Abstract
The esophagus is an organ which consists of a fibromuscular tube which enables the passage of food. This transmission is aided by peristaltic contractions, from the pharynx to the stomach, where the process of digestion reaches the acme. Efficient transport through the esophagus requires the organ either to be patent and well canalized or to have adequate motility. This consists of coordinating sequential contraction that mobilizes the bolus from above and clears acid and bile reflux from below. Dysfunction of this integrated muscular motion reduces progression of bolus and causes a distressing sense of dysphagia, chest pain, and regurgitation, or leads to other severe conditions (i.e., ab ingestis pneumonia).
In this scenario modern imaging techniques, either invasive or noninvasive, concede an early and accurate diagnosis that radically changed the approach to those disorders. In fact a predominant role is played by contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI), but conventional radiology (barium esophagography; endoscopic ultrasonography, EUS) is still essential. Among those techniques, dynamic MRI of the esophagus has developed a great potential, with the introduction of ultrafast MR sequences, which have decreased scan times, granting a minor exposure to ionizing radiation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Esophagus
- Esophagus congenital malformation
- Esophagus motility
- Hiatus hernia
- Esophageal trauma
- Esophageal atresia
- Achalasia
- Esophagitis, reflux
17.1 Congenital Anomalies of the Esophagus
17.1.1 Esophageal Atresia and Tracheoesophageal Fistula
Congenital esophageal anomalies represent a complex of anatomical alteration characterized by the uncomplete formation of esophagus and/or an abnormal communication between the esophagus and other anatomical structures. This combination of pathologies may occur in 1 per 3000–5000 births.
The rare defects of development of the esophagus must be corrected early after the birth because of incompatibility with life. They are often found because of an early regurgitation after feeding. The exact cause is still undefined. It is known that it occurs as a developmental disorder in formation and separation of the primitive foregut into trachea and esophagus. This results from the fusion of both lateral mesodermal ridges of the foregut approximately around fourth week of gestation.
If the lateral walls fail to meet, they result in tracheoesophageal fistula; if these lateral walls turn dorsally, and compress the esophageal lumen, it will result in atresia.
Other causes can be intrauterine anoxia (can lead to a focal reduced perfusion and necrosis resulting in fistulae or atresia).
The esophageal agenesia is the absence of the esophagus and is not compatible with life. While this condition is extremely rare, more common defects are the esophageal atresia and tracheoesophageal fistula, often present at the same time [1].
Those anomalies can be associated with other gastrointestinal malformations in 25% of cases, such as imperforate anus, pyloric stenosis, duodenal atresia, and annular pancreas. Less frequent are cardiac, genitourinary, and vertebral changes.
Five different types of atresia have been identified, depending on the presence or absence of tracheoesophageal fistula and its location (Fig. 17.1):
-
Type A corresponds to pure esophageal atresia without fistula (8%).
-
Type B is esophageal atresia and fistula from trachea to the proximal esophageal pouch (>1%).
-
Type C is esophageal atresia with fistula between the trachea and the main bronchus to the distal segment; this is the most common type (84%).
-
Type D is esophageal atresia with both proximal and distal fistulae (3%).
-
Type E is tracheoesophageal fistula without atresia (4%); this type of anomaly must be suspected in feeding difficulties in infants, occasionally related with choking.
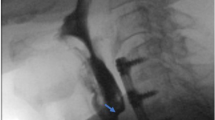
The inability to pass a rigid nasogastric tube from patient’s mouth to the stomach is diagnostic of EA and/or TEF. However this finding should be confirmed with radiographic visualization of the tube coiled in the proximal esophageal segment.
The radiological diagnosis (Fig. 17.2a, b) is based on chest radiographs on the posteroanterior and lateral projection which reveal a blind proximal pouch distended with air. The radiograph should always include the abdomen, in order to demonstrate the presence of air in the gastrointestinal tract. In types A and B, there is complete absence of gas in the stomach and intestinal tract, although in types C and D the gastrointestinal tract is distended with air.
Esophageal atresia. Chest radiograph of a newborn child revealed esophageal atresia without tracheoesophageal fistula. Should be noted (in a) the Replogle tube in the upper pouch (arrows) and the absence of GI air below the diaphragm (arrowheads), which is an indirect sign of the absence of tracheoesophageal fistula. After barium swallow (in b) is seen a narrowing of the esophagus lumen (arrowheads) with a mild dilatation of the upper pouch (arrows)
Confirmation of diagnosis is achieved by a contrast swallow study. Barium agents allow the best visualization of a fistula; however if TEF is suspected, barium is not indicated because of the risk of mediastinitis and chemical pneumonia. The contrast of choice is an isotonic, nonionic iodate contrast agent. Main findings are the demonstration of esophageal obstruction and fistula, together with opacification of the bronchus and distal airways.
CT scan is not typically used in the evaluation of EA and TEF; however, CT does allow 3-dimensional (3D) examination of the esophagus in relation to its adjacent structures. Axial images can be difficult to interpret, in which a fistula may be missed; however the sagittal reformation obtained from thin-section multidetector scans has been used in newborns to accurately diagnose EA and TEF. This method enables visualization of the entire length of the esophagus, complete with atresias, fistulas, and gap length.
Three-dimensional CT with virtual endoscopy facilitates the understanding of complex anatomic relationships and evaluation of transverse stenoses.
17.1.2 Esophageal Duplications
Duplication of the esophagus is the second most common duplication of the gastrointestinal tract after that of the ileum. Several theories have been proposed to explain the embryologic basis for gastrointestinal tract duplications.
The most held theory was proposed by Bremer, according to which at the fifth week of intrauterine life, the foregut epithelium produces secretions that form vacuoles. Those vacuoles line up longitudinally to form the new lumen; if some vacuoles fail to coalesce, it will result in a cyst that migrates into the esophageal wall and becomes surrounded by the muscular layers. The duplicated segment has a thick wall of smooth muscle and is lined with alimentary tract mucosa.
It is possible to distinguish a partial and a complete esophageal duplication; the former is the second most common gastrointestinal duplication after the ileum and the latter is a rare malformation, often associated with gastric duplication.
Most often, duplications are spherical cysts that rarely make an impression on the esophagus. On plain chest radiographs, they are usually seen as posterior mediastinal masses. Barium swallow shows the esophagus to be displaced to the cyst’s opposite side or an intramural extramucosal mass. At CT, a duplication is sharply marginated, has a homogeneous near-water density (9–18 HU), and is not enhanced after intravenous contrast material injection.
The MRI shows relatively low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Fig. 17.3a, b), and no significant enhancement in postcontrast sequences.
Endoscopic sonography has proved to be a reliable method for the diagnosis of this lesion because it can demonstrate contiguity of the muscularis propria of the esophagus with the muscle layer of the cyst wall.
17.1.3 Other Minor Congenital Anomalies
Less frequent congenital anomalies are diaphragm, stenosis, and congenital brachy-esophagus. Those alterations can be easily demonstrated with barium swallow:
Diaphragms are incomplete endoluminal thin membranes that can be mostly seen in the upper tract of the esophagus; generally these membranes are seen in particular conditions such as Plummer-Vinson syndrome (esophagitis, sideropenic anemia, and gastric achlorhydria).
Congenital stenosis (CES) is an esophageal benign lesion that narrows the organ; those lesions can easily be demonstrated by a barium swallow that demonstrates the narrowing.
Brachy-esophagus is an incomplete enlargement of the esophagus that leads to a hiatal hernia.
17.2 Acquired Malformations
17.2.1 Esophageal Diverticula
Rings, webs, and diverticula are among the most common acquired anomalies of the esophagus. Although these structural lesions are often asymptomatic, patients can develop significant problems with dysphagia, regurgitation, and aspiration.
Most well-known classifications of diverticula are by location in the esophagus: upper (pharyngoesophageal, Killian-Jamieson, or Zenker), middle, or lower (epiphrenic) and on the basis of histopathology in true diverticula (contain all layers of the intestinal tract wall) and pseudodiverticula (herniation of mucosa and submucosa through a defect in the muscular wall occurs). Of the diverticula, Zenker diverticula are the most common type to cause symptoms. Zenker diverticula are an acquired pulsion type of diverticula formed by the herniation of mucosa through an area of weakness in the Killian triangle of the hypopharynx.
A special type of pseudodiverticula, believed to represent dilated excretory ducts of esophageal submucosal glands, is observed in the condition of esophageal intramural pseudodiverticulosis.
Most esophageal diverticula occur in middle-aged adults and elderly people, in particular Zenker diverticula typically present in people older than 50. Oropharyngeal dysphagia to solids is the most common symptom. Regurgitation and aspiration may be related to large midesophageal and epiphrenic diverticula.
Most diverticula are caused by an underlying motility disorder of the esophagus. Structural lesions, including a noncompliant cricopharyngeus muscle (i.e., Zenker diverticulum), incomplete or uncoordinated relaxation of the lower esophageal sphincter, or strictures, may play a role as well.
17.2.2 Imaging Findings
Esophageal diverticula often are asymptomatic; therefore, radiographic studies detect many esophageal diverticula incidentally. On standard chest radiographs and CT scans, large diverticula of the esophagus and hypopharynx also may manifest as air-filled and/or fluid-filled structures communicating with the esophagus. Barium radiography is still considered the gold standard exam even because barium swallow also may provide clues to underlying motility disturbances that may be involved in diverticular formation. Diagnosis of esophageal intramural pseudodiverticulosis is made best using barium radiography. Diagnosis of Zenker diverticulum is made best using barium swallow, which should include lateral views of the pharyngoesophageal junction. Because of its location Killian-Jamieson diverticula could be detected on ultrasonography miming thyroid nodules. Also a Zenker diverticulum reportedly can be distinguished from a thyroid nodule on ultrasound by the sign of air in the diverticulum. There also has been a study of the use of swallow contrast-enhanced ultrasound to detect Zenker diverticulum that appeared as a pouch-shaped structure at the posterior pharyngoesophageal junction which retained ultrasound contrast agent for longer than 3 min. All patients underwent a barium esophagram as the gold standard. The authors explained that contrast-enhanced ultrasound provides advantages of bedside availability and no radiation exposure. When incidentally imaged on computed tomography (CT) and magnetic resonance imaging (MRI) scan, a Zenker diverticulum appears as a structure arising posteriorly from the hypopharynx and is filled with gas, fluid, oral contrast material, or a mixture of these [2, 3].
17.3 Dyskinesia
Esophageal motility disorders consist of a complex array of disturbances in normal esophageal function associated with dysphagia, gastroesophageal reflux, and noncardiac chest pain.
Primary motility disorders consist of achalasia, diffuse esophageal spasm (DES), “nutcracker esophagus,” hypertensive lower esophageal sphincter, and nonspecific esophageal motility dysfunction (NEMD). A host of secondary and miscellaneous motility disorders also affect the esophagus, including scleroderma and other connective tissue diseases, diabetes mellitus, Chagas disease, chronic idiopathic intestinal pseudo-obstruction, and neuromuscular disorders of striated muscle. Gastroesophageal reflux disease (GERD) may also be promoted by associated motility disturbances. In this chapter will discuss imaging findings in patients with achalasia.
Deglutition is a neuromuscular act coordinated by neural centers located in the brainstem and controlled by a widespread network of cortical regions; it is a complex function coordinated by voluntary and involuntary muscles of the oropharynx, larynx, and upper digestive pathway. The swallowing process is generally divided into three phases: (1) the voluntary phase, the onset of deglutition; (2) the pharyngeal phase, consisting of bolus passage from the mouth to the superior esophageal sphincter through the pharynx; and (3) the esophageal phase, characterized by bolus progression to the stomach through the esophagus [4]. The last two phases are involuntary. The esophageal phase starts with the opening of the superior esophageal sphincter. The primary function of the esophagus is to transport and direct the bolus from the pharynx to the stomach. The sequence of the muscular contractions is known as peristalsis. In normal subjects, two types of peristaltic activity are described: primary and secondary peristalsis.
The identification of the deglutition phases has been described in dynamic MR studies [using the different dynamic sequences such us turbo-FLASH, TrueFISP, and EPI combined with an oral positive contrast agent]. Turbo-FLASH and echo planar (EPI) MRI sequences demonstrated a sufficient temporal resolution for dynamic imaging of the deglutition process. However, detailed analysis is still restricted, mainly by the significantly lower SNR of high-speed MRI compared with conventional MRI, and by the occurrence of shape distortions in regions of large susceptibility gradients. The temporal resolution provided by ultrafast MRI is essential for the analysis of deglutition. Hagen et al. obtained good-quality images using turbo-FLASH sequences for dynamic oropharyngeal evaluation with temporal resolution of 0.2 s/slice and 0.3 s/slice [5].
Gd-based oral contrast agents are used for MRI evaluation of bolus transit along the pharyngeal and esophageal tract. Gd-DTPA-based contrast agent provides optimum signal intensity, while its combination with semifluid yoghurt (1:100) offered barium-like physical properties, improving at the same time patient comfort and granting good compliance during the examination [6]. The bolus is injected in the patient’s mouth by the doctor or technician before the start of the dynamic sequence by a syringe (20 mL) and oral silicone cannula [6,7,8].
Esophageal motility imaging protocol is divided into the following:
-
Phase 1: A breath-hold half Fourier single-shot turbo spin echo (HASTE) T2-weighted sequence orientated on coronal and axial planes is used to visualize the position of the esophagus and the gastroesophageal junction.
-
Phase 2: Dynamic examination is performed with a single-slice sagittal slab (10mm thickness) T1-weighted (turbo-FLASH) sequence. This slice is positioned at the center of the esophageal lumen, in order to depict the transit of contrast agent boluses through the esophageal lumen. The parameters of the turbo-FLASH sequence are modified to obtain a temporal resolution of approximately 3–4 images/s. Immediately before the sequence starts, a small amount of contrast agent (10–15mL) is administered directly into the oral cavity. Patients are instructed to swallow the entire bolus immediately after the onset of the gradient pulsations. Patients with severe deglutition disorders are at high risk for airway aspiration of oral contrast agent; in such situations oral contrast media should not be used. Five series of dynamic acquisitions are obtained: four acquired on the median sagittal and coronal plane to visualize esophageal motility and one on the oblique axial plane to depict gastroesophageal junction functionality.
17.3.1 Imaging Findings
In patients with achalasia, the main findings are the stenotic appearance of the distal esophagus with and/or without wall thickening and the dilatation of the segments above (Fig. 17.4a, b); poor relaxation of the gastroesophageal junction; inefficient peristalsis replaced by “to-and-fro” movements (repeated upward/downward movement of the bolus in the esophageal lumen caused by abnormal peristalsis and unrelaxed gastroesophageal junction); and the increased bolus transit time (up to 20 s). In advanced stages, the main findings are represented by a marked and widespread distension of the esophageal lumen (maximum caliber >60 mm) with tertiary peristalsis that is unable for activity and to fully empty the organ [9,10,11].
MRI findings (Fig. 17.5a–f) in patients with esophageal body motility incoordination (EBMU) are intermittent progression of the contrast bolus along the lumen with tertiary peristalsis, increased transit time (12–15 s), and reduction with a “corkscrew” appearance [12, 13].
MRI of achalasia: T2W HASTE images acquired on axial (a), coronal (b), and sagittal (c) plane. The exam revealed distension of the esophageal lumen (40 mm), narrowing of the distal esophagus, and replacement of the normal peristalsis by tertiary activity. On T1W F.L.A.S.H. sequences, after contrast bolus administration the gastroesophageal junction is closed and the bolus cannot progress (d–f)
In ineffective esophageal motility (IEM)/scleroderma of the esophagus (SE), a weak peristaltic activity, enlarged diameter, and mildly reduced esophageal clearance are considered significant diagnostic features of gastroesophageal junction incontinence with reflux and diminished clearance with delayed cleansing time (>18 s) [14, 15].
An additional application of MR-fluoroscopy is the follow-up of patients affected by achalasia after conservative treatment such as balloon dilatation of the distal esophagus. In this patient, population dynamic MRI is also adequate in the posttreatment follow-up to evaluate the efficacy therapy [8, 16,17,18].
17.4 Esophagitis
Common forms of esophagitis include reflux esophagitis, infectious esophagitis, pill esophagitis, eosinophilic esophagitis, and radiation and chemoradiation esophagitis. Candida esophagitis is the most common type of infectious esophagitis [19]. The prognosis is good with rapid diagnosis and proper treatment [16]. Although barium studies and endoscopy are more sensitive modalities for detecting this condition, the CT finding should suggest the diagnosis of esophagitis in the proper clinical setting. It has been suggested that 3 or 5 mm be considered the maximum normal values for esophageal wall thickness on CT and that a greater wall thickness may represent a sign of esophagitis. Wall thickening in esophagitis was concentric and circumferential in all patients and involved a relatively long segment of the esophagus. Esophageal wall thickening was found in all types of esophagitis, so a specific cause of esophagitis cannot be suggested on the basis of CT findings. Occasionally, malignant esophageal tumors may be manifested on CT by concentric wall thickening over relatively long segments so that further investigation with double-contrast esophagography or endoscopy may be required in patients who do not have typical clinical features of esophagitis.
17.5 Trauma
17.5.1 Perforation
Causes of perforations are divided into iatrogenic (75%: endoscopy and tracheal intubation are the most common causes) and non-iatrogenic (25%, including compression, foreign bodies, and neoplastic expansion). The cricopharyngeal area (UES) is the narrowest part of the esophagus where the perforations occur during endoscopy. The second restricted area is located close to aortic button and left bronchus and is the seat of the perforations by foreign bodies. The third zone is defined esophagogastric junction, and is involved in perforation especially during expansion maneuvers.
Symptoms of traumatic injury are often not specific: sudden violent pain, dysphagia, dyspnea, fever, subcutaneous emphysema, and shock.
The diagnosis is clinical and instrumental. When perforation is suspected, the imaging evaluation should generally commence with esophagography while the patient swallows a water-soluble contrast material (Fig. 17.6a, b). If no extra-esophageal leakage is observed, esophagography should be repeated during the oral administration of barium, which has a greater sensitivity for the detection of small perforations. In patients with penetrating trauma to the neck or chest, CT should be performed before contrast-enhanced esophagography, given the significant risk of damage to critical vascular structures. CT may be useful also in patients who are too ill to cooperate in esophagography or as a complement to contrast-enhanced luminal studies, to further delineate the extent of disease, assess complications, and guide therapy. In patients with acute chest pain, CT is used to exclude serious conditions such as aortic dissection and pulmonary embolism. CT findings of esophageal injury include esophageal wall thickening, periesophageal gas and fluid collections (Figs. 17.7a–f and 17.8a–d), contrast material extravasation, mediastinal fluid collection, mediastinal inflammation, focal esophageal wall defect, and pleural effusion [20,21,22]. Therapy is always urgent and surgical; it includes where possible the esophagus raffia.
Tracheoesophageal fistula. A 43-year-old man was involved in a car accident and transferred to intensive care unit. A chest X-ray (a) demonstrated a tracheal tube and left hydropneumothorax. A thoracic CT scan on axial (b), sagittal (c), and coronal (d) planes showed a short fistula (arrow) located at the level of the fourth and fifth thoracic vertebrae, and left hydropneumothorax (*). In figure (e) is shown a 3D endoscopic reconstruction of the fistula (arrow). Figure (f) shows post-processed 3D reconstruction of lungs and tracheas with mild posttraumatic laryngocele (arrowhead)
17.5.2 Mallory-Weiss Tear and Other Mucosal Lacerations
A Mallory-Weiss tear is a longitudinal mucosal laceration observed in the distal esophagus or across the gastroesophageal junction. It occurs in the setting of retching or vomiting, frequently after excessive alcohol consumption or as a complication of endoscopy. Some degree of hematemesis is invariably present and is an indication for upper endoscopy. Similar linear mucosal lacerations occurring elsewhere in the esophagus as a result of forceful swallowing of an impacted foreign body or food bolus may pose a diagnostic dilemma. A mucosal laceration without transmural perforation is likely to be radiographically occult. However, to the attentive observer, CT images of the esophagus in patients with chest pain occasionally show evidence of hemorrhage or foci of extraluminal gas at a site of mucosal injury [23]. Unless bleeding persists, the treatment of a Mallory-Weiss tear, like that of other mucosal lacerations, is supportive.
17.5.3 Foreign Bodies
The inability of food to progress towards the stomach depends on the size of foreign bodies or on the presence of tips that favor anchor to the esophagus wall. Most commonly foreign bodies stop in thoracic esophagus and preferably at the level of physiological narrowing. Symptoms could be pain, drooling, and dysphagia, and symptoms of perforation (if complicated). In cervical foreign body we could see phlegmon of the neck with subcutaneous emphysema and in chest foreign body an esophageal-mediastinal fistula with acute mediastinitis or esophageal-pleural. Diagnosis is by X-ray if the foreign body is metallic; if it is not radio-opaque examination with contrast reveals the location of the ingested body. Esophagoscopy allows to establish the seat of the foreign body and its removal and allows to detect the extent of a possible esophageal laceration. Once the presence of a foreign body is radiologically confirmed in the esophagus, but also in the clinical suspicion, it is necessary to intervene endoscopically early, regardless of location, size, and potential damaging [24]. Surgical excision, via cervicotomy or thoracotomy, is limited to cases where the endoscopy fails. The CT appearance of foreign-body impaction is variable, depending on the item ingested, site of impaction, and presence of an underlying pathologic esophageal process or associated complication. Axial and sagittal contrast-enhanced chest and upper abdomen CT image helps confirm the presence of a foreign body. Chronic impaction of a foreign body in the esophagus may produce erosion, fistula formation, and inflammatory reaction that are indistinguishable from those produced by a neoplastic process.
17.6 Esophageal Varices
Esophageal varices are abnormally enlarged veins, usually seen in lower part of the esophagus; these alterations are a spy of a portal hypertension that occurs in severe liver disease. These enlarged veins link the portal blood flow to the cava vein system, bypassing the liver. Esophageal varices are graded according to their size and appearance, as follows:
Small (grade 1): small straight varices
Medium (grade 2): enlarged tortuous varices occupying less than one-third of the lumen
Large (grade 3): large coil-shaped varices occupying more than one-third of the lumen
The classification enlisted above is based on endoscopic appearance, which remains the gold standard in diagnosis, because of the direct visualization, and eventually for the possibility of treatment.
Diagnostic imaging can give an important support in the diagnosis, by using contrast imaging.
Nowadays, barium swallow technique is not considered the first-line exam to evaluate varices. However barium swallow may demonstrate large- and medium-grade varices as incidental finding. Those veins are not directly seen but images can demonstrate a serpiginous filling defects, involving the inferior third of the esophagus.
Better than barium swallow, contrast-enhanced CT (Fig. 17.9a–d) scanning and MRI are identical in their usefulness in diagnosing esophageal varices because of its direct visualization. These modalities have the advantage over endoscopy because of the possibility in evaluating the surrounding anatomic structures and the extent of esophageal varices, both above and below the diaphragm [25,26,27].
17.7 Hiatus Hernia
Hiatal hernia (or paraesophageal hernia) occurs when part of the stomach protrudes into the thoracic cavity through the esophageal hiatus of the diaphragm. A hernia may occur through a congenitally large esophageal hiatus; however, acquired hernias through the esophageal hiatus are more common. Approximately 99% of hiatal hernias are sliding, and the remaining 1% are paraesophageal, but it’s very important to diagnose the last one because they are potentially life threatening because of the risk of volvulus and incarceration.
17.7.1 Imaging Findings
Most hiatal hernias are found incidentally, and they are usually discovered on routine chest radiographs or computed tomography (CT) scans performed for unrelated symptoms. When symptomatic, patients may experience heartburn, dyspepsia, or epigastric pain.
An upper GI barium series is the preferred examination in the investigation of suggested hiatal hernia and its sequelae. CT scans are useful when more precise cross-sectional anatomic localization is desired and a hiatal hernia appears as a retrocardiac mass with or without an air-fluid level. The mass can usually be traced into the esophageal hiatus on sequential cuts. Herniation of omentum through the esophageal hiatus may result in an increase in fat surrounding the lower esophagus (Fig. 17.10a–c). CT scanning is particularly useful in the accurate anatomic depiction of a totally intrathoracic stomach, especially in patients in whom volvulus of the stomach is suspected. CT scanning is also useful for staging purposes in patients in whom a carcinoma complicates a hiatal hernia [28].
Hiatal hernia: 82-year-old woman referred to our department for a routine angio-CT scanner to study the abdominal aorta. As collateral finding marked distention and distortion of the stomach were seen with herniation of a portion of the fundus into the lower thorax. The hiatal hernia is seen on the axial (a), volume-rendering oblique reconstruction (b, c)
The use of magnetic resonance imaging (MRI) and radionuclide studies is anecdotal. MRI has helped to achieve a diagnosis of a paraesophageal omental hernia, in which a retrocardiac mass was shown as a fatty tumor, with contiguous blood vessels extending from the abdominal portion into the thoracic portion.
Ultrasonography is a sensitive means of diagnosing gastroesophageal reflux, and it is particularly attractive for use in young patients because it is noninvasive and does not require the use of ionizing radiation.
Abbreviations
- CES:
-
Congenital esophageal stenosis
- CT:
-
Computed tomography
- DES:
-
Diffuse esophageal spasm
- EA:
-
Esophageal atresia
- Gd:
-
Gadolinium
- GERD:
-
Gastroesophageal reflux disease
- HU:
-
Hounsfield unit
- IEM:
-
Ineffective esophageal motility
- MRI:
-
Magnetic resonance imaging
- NEMD:
-
Nonspecific esophageal motility dysfunction
- SE:
-
Scleroderma of the esophagus
- TEF:
-
Tracheoesophageal fistula
- UES:
-
Upper esophageal sphincter
References
Berrocal T, Madrid C, Novo S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics. 2003;24:e17. https://doi.org/10.1148/rg.e17.
Ekberg O. Normal anatomy and techniques of examination of the esophagus: fluoroscopy, CT, MRI, and scintigraphy. In: Freeny PC, Stevenson GW, editors. Margulis and Burhenne’s alimentary tract radiology. 5th ed. St. Louis: Mosby; 1994. p. 168–85.
Herbella FA, Patti MG. Modern pathophysiology and treatment of esophageal diverticula. Langenbecks Arch Surg. 2012;397(1):29–35. Accessed Jan 2012.
Vaz M, Raj T, Anura K. Guyton & Hall textbook of medical physiology. 2nd ed. New Delhi: Elsevier; 2016. p. 747–8.
Mason RJ, Bremner CG, DeMeester TR, Crookes PF, Peters JH, Hagen JA, DeMeester SR. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg. 1998;228(4):598–608.
Panebianco V, Tomei E, Anzidei M, Habib FI, Catalano C, Lisi D, Laghi A, Passariello R. Functional MRI in the evaluation of oesophageal motility: feasibility, MRI patterns of normality, and preliminary experience in subjects with motility disorders. Radiol Med. 2006;111(7):881–9. Accessed 11 Oct 2006.
Covotta F, Piretta L, Badiali D, Laghi A, Biondi T, Corazziari ES, Panebianco V. Functional magnetic resonance in the evaluation of oesophageal motility disorders. Gastroenterol Res Pract. 2011;2011:367639. https://doi.org/10.1155/2011/367639. Accessed 29 Aug 2011.
Panebianco V, Habib FI, Tomei E, Paolantonio P, Anzidei M, Laghi A, Catalano C, Passariello R. Initial experience with magnetic resonance fluoroscopy in the evaluation of oesophageal motility disorders. Comparison with manometry and barium fluoroscopy. Eur Radiol. 2006;16(9):1926–33. Accessed 26 April 2006.
Vaezi MF, Richter JE. Duodenogastroesophageal reflux and methods to monitor nonacidic reflux. Am J Med. 2001;111(Suppl 8A):160S–8S.
Fiorentino E, Barbiera F, Runza G, Pangaro A, Rapisarda E, Latteri S, Valenti A, La Rocca A, Maiorana A, Mastrosimone A. Digital cineradiology in the diagnosis and surgical treatment of pharyngo-oesophageal junction motor disorders. Chir Ital. 2004;56(4):495–500.
Schima W, Ryan JM, Harisinghani M, Schober E, Pokieser P, Denk DM, Stacher G. Radiographic detection of achalasia: diagnostic accuracy of videofluoroscopy. Clin Radiol. 1998;53(5):372–5.
Schima W, Stacher G, Pokieser P, Uranitsch K, Nekahm D, Schober E, Moser G, Tscholakoff D. Esophageal motor disorders: videofluoroscopic and manometric evaluation—prospective study in 88 symptomatic patients. Radiology. 1992;185(2):487–91.
Prabhakar A, Levine MS, Rubesin S, Laufer I, Katzka D. Relationship between diffuse esophageal spasm and lower esophageal sphincter dysfunction on barium studies and manometry in 14 patients. AJR Am J Roentgenol. 2004;183(2):409–13.
Nelson JB, Castell DO. Esophageal motility disorders. Dis Mon. 1988;34(6):297–389.
Ipsen P, Egekvist H, Aksglaede K, Zachariae H, Bjerring P, Thommesen P. Oesophageal manometry and video-radiology in patients with systemic sclerosis: a retrospective study of its clinical value. Acta Derm Venereol. 2000;80(2):130–3.
Boyce HW Jr, Boyce G. Esophagus: anatomy and structural anomalies. In: Yamada T, editor. Textbook of gastroenterology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. p. 1148–65.
Bergeron JL, Long JL, Chhetri DK. Dysphagia characteristics in Zenker’s diverticulum. Otolaryngol Head Neck Surg. 2013;148(2):223–8. Accessed Feb 2013.
James B, Nelson MD, Donald O, Castell MD. Esophageal motility disorders. (2015). http://www.uptodate.com/contents/. Accessed 19 Dec 2014.
Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7(12):1305–13; quiz 1261. Accessed Dec 2009.
Wu JT, Mattox KL, Wall MJ Jr. Esophageal perforations: new perspectives and treatment paradigms. J Trauma. 2007;63(5):1173–84.
Port JL, Kent MS, Korst RJ, Bacchetta M, Altorki NK. Thoracic esophageal perforations: a decade of experience. Ann Thorac Surg. 2003;75(4):1071–4.
Rubesin SE, Levine MS. Radiologic diagnosis of gastrointestinal perforation. Radiol Clin North Am. 2003;41(6):1095–115.
Young CA, Menias CO, Bhalla S, Prasad SR. CT Features of esophageal emergencies. Radiographics. 2008;28:1541–53.
Società Italiana di Endoscopia Digestiva (S.I.E.D.). Linee guida per la rimozione endoscopica di corpi estranei. Clinical Guideline 1995.
De Macedo GF, Ferreira FG, Ribeiro MA, Szutan LA, Assef MS, Rossini LG. Reliability in endoscopic diagnosis of portal hypertensive gastropathy. World J Gastrointest Endosc. 2013;5(7):323–31. Accessed 16 Jul 2013.
Furuichi Y, Kawai T, Ichimura S, Metoki R, Miyata Y, Oshima T, et al. Flexible imaging color enhancement improves visibility of transnasal endoscopic images in diagnosing esophageal varices: a multicenter prospective blinded study. J Dig Dis. 2012;13(12):634–41.
Kim SH, Kim YJ, Lee JM, Choi KD, Chung YJ, Han JK, et al. Esophageal varices in patients with cirrhosis: multidetector CT esophagography—comparison with endoscopy. Radiology. 2007;242(3):759–68.
Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22(4):601–16.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Indino, E.L., di Gaeta, A., Andreoli, G., Del Monte, M., Panebianco, V. (2018). Imaging of Nonneoplastic Esophageal Pathologies. In: Anzidei, M., Anile, M. (eds) Diagnostic Imaging for Thoracic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-89893-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-89893-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89892-6
Online ISBN: 978-3-319-89893-3
eBook Packages: MedicineMedicine (R0)