Abstract
Previous data showed the lack of efficacy of an adrenoceptor antagonist to revert hypertension induced by chronic intermittent hypoxia (CIH). We hypothesized that, in addition to sympathetic activation, CIH may change the availability and dynamics of cysteine. Temporal variation in total cysteine and its fractions, free reduced, free oxidized and protein-bound (CysSSP), were measured in homogenates of kidney cortex and medulla of Wistar rats. Animals were exposed to CIH for 14, 21 and 60 days and cysteine fractions and fibronectin gene expression were assessed at these time-points. Two different phases in cysteine dynamics were identified. An early phase (14d) characterized by an increase in cysteine oxidation and CysSSP forms. Late events (>21d) were characterized by a global reduction in cysteine, minimum level of CysSSP and maximum overexpression of fibronectin in kidney cortex. In conclusion, cysteine dynamics is influenced by the duration of CIH exposure: first there is a cysteine disulfide stress-like adaptive response followed by a progressive loss of cysteine availability and a decrease in CysSSP fraction. Kidney fibrosis associated to an unbalance in cysteine dynamics might contribute to the inefficacy of available antihypertensive drugs in patients with delayed diagnosis of sleep apnea.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Cysteine
- Protein S-cysteinylation
- Disulfide stress
- Kidney fibrosis
- Systemic hypertension
- Antihypertensive drug response
10.1 Introduction
We have previously reported the lack of efficacy of carvedilol (CVDL) in reversing hypertension induced by chronic intermittent hypoxia (CIH) in rats (Diogo et al. 2015). CVDL is an unselective beta-blocker with intrinsic anti-α1-adrenergic and antioxidant activities. Neither dose, bioavailability, nor absence of beta-blocking activity were responsible for our findings (Diogo et al. 2015). A plausible explanation is that CVDL would be unable to stop oxidative stress and kidney inflammation in response to CIH. More, the chronicity of intermittent hypoxia promotes progressive cardiometabolic disease and irreversible renal damage (Wu et al. 2015, Sun et al. 2012) that compromise the antihypertensive effect of drugs, mainly when treatment is prescribed too late. The temporal variation in factors involved in kidney oxidative stress and inflammation during CIH is unknown. Additionally, the contribution of cysteine to the mechanisms underlying the regulation of blood pressure and kidney function has been supported by several reports (Vasdev et al. 2009). Evidence on cysteine effects in fibrosis is scarce (Horie et al. 2003). Mechanisms have been mostly investigated through N-acetylcysteine and cysteine supplementation (Vasdev et al. 2009), supporting that its low availability is related to kidney function, particularly in early phases of injury.
Cysteine availability is a net contribution of three pools which can dynamically exchange one- to-one, depending on the redox status of tissue involved. The protein-bound fraction (S- cysteinylated proteins, CysSSP) is generated by a reversible post-translational modification through a disulfide bound between free reduced low molecular weight cysteine and cysteine residues of proteins (Rossi et al. 2009). The free cysteine pool includes the reduced (CysSH) and oxidized cysteine fractions (CysSSX). The oxidized free cysteine pool contains mixed disulfides, like cysteinyl-glutathione or the combination of two cysteines (cystine).
Herein, we hypothesized that the duration of intermittent hypoxia exposure changes the dynamics of cysteine in the kidney and decreases its availability, favoring kidney fibrosis and consequently compromising the management of hypertension.
10.2 Methods
10.2.1 Animals
Thirty-five male Wistar Crl:WI (Han) (Rattus norvegicus L.), aged 10–13 weeks, with mean body weights of 293 ± 7 g were housed in polycarbonate cages with wire lids and maintained under standard laboratory conditions as follows: artificial 12 h light/dark cycles, room temperature (22 ± 2.0 °C) and relative humidity of 60 ± 10%. Rats were given reverse osmosis water ad libitum and standard laboratory diet (SDS diets RM1). This diet has 2.2 g/Kg of methionine and 2.4 g/kg of cystine (SDS, Special Diets Services, UK).
All applicable institutional and governmental regulations concerning the ethical use of animals were followed and the present study was also approved by the Institutional Ethics Committee of the NOVA Medical School for animal care and use in research.
10.2.2 Chronic Intermittent Hypoxia (CIH) Exposure
Rats were randomly assigned and divided into six groups: Group 1 – CIH 14 days (n = 6), Group 2 – normoxic conditions (Nx) 14 days (n = 5), Group 3 – CIH 21 days (n = 6), Group 4 – Nx 21 days (n = 6), Group 5 – CIH 60 days (n = 6) and Group 6 – Nx 60 days (n = 6). A period of 2 days was provided for chamber acclimatization under normoxic conditions (21% O2 + 79% N2) before the exposure to CIH. Normoxic rats were exposed for 14, 21 or 60 days, respectively, to 21% O2 and 79% N2 in the same room as the CIH animal groups. Animals were kept in a eucapnic atmosphere inside medium A-chambers (Biospherix Ltd., NY, USA). O2 concentration inside the chambers was controlled using 100% nitrogen (N2) and 100% O2 via electronically regulated solenoid switches, which gradually lowered the O2 in the chamber from 21% to 5% O2 (OxyCycler AT series, Biospherix Ltd). Chambers were infused with 100% N2 for 3.5 min to quickly reduce the O2 concentration to 5%; then the chambers were infused with 100% O2 for 7 min to restore O2 to ambient levels of 21%, until the next CIH cycle. Each CIH cycle lasted 10.5 min (normoxic period: 3.5 min; hypoxic period: 7 min) and the rats were exposed during their sleep period (light phase of light/dark cycle) to 5.6 CIH cycles/h, 10.5 h/day (9:30–8:00 pm), for 14, 21 or 60 days. During the remaining time, the chambers were ventilated with room air (21% of O2).
10.2.3 Gene Expression Analysis
Left kidney cortex and medulla samples were collected and homogenized in Trizol® (Life Technologies) using a tissue homogenizer (Heidolph DIAX 900). After total RNA extraction, cDNA was synthesized from 1 μg of RNA. Quantitative real time PCR (qPCR) was carried out in a final volume of 8.3 μL, with 4 μL of SYBR® Green Master Mix, 10 μM of each primer, plus 1 μL of cDNA for each sample. Relative quantification (arbitrary units) of fibronectin gene expression was assessed in kidney samples. Rat specific primers were used for the housekeeping gene β-actin. Fold change was calculated in CIH-exposed rats in relation to the normoxic group for each time-course.
10.2.4 Determination of Cysteine Fractions
Cysteine fractions were quantified in right kidney cortex and medulla homogenates according to Grilo and co-authors by high performance liquid chromatography with fluorescence detection (Grilo et al. 2017).
10.2.5 Statistical Analysis
Data are presented as the ratio of CIH/Nx groups or as percentage of effect whenever applicable and as mean values with their standard deviations. Differences were considered significant at p < 0.05 and obtained using Two-way ANOVA with Bonferroni multiple comparison test or Student t-test whenever applicable. GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA, USA) was used to perform all the statistical analysis.
10.3 Results
We have previously characterized the time-course of blood pressure evolution with the current paradigm of CIH (Diogo et al. 2015). Under this paradigm, hypertension is established at day 14, the plateau of increased blood pressure is attained at day 21 and carvedilol was administered from day 35 until day 60.
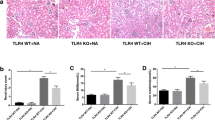
To clarify the degree of irreversible compromise of the kidney, we assessed fibronectin gene expression at day 21 and day 60 (Fig. 10.1 ). At day 21 there was a slight increase in fibronectin in both cortex and medulla (p < 0.05) that was remarkably higher at day 60 in kidney cortex (p < 0.001).
Fibronectin gene expression upon exposure to CIH for 21 and 60 days. Gene expression was normalized with β-actin and quantified by qPCR in renal tissue homogenates. Data are presented as the ratio of chronic intermittent hypoxia (CIH) by normoxia (Nx) groups. (*) Two-way ANOVA with Bonferroni post-test vs. day 21; (+) Student t-test Nx vs. CIH at each represented time-point. Grey bar – 21 days; black bar – 60 days
Renal cortex and renal medulla of animals not exposed to CIH presented a total cysteine content of 5.73 ± 1.16 and 4.83 ± 1.01 μM/mg tissue, respectively. In these tissues the major fraction was represented by reduced cysteine (2.37 ± 1.14 vs. 2.77 ± 0.64 μM/mg tissue) followed by free oxidized (2.10 ± 0.95 vs. 1.1 ± 0.73 μM/mg tissue) and CysSSP (1.26 ± 0.92 vs. 0.97 ± 0.5 μM/mg tissue).
The total content of cysteine (free + protein bound) was quantified in order to investigate the impact of CIH exposure on cysteine availability. At day 14 the total cysteine availability in both renal cortex and medulla was similar to normoxia. It decreased at day 21, attaining its minimum in kidney medulla and remaining low until day 60 (Fig. 10.2a, p < 0.001).
Cysteine dynamics in kidney cortex and medulla upon 14, 21 and 60 days of CIH exposure. (a) Total content of cysteine in kidney cortex and medulla. (b) S-cysteinylation (CysSSP) of proteins in kidney homogenates. (c) CysSH/CysSSX in kidney homogenates. Data are presented as percentage of effect from normoxia (Nx) upon CIH exposure. (*) Two-way ANOVA with Bonferroni post-test vs. day 14. (+) Student t-test Nx vs. CIH at each timepoint. White bar – 14 days; grey bar – 21 days; black bar – 60 days
Further, we addressed if changes in cysteine availability were accompanied by variation in cysteine dynamics. Although no loss in total cysteine availability was noted at day 14, it was already apparent an increase in CysSSP fraction at this time point (Fig. 10.2b, p < 0.05). This initial increase in CysSSP was maximal at day 14 of CIH and observed in both cortex (p < 0.05) and medulla (p < 0.05). Further, this cysteine fraction decreased over-time in both kidney cortex and kidney medulla, reaching its minimum at day 60 at kidney cortex (p < 0.001). Also, at both kidney cortex and medulla at days 14 and 21 the redox couple CysSH/CysSSX shifted towards an oxidized status in CIH-group and recovered to control levels at day 60 (Fig. 10.2c).
10.4 Discussion
Our data shows that CIH influences both availability and dynamics of cysteine in kidney Tissues in a time-dependent and biphasic manner. Increased cysteine oxidation is an early event in this time-course, which is followed by a latter gradual decrease in protein S- cysteinylation and simultaneously a recovery of the cysteine redox couple to normoxic levels. Our hypothesis-oriented research was motivated by the fact that the kidney is exposed to relatively high concentrations of cysteine (Stipanuk et al. 2002) and the availability of cysteine highly increases in developing kidney from term to adult life (Foreman and Segal 1987). In fact, the kidney is vital in preventing the loss of cysteine from the organism (Kowalczyk-Pachel et al. 2016) and is an important source of this amino acid with antioxidant, anti-inflammatory and anti-fibrotic properties (Vasdev et al. 2009, Horie et al. 2003). Also, cysteine has been described as a blood pressure regulator and a renoprotective factor (Vasdev et al. 2009). Cysteine availability in kidney also regulates cysteine metabolism (Stipanuk et al. 1992).
We must highlight that in the present work we measured the net content of cysteine and its fractions that, to our best knowledge, was investigated for the first time in kidney and particularly in CIH model. However, total cysteine content was of the same magnitude of that reported by others (Stipanuk et al. 2002). This allowed us to distinguish cysteine dynamics and relate it to time-course establishment of hypertension and progression to kidney fibrosis. For instance, fibrosis is concomitant with a decrease in cysteine availability and proceeded by early events in its dynamics that were not reflected by changes in the total amount of cysteine.
Early after CIH exposure (day 14), the CysSH/CysSSX ratio, which is a pivotal node for redox signaling (Jones et al. 2004), shifted towards a more oxidized state, accompanied by an increase in CysSSP. Scarce information exists on the physiological role of CysSSP (Rossi et al. 2009). Nevertheless, S-thiolation events like CysSSP, have a key role regulating several signaling pathways, which may lead to adaptive responses in order to surpass kidney injury (Eaton et al. 2003).
Increases in cysteine free oxidized and in CysSSP fractions were previously described as “disulfide stress” in an acute inflammation model of pancreatitis (Moreno et al. 2014). This disulfide stress would promote redox signaling (Jones et al. 2004) with several targets including ribonuclease inhibitor, APE1/Ref1 (Naganuma et al. 2010), Keap1(Miyazaki et al. 2014), phosphatases and protein disulfide isomerases (Wang and Asghar 2017) that have important roles in kidney and blood pressure regulation.
On the other hand, maximal fibrosis was coincident with minimum values of CysSSP. A plausible hypothesis is that longer exposure to intermittent hypoxia would inhibit the enzymes involved in S-thiolation process (Wouters, Fan, and Haworth 2010). Besides its redox regulatory function (Banks et al. 2008), CysSSP can ensure protection of proteins against irreversible oxidation (Auclair et al. 2013), which is often associated with permanent loss of protein function and the accumulation of insoluble aggregates (Rossi et al. 2009, Dalle-Donne et al. 2007, Grilo et al. 2017), suggesting that at later stages proteins are more vulnerable to this pro-fibrotic process (Li et al. 2007).
Our findings show that when treatment with carvedilol was initiated to revert hypertension induced by CIH a significant degree of fibrosis was already established, and the variations in cysteine redox dynamics might not be targeted by this adrenergic blocker.
References
Auclair JR, Johnson JL, Liu Q, Salisbury JP, Rotunno MS, Petsko GA, Ringe D, Brown RH Jr, Bosco DA, Agar JN (2013) Post-translational modification by cysteine protects Cu/Zn-superoxide dismutase from oxidative damage. Biochemistry 52(36):6137–6144
Banks DD, Gadgil HS, Pipes GD, Bondarenko PV, Hobbs V, Scavezze JL, Kim J, Jiang XR, Mukku V, Dillon TM (2008) Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm Sci 97(2):775–790
Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A (2007) S-glutathionylation in protein redox regulation. Free Radic Biol Med 43(6):883–898
Diogo LN, Pereira SA, Nunes AR, Afonso RA, Santos AI, Monteiro EC (2015) Efficacy of carvedilol in reversing hypertension induced by chronic intermittent hypoxia in rats. Eur J Pharmacol 765:58–67
Eaton P, Jones ME, McGregor E, Dunn MJ, Leeds N, Byers HL, Leung K-Y, Ward MA, Pratt JR, Shattock MJ (2003) Reversible cysteine- targeted oxidation of proteins during renal oxidative stress. J Am Soc Nephrol 14(suppl 3):S290–S296
Foreman JW, Segal S (1987) Cysteine and glutathione levels in developing rat kidney and liver. Pediatr Res 22(5):605–608
Grilo NM, Joao Correia M, Miranda JP, Cipriano M, Serpa J, Matilde Marques M, Monteiro EC, Antunes AMM, Diogo LN, Pereira SA (2017) Unmasking efavirenz neurotoxicity: time matters to the underlying mechanisms. Eur J Pharm Sci 105:47–54
Horie T, Sakaida I, Yokoya F, Nakajo M, Sonaka I, Okita K (2003) L-cysteine administration prevents liver fibrosis by suppressing hepatic stellate cell proliferation and activation. Biochem Biophys Res Commun 305(1):94–100
Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM Jr, Kirlin WG (2004) Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 18(11):1246–1248
Kowalczyk-Pachel D, Iciek M, Wydra K, Nowak E, Górny M, Filip M, Włodek L, Lorenc-Koci E (2016) Cysteine metabolism and oxidative processes in the rat liver and kidney after acute and repeated cocaine treatment. PLoS One 11(1):e0147238
Li HY, Hou FF, Zhang X, Chen PY, Liu SX, Feng JX, Liu ZQ, Shan YX, Wang GB, Zhou ZM (2007) Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol 18(2):528–538
Miyazaki Y, Shimizu A, Pastan I, Taguchi K, Naganuma E, Suzuki T, Hosoya T, Yokoo T, Saito A, Miyata T (2014) Keap1 inhibition attenuates glomerulosclerosis. Nephrol Dial Transplant 29(4):783–791
Moreno M-L, Escobar J, Izquierdo-Álvarez A, Gil A, Pérez S, Pereda J, Zapico I, Vento M, Sabater L, Marina A (2014) Disulfide stress: a novel type of oxidative stress in acute pancreatitis. Free Radic Biol Med 70:265–277
Naganuma T, Nakayama T, Sato N, Zhenyan F, Soma M, Yamaguchi M, Shimodaira M, Aoi N, Usami R (2010) Haplotype-based case–control study on human apurinic/apyrimidinic endonuclease 1/redox effector factor-1 gene and essential hypertension. Am J Hypertens 23(2):186–191
Rossi R, Giustarini D, Milzani A, Dalle-Donne I (2009) Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J Cell Mol Med 13(9B):3131–3140
Stipanuk MH, Coloso RM, Garcia RA, Banks MF (1992) Cysteine concentration regulates cysteine metabolism to glutathione, sulfate and taurine in rat hepatocytes. J Nutr 122(3):420–427
Stipanuk MH, Londono M, Lee J-I, Mindy H, Anthony FY (2002) Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr 132(11):3369–3378
Sun W, Yin X, Wang Y, Tan Y, Cai L, Wang B, Cai J, Fu Y (2012) Intermittent hypoxia-induced renal antioxidants and oxidative damage in male mice: hormetic dose response. Dose-Response 11(3):385–400
Vasdev S, Singal P, Gill V (2009) The antihypertensive effect of cysteine. Int J Angiol 18(1):7–21
Wang X, Asghar M (2017) Protein disulfide isomerase regulates renal AT1 receptor function and blood pressure in rats. Am J Physiol Renal Physiol 313(2):F461–F466
Wouters MA, Fan SW, Haworth NL (2010) Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal 12(1):53–91
Wu H, Zhou S, Kong L, Chen J, Feng W, Cai J, Miao L, Tan Y (2015) Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol Lett 232(2):340–348
Funding
iNOVA4Health – UID/Multi/04462/2013 (Ref: 201601-02-021).
FCT – PD/BD/114257/2016(NRC), PD/BD/105892/2014 (CGD) and SFRH/BD/130911/2017 (MJC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Coelho, N.R. et al. (2018). Cysteine Oxidative Dynamics Underlies Hypertension and Kidney Dysfunction Induced by Chronic Intermittent Hypoxia. In: Gauda, E., Monteiro, M., Prabhakar, N., Wyatt, C., Schultz, H. (eds) Arterial Chemoreceptors. Advances in Experimental Medicine and Biology, vol 1071. Springer, Cham. https://doi.org/10.1007/978-3-319-91137-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-91137-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91136-6
Online ISBN: 978-3-319-91137-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)