Abstract
Until the mid-twentieth century, the clinical and pathological features of melanoma were not well documented, prognostic factors were poorly understood, and the evidence base for management strategies was sadly deficient. Aggressive treatment approaches for both the primary melanoma site and the regional lymph nodes had been established by the early 1900s, and continuing as the standard of care until the early 1980s. Several clinical trials failed to demonstrate a survival benefit for patients having a very wide excision of their primary melanoma, or those having elective lymph node dissection (ELND), although in two of the largest studies there was a trend in favor of ELND. In 1992, Morton et al. reported the technique of sentinel node biopsy (SNB). This identified node-positive patients most likely to benefit from a completion lymph node dissection (CLND). A large international trial, MSLT-I, showed no overall survival benefit for SNB, but did show a substantial survival benefit for SN-positive patients treated by immediate CLND compared to patients whose regional nodes were simply observed, and treated by CLND only if nodal metastases later became apparent. Initial results of a second trial initiated by Morton, MSLT-II, indicate that routine CLND in SN-positive patients confers no additional survival benefit. In the last decade, targeted inhibitors of the MAP kinase pathway and immune checkpoint inhibitors have produced remarkable improvements in the previously dismal survival outcome for patients with systemic melanoma metastases. Furthermore, the role of these agents as neoadjuvant and adjuvant therapies in high-risk patients is currently being assessed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Melanoma in Antiquity and Through the Ages
Melanoma classically occurs in fair-skinned individuals of European origin. It is therefore surprising that the earliest evidence of melanoma has been found in the skeletons and skin of pre-Columbian, non-European mummies discovered in the foothills of the Peruvian Andes [1]. As well as widespread metastases in bone, rounded melanotic masses were identified in the skin of these mummies. Carbon dating has indicated that these individuals died around 2400 years ago.
At about the same time in Europe, in the fifth century BC, the Greek physician Hippocrates described a condition that he referred to as the “fatal black tumor ,” almost certainly melanoma. This condition was subsequently reported in the writings of Rufus of Ephesus in the first century AD, but there was no further mention of it until references were made to “fatal black tumor,” and “black fluid in the body” in European reports from the seventeenth and eighteenth centuries, including the writings of Highmore in 1651 [1], Bartholin in 1677 [2], Bonet in 1679, and Henrici and Nothnagel in 1757 [2]. In 1804, René Laennec, inventor of the stethoscope, used the term “melanose” to describe a distinct disease entity [3]. Laennec’s mentor in Paris was an anatomist and surgeon Baron Guillaume Dupuytren, and in 1812 they published detailed descriptions of “la melanose”. Jean Cruveilhier, author of the famous treatise Anatomie pathologique du corps humain, was another student of Dupuytren, and published the earliest descriptions of melanoma of the hand, foot, and vulva [2].
In 1858, Oliver Pemberton, a surgeon from Birmingham, England, reported a series of 60 patients with metastatic melanoma treated over a 37-year period, describing their clinical features and the sites to which metastasis occurred. Based on Greek words “melas” (dark) and “oma” (tumor), the English word “melanoma” is believed to have been first introduced by a Scottish pathologist, Robert Carswell, in 1838 [4, 5]. Carswell produced a monograph entitled Illustrations of the Elementary Forms of Disease, in which he depicted examples of melanomas. However, in the mid-1900s, this disease was often referred to as “melanoblastoma” [6], presumably because it was observed to behave more like a sarcoma than a skin cancer [4, 5].
Melanoma Pathology: Classical and Modern
The first illustrated descriptions of both primary and metastatic melanomas, notably those of Cruveilhier in 1829, were derived from autopsies. In 1853, a surgeon at St Bartholomew’s Hospital in London, James Paget, published a report documenting 25 cases of melanoma, and he appears to have been the first to point out that melanoma can progress from a radial growth phase to a vertical growth phase [7]. In 1894, one of the first pathological descriptions of an excised melanoma was published by the British surgeon, Jonathan Hutchinson. He described a case of lentigo maligna melanoma in which an excised amelanotic nodule was examined pathologically by his son (Jonathan Hutchinson, Jr.), who identified a malignant tumor composed of spindle-shaped cells resembling a sarcoma and which he therefore called “melanotic sarcoma.”
It was not until the 1900s that detailed descriptions of the histopathological features of melanoma were published. In 1948, a monograph entitled Biology of Melanomas was published by the New York Academy of Sciences detailing the state of knowledge at the time. In early 1967 a committee of Australian pathologists, led by the eminent melanoma researcher Vincent McGovern, published recommendations for melanoma classification and terminology [8]. Additionally, in 1967, the US pathologist, Dr. Wallace Clark, published his landmark paper on the histogenetic classification of melanoma that became the basis of previous and current versions of the WHO Classification of Melanoma [9]. He described three types of melanoma (lentigo maligna melanoma, superficial spreading melanoma, and nodular melanoma), and also suggested that they had differing prognoses. Subsequent studies provided some evidence in support of this assertion [10,11,12]. However, in their 1969 publication, Clark et al. showed that the prognosis of melanoma was primarily related to the depth of invasion, which they categorized according to five levels of the skin (now known as Clark-McGovern levels, reflecting the contributions that both these pathology doyens made to establishing level of invasion as an important prognostic factor for patients with primary melanomas) [10,11,12].
Although it had been documented as early as 1953 that the depth of invasion appeared to be associated with prognosis [13], it was not until the publication in 1970 of Alexander Breslow’s landmark paper on the prognostic significance of tumor thickness that the vertical (Breslow’s) tumor thickness was established as a very strong prognostic factor [14]. Breslow defined tumor thickness as the vertical depth from the top of the granular layer of the epidermis to the deepest invasive cell. Melanomas <0.76 mm thick and Clark level II tumors were noted to be associated with a more favorable prognosis. The pioneering work of Breslow remains relevant today, with tumor thickness now confirmed in multiple studies to be the strongest predictor of outcome in patients with clinically localized primary melanomas. It has been, and remains, an important factor in successive versions of the internationally accepted American Joint Committee on Cancer (AJCC) Melanoma Staging System [15].
At the International Pigment Cell Conference and the International Cancer Conference held concurrently in Sydney in 1972, an international group of pathologists, chaired by Vincent McGovern, met to develop a consensus on the classification and histopathological reporting of melanoma. The classification, published in 1973 [16], was based on Clark’s original proposal and listed two forms of noninvasive “in situ” melanoma (Hutchinson’s melanotic freckle and superficial spreading melanoma), and three forms of invasive melanoma (invasive melanoma with adjacent intraepidermal component of Hutchinson’s melanotic freckle type, invasive melanoma with an adjacent intraepidermal component of superficial spreading type, and invasive melanoma without an adjacent intraepidermal component).
In 1977 Arrington, Reed et al. described the features of melanomas involving acral skin as a distinct subtype of melanoma that has subsequently become known as acral lentiginous melanoma [17].
The 1972 Sydney classification was revised at an international workshop held in Sydney in 1982 [18]. The original categories were retained (albeit with some slightly different terminologies) but new categories were added: melanoma with an adjacent component of acral lentiginous type, melanoma with an adjacent component of mucosal lentiginous type, and melanoma of unclassifiable histogenetic type.
In the 1960s, and for the next 25–30 years, the disease was usually referred to as “malignant” melanoma , but use of this adjective is now strongly discouraged because it is confusing to patients and to many physicians because there is no nonmalignant form of invasive melanoma. In 2013, Bahmer and Bahmer referred to melanoma in more dramatic terms, describing it as “the black death of modern times”! [19].
In the early years of the twenty-first century, great technological advances facilitated a new understanding of the molecular pathogenesis of a wide variety of diseases, including melanoma [20]. In 2005, Boris Bastian and colleagues published their landmark study showing the presence of common oncogenic somatic gene mutations in BRAF, NRAS, and CKIT in many melanomas. They also showed associations of these mutations with the anatomical site of the primary tumor and the degree of chronic sun damage [21]. These discoveries paved the way for the development of a molecular classification of melanoma that was subsequently expanded into four major melanoma molecular subclasses by The Cancer Genome Atlas Melanoma Project: BRAF mutant, NRAS mutant, NF1 mutant, and triple-wild-type tumors [22]. These advances also led to the development of new therapies that targeted specific gene mutations in melanoma patients, resulting in new treatment options for those with advanced-stage disease.
Recognition of Genetic and Environmental Factors Predisposing to Melanoma
Hereditary Predisposition to Melanoma
In 1820, William Norris, a British general practitioner, noted that satellite lesions frequently occurred around a primary cutaneous melanoma (which he referred to as “fungoid disease”), and observed that spread could occur to distant sites including the lungs, liver, brain, and bone [23]. Norris also appears to have been the first to point out that patients with melanoma sometimes have a family history of melanoma, suggesting that there was an inherited predisposition [24]. Norris later commented on the fact that most of his patients with the condition had a fair complexion and light hair. He based this observation on his clinical experience that a father of one of his melanoma patients later died of an apparently similar disease. Furthermore, the patient’s children had multiple nevi on their bodies, as did the patient himself. (It seems likely that they had the atypical multiple mole syndrome, now a well-recognized entity partially attributed to germline CDKN2A mutations; we now know that melanoma occurs by 80 years of age in 58–92% of individuals with these mutations [25]). Thus, Norris appears to have been the first to propose that melanomas could develop from preexisting nevi. Although Norris had suggested that melanomas typically occurred in individuals with fair skin and light hair, a rare case of what we now know as melanoma occurring in a patient with dark skin, a native of Madagascar, was reported by Pemberton in 1858 [26].
The Role of Exposure to Sunshine in Melanoma Development
Sun worshippers have existed for millennia [27]. In some ancient civilizations, sun gods were regarded as the source of all goodness and life, and “Sunday” was designated the special day to worship the sun. For the Greeks, Apollo was the god of the sun and his son Asclepius the god of medicine. It is therefore not surprising that sun bathing was widely practiced in the Asclepian health clinics in the first century BC.
It was not until the late nineteenth century that there was any suggestion that sunshine might not always be beneficial to health. The first documented proposal that sunshine could be harmful is attributed to Unna [28], who in 1893 described severe damage to the skin of sailors caused by prolonged and intense solar exposure. In the early twentieth century, it was specifically noted that skin cancer was more common in geographical areas where there was much sunshine, particularly in outdoor workers.
In 1956, a landmark report was published by the Australian researcher H.O. Lancaster [29], containing data supporting the concept that sunshine was involved in the initiation of cutaneous melanoma. Lancaster indicated that his studies had been undertaken to test the earlier proposal by AGS Cooper, director of the Queensland Radium Institute in Brisbane, that sunlight was an important predisposing factor for all forms of skin cancer. He had observed that skin cancer was far more common in northern Queensland than southern Queensland. Cooper did not publish his results until 1959 [30]. In this article, he noted that a similar observation about latitude and skin cancer incidence had been made in the United States, in the southern cities of New Orleans and Dallas, respectively, and published by Sarnat and Schour in 1950 [31].
A subsequent paper by Lancaster and Nelson, published in 1957 [32], explored in more detail the concept as it applied specifically to melanoma. They noted that those most commonly affected by melanoma in Australia had a fair complexion and did not tan readily, but instead sunburned easily and developed freckles. Lancaster and Nelson clearly documented the fact that melanoma, as well as other types of skin cancer, developed more often in white Australians who lived in the northern state of Queensland than in the southern states of New South Wales and Victoria, with a remarkable latitudinal gradient correlation with melanoma incidence rates. Population-based incidence data for cutaneous melanoma have been collected in Australia and most other developed countries since the 1960s, with a steady increase in observed incidence worldwide. This is attributed mainly to increased UV exposure, mainly from sunshine but also from artificial sources of UV including skin tanning equipment.
Recognition of Melanoma as a Treatable Entity in the Eighteenth and Nineteenth Centuries
In 1844 Samuel Cooper, a London surgeon, recommended early surgical removal of malignant pigmented tumors, but as Norris had done previously he emphasized the apparently untreatable situation of patients with locally advanced and metastatic disease [1, 2, 33]. Subsequently, Norris discussed treatment options in more detail, recommending wide excision of the skin and subcutaneous tissue around a primary melanoma to minimize the risk of recurrence [23].
The first known surgical resection of metastatic melanoma appears to have been undertaken in 1787 [4] by a British surgeon, John Hunter, in London [2]. He reported resection of a recurrent tumor mass behind the angle of the mandible of a 35-year-old man, and described it as a “cancerous fungus excrescence” [34]. It is not clear whether Hunter knew what condition he was dealing with, but the preserved tumor is still on display in the Hunterian Museum of the Royal College of Surgeons in London, subsequently confirmed to be melanoma [35]. The fate of the patient is unknown. The first reported groin dissection for melanoma was in 1851 by Ferguson [36]. Shortly afterwards, in 1857, Jonathan Hutchinson provided the first definite description of subungual melanoma, and stated that early amputation was required [37].
Establishment of Surgical Treatment Paradigms for Melanoma in the Late Nineteenth and Early Twentieth Centuries
Herbert Snow, of the Marsden Hospital in London, was the first to propose surgical clearance of the regional lymph nodes as part of the initial management of patients with primary cutaneous melanoma (which he called “melanotic cancerous disease”). Snow suggested that the regional lymph nodes functioned as “traps” (filters) to prevent the spread of cancer cells into the bloodstream. What is today referred to as an elective lymph node dissection (ELND) was described by Snow as “anticipatory gland dissection” in 1892 [38]. In 1903 Frederick Eve [39] also recommended excision of regional lymph nodes as well as wide excision of the primary melanoma site, whether or not there was any clinical evidence of metastatic disease in the nodes. In 1907, William Sampson Handley emphasized that generous wide excision of primary melanomas (which he referred to as “melanotic growths”) was required for effective treatment, in combination with surgical clearance of the regional lymph nodes, and amputation in selected cases [40]. Handley noticed the lymphatic spread of melanoma at the time of autopsy in the leg of a woman who had apparently died of advanced metastatic melanoma. Based on this single case, he recommended not only removal of two inches of skin around the primary melanoma site, with an even wider excision of subcutaneous tissue down to the level of muscle fascia, but also removal of the regional lymph nodes. This treatment plan went unchallenged for the subsequent 50 years.
Pringle, in 1908, extended Handley’s concept of lymphatic spread of melanoma, and proposed wide excision of the primary melanoma site and in continuity excision of a generous strip of skin, subcutaneous tissue, and fascia between the primary site and involved regional lymph nodes, all as a single operative specimen [41].
ELND, as part of the primary definitive treatment of melanomas more than 1.5 mm in Breslow’s thickness, continued to be performed at many major melanoma treatment centers worldwide. This did not come under debate until the mid-1980s, when its value had become a matter of ongoing controversy. Those who advocated ELND based their recommendation mainly on retrospective studies that may have been subject to selection bias. Prospective clinical trials had not been performed at this point, with little evidence of a true benefit. There were two large, randomized, multicenter clinical trials led by Charles Balch in the United States and Pino Cascinelli in Italy (the Intergroup Melanoma Surgical Trial [42] and the WHO Melanoma Group Trial Number 14 [43]), both failing to demonstrate a statistically significant overall survival benefit. However, both studies showed a nonsignificant trend in favor of ELND.
The History of Radiation Therapy for Melanoma
The use of radiation therapy in the treatment of melanoma has a checkered history, with periods of enthusiasm alternating with bouts of skepticism. Following the discovery of radiation and radioactivity by Roentgen [44], Becquerel [45], and the Curies [46] in 1895–1896, radiation was applied enthusiastically to a wide range of medical conditions, including cancers [47]. However, its inappropriate use and the occurrence of late complications led to disrepute. Early reports of radiation treatment for melanoma were particularly disappointing, leading to the conclusion that melanomas responded poorly to radiation. In retrospect, many of these early treatments were for advanced, incurable melanomas for which meaningful responses would have been unlikely.
The concept that melanoma was a radioresistant tumor was strengthened by in vitro studies of the response of melanoma cell lines by Dewey and Barranco [48, 49]. Both reported that fewer melanoma cells than non-melanoma cells were killed by low doses of radiation. However, enhanced cell killing occurred for higher radiation doses than the usual daily amounts administered. This response to radiation was viewed as an intrinsic characteristic of melanoma cells, which had a high capacity for repair of radiation damage, but which could be overcome by delivering higher daily doses of radiation. A large number of in vivo studies ensued, indicating that high response rates resulted from a hypofractionated treatment schedule (a small number of large fractions of radiation). This became the prevailing methodology for both therapeutic and adjuvant radiation therapy in the late twentieth century [50].
There was, however, growing evidence of a marked heterogeneity in melanoma response to radiation therapy from both in vitro and in vivo studies. In vitro cell line and xenograft studies showed that melanomas had a wide range of radiation sensitivities, from highly sensitive to highly resistant types. A randomized clinical trial failed to show an improvement for an extreme hypofractionated treatment schedule (8 Gray × 4 fractions) compared with a mildly hypofractionated schedule (2.5 Gray × 20 fractions). It has now become clear that most melanomas are somewhat less sensitive to radiation than other common cancer types, such that mildly hypofractionated schedules are appropriate. These have become the most common treatment schedules for adjuvant radiation therapy following resection of primary melanomas with desmoplastic features or positive margins, and following regional lymph node dissection. A Phase III clinical trial clearly showed evidence of substantially lower node field recurrence rates (18% vs. 31%) in patients at high risk of regional recurrence [51].
Novel strategies to improve the response of melanomas have included the combination of radiation with hyperthermia [52]; however, uniform and selective heating of deeply placed tumors was difficult. This was complicated by the lack of specialized equipment being readily available except for a few institutions. Other methods have included selective targeting of melanoma cells using boron neutron capture therapy (BNCT) [53] or alpha-emitting isotopes attached to melanoma-selective probes (TAT-targeted alpha therapy) [54]. None of these approaches has become widely available for clinical practice. By contrast, proton therapy has been used with great success for uveal melanomas for several decades in selected institutions [55, 56].
The modern era of radiation therapy is dominated by continued improvements in the delivery of radiation from linear accelerators. Dose rates have increased, reducing concerns regarding tumor and organ motion; multileaf collimators (MLCs) have replaced single-square apertures, allowing greater conformality of tumor shape from different angles; “onboard” imaging devices (plain X-ray, CT, or MRI scans) allow real-time imaging of tumors and normal anatomy. Inverse treatment planning allows dose sculpting around critical normal tissues, and dynamic motion of all moving components permits safety margins around tumors to be reduced. This allows for higher radiation doses to be delivered to the tumors without causing unacceptable complications.
The availability of these advances has led to the development of “stereotactic” radiation therapy, in which very high radiation doses, delivered as single treatments, or as a highly hypofractionated schedule with great precision, are used to ablate tumors. This technology was used initially for the treatment of intracranial metastases, called stereotactic radiosurgery (SRS), and is delivered using linear accelerators, the Gamma Knife or the CyberKnife. Studies indicated that the differing radiosensitivities observed for conventionally fractionated treatments did not persist following ablative therapy, with high response rates for all tumor types, including melanoma [57]. This technology is increasingly applied to tumors throughout the body, called “stereotactic body radiation therapy (SBRT).”
With the recent availability of effective systemic therapies for locally recurrent and metastatic melanoma, a new role for radiation therapy is emerging. The success of immunotherapy depends on the activation of cytotoxic T lymphocytes. Priming doses of radiation are used to disrupt melanoma cells, releasing melanoma antigens and thereby facilitating the antitumor immune response to immunotherapy or as an abscopal radiation response at distant sites [58].
The Evolution of Locoregional Techniques for Treating Melanoma Metastases: Isolated Limb Perfusion, Isolated Limb Infusion, Intralesional and Topical Therapies
Until the mid-twentieth century, patients with locally advanced or extensive metastatic melanoma involving a limb were usually treated by amputation. This dramatic and disabling surgery was able to be avoided in most cases after the introduction, in 1958, of the isolated limb perfusion (ILP) technique by Creech and Krementz et al. in Louisiana [59]. This procedure involved temporary isolation of the limb vasculature from the general circulation, and then perfusion of the “isolated” limb with cytotoxic drugs via large-bore catheters inserted surgically into the major vessels of the groin or axilla and connected to a modified heart-lung external circuit. Overall response rates of around 80% were achieved with ILP, and use of the technique meant that limb amputation was able to be avoided in most patients [60].
The ILP technique was complex and technically challenging. Consequently, it was only performed in a few specialized centers. In 1994, Thompson et al. in Sydney described a similar but much simpler technique that they called isolated limb infusion (ILI) [61]. Percutaneously inserted arterial and venous catheters were used, and after inflating a pneumatic tourniquet around the proximal limb the infused drug was circulated using a syringe and a three-way tap via a simple external circuit containing a heating device without an oxygenator. The results obtained by ILI were similar to those achieved by ILP, and ILI is now being used at many melanoma treatment centers around the world [62].
Another form of treatment that has been used to treat melanoma in transit metastases is intratumoral injection. A wide variety of agents have been injected. Reports dating as far back as 1896 [63] have documented intratumoral injection of bacillus prodigiosus, BCG, and the cytotoxic drug thiotepa [64,65,66,67]. In the 1980s, there were reports of intralesional injection of interleukin-2 [68], and more recently of Rose Bengal (PV-10) [69] and talimogene laherparepvec (TVEC, “Imlygic”) [70]. Good local control of injected lesions has been reported for each of the latter three agents. Involution of nearby, non-injected lesions can also occur, and occasionally there is an abscopal effect on distant metastatic disease. These effects on non-injected tumor deposits are attributed to an antitumoral immune response enhanced by the release of antigens from tumor cells damaged or killed by the intratumoral injection.
Another local therapy technique for melanoma recurrences is electrochemotherapy (electroporation) [71,72,73,74,75]. First reported in 1987 [76], this technique involves intratumoral or systemic administration of a cytotoxic drug (usually bleomycin), and then application of an electrical field to tumor deposits using an array of needle electrodes. Transient permeability of the tumor cell membrane to the drug occurs, resulting in cell death [72]. Yet another form of local therapy for recurrent melanoma involves the topical application of diphencyprone. This was first reported by Damian and Thompson in 2007 [77] and is often effective in controlling or eliminating extensive but superficial melanoma recurrences, sometimes with an abscopal effect on metastases at remote sites.
Early Studies of Lymphatic Anatomy and Physiology Leading to Understanding of the Sentinel Node Concept
In 280 BC, the existence of the lymphatic system was first noted by Erasistratus, who described vessels that contained milky fluid and terminated in mesenteric lymph nodes, and considered them to be a form of blood vessel. Nearly two centuries later, lymphatic vessels in the mesentery were rediscovered by Gasparo Aselli in Italy. He too proposed that they were lacteal “veins.” In the early part of the seventeenth century, several investigators including Vessling, Folius, Tulp, Wallee, and Pecquet confirmed the existence of lymphatic vessels [78]. The Danish polymath Thomas Bartholin reported the existence of the thoracic duct in 1652, and appears to have been the first to clearly state that the lymphatic system was separate from the vascular system [79]. He proposed (correctly) that lymph originated from the blood by filtration. He was also the first to introduce the term “lymphatic”, referring to the lymph vessels that he observed as “vasa lymphatica.” Subsequently, a number of investigators attempted to map the lymphatic system, including Nuck (1650–1692), a professor of anatomy in the Netherlands, who injected mercury mixed with tin and lead into lymphatics to demonstrate their course towards lymph nodes [80].
The function of lymphatics and lymph nodes was essentially unknown until the studies of the famous German pathologist, Rudolf Ludwig Carl Virchow, in the mid-nineteenth century. In 1863, he proposed that lymph from any particular body site drained through lymphatics to specific lymph nodes, and then onwards to other lymph nodes [81]. This proposal was based on autopsy findings in a sailor who had a tattoo on the skin of his arm and was found to have obvious carbon pigment in a single axillary lymph node. The sentinel node concept is thus at least 150 years old!
Sentinel Lymph Node Biopsy: A New Gold Standard for Melanoma Staging
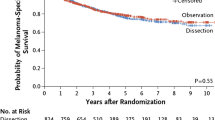
As previously discussed, “elective” lymph node dissection was widely practiced in patients with melanomas >1.5 mm in thickness until the 1980s. It was known that only about 20% of patients could possibly benefit, because only this percentage had metastatic disease in their regional nodes, but there was no way of identifying them. In 1992, Morton and Cochran et al. published a landmark article [82] describing the technique of sentinel node biopsy (SNB). This was practice-changing, and allowed the 20% of node-positive patients to be identified and offered a completion lymph node dissection. In 1994, a large international study, the first Multicenter Selective Lymphadenectomy Trial (MSLT-I), was commenced [83], and 2001 patients were randomized. The final results, published in 2014 [84] with 10 years minimum follow-up, showed no overall survival benefit for SN biopsy. However, there was a substantial survival benefit (compared with patients who were simply observed) in the subgroup of patients who had a positive SN followed by an immediate completion lymph node dissection (CLND). The great advantage of SN biopsy for all patients, however, was that it provided much better staging than had been possible previously, and allowed more accurate estimates of prognosis to be made. Consequently, SN status has become an essential part of the AJCC/UICC Melanoma Staging System. Whether CLND is necessary in all patients found to be SN positive is being assessed in another trial designed by Morton, the Second Multicenter Selective Lymphadenectomy Trial (MSLT-II). Initial results indicate that routine CLND in SN-positive patients does not improve survival outcome [85].
Reliable SN identification requires careful lymphatic mapping, and although Morton’s initial studies were with blue dye only, the importance of high-quality lymphoscintigraphy soon became apparent. Detailed lymphoscintigraphic studies by Uren, Thompson et al. in the 1990s, undertaken in the course of SN mapping for melanoma and breast cancer, provided important new insights into lymphatic anatomy that had major implications for melanoma surgery [86, 87].
Treatment of Metastatic Melanoma with Conventional Systemic Therapies: 1960 to 2010
Chemotherapy
For half a century, following the introduction of cytotoxic chemotherapy for solid human cancers in the 1950s, efforts in treating metastatic melanoma focused largely on attempts to use this class of drugs to achieve standard clinical endpoints: tumor shrinkage (response), durable response, improved progression-free survival, and prolonged overall survival. Clinical trialists recognized early that, as with other solid cancers, the most relevant of these was overall survival. Metastatic melanoma is relatively resistant to treatment with cytotoxic drugs, with no convincing evidence from randomized, controlled trials that any form of chemotherapy prolongs overall survival. Partial responses to single agents occurred in less than 25% of treated patients, and complete responses in less than 5% [88]. The median duration of response was 5–6 months. Although other cytotoxic drugs had similar or possibly slightly superior response rates, the s tandard of care throughout this period was single-agent dacarbazine (DTIC), yet in only 2% of patients treated with DTIC were long-term complete responses observed [89].
Immunotherapy
Adoptive immunotherapy using expanded pools of autologous tumor-infiltrating lymphocytes, when infused into selected immune-conditioned patients, showed clear activity against metastatic melanoma and, in a subgroup of selected patients, long-term disease control was observed [90]. This work by Steven Rosenberg at the National Cancer Institute, Surgery Branch in Bethesda, Maryland, as well as many others, clearly and convincingly established the proof of principle that immunotherapy had a central role in the treatment of metastatic melanoma. However, the intensive and costly nature of the original protocols and its associated toxicity led to its utilization only in highly selected centers.
Trials with interleukin-2, reported by Atkins et al., showed long-term disease control in a subgroup of patients with metastatic melanoma, leading to FDA approval of this cytokine [91]. Toxicity and lack of randomized, phase 3 data resulted in the regimen being confined to just a few centers, mostly in the United States. Other cytokines, like interferon-alpha, had limited activity as single agents, and when interferon-alpha and interleukin-2 were combined with chemotherapy (“biochemotherapy”) there was no survival advantage over single-agent dacarbazine [92], despite substantially greater toxicity.
The Early-Twenty-First-Century Revolution in Melanoma Treatment: Targeted Therapies and Immune Checkpoint Inhibitors
The frequency and potential importance of driver BRAF mutations in melanoma were known from the late 1990s, together with the fact that the MAP kinase pathway was constitutively activated in more than 90% of cases. Early attempts to use small molecules to block MAP kinase activation were made with fairly nonspecific “pan”-RAF inhibitors, such as sorafenib. These showed minimal single-agent clinical activity and a brief signal of synergy between sorafenib with other cytotoxic drugs failed to stand up to phase 3 testing [93]. It took a decade to develop potent, selective drugs to target and inhibit mutant BRAF and its downstream substrate kinase, MEK. The first of these selective agents, vemurafenib, was a landmark development, with the results of a phase 1 trial first presented in 2010 [94]. Its spectacular induction of rapid metabolic responses in metastatic melanoma resulted in front-page headlines in the New York Times. In around 80% of patients with a specific point mutation within the BRAF gene, there was substantial or even complete regression of bulky metastatic disease, far superior to dacarbazine, and a significant improvement in all clinical endpoints, including overall survival [95].
Subsequent refinements in MAP kinase targeting included the development of other effective mutant BRAF inhibitors like dabrafenib and encorafenib, with similar activity to vemurafenib, but with markedly different toxicity profiles. It was also demonstrated that dabrafenib was active against brain metastases [96], and that there was an extension of progression-free and overall survival when MEK inhibitors were combined with inhibitors of mutant BRAF [97]. The combination of BRAF and MEK inhibitors had the additional advantage of abrogating premalignant and malignant keratopathy from single-agent BRAF inhibitors. However, despite the massive progress achieved with the introduction of MAPK inhibitors, nearly all treated patients eventually developed resistance to them, with >50% showing disease progression within 12 months. This was due to reactivation of the MAPK pathway via alternative pathways. It was immediately recognized by clinicians and researchers worldwide that another strategy was clearly required.
Fortunately, in parallel developments, clinical trials were under way using monoclonal antibodies targeting the complex synapse between, on the one hand, T cells and antigen-presenting cells (the afferent arm of the immune response) and, on the other hand, T cells and melanoma cells (the efferent arm). The first of these so-called immune checkpoint inhibitors, ipilimumab, targeted the CTLA4 receptor, a cell surface molecule on T cells which mediates inhibition of T-cell activation in the afferent immune response. Despite achieving only low levels of RECIST tumor response, long-term disease control was achieved in a subset of patients with metastatic melanoma treated with ipilimumab. Phase 3 trials confirmed an overall survival benefit over dacarbazine, and pooled trial results showed a plateau on the Kaplan-Meier curve, with around 26% of treatment-naïve patients exhibiting progression-free survival at up to 10 years of follow-up [98].
A further landmark discovery was the demonstration that monoclonal antibodies inhibiting the synapse between the programmed death receptor 1(PD1) on T cells and its ligand, PDL1 on tumor cells, resulted in profound T-cell activation in many patients, with substantial consequent tumor cell death. The first two anti-PD1 drugs to be approved by the FDA were pembrolizumab (initially named lambrolizumab) and nivolumab (reviewed by Lee et al. [99]). Both drugs showed RECIST tumor responses in 30–40% of treated melanoma patients, with significant improvements in progression-free and overall survival in phase 3 trials. Patients achieving a complete response to therapy rarely relapsed within 3 years of follow-up. Further improvements in response rates and other clinical endpoints were obtained with combinations of the checkpoint inhibitors, ipilimumab/nivolumab and ipilimumab/pembrolizumab. Many more immune checkpoint inhibitors are in clinical development, with a vast array of potential combinations in the pipeline.
Summary and Conclusions
Cutaneous melanoma has been recognized as an entity for more than two millennia. Once considered to be an uncommon disease, its incidence worldwide has increased substantially since detailed population-based statistics began to be collected in the 1960s, and continues to increase. Surgery was the mainstay of treatment throughout the twentieth century, and even today 80% of patients are still cured by surgery alone. The extent of surgical intervention is clearly less invasive than previously, minimizing the potential for serious postsurgical side effects and complications. Whereas previously the outlook was dismal for those who developed inoperable metastatic disease, effective systemic therapies in the form of immunotherapy and checkpoint inhibitors have revolutionized our current approach to patients with metastatic melanoma. It is now a reality that a select group of patients with metastatic melanoma can achieve long-term, often lifetime, disease-free survival after such treatment. The history of melanoma continues to be written, as incredible advances in our treatment of this disease continue to be made.
References
Rebecca VW, Sondak VK, Smalley KS. A brief history of melanoma: from mummies to mutations. Melanoma Res. 2012;22:114–22.
Rotte A, Bhandaru M. Melanoma—introduction, history and epidemiology. Immunotherapy of melanoma. Cham: Springer International Publishing AG; 2016. p. 3–20.
Laennec RTH. Extrait au memoire de M Laennec, sur les melanoses. Paris: Bull L’Ecole Societie de Medicine; 1812; p. 24.
Gorantla VC, Kirkwood JM. State of melanoma: an historic overview of a field in transition. Hematol Oncol Clin North Am. 2014;28:415–35.
Urteaga O, Pack GT. On the antiquity of melanoma. Cancer. 1966;19:607–10.
McGovern VJ. Melanoblastoma. Med J Aust. 1952;1:139–42.
Paget J. Lectures on surgical pathology. In: Turner W, editor. London: Longman, Brown, Green and Longman; 1853. p. 639.
Moles and malignant melanoma: terminology and classification. Med J Aust. 1967;1:123–5.
de Vries E, Bray F, Coebergh JW, et al. Melanocytic tumours. In: PE LB, Burg G, Weedon D, Sarasin A, editors. World Health Organisation classification of tumours: pathology and genetics of skin tumours. Lyon: International Agency for Research on Cancer (IARC) Press; 2006. p. 49–120.
Clark WH Jr, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–27.
McGovern VJ. The classification of melanoma and its relationship with prognosis. Pathology. 1970;2:85–98.
Mihm MC Jr, Clark WH Jr, From L. The clinical diagnosis, classification and histogenetic concepts of the early stages of cutaneous malignant melanomas. N Engl J Med. 1971;284:1078–82.
Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953;6:1–45.
Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–8.
Gershenwald JE, Scolyer RA, Hess KR, et al. for members of the American Joint Committee on Cancer Melanoma Expert P, the International Melanoma D, Discovery P. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472–492
McGovern VJ, Mihm MC Jr, Bailly C, et al. The classification of malignant melanoma and its histologic reporting. Cancer. 1973;32:1446–57.
Arrington JH 3rd, Reed RJ, Ichinose H, et al. Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma. Am J Surg Pathol. 1977;1:131–43.
McGovern VJ, Cochran AJ, Van der Esch EP, et al. The classification of malignant melanoma, its histological reporting and registration: a revision of the 1972 Sydney classification. Pathology. 1986;18:12–21.
Bahmer FA, Bahmer JA. Cutaneous melanoma—“black death” of modern times? Traces in contemporary literature. Hautarzt. 2013;64:864–7.
Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma in the era of molecular profiling. Lancet. 2009;374:362–5.
Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47.
Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–96.
Norris W. Eight cases of melanosis with pathological and therapeutic remarks on that disease. London: Longman and Robarts; 1857.
Norris W. Case of fungoid disease. Edinburgh Med Surg J. 1820;16:562–5.
Begg CB, Orlow I, Hummer AJ, et al. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst. 2005;97:1507–15.
Pemberton O. On melanosis. Observations on the history, pathology and treatment of cancerous disease. London: John Churchill; 1858. p. 1–38.
Davis NC, Shaw HM, McCarthy WH. Melanoma: an historical perspective. In: Thompson JF, Morton DL, Kroon BBR, editors. Textbook of melanoma. London, UK: Martin Dunitz; 2004. p. 1–12.
Randle HW. Suntanning: differences in perceptions throughout history. Mayo Clin Proc. 1997;72:461–6.
Lancaster HO. Some geographical aspects of the mortality from melanoma in Europeans. Med J Aust. 1956;43:1082–7.
Cooper AGS. The contribution of radiotherapy to the problem of skin cancer in Queensland. Acta Radiol Suppl. 1959;188:61–70.
Sarnat BG, Schour I. Oral and facial cancer. Chicago: The Yearbook Publishers, Inc.; 1950.
Lancaster HO, Nelson J. Sunlight as a cause of melanoma; a clinical survey. Med J Aust. 1957;44:452–6.
Cooper S. The first lines of the theory and practice of surgery. London: Longman; 1840.
Home E. Observations on cancer, connected with histories of the disease. London: W. Bulmer and Co.; 1805.
Bodenham DC. A study of 650 observed malignant melanomas in the south-west region. Ann R Coll Surg Engl. 1968;43:218–39.
Fergusson A. Recurrence of a melanotic tumour: removal. Lancet. 1851;57:622.
McCarthy WH, Shaw HM. The surgical treatment of primary melanoma. Hematol Oncol Clin North Am. 1998;12:797–805.
Snow H. Melanotic cancerous disease. Lancet. 1892;140:872–4.
Eve F. A lecture on melanoma. Practitioner. 1903;70:165–74.
Handley WS. The pathology of melanotic growths in relation to their operative treatment. Lancet. 1907;169:927–33.
Pringle JH. A method of operation in melanotic tumours of the skin. Edinb Med J. 1908;23:496–9.
Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87–97.
Cascinelli N, Morabito A, Santinami M, et al. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–6.
Röntgen WC, Stokes GGB, Thomson JJ. Röntgen rays—memoirs by Röntgen, Stokes and JJ Thomson. New York and London: Harper & Brothers Publishers; 1899.
Becquerel H. Sur les radiations émises par phosphorescence. C R Acad Sci. 1896;122:420–1.
Curie P, Curie M, Bémont MG. Sur une nouvelle substance fortement redio-active, contenue dans la pecheblende. C R Acad Sci. 1898;127:1215–7.
Coutard H. The results and methods of treatment of cancer by radiation. Ann Surg. 1937;106:584–98.
Dewey DL. The radiosensitivity of melanoma cells in culture. Br J Radiol. 1971;44:816–7.
Barranco SC, Romsdahl MM, Humphrey RM. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971;31:830–3.
Johanson CR, Harwood AR, Cummings BJ, et al. 0-7-21 radiotherapy in nodular melanoma. Cancer. 1983;51:226–32.
Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13:589–97.
Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperth. 1996;12:3–20.
Larsson BS, Larsson B, Roberto A. Boron neutron capture therapy for malignant melanoma: an experimental approach. Pigment Cell Res. 1989;2:356–60.
Raja C, Graham P, Abbas Rizvi SM, et al. Interim analysis of toxicity and response in phase 1 trial of systemic targeted alpha therapy for metastatic melanoma. Cancer Biol Ther. 2007;6:846–52.
Lawton AW. Proton beam therapy for uveal melanoma. Ophthalmology. 1989;96:138–9.
Marucci L, Ancukiewicz M, Lane AM, et al. Uveal melanoma recurrence after fractionated proton beam therapy: comparison of survival in patients treated with reirradiation or with enucleation. Int J Radiat Oncol Biol Phys. 2011;79:842–6.
Yaeh A, Nanda T, Jani A, et al. Control of brain metastases from radioresistant tumors treated by stereotactic radiosurgery. J Neuro-Oncol. 2015;124:507–14.
Okwan-Duodu D, Pollack BP, Lawson D, et al. Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma. Am J Clin Oncol. 2015;38:119–25.
Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–32.
Kapma MR, Vrouenraets BC, Nieweg OE, et al. Major amputation for intractable extremity melanoma after failure of isolated limb perfusion. Eur J Surg Oncol. 2005;31:95–9.
Thompson JF, Waugh RC, Saw RPM, et al. Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Regional Cancer Treatment. 1994;7:188–92.
Kroon HM, Coventry BJ, Giles MH, et al. Australian multicenter study of isolated limb infusion for melanoma. Ann Surg Oncol. 2016;23:1096–103.
Coley WB. The therapeutic value of the mixed toxins of the streptococcus of erysipelas and bacillus prodigiosus in the treatment of inoperable malignant tumors. With a report of 160 cases. Am J Med Sci. 1896;112:251–81.
Nathanson L, Schoenfeld D, Regelson W, et al. Prospective comparison of intralesional and multipuncture BCG in recurrent intradermal melanoma. Cancer. 1979;43:1630–5.
Krown SE, Hilal EY, Pinsky CM, et al. Intralesional injection of the methanol extraction residue of bacillus Calmette-Guerin (MER) into cutaneous metastases of malignant melanoma. Cancer. 1978;42:2648–60.
Mastrangelo MJ, Sulit HL, Prehn LM, et al. Intralesional BCG in the treatment of metastatic malignant melanoma. Cancer. 1976;37:684–92.
Oratz R, Hauschild A, Sebastian G, et al. Intratumoral cisplatin/adrenaline injectable gel for the treatment of patients with cutaneous and soft tissue metastases of malignant melanoma. Melanoma Res. 2003;13:59–66.
Adler A, Stein JA, Kedar E, et al. Intralesional injection of interleukin-2-expanded autologous lymphocytes in melanoma and breast cancer patients: a pilot study. J Biol Response Mod. 1984;3:491–500.
Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional rose Bengal. Melanoma Res. 2008;18:405–11.
Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–8.
Allen BJ, Raja C, Rizvi S, et al. Intralesional targeted alpha therapy for metastatic melanoma. Role of electrochemotherapy in the treatment of metastatic melanoma and other metastatic and primary skin tumors [Review]. Cancer Biol Ther. 2006;5:118–9.
Byrne CM, Thompson JF, Johnston H, et al. Treatment of metastatic melanoma using electroporation therapy with bleomycin (electrochemotherapy). Melanoma Res. 2005;15:45–51.
Campana LG, Testori A, Mozzillo N, et al. Treatment of metastatic melanoma with electrochemotherapy. J Surg Oncol. 2014;109:301–7.
Dolinsek T, Prosen L, Cemazar M, et al. Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol Oncol. 2016;50:274–9.
Kunte C, Letule V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176(6):1475–85.
Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res. 1987;78:1319–21.
Damian DL, Thompson JF. Treatment of extensive cutaneous metastatic melanoma with topical diphencyprone. J Am Acad Dermatol. 2007;56:869–71.
Delamere G, Poirier P, Charpy A. The lymphatics: general anatomy of the Lymphatics, with special study of the Lymphatics in different parts of the body. Westminster: Archibald Constable & Co. Ltd; 1903.
Bartholin T. De lacteis thoracicis in homine brutisque nuperrime observatis, historia anatomica. Martzan, M: Copenhagen, Denmark; 1652.
Nieweg OE, Uren RF, Thompson JF. The history of sentinel lymph node biopsy. Cancer J. 2015;21:3–6.
Virchow R. Die Krankhaften Geschwulste. Berlin, Germany: Hirschwald; 1863.
Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9.
Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17.
Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609.
Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376:2211–2222.
Thompson JF, Uren RF, Shaw HM, et al. Location of sentinel lymph nodes in patients with cutaneous melanoma: new insights into lymphatic anatomy. J Am Coll Surg. 1999;189:195–204.
Uren RF, Howman-Giles RB, Shaw HM, et al. Lymphoscintigraphy in high-risk melanoma of the trunk: predicting draining node groups, defining lymphatic channels and locating the sentinel node. J Nucl Med. 1993;34:1435–40.
Hill GJ 2nd, Krementz ET, Hill HZ. Dimethyl triazeno imidazole carboxamide and combination therapy for melanoma. IV. Late results after complete response to chemotherapy (Central Oncology Group protocols 7130, 7131, and 7131A). Cancer. 1984;53:1299–305.
Coates AS, Segelov E. Long term response to chemotherapy in patients with visceral metastatic melanoma. Ann Oncol. 1994;5:249–51.
Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9.
Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16.
Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the eastern cooperative oncology group. J Clin Oncol. 2008;26:5748–54.
Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31:373–9.
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16.
Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–901.
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14.
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of Ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94.
Lee J, Kefford R, Carlino M. PD-1 and PD-L1 inhibitors in melanoma treatment: past success, present application and future challenges. Immunotherapy. 2016;8:733–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Thompson, J.F., Kefford, R., Stevens, G., Scolyer, R. (2018). History of Melanoma. In: Riker, A. (eds) Melanoma. Springer, Cham. https://doi.org/10.1007/978-3-319-78310-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-78310-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-78309-3
Online ISBN: 978-3-319-78310-9
eBook Packages: MedicineMedicine (R0)