
11.5.2 Transmission Electron Microscopy Measurements
The interaction between the microorganisms and the different nanomaterials can be illustrated using bright-field TEM imaging of the bacteria treated with various NMs. Irrespective of the type of bacteria used, it was noticed that most of the nanoparticles were found attached to the surface of the bacterial cell wall, implying their higher affinity toward the cells. Although TEM imaging provides a direct measure of nanoparticle interactions with the microorganisms, the potential for imaging artifacts cannot be eliminated.
11.5.3 Atomic Force Microscopy (AFM) Measurements
AFM can be used to photograph fully hydrated samples and might correctly photo each nanoparticles and microorganisms in situ on most surfaces, including both difficult and gentle surfaces. Unlike electron microscopy strategies or STM strategies, the pattern may be either a conductor or insulator and no staining is needed with a purpose to obtain evaluation. AFM may also offer future insights into nanoantimicrobial substances and the interest of such materials. One of the limitations of AFM is that chemical specificity is lacking and capabilities are recognized based on size and form. Another limitation is that while molecular scale surface topographic details are well resolved, AFM has a limited ability to image features that do not provide sufficient topographical contrast such as peptides in a membrane. In tandem with developments in electron microscopy and scanning probe microscopy methods , optical microscopy techniques continue to evolve rapidly, and techniques such as confocal microscopy provide unprecedented insights into microbial interactions with nanomaterials.
11.5.4 Confocal Microscopy
The primary concept of confocal laser scanning microscopy (CLSM) and multiphoton strategies are integral techniques for visible characterization of nanomaterial-based antimicrobial materials. Particularly, CLSM facilitated the exploration of microbial habitats and allowed the commentary of host-associated microorganisms in situ with an unprecedented accuracy (Cardinale 2014). External modifications in mobile membrane integrity can be monitored to unravel the mechanisms of antimicrobial interest and the modifications attributable to contact with nanomaterials.
11.6 Reactive Oxygen Species Generation
ROS are chemically reactive molecules together with peroxides that incorporate oxygen. ROS are equally reactive because of the presence of unpaired valence shell electrons. ROS form as a herbal derivative of the everyday metabolism of oxygen and have vital roles in mobile signaling, homeostasis, and furthermore apoptosis. However, during times of environmental stress, in the present case in the form of nanoparticles, ROS levels are known to increase drastically which might result in significant damage to cell structures. This cumulates into the event known as oxidative stress.
ROS production can be monitored using various analytical methods such as XTT assay that yields a colorimetric signal when reduced by superoxides. ROS are best known to implicate toxicity to several prokaryotic and eukaryotic systems upon interaction with metal/metal oxide nanoparticles. ROS in the form of either superoxide radical (O2−), hydrogen peroxide (H2O2), or hydroxyl radical (OH) causes oxidative stress, thereby causing damage to DNA, cell membranes, and cellular proteins, and finally leading to cell death. The presence of ROS was observed using an XTT assay , which yields a colorimetric signal when decreased by superoxides. ROS quantification flow cytometric assay was used to evaluate the production of free intracellular radicals as reported (Raimondi et al. 2008; Prasad et al. 2017b).
11.7 Omics Methodologies
The suffix “omics” stands for “as a whole” and omics technology consists of genomics, transcriptomics, proteomics, and metabolomics credited to modern breakthroughs in genome sequencing, bioinformatics, and analytic equipment including liquid and gas chromatography and mass spectrometry, in conjunction with high-throughput technology (Fig. 11.5). Omics technologies have provided crucial insights into processes related to microbial physiology, virulence, and stress and mechanisms of action (MOA) of nanoantimicrobial materials (Tang 2015; Fröhlich 2017). These methods differ from the microscopic observation of phenotypes in the way that they can provide primarily mechanistic information and may identify the pathway of toxicity for microbial pathogens. One advantage might be the identification of new targets and markers for NP toxicity. Another benefit of the omics strategies might be their lower interference with NPs (Fröhlich 2017). DNA- and RNA-based research, genomics record analyses, transcriptomics, metagenomics, metabolomics, next-generation sequencing (NGS) technologies, and proteomics processes have proved to be precious techniques to examine plant-pathogen interactions and their associations. Various “omics” methods are a promising approach to recognize the advantages and the pathogenic effect of microbes in crop development. The plant native immunity has always been an important aspect of research and leads to some interesting information like the adaptation of unique immune mechanisms of plants against pathogens (Imam et al. 2016). Proteomics and metabolomics, pooled with systems biology, are outstanding tools to screen the results and toxicity mechanisms elicited by NPs. However, the metabolomics approach remains terribly difficult and still comparatively new in the field of nanotoxicology.
Omics techniques used for the study of nanotoxicology include genomics, epigenomics (miRNomics and DNA modifications), transcriptomics, proteomics, and metabolomics. Genomics investigates genes and their functions through the use of recombinant DNA, DNA sequencing, and bioinformatics to analyze the function and structure of the genome. Proteomic and transcriptomic tools: target identification can be performed by evaluating the differential expression of genes in microbial strains treated or untreated with NMs; while metabolomic assay: the metabolic profile of a microbe treated with NMs can be compared with the profile of untreated strains
11.7.1 Genomics Assays
The mode of action of a nanomaterial compound on DNA integrity can be evaluated through DNA-binding evaluation, in which a pattern of purified plasmid DNA is blended with different concentrations of the examined compound. Pathways that might describe contrary effects on DNA are regulation of DNA destruction and repair of nucleic acid metabolism. Epigenetic fluctuations are involved in the transformation and mutation of cells and, thus, may assist as indicator for genotoxicity. With the advent of next genertions sequence technologies which results in the completion of genome sequencing and re-sequencing of the over-whelming numbers of plant and their pathogens generating huge amount of data, we are witnessing an era of genomics and post-genomics with a challenge to translate these plethora of information for the crop improvements with broader disease resistance spectra (Knief 2014). In the post-genomic era, the translational genomics presented a better solution in crop improvement against pathogenic bacteria, fungi, and viruses and prepare these crops in current thwarting climatic conditions (Knief 2014). DNA sequencing and bioinformatics investigate characteristic and shape of the genome. The purpose is to identify a selected sensitivity of people to a given toxin as opposed to the screening for toxicity of compounds or NPs. The epigenome may be altered via toxicants and, consequently, is useful for toxicity screening.
11.7.2 Transcriptomics Analysis
To evaluate the genetic-based response mechanisms , the global transcriptomic response of microorganisms upon exposure to nanomaterials can be assessed using whole-genome microarray analysis and compared to treatments with NMs or Milli-Q water control. Presently, DNA microarrays are the technology of choice for large-scale studies of gene expression. Microarray technology was developed using the information available from the genome projects and is based on the hybridization of cDNA (complementary DNA produced from mRNA) to oligonucleotide probes incorporated into a slide. Each probe has a sequence of a specific gene from the microorganism (Nambiar et al. 2010). Transcriptomics basically cognize DNA sequencing using NGS approaches and also quantitatively measure the expression of mRNA, and their variations occurring under diverse stress situations. The use of mRNA sequencing evaluation and microarray approach to generate transcriptome level facts is one of the important methodologies employed for studying plant-microbe interactions (Budak and Akpinar 2015). Furthermore, the development in bioinformatics methods , both as hardware and software program for the evaluation of statistics, allows us to enhance our information on ever-increasing management and mining of such big datasets. RNA-seq, a recently evolved approach for transcriptome profiling by way of deep-sequencing technologies, offers a precise dimension of the extent of gene transcripts and their isoforms than different strategies.
11.7.3 Proteomics Analysis
Proteomics is the systematic assessment of all proteins expressed through one particular mobile, tissue, organic fluid, or organism in a given time period. It is used to identify and quantify proteins targeted in selected biological parameters and also can be implemented to determine post-translational modifications, in addition to cellular origin and location of movement (Yates et al. 2009). Different methods that are being applied to promote our understanding of proteins are gel-based techniques like two-dimensional gel electrophoresis (2DE) and fluorescence two-dimensional difference gel electrophoresis (2DDIGE) and gel-free techniques like isotope-coded affinity tags (ICAT), isobaric tags for relative and absolute quantitation (iTRAQ), multidimensional protein identification technology (MudPIT), and the commonly used primary tool mass spectrophotometry (MS) and MALDI-TOF. High sensitivity is the advantage of MS.
11.7.4 Metabolomics Assays
The final “omics” technology to be provided in this review is metabolomics, which is defined as the examination of the global profile of metabolites found in a biological system under sure conditions and time (Nambiar et al. 2010). The approaches followed to generate metabolic signatures are usually nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), the combination of chromatography with MS that helps to detect more number of complex compounds. Widely used methods are combinations of gas chromatography (GC)-MS and liquid chromatography (LC)-MS (Zhang et al. 2012). Metabolomics techniques had been significantly accepted to discover the responses of microorganisms to numerous environmental stressors inclusive of heavy metals, temperature, and natural compounds (Lankadurai et al. 2013). Metabolomics may represent a promising approach to explain the mechanism of diverse nanomaterials. Datasets from diverse studies like genomics, transcriptomics, proteomics and metabolomics need to be combined using bioinformatics and statistical tools that will help to identify and integrate key biological processes.
11.8 Conclusion and Future Perspectives
Understanding the inactivation mechanisms is the key to the increasing use of nanoparticles (NPs) and enhances the practicability of their application aganist numerous plant pathogens under extraordinary environments. The potential mechanism of toxicity has been attributed to numerous possible techniques, and the dissolution or release of ions from the nanoparticles elicits either inflammatory response, mitochondrial dysfunction, disruption of mobile membrane integrity, oxidative strain, protein or DNA binding and harm, or reactive oxygen species (ROS) technology, affecting the proteins and phosphate lipids and in the long run inflicting cellular death. Particular emphasize become given to antimicrobial mechanisms with attention on formation of reactive oxygen species (ROS) consisting of hydrogen peroxide (H2O2), OH− (hydroxyl radicals), and O2 −2 (peroxide). ROS has been a chief factor for several mechanisms consisting of cell wall harm due to NPs-localized interaction and more advantageous membrane permeability. Use of highly sophisticated techniques such as high resolution microscopic (AFM, FE-SEM, TEM, and XRD), spectroscopy (DLS, ESR spectroscopy, fluorescence spectroscopy, inductively coupled plasma optical emission spectroscopy, UV-vis), and molecular and biochemical techniques have provided deep mechanistic insights approximately in the mode of action of antimicrobial activity of AgNPs (Kim et al. 2007; Rai and Ingle 2012). The emerging discipline of nanotoxicogenomics (Waters et al. 2009) which attempts to correlate global gene expression profiles of cells or tissues exposed to NPs with organic/toxicological responses to the usage of cDNA microarray technology may offer beneficial information in this regard. Genotoxicity is a harmful impact which affects DNA integrity through the movement of harm-inducing markers (genotoxins) together with chemicals and radiation. There are many markers which can act as genotoxins, and they may be categorized as bodily (e.g., UV, X-rays), chemical (e.g., benzopyrene, ethidium bromide), and organic (e.g., virus, transposons). These agents can also be described according to their action mechanism such as oxidants (e.g, hydrogen peroxide), alkylating (e.g, methyl methanesulfonate), inductors of DNA breaking (e.g, ionizing radiation), and aneugenic agents which affect chromosome division (e.g, taxanes) (Parry and Parry 2012). As DNA damage may also both provoke and sell carcinogenesis or effect fertility, the genotoxicology technological know-how has become a crucial area governing regulatory health risk evaluation. Mechanistic investigations of these interactions may even enable a correct evaluation of fate and results within the crop or cropping system as a way to deal with issues over threat and meals safety. Great efforts have also been made to recognize the nanoantimicrobial mechanisms; researchers are still looking to recognize the mechanism of antimicrobial activity of nanostructures, and consequently they may be investigating the morphological changes in microbes as a result of nanoantimicrobials. No-clear cut conclusion for nanoantimicrobial mechanism through; damage through membrane disruption, DNA transformation, ROS production or other mechanisms, we still want for in addition research to find the exact mechanism.
References
Abbaszadegan A, Ghahramani Y, Gholami A, Hemmateenejad B, Dorostkar S, Nabavizadeh M, Sharghi H (2015) The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater ID 720654
Abd-Elsalam KA, Vasil’kov AY, Said-Galiev EE, Rubina MS, Khokhlov AR, Naumkin AV, Shtykova EV, Alghuthaymi MA (2017) Bimetallic and chitosan nanocomposites hybrid with trichoderma: novel antifungal agent against cotton soil-borne fungi. Euro J Plant Pathol. https://doi.org/10.1007/s10658-017-1349-8
Abkhoo J, Panjehkeh N (2017) Evaluation of antifungal activity of silver nanoparticles on Fusarium oxysporum. Int J Inf Secur 4(2):e41126. https://doi.org/10.5812/iji.41126
Ahmed IIS, Yadav DR, Lee YS (2016) Applications of nickel nanoparticles for control of fusarium wilt on lettuce and tomato. Int J Innov Res Sci Eng Technol 5:7378–7385
Akhavan O, Ghaderi E, Esfandiar A (2011) Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J Phys Chem B 115:6279–6288
Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10:1066. https://doi.org/10.3390/ma10091066
Allahverdiyev AM, Abamor ES, Bagirova M, Baydar SY, Ates SC, Kaya F, Kaya C (2013) Investigation of antileishmanial activities of Tio2@Ag nanoparticles on biological properties of L. tropica and L. infantum parasites, in vitro. Experimental Parasitol 135:55–63
Alonso A, Vigués N, Muñoz-Berbel X, Macanás J, Muñoz M, Mas J, Muraviev DN (2011) Environmentally-safe bimetallic Ag@Co magnetic nanocomposites with antimicrobial activity. Chem Commun (Camb) 47:10464–10466
Ansari MA, Khan HM, Khan AA, Pal R, Cameotra SS (2013) Antibacterial potential of Al2O3 nanoparticles against multidrug resistance strains of Staphylococcus aureus isolated from skin exudates. J Nanopart Res 15:1970
Arciniegas-Grijalba PA, Patin˜o-Portela MC, Mosquera-Sa´nchez LP, Guerrero-Vargas JA, Rodrı´guez-Pa´ez JE (2017) ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl Nanosci 7:225–241
Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 4:634–664
Aziz N, Fatma T, Varma A, Prasad R (2014) Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. J Nanopart:689419. https://doi.org/10.1155/2014/689419
Aziz N, Faraz M, Pandey R, Sakir M, Fatma T, Varma A, Barman I, Prasad R (2015) Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial and photocatalytic properties. Langmuir 31:11605–11612. https://doi.org/10.1021/acs.langmuir.5b03081
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Baruah S, Dutta J (2009) Nanotechnology applications in pollution sensing and degradation in agriculture: a review. Environ Chem Lett 7:161–204
Benhamou N (1992) Ultrastructural and cytochemical aspects of chitosan on Fusarium oxysporum f. sp. radices-lycopersici, agent of tomato crown and root rot. Phytopathology 82:1185–1193
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Borkow G, Gabbay J (2005) Copper as a biocidal tool. Curr Med Chem 12:2163–2175
Brady-Est´evez AS, Kang S, Elimelech M (2008) A single walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 4:481–484
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet M (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870
Brunel F, Gueddari NE, Moerschbacher BM (2013) Complexation of copper (II) with chitosan nanogels: toward control of microbial growth. Carbohydr Polym 92:1348–1356
Budak H, Akpinar BA (2015) Plant miRNAs: biogenesis, organization and origins. Funct Integr Genomics 15:523–531
Butt HJ, Berger R, Bonaccurso E, Chen Y, Wang J (2007) Impact of atomic force microscopy on interface and colloid science. Adv Colloid Interf Sci 133:91–104
Canale Rappussi MC, Pascholati SF, Aparecida Benato E, Cia P (2009) Chitosan reduces infection by Guignardia citricarpa in postharvest “Valencia” oranges. Braz Arch BioTechnol 52:513–521
Carbon J, David H (1968) Thiobases in Escherichia coli transfer RNA-2- thiocytosine and 5-methylaminomethyl-2-thiouracil. Science 161(3846):1146–1147
Cardinale M (2014) Scanning a microhabitat: plant-microbe interactions revealed by confocal laser microscopy. Front Microbiol 5:94. https://doi.org/10.3389/fmicb.2014.00094
Carré G, Hamon E, Ennahar S, Estner M, Lett MC, Horvatovich P, Gies JP, Kellerb V, Kellerb N, Andrea P (2014) TiO2 Photocatalysis damages lipids and proteins in Escherichia coli. Appl Environ Microbiol 80:2573–2581
Cataldo F, Da Ros T (2008) Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes. Springer Science & Business Media, Dordrecht
Chandra S, Chakarborty N, Dasgupta A, Sarkar J, Panda K, Acharya K (2015) Chitosan nanoparticle: a positive modulator of innate immune responses in plants. Sci Rep 5:1–13
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871
Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Watkins R (2008) Applications and implications of nanotechnologies for the food sector. Food Addit Contam 25:241–258
Chen JP, Peng H, Wang X, Shao F, Yuan Z, Han H (2014) Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 6:1879–1889
Chen Q, Ma Z, Liu G, Wei H, Xie X (2016) Antibacterial activity of cationic cyclen-functionalized fullerene derivatives: membrane stress. Dig J Nanomater Biostruct (DJNB) 11:753–761
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Choi JY, Kim KH, Choy KC, Oh KT, Kim KN (2007) Photocatalytic antibacterial effect of TiO 2 fim formed on Ti and TiAg exposed to Lactobacillus acidophilus. J Biomed Mater Res Part B 80:353–359
Chou KS, Chen CC (2007) Fabrication and characterization of silver core and porous silica shell nanocomposite particles. Microporous and Mesoporous Mater 98:208–213
Chung YC, Chen CY (2008) Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour Technol 99:2806–2814
Chung YC, Su YP, Chen CC, Jia G, Wang HL, Wu JC, Lin JG (2004) Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin 25:932–936
Chwalibog A, Sawosz E, Hotowy A, Szeliga J, Mitura S, Mitura K et al (2010) Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int J Nanomed 5:1085–1094
Cioffi N, Rai M (2012) NanoAntimicrobials: progress and prospects springer. Springer-Verlag, Berlin, Heidelberg
Cioffi N, Torsi L, Ditaranto N, Sabbatini L, Zambonin PG, Tantillo G, Ghibelli LD, Alessio M, Bleve-Zacheo T, Traversa E (2004) Antifungal activity of polymer-based copper nano-composite coatings. Appl Phys Lett 85:2417–2419
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 17:5255–5262
Come V, Deschamps A, Mertial A (2003) Bioactive packaging materials from edible chitosan polymer-antimicrobial activity assessment on dairy-related contaminants. J Food Sci 68:2788–2792
Cota-Arriola O, Cortez-Rocha MO, Burgos-Hernández A, Ezquerra-Brauer JM, Plascencia-Jatomea M (2013) Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: development of new strategies for microbial control in agriculture. J Sci Food Agric 93:1525–1536
Cui Y, Zhao Y, Tian Y, Liu W, Ma W, Jiang X (2012) The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33:2327–2333
Cui J, Yang Y, Zheng M, Liu Y, Xiao Y, Lei B, Chen W (2014) Facile fabrication of graphene oxide loaded with silver nanoparticles as antifungal materials. Mater Res Express 1:045007
Das R, Gang S, Nath SS, Bhattacharjee R (2010) Linoleic acid capped copper nanoparticles for antibacterial activity. Bionanosci J 4:82–86
Das SN, Madhuprakash J, Sarma PVSRN, Purushotham P, Suma K, Manjeet K, Podile AR (2015) Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants. Crit Rev Biotechnol 35:29–43
De Faria AF, De Moraes ACM, Alves OL (2014) Toxicity of nanomaterials to microorganisms: mechanisms, methods, and new perspectives. In: Duran N, Guterres SS, Alves OL (eds) Nano medicine and Nanotoxicology. Springer, New York, pp 363–405
De Filpo G, Palermo AM, Rachiele F, Nicoletta FP (2013) Preventing fungal growth in wood by titanium dioxide nanoparticles. Int Biodeterior Biodegradation 85:217–222
De La Torre-Roche R, Hawthorne J, Deng YQ, Xing BS, Cai WJ, Newman LA, Wang Q, Ma XM, Hamdi H, White JC (2013) Multiwalled carbon nanotubes and C-60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ Sci Technol 47:12539–12547
Dizaj SM, Mennati A, Jafari S, Khezri K, Adibkia K (2015) Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull 5:19–23
Donsì F, Annunziata M, Sessa M, Ferrari G (2011) Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci Technol 44:1908–1914
Durán N, Durán M, Bispo M, Jesus d, Seabra AB, Fávaro WJ, Nakazato G (2016) Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine 12:789–799
El Hadrami A, Adam LR, El Hadrami I, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8:968–987
Fakruddin MD, Hossain Z, Afroz H (2012) Prospects and applications of nanobiotechnology: a medical perspective. J Nanobiotechnol 10:31
Fondevilla S, Rubiales D (2012) Powdery mildew control in pea. A review. Agron Sustain Dev 32:401–409
Foster HA, Ditta IB, Varghese S, Steele A (2011) Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol 90:1847–1868
Fröhlich E (2017) Role of omics techniques in the toxicity testing of nanoparticles. J Nanobiotechnol 15:84. https://doi.org/10.1186/s12951-017-0320-3
Fu PP, Xia Q, Hwang HM, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22:64–75
García-Rincóna J, Vega-Pérezb J, Guerra-Sánchezb MG, Hernández-Lauzardo AN, Peña-Díazc A, Velázquez-Del Vallea MG (2010) Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pestic Biochem Phys 97:275–278
Goswami A, Roy I, Sengupta S, Debnath N (2010) Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films 519:1252–1257
Goy RC, Britto D, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polímeros 19:241–247
Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1548
Gupta A, Silver S (1998) Silver as a biocide: will resistance become a problem? Nat Biotechnol 16:888–890
Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim JH (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine 7:5901–5914
Hadwiger LA (1999) Host-parasite interactions: elicitation of defense responses in plant with chitosan. Experientia Suppl 87:185–200
Haghighi F, Roudbar Mohammadi S, Mohammadi P, Eskandari M, Hosseinkhani S (2012) The evaluation of Candida albicans bio¿lms formation on silicone catheter, PVC and glass coated with titanium dioxide nanoparticles by XTT method and ATPase assay. Bratisl Lek Listy 113:711–715
Hamouda T, Baker J (2000) Antimicrobial mechanism of action of surfactant lipid preparations in enteric gram-negative bacilli. J Appl Microbiol 89:397–403
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166:207–215
He Y, Ingudam S, Reed S, Gehring A, Strobaugh TPN, Irwin P (2016) Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J Nanobiotechnol 14:54. https://doi.org/10.1186/s12951-016-0202-0
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol 71:235–244
Hernández-Lauzardo A, Velázquez M, Guerra-Sánchez M (2011) Current status of action mode and effect of chitosan against phytopathogens fungi. Afr J Microbiol Res 5:4243–4247
Hoseinzadeh A, Habibi-Yangjeh A, Davari M (2016) Antifungal activity of magnetically separable Fe3O4/ZnO/AgBr nanocomposites prepared by a facile microwave-assisted method. Prog Nat Sci: Mater Int J 26:334–340
Hossain F, Perales-Perez OJ, Hwang S, Román F (2014) Antimicrobial nanomaterials as water disinfectant: applications, limitations and future perspectives. Sci Total Environ 466–467:1047–1059
Huang S, Wang L, Liu L, Hou Y, Li L (2015) Nanotechnology in agriculture, livestock, and aquaculture in China. A review. Agron Sustain Dev 35:369–400
Huh AJ, Kwon YJ (2011) “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 156:128–145
Imada K, Sakai S, Kajihara H, Tanaka S, Ito S (2016) Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol 65:551–560
Imam J, Singh PK, Shukla P (2016) Plant microbe interactions in post genomic era: perspectives and applications. Front Microbiol 7:1488. https://doi.org/10.3389/fmicb.2016.01488
Ingle AP, Duran N, Rai M (2013) Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 98:1001–1009
Ismail M, Prasad R, Ibrahim AIM, Ahmed ISA (2017) Modern prospects of nanotechnology in plant pathology. In: Prasad R, Kumar M, Kumar V (eds) Nanotechnology. Springer Nature Singapore Pte Ltd, Singapore, pp 305–317
Issam ST, Adele MG, Adele CP, Stephane G, Veronique C (2005) Chitosan polymer as bioactive coating and film against Aspergillus niger contamination. J Food Sci 70:100–104
Jastrzębska AM, Kurtycz P, Olszyna AR (2012) Recent advances in graphene family materials toxicity investigations. J Nanopart Res 14(12):1–21
Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao KV (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc 90:78–84
Jayaseelan C, Ramkumar R, Abdul Rahuman A, Perumal P (2013) Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crop Prod 45:423–429
Je JY, Kim SK (2006) Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J Agric Food Chem 54:6629–6633
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ Pollut 157:1619–1625
Jo Y, Kim BH, Jun G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043
Jo DH, Kim JH, Lee TG, Kim JH (2015) Review article: size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 11:1603–1611
Joshi N, Jain N, Pathak A, Singh J, Prasad R, Upadhyaya CP (2018) Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J Sol-Gel Sci Techn. https://doi.org/10.1007/s10971-018-4666-2
Jyoti S, Satendra S, Sushma S, Anjana T, Shashi S (2007) Antistressor activity of Ocimum sanctum (Tulsi) against experimentally induced oxidative stress in rabbits. Methods Find Exp Clin Pharmacol 29:411–416
Kairyte K, Kadys A, Luksiene Z (2013) Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. Photochem J Photobiol B 128:78–84
Kang S, Pinault M, Pfefferle LD, Elimelech M (2007) Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23:8670–8673
Karthikeyan R, Amaechi BT, Rawls HR, Lee VA (2011) Antimicrobial activity of nanoemulsion on cariogenic Streptococcus mutans. Arch Oral Biol 56:437–445
Katiyar D, Hemantarajan A, Sing B (2015) Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Ind J Plant Physiol 20:1–9
Kaur K (2016) Nanoemulsions as delivery vehicles for food and pharmaceuticals. In: Grumezescu AM (ed) Emulsions. Nanotechnology in the Agri-Food Industry, vol. 3. https://doi.org/10.1016/B978-0-12-804306-6.00018-0
Kaur P, Thakur R, Choudhary A (2012) An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int J Sci Technol Res 1:83–86
Kim J, Cho H, Ryu S, Choi M (2000) Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion. Arch Biochem Biophys 382:72–80
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee J, Kim SH, Park YK, Park YH, Hwang CY et al (2007) Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med 3(95):101–157
Kim SW, Kim KS, Lamsal K, Kim YJ, Kim SB, Jung M, Sim SJ, Kim HS, Chang SJ, Kim JK, Lee YS (2009) An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J Microbiol Biotechnol 19:760–764
Kim SH, Lee HS, Ryu DS, Choi SJ, Lee DS (2011) Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Kor Microbiol Biotechnol 39:77–85
Kleandrova VV, Luan F, Speck-Planche A, Cordeiro M (2015) Review of structures containing fullerene-C60 for delivery of antibacterial agents. Multitasking model for computational assessment of safety profiles. Curr Bioinforma 10:565–578
Knief C (2014) Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front Plant Sci 5:216. https://doi.org/10.3389/fpls.2014.00216
Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H, Takahashi Y, Uruga T (2007) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N (2016) Biosynthesized metallic nanoparticles using for pharmacological applications. Saudi Pharmaceutical J 24:473–484
Kuzma J (2007) Moving forward responsibly oversight for the nanotechnology-biology interface. J Nanopart Res 9:165–182
Lankadurai BP, Nagato EG, Simpson MJ (2013) Environmental metabolomics: an emerging approach to study organism responses to environmental stressors. Environ Rev 21:180–205
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, moleculartargets and applications. Nat Rev Microbiol 11:371–384
Leuba KD, Durmus NG, Taylor EN, Webster TJ (2013) Short communication: carboxylate functionalized superparamagnetic iron oxide nanoparticles (SPION) for the reduction of S. aureus growth post biofilm formation. Int J Nanomedicine 8:731–736
Leung YH, Ng AMC, Xu X, Shen Z, Gethings LA, Wong MT, Chan CM, Guo MY, Ng YH, Djurišić AB, Lee PK (2014) Mechanisms of antibacterial activity of MgO: non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 10:1171–1183
Li XF, Feng XQ, Yang S, Wang TP (2008) Effects of molecular weight and concentration of chitosan on antifungal activity against Aspergillus niger. Iran Polym J 17:843–852
Li Y, Zhang W, Niu J, Chen Y (2012a) Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 6:5164–5173
Li Y, Yu S, Wu Q, Tang M, Pu Y, Wang D (2012b) Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans. Hazard Mater J 219–220:221–230
Li B, Liu BP, Shan CL, Ibrahim M, Lou YH, Wang YL, Xie GL, Li HY, Sun GC (2013a) Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag Sci 69:312–320
Li C, Wang X, Chen F, Zhang C, Zhi X, Wang K, Cui D (2013b) The antifungal activity of graphene oxide –silver nanocomposites. Biomaterials 34:3882–3890
Li JG, Zhu WH, Zhang M, Zheng X, Di Z et al (2014) Antibacterial activity of large area monolayer graphene film manipulated by charge transfer. Sci Rep 4:4359–4367
Lin X, Li J, Ma S, et al (2014) Toxicity of TiO2 nanoparticles to Escherichia coli: effects of particle size, crystal phase and water chemistry. Rozhkova EA, ed. PLoS One. 2014;9(10):e110247. doi:https://doi.org/10.1371/journal.pone.0110247
Liu XF, Guan YL, Yang DZ, Li Z, Yao KD (2001) Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci 79:1324–1335
Liu H, Du YM, Wang XH, Sun LP (2004) Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol 95:147–155
Liu J, Qiao SZ, Hu QZ, Lu GQ (2011a) Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small 7:425–443
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y (2011b) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5:6971–6980
Liu J, Zhao Z, Feng H, Cui FJ (2012) One-pot synthesis of Ag–Fe3O4 nanocomposites in the absence of additional reductant and its potent antibacterial properties. Mater Chem 22:13891–13894
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H et al (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5:916–924
Ma L, Li J, Y Y, Yu CM, Wang Y, Li XM, Li N (2014) Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. Int J Agric Biol 16:766–770
Makhluf S, Dror R, Nitzan Y et al (2005) Microwave-assisted synthesis of nanocrystalline MgO and its use as bacteriocide. Adv Funct Mater 15:1708–1715
Malarkodi C, Rajeshkumar S, Paulkumar K, Gnanajobitha G, Vanaja M, Annadurai G (2013) Biosynthesis of semiconductor nanoparticles by using sulfur reducing bacteria Serratia nematodiphila. Adv Nano Res 1:83–91
Mansilla AY, Albertengo L, Rodríguez MS, Debbaudt A, Zúñiga A, Casalongué CA (2013) Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl Microbiol Biotechnol 97:6957–6966
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Markowska-Szczupak A, Ulfig K, Morawski AW (2011) The application of titanium dioxide for deactivation of bioparticulates: an overview. Catal Today 169:249–257
Marquez IG, Akuaku J, Cruz I, Cheetham J, Golshani A, Smith ML (2013) Disruption of protein synthesis as antifungal mode of action by chitosan. Int J Food Micobiol 164:108–112
Matˇeejka V, Tokarsk´y J (2014) Photocatalytical nanocomposites: a review. J Nanosci Nanotechnol 14:1597–1616
Mathur A, Raghavan A, Chaudhury P, Johnson JB, Roy R, Kumari J, Chaudhuri G, Chandrasekaran N, Suraishkumar GK (2015) Cytotoxicity of titania nanoparticles towards waste water isolate Exiguobacterium acetylicum under UVA, visible light and dark conditions. Environ J Chem Eng 3:1837–1846
Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T (2003) Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol 69:4278–4281
Meng XH, Tian S (2009) Effects of preharvest application of antagonistic yeast combined with chitosan on decay and quality of harvested table grape fruit. J Sci Food Agric 89:1838–1842
Meng XH, Qin GZ, Tian SP (2010) Influences of preharvest spraying Cryptococcus laurentii combined with chitosan coating on postharvest disease and quality of table grapes in storage. LWT – Food Sci Technol 43:596–601
Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ (2016) Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem 194:410–415
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT et al (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Nair R, Kumar D (2013) Plant diseases—control and remedy through nanotechnology. In: Tuteja N, Gill S (eds) Crop improvement under adverse conditions. Springer, New York, pp 231–243
Nambiar PR, Gupta RR, Misra V (2010) An “omics” based survey of human colon cancer. Mutat Res 693:3–18
Navrotsky A (2000) Technology and applications nanomaterials in the environment agriculture and technology (NEAT). J Nanopart Res 2:321–323
Niazi JH, Gu MB (2009) Toxicity of metallic nanoparticles in microorganisms- a review. In. 452. Atmospheric and biological environmental monitoring, Kim YJ, Platt U, Gu MB, Iwahashi H, 453. Eds. Springer Netherlands: Dordrecht, 2009, pp 193–206
Ocsoy I, Paret LM, Ocsoy AM, Kunwar S, Chen T, You M, Tan W (2013) Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ASC Nano 7:8972–8980
Pandian CJR, Palanivel S, Dhanasekaran (2016) Screening antimicrobial activity of nickel nanoparticles synthesized using Ocimum sanctum leaf extract. J Nanoparticle Article ID 4694367, 13. https://doi.org/10.1155/2016/4694367
Parry JM, Parry EM (2012) Genetic toxicology: principles and methods. Humana Press, New York
Paspaltsis I, Kotta K, Lagoudaki R, Grigoriadis N, Poulios I, Sklaviadis T (2006) Titanium dioxide photocatalytic inactivation of prions. J Gen Virol 87:3125–3130
Pelgrift RY, Friedman AJ (2013) Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 65:1803–1815
Perez Espitia PJP, Soares NFF, Coimbra JSR, Andrade NJ, Cruz RS, Medeiros EAA (2012) Zinc oxide nanoparticles: synthesis antimicrobial activity and food packaging applications. Food Bioprocess Technol 5:1447–1464
Peter KS, Rosalyn L, George LM, Klabunde JK (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18:6679–6686
Petrus E, Petrus E, Tinakumari S, Chai L, Ubong A, Tunung R, Elexson N, Chai LF, Son R (2011) A study on the minimum inhibitory concentration and minimum bactericidal concentration of nano colloidal silver on food borne pathogens. Int Food Res J 18:55–66
Pichyangkura R, Chadchawan S (2015) Biostimulant activity of chitosan in horticulture. Sci Hortic 196:49–65
Pontón J (2008) La pared celular de los hongos y el mecanismo de accio’n de la anidulafungina. Rev Iberoam Micol 25:78–82
Prasad R (2014) Synthesis of silver nanoparticles in photosynthetic plants. J Nanopart:963961 https://doi.org/10.1155/2014/963961
Prasad R, Swamy VS, Varma A (2012) Biogenic synthesis of silver nanoparticles from the leaf extract of Syzygium cumini (L.) and its antibacterial activity. Int J Pharma Bio Sci 3(4):745–752
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afri J Biotechnol 13:705–713
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prasad R, Bhattacharyya A, Nguyen QD (2017a) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Prasad R, Gupta N, Kumar M, Kumar V, Wang S, Abd-Elsalam KA (2017b) Nanomaterials act as plant defense mechanism. In: Prasad R, Kumar V, Kumar M (eds) Nanotechnology. Springer, Singapore, pp 253–269
Priyanka KP, Harikumar VS, Balakrishna KM, Varghese T (2017) Inhibitory effect of TiO2 NPs on symbiotic arbuscular mycorrhizal fungi in plant roots. IET Nanobiotechnol 111:66–70
Rabea EI, Badawy MEI, Steurbaut W, Stevens CV (2009) In vitro assessment of N-(benzyl) chitosan derivatives against some plant pathogenic bacteria and fungi. Eur Polym J 45:237–245
Raghupathi KR, Koodali RT, Manna AC (2011) Size dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020–4028
Rai M, Ingle A (2012) Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol 94:287–293
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotech Adv 27:76–83
Rai A, Prabhune A, Perry CC (2010) Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem 20:6789–6798
Raimondi S, Zanni E, Talora C, Rossi M, Palleschi C, Uccelletti D (2008) SOD1, a new Kluyveromyces lactis helper gene for heterologous protein secretion. Appl Environ Microbiol 74:7130–7137
Roda F, Al-Thani PNK, Al-Maadeed MA (2014) Garphene oxide as antimicrobial against two gram-positive and two gram-negative bacteria in addition to one fungus. Online J Biol Sci 14:230–239
Roopan SM, Surendra TV, Elango G et al (2014) Biosynthetic trends and future aspects of bimetallic nanoparticles and its medicinal applications. Appl Microbiol Biotechnol 98:5289–5300
Ruparelia JP, Chatterjee A, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716
Sadiq IM, Chowdhury N, Chandrasekaran A, Mukherjee (2009) Antimicrobial sensitivity of E. coli to alumina NPs. Nanomed Nanotechnol 5:282–286
Sadiq IM, Pakrashi S, Chandrasekaran N, Mukherjee A (2011) Studies on toxicity of aluminium oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp and Chlorella sp. J Nanopart Res 13:3287–3299
Sadjad S, Ali K, Hossein M (2017) Nano-bio control of bacteria: a novel mechanism for antibacterial activities of magnetic nanoparticles as a temporary nanomagnets. J Mol Liquids 251. https://doi.org/10.1016/j.molliq.2017.12.036
Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A (2013) Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol 62:677–683
Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, Raliya R, Biswas P (2015) Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromolecules 75:346–353
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnol 2:3–3
Samberg ME, Orndorff PE, Monteiro-Riviere NA (2011) Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology 5(2):244–253
Sang WK, Jin HJ, Kabir L et al (2012) Antifungal effects of silver nanoparticles (Ag NPs) against various plant pathogenic fungi. Mycobiology 40:415–427
Santos CMJ, Mangadlao F, Ahmed A, Advincula LRC et al (2012) Graphene nanocomposite for biomedical applications: fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 23:395101–395101
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29
Sawai J, Yoshikawa T (2004) Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J Appl Microbiol 96:803–809
Sawai JE, Kawada F, Kanou H, Igarashi A, Hashimoto T, Kokugan M, Shimizu (1996) Detection of active oxygen generated from ceramic powders having antibacterial activity. J Chem Eng Jpn 29:627–633
Sawai J, Kojima H, Igarashi H, Hasimoto A, Shoji S, Takehara A, Sawaki T, Kokugan T, Shimizu M (1997) Escherichia coli damage by ceramic powder slurries. J Chem Eng Jpn 30:1034–1039
Sawai J, Kojima H, Igarashi H, Hasimoto A, Shoji S, Sawaki T, Hakoda A, Kawada E, Kokugan T, Shimizu M (2000) Antibacterial characteristics of magnesium oxide powder. World J Microbiol Biotechnol 16:187–194
Sawai J, Shuga S, Kojima H (2001) Kinetic analysis of death of bacteria in CaO powder slurry. Int Biodeterior Biodegradation 47:23–26
Sawangphruk M, Srimuk P, Chiochan P, Sangsri T, Siwayaprahm P (2012) Synthesis antifungal activity of reduced graphene oxide nanosheets. Carbon 50:5156–5161
Sayed HH, Shamroukh AH, Rashad AE (2006) Synthesis and biological evaluation of some pyrimidine, pyrimido [2,1-b] [1,3] thiazine and thiazolo [3,2-a] pyrimidine derivatives. Acta Pharma 56:23–144
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF (2010) Metal-based nanoparticles and their toxicity assessment. WIREs Nanomed Nanobiotechnol 2:554–568
Shah MA, Towkeer A (2010) Principles of nanosciences and nanotechnology. Narosa Publishing House, New Delhi
Shankramma K, Yallappa S, Shivanna MB, Manjanna J (2016) Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl Nanosci 6:983–990
Shao X, Wang H, Xu F, Cheng S (2013) Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biol Technol 7:94–101
Sharma P, Sharma A, Sharma M, Bhalla N, Estrela P, Jain A, Thakur P, Thakur A (2017) Nanomaterial fungicides: in vitro and in vivo antimycotic activity of cobalt and nickel nanoferrites on phytopathogenic fungi. Glob Chall 1(1700041):1–7
Sharon M, Choudhary AK, Kumar R (2010) Nanotechnology in agricultural diseases and food safety. J Phytology 2:83–92
Shrivastava S, Prasad R, Varma A (2014) Anatomy of root from eyes of a microbiologist. In: Morte A, Varma A (eds) Root engineering, vol 40. Springer, Berlin/Heidelberg, pp 3–22
Sierra-Fernandez A, De la Rosa-García SC, Gomez-Villalba LS, Gómez-Cornelio S, Rabanal ME, Fort R, Quintana P (2017) Synthesis, photocatalytic, and antifungal properties of MgO, ZnO and Zn/Mg oxide nanoparticles for the protection of calcareous stone heritage. ACS Appl Mater Interfaces 929:24873–24886
Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH (2009) Nanogenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30:3891–3914
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett 7:219–242
Sonawane RS, Hegde SG, Dongare MK (2003) Preparation of titanium (VI) oxide thin film photocatalyst by sol–gel dip coating. Mater Chem Phys 77(3):744–746
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci 275:177–182
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18:6679–6686
Sudarshan NR, Hoover DG, Knorr D (1992) Antibacterial action of chitosan. Food Biotechnol 6:257–272
Tang Y (2015) Non-genomic omic techniques. In: Tang Y, Sussman M, Liu D, Poxton I, Schwartzman J (eds) Molecular Medical Microbiology. Academic Press, London, pp 399–406
Tang Z-X, Lv B-F (2014) MgO nanoparticles as antibacterial agent: preparation and activity. Braz J Chem Eng 31(3):591–601
Tarafdar JC, Sharma S, Raliya R (2013) Nanotechnology: interdisciplinary science of applications. Afr J Biotechnol 12(3):219–226
Tavaria FK, Costa EM, Gens EJ, Malcata FX, Pintado ME (2013) Influence of abiotic factors on the antimicrobial activity of chitosan. J Dermatol 40:1014–1019
Theron J, Walker JA, Cloete TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34:43–69
Torres R, Valentines MC, Usall J, Vinas I, Larrigaudiere C (2003) Possible involvement of hydrogen peroxide in the development of resistance mechanisms in “golden delicious” apple fruit. Postharvest Biol Technol 27:235–242
Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H, Zhou R (2013) Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol 8:594–601
Usha R, Prabu E, Palaniswamy M, Venil CK, Rajendran R (2010) Synthesis of metal oxide nanoparticles by Streptomyces sp for development of antimicrobial textiles. Global J Biotechnol Biochem 5:153–160
Usman MS, Ibrahim NA, Shameli K, Zainuddin N, Junus WMZW (2012) Copper nanoparticles mediated by chitosan: synthesis and characterization via chemical methods. Molecules 17:14928–14936
Vahabi K, Mansoori GA, Karimi S (2011) Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Inscience J 1:65–79
Van SN, Minh HD, Anh DN (2013) Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal Agric Biotechnol 2(4):289–294
Van-Loon LC, Van-Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Vidic J, Stankic S, Haque F, Ciric D, Goffic R, Vidy A, Jupille J, Delmas BJ (2013) Selective antibacterial effects of mixed ZnMgO nanoparticles. J Nanopart Res 15:1–10
Wang SY, Gao H (2012) Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x ananassa Duch.). LWT – Food Sci Technol 52:71–79
Wang SR, Lawson PC, Ray H, Yu (2010) Toxic effects of gold nanoparticles on Salmonella typhimurium bacteria. Toxicol Ind Health 27:547–554
Wang X, Liu X, Chen J, Han H, Yuan Z (2014) Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 68:798–806
Wang L, Hu C, Shao L (2017a) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227–1249
Wang X, Zhou Z, Chen F (2017b) Surface modification of carbon nanotubes with an enhanced antifungal activity for the control of plant fungal pathogen. Materials 10:1375. https://doi.org/10.3390/ma10121375
Wani AH, Shah MA (2012) A unique and profound effect of MgO and ZnO nanoparticles on some plant pathogenic fungi. J App Pharm Sci 2:40–44
Waters KM, Masiello LM, Zangar RC et al (2009) Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol Sci 107:553–569
Xie Y, He Y, Irwin PL, Jin T, Shi X (2011) Antibacterial activity and mode of action of ZnO. Appl Environ Microbiol 77:2325–2331
Xing K, Chen XG, Liu CS, Cha DS, Park HJ (2009) Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int J Food Microbiol 132:127–133
Xing K, Zhu X, Peng X, Qin S (2015) Chitosan antimicrobial and eliciting properties for pest control in agriculture a review. Agron Sustain Dev 35:569–588
Xu FF, Imlay JA (2012) Silver (I), mercury (II), cadmium (II), and zinc (II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl Environ Microbiol 78:3614–3621
Yamamoto O, Sawai J, Sasamoto T (2000) Change in antibacterial characteristics with doping amount of ZnO in MgO-ZnO solid solution. Int J Inorg Mater 2:451–454
Yamanaka M, Hara K, Kudo J (2005) Bactericidal actions of a silver ion solution on Escherichia coli studied by energy filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol 71:7589–7593
Yates JR, Ruse CI, Nakorchevsky A (2009) Proteomics by mass spectrometry: approaches, advances, and applications. Annu Rev Biomed Eng 11:49–79
Yen MT, Yang JH, Mau JL (2008) Antioxidant properties of chitosan from crab shells. Carbohydr Polym 74:840–844
Yin JJ, Liu J, Ehrenshaft M, Roberts JE, Fu PP, Mason RP, Zhao B (2012) Phototoxicity of nano titanium dioxides in HaCaT keratinocytes generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol 263:81–88
Yu JC, Tang HY, Yu JG (2002) Bactericidal and photocatalytic activities of TiO2 thin films prepared by sol–gel and reverse micelle methods. J Photochem Photobiol A Chem 3:211–219
Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M (2016) Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep 26667(36):6
Zakrzewska A, Boorsma A, Brul S, Hellingwerf KJ, Klis FM (2005) Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryot Cell 4:703–715
Zhang L, Jiang Y, Ding Y, Povey M, York D (2007) Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res 9:479–489
Zhang H, Li R, Liu (2011) Effects of chitin and its derivative chitosan on postharvest decay of fruits: a review. Int J Mol Sci 12:917–934
Zhang A, Sun H, Wang P, Han Y, Wang X (2012) Modern analytical techniques in metabolomics analysis. Analyst 137:293–300
Zhao D, Wang J, Sun BH, Sun BH, Gao JQ, Xu R (2000) Development and application of TiO2 photocatalysis as antimicrobial agent. J Liaoning Univ (Nat Sci Ed) 2:173–174
Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X (2010) Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J Amer Chem Soc 132(35):12349–11256
Zhou R, Gao H (2014) Cytotoxicity of graphene: recent advances and future perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol 6:452–474
Acknowledgment
This research was supported by the Science and Technology Development Fund (STDF), Joint Egypt (STDF)-South Africa (NRF) Scientific Cooperation, Grant ID 27837 to Kamel Abd-Elsalam.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Mostafa, M., Amal-Asran, Almoammar, H., Abd-Elsalam, K.A. (2018). Nanoantimicrobials Mechanism of Action. In: Abd-Elsalam, K., Prasad, R. (eds) Nanobiotechnology Applications in Plant Protection. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-91161-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-91161-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91160-1
Online ISBN: 978-3-319-91161-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)