Abstract
Osteochondral lesions represent one of the major causes of disabilities in the world. These defects are due to degenerative or inflammatory arthritis, but both affect the articular cartilage and the underlying subchondral bone. Defects from trauma or degenerative pathology frequently cause severe pain, joint deformity, and loss of joint motion. Osteochondral defects are a significant challenge in orthopedic surgery, due to the cartilage complexity and unique structure, as well as its exposure to high pressure and motion. Although there are treatments routinely performed in the clinical practice, they present several limitations. Tissue engineering can be a suitable alternative for osteochondral defects since bone and cartilage engineering had experienced a notable advance over the years. Allied with nanotechnology, osteochondral tissue engineering (OCTE) can be leveled up, being possible to create advanced structures similar to the OC tissue. In this chapter, the current strategies using nanoparticles-based systems are overviewed. The results of the studies herein considered confirm that advanced nanomaterials will undoubtedly play a crucial role in the design of strategies for treatment of osteochondral defects in the near future.
*These authors contributed equally to this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
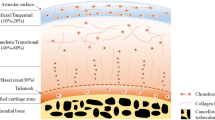
Osteochondral tissue defects affect both the articular cartilage and the underlying subchondral bone (Fig. 9.1). Usually, these defects are due to degenerative or inflammatory arthritis and represent one of the major causes of disabilities in the world [1, 2].
Osteochondral tissue structure . (A) Superficial zone , (B) middle zone, (C) deep zone and calcified cartilage, and (D) subchondral bone . (Adapted with permission [10])
Osteochondral lesions lead to the formation of fibrocartilage with different biomechanical properties from the native hyaline cartilage and do not protect the subchondral bone from further degeneration. Damage from trauma or degenerative pathology frequently causes severe pain, joint deformity, and loss of joint motion [3]. Osteochondral tissue repair requires an advanced knowledge of how bone and cartilage interact, that is, comprehension of the osteochondral interface and its combined yet separate mechanical strengths, structure, and biology [4, 5].
Osteochondral defects are a big challenge in orthopedic surgery , due to the cartilage complexity and unique structure, as well as its exposure to high pressure and motion. Current clinical treatments comprise palliative methods (arthroscopic debridement), intrinsic repair enhancement (microfracture), tissue transplantation (osteochondral autograft), and cell-based tissue repair (autologous chondrocyte implantation) [6].
Despite the progress in clinical treatment , there are several limitations, such as the amount of material available, the donor site morbidity, and the difficulty to match the topology of the grafts with the injured site [7]. Furthermore, the absence of a scaffold able to guide cell differentiation and support the secretion of a structurally coherent ECM, simultaneously with the inherent predisposition of chondrocytes to dedifferentiate into fibroblast-like phenotypes upon ex vivo expansion, represents a big limitation of current clinical treatments [8]. Clinical treatments generally need long postoperative treatments, with limited mechanical loading, until tissue remodeling is achieved at the defect site, and, although patients initially demonstrate improvement, studies show that the functionality of the tissue is not improved in the long term [9].
Tissue engineering takes advantage of biomaterials, scaffolds, and cells to regenerate injured tissue, and it is a suitable alternative for osteochondral defects. The study of both bone engineering and cartilage engineering has experienced a great advance over the years, and it is improving the understanding of tissue engineering materials and biology [10].
Strategies to osteochondral repair using tissue engineering typically involve nanoparticles-based system. These systems play a key role in the repair of osteochondral defects and must have a number of criteria such as possess similar mechanical properties as the native tissue; support the growth and proliferation of cells; be biocompatible, non-immunogenic in vivo; and remain integrated in the defect while subjected to repetitive physiological loads, till the tissue repair is complete [11].
Herein, the current osteochondral tissue engineering (OCTE ) strategies using nanoparticles-based systems are overviewed.
2 Nanoparticles-based Systems for Osteochondral Repair
Nanotechnology opens up a new era for tissue engineering (TE), with nanoparticles (NPs) being applied in several approaches, from scaffold construction to drug delivery or cell tracking. This versatility is a result of the several NP available and countless functionalization reactions that can be used to tailor NP for a desired application. These include antibodies, labeling probes, hydrophobic or hydrophilic molecules, DNA, and/or oligonucleotides [12].
Considering its nature, NP can be divided as organic and inorganic. Organic NP can include liposomes and polymeric NP. These are mostly used as delivery systems or reservoirs . On the other hand, inorganic NPs comprise silica and metallic NP, bioceramic and bioactive glass NP, carbon nanotubes, and quantum dots (QD) [13]. Although all of them are of inorganic nature, they have distinct properties and, consequently, different applications that will be further discussed in this chapter.
2.1 NP for Scaffolding Applications
Anatomically, the bone can be considered as a hierarchical complex nanocomposite, with an organic extracellular matrix, strengthened by inorganic calcium phosphate NPs, namely, hydroxyapatite (HA) crystals [14]. Hence, bioceramic NPs, like HA or tricalcium phosphate (TCP) , are broadly applied in biomedical field alone or combined with natural [15, 16] or synthetic polymers [17, 18]. This combination results into a nanocomposite [19], usually with a similar structure to the one observed in bone. These biomaterials usually hold superior mechanical properties [20] which are very attractive for bone tissue engineering strategies. Indeed, bone-inspired hybrid scaffolds , comprising an organic and an inorganic portion with similar size and functionality as natural nanosized inorganic ceramic particles, have been considered as a potential approach to mimic the bone part of OC region.
Following this rationale, Nowicki et al. [18] combined fused deposition modeling technique (FDM) and nanocrystalline HA (nHA) to design a scaffold with tunable porosity and improved bone marrow human mesenchymal stem cell (hMSC) adhesion, growth, and osteochondral differentiation. The nanocomposite scaffold was constituted by a mix of poly(ethylene glycol) (PEG) and PEG diacrylate, as organic phase, and an nHA equivalent of 60% wt of PEG-DA as inorganic part. Bare scaffolds, i.e., without inorganic phase, were also studied to mimic the cartilaginous part of the OC region. Using a supplemented media with chondrogenic factors as culture media, it was possible to observe that the presence of nHA enhanced the osteogenic differentiation of hMSC but inhibited chondrogenic differentiation. On the other hand, cells cultured in scaffolds void of nHA showed enhanced chondrogenic differentiation rather than osteogenic.
In another approach, Amadori et al. [16] combined gelatin, a natural polymer, with nHA, using a gradient of these NPs to better mimic the OC anatomy. The scaffold is obtained using a bottom-up strategy where different layers of gelatin, combined with a decreasing gradient of nHA (50% wt., 30% wt., and 0% wt.), are glued together to obtain a multilayer scaffold. Such scaffolds showed interconnected porosity, which was not affected by the presence of nHA. Conversely, the mechanical performance of the resulting scaffolds was greatly improved by the presence of an inorganic phase. As expected, the presence of nHA pushed hMSC, seeded on these scaffolds, into an osteogenic lineage, while on the layer without nanoparticles, cells differentiated into a chondrogenic lineage.
Another interesting method was considered by Mellor et al. [21]. After concluding that an elevated extracellular concentration in calcium promoted the osteogenic differentiation of human adipose stem cells (hASC) but inhibited chondrogenic differentiation, the authors engineered a scaffold with site-specific calcium concentrations. For that, the authors used polylactic acid nanofibers, obtained by electrospinning, containing either 0% or 20% of TCP nanoparticles corresponding to low and elevated calcium concentration, respectively. Scaffolds were then seeded with hASC and cultured in presence of chondrogenic differentiation medium. Under such conditions, hASC differentiated locally into cartilage in the layers with no TCP and generated calcified tissue in layers containing 20% TCP, as anticipated.
2.2 NP for Imaging
Another application of NP is as imaging tracking agents. Either incorporated within a scaffold or internalized by cells, NPs appear as a good alternative for noninvasive tracking of TE constructs [13]. Different NPs can be used as contrast agents, being the most common: the magnetic nanoparticles [22, 23], gold NP [24], mesoporous silica NP [25], and quantum dots (QDs) [26]. The detection method is dependent on the type of NP used and can include magnetic resonance (MRI) , computed tomography (CT) , and photo-acoustic imaging.
Magnetic nanoparticles (MNP) are composed by magnetic elements such as iron, nickel, cobalt, and their oxides [27, 28]. Typically, MNP are detected using MRI techniques due to their magnetism and strong contrast enhancement effects [27]. Since MNP can be functionalized at surface level, these particles can be modified to recognize specific targets or to include fluorescent probes for multimodal imaging. Thence, these NPs are a very versatile tool for imaging processes, cell tracking and isolation, biosensors, guided drug and gene delivery, 3D cell organization, and hyperthermia [28]. Nevertheless, care must be taken when a biological application of NP is envisaged, as particles can be toxic for cells or may alter their phenotype [29].
To access whether MNP can be used together with rabbit chondrocytes, Su et al. [30] labeled these cells with commercial iron oxide magnetic nanoparticles and further analyzed cell proliferation, viability, and differentiation capacity. After finding an optimal concentration (250 μg/ml), the authors concluded that, at this concentration, MNP did not affect cell morphology, viability, or phenotype. Additionally, the authors took advantage of the magnetic properties of NP to guide cells inside of a biphasic scaffold made by type II collagen-chitosan/PLGA.
2.3 NP as Delivery Vehicles
Nanoparticles-based systems have the potential to provide more effective tissue regeneration when compared to the existing therapies. Systems containing nanoparticles loaded with bioactive agents can be used for their local delivery, enabling site-specific pharmacological effects, for instance, the induction of cell proliferation and differentiation and, therefore, neo-tissue formation, such as cartilage repair and bone regeneration [31].
As aforementioned, magnetic NP can be used to take advantage of their inherent magnetic properties, but they can also be functionalized to deliver specific molecules to cells. Taking advantage of this, Zhang et al. [32] developed a new Fe3O4 magnetic nanoparticle coated with nanoscale graphene oxide to label stem cells and delivery growth factors. These nanoparticles were successfully fabricated, with an average diameter of 10 nm, and exhibited a core-shell structure and kept high-saturation magnetization values. The Fe3O4 magnetic nanoparticle coated with nanoscale graphene oxide did not affect the viability and proliferation of dental-pulp stem cells (DPSCs) , and nanoparticle-labeled cells could be organized via magnetic force to form multilayered cell sheets with different patterns. When compared to traditional Fe3O4 nanoparticles, the graphene oxide coating gives abundance of carboxyl groups to bind and deliver growth factors. With these Fe3O4 magnetic nanoparticle coated with nanoscale graphene oxide, bone-morphogenetic-protein-2 (BMP2) is favorably included into DPSC sheets to promote more bone formation. Moreover, an integrated osteochondral complex is also constructed using a combination of DPSCs/TGF-β3 and DPSCs/BMP2. This study showed that Fe3O4 magnetic nanoparticle coated with nanoscale graphene oxide supports a novel magnetically controlled vehicle for stem cells and growth factors to construct protein-immobilized cell sheets, and they have promising potential for future use in regenerative medicine.
Other recent study developed a bilayer scaffold, in order to promote the regeneration of osteochondral tissue within a single integrated construct. For the subchondral bone layer, organic type I collagen and the inorganic hydroxyapatite were used to mimic the bone matrix. A composite scaffold was made through mineralization of hydroxyapatite nanocrystals, with oriented growth on collagen fibrils. For that, a multi-shell NP system with different layers was developed, comprised by a calcium phosphate core and a DNA/calcium phosphate shell conjugated with polyethyleneimine to operate as nonviral vectors for delivery of plasmid DNA encoding BMP2 and TGF-β3. For this, it was used microbial transglutaminase as a cross-linking agent to cross-link the bilayer scaffold. The results showed that the produced scaffold has the capacity to promote transfection of human mesenchymal stem cells (hMSCs) and the functional osteochondral tissue formation. Moreover, the sustained release of plasmids, BMP2 and TGF-b3, from gene-activated matrix could induce prolonged transgene expression and stimulate hMSC differentiation into osteogenic and chondrogenic lineages by spatial and temporal control manner. This system may increase the functionalization composite graft to accelerate healing process for osteochondral tissue regeneration [33]. This work is a great example of the versatility of NP, since they were used not only to mimic the natural anatomy of the subchondral bone layer but also to simultaneously deliver nonviral vectors, improving the outcome of the system.
Polymeric NPs have been also greatly used in recent years, owing to their bulk physical properties, tunable architecture, and biodegradability. Synthesis of polymeric NP is commonly an easy and flexible process, allowing the incorporation of a wide range of molecules [12]. It is possible to produce NP with different shapes including nanofibers [34] and spherical NPs [35], either nanocapsules or nanospheres. Typically, this type of particles is used for transport and/or delivery of molecules, since they are characterized by a high drug-loading capacity and can be easily functionalized to perform an active targeting. Considering the above, Wang et al. [36] studied chitosan nanoparticles and electrospun fiber scaffolds as a sustained release system of Nel-like molecule-1(Nell-1) growth factor, protecting its bioactivity. Then, the effect and process of Nell-1 on inducing human bone MSC (hBMSC) differentiate toward chondrocytes were analyzed. The results showed that scaffolds mimicked the oriented structure of native articular cartilage by regulating cell adhesion and distribution . In release and bioactivity protection study, the incorporation of Nell-1 into chitosan nanoparticles significantly prolonged the release time and increased the bioactivity of the released growth factor, as compared to the scaffolds with free Nell-1. Furthermore, in vitro chondrogenesis study proved that Nell-1 increases hBMSC chondrogenic differentiation and extracellular matrix production. This study showed the potential ability of Nell-1 integrated dual release scaffold for cartilage tissue engineering.
3 Conclusions
In this chapter, several alternatives that can be potentially used for the treatment osteochondral defects, rather than traditional treatments, are presented. Many techniques using nanoparticles have been developed for the treatment osteochondral tissues. The results of recent studies allow confirming that the use of nanoparticle systems is very promising, due to their physicochemical and biological properties for OCTE.
Undoubtedly, nanotechnology is a breakthrough in the TE field, and OCTE is not an exception. Being a very complex tissue, the application of NP can be justified by different aims, which is only possible because of the large variability concerning NP synthesis, nature, and properties.
As part of the OC tissue is composed by bone, this field of TE particularly benefits of NP usage, as they can mimic the nanosized inorganic phase present in the bone. The reinforced scaffolds present not only better mechanical properties but also a commitment for the osteogenic lineage. Despite the encouraging results of most the studies herein discussed, it is still a great challenge to pass these nanosystems to clinical applications. Yet it is possible to state that advanced nanomaterials, in the future, will certainly play a crucial role on the design of strategies for treatment of osteochondral defects.
References
Castro NJ, Hacking SA, Zhang LG (2012) Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng 40(8):1628–1640
Khan MS, Vishakante GD, Siddaramaiah H (2013) Gold nanoparticles: a paradigm shift in biomedical applications. Adv Colloid Interface Sci 199–200:44–58. https://doi.org/10.1016/j.cis.2013.06.003
Panseri S, Russo A, Cunha C et al (2012) Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg SportTraumatol Arthrosc 20(6):1182–1191
Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP (2012) Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng Part A 18:1376. https://doi.org/10.1089/ten.tea.2011.0076
Nukavarapu SP, Amini AR (2011) Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Boston, MA, USA.
Nejadnik H, Daldrup-Link HE (2012) Engineering stem cells for treatment of osteochondral defects. Skeletal Radiol 41:1
Martin I, Miot S, Barbero A et al (2007) Osteochondral tissue engineering. J Biomech 40(4):750–765
Makris EA, Gomoll AH, Malizos KN et al (2014) Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11:21. https://doi.org/10.1038/nrrheum.2014.157
Camarero-Espinosa S, Cooper-White J (2017) Tailoring biomaterial scaffolds for osteochondral repair. Int J Pharm 523:476. https://doi.org/10.1016/j.ijpharm.2016.10.035
Nukavarapu SP, Dorcemus DL (2013) Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv 31:706. https://doi.org/10.1016/j.biotechadv.2012.11.004
Degoricija L, Bansal PN, Söntjens SHM et al (2008) Hydrogels for osteochondral repair based on photocrosslinkable carbamate dendrimers. Biomacromolecules 9:2863. https://doi.org/10.1021/bm800658x
Nicolas J, Mura S, Brambilla D et al (2013) Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev 42:1147–1235. https://doi.org/10.1039/C2CS35265F
Vieira S, Vial S, Reis RL, Oliveira JM (2017) Nanoparticles for bone tissue engineering. Biotechnol Prog 33:590–611. https://doi.org/10.1002/btpr.2469
Alves Cardoso D, Jansen JA, Leeuwenburgh SCG (2012) Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J Biomed Mater Res Part B Appl Biomater 100B:2316–2326. https://doi.org/10.1002/jbm.b.32794
Yan L-P, Silva-Correia J, Correia C et al (2012) Bioactive macro/micro porous silk fibroin/nano-sized calcium phosphate scaffolds with potential for bone-tissue-engineering applications. Nanomedicine 8:359–378. https://doi.org/10.2217/nnm.12.118
Amadori S, Torricelli P, Panzavolta S et al (2015) Multi-layered scaffolds for osteochondral tissue engineering: in vitro response of co-cultured human mesenchymal stem cells. Macromol Biosci 15:1535–1545. https://doi.org/10.1002/mabi.201500165
Gaharwar AK, Dammu SA, Canter JM et al (2011) Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 12:1641–1650. https://doi.org/10.1021/bm200027z
Nowicki MA, Castro NJ, Plesniak MW, Zhang LG (2016) 3D printing of novel osteochondral scaffolds with graded microstructure. Nanotechnology 27:414001. https://doi.org/10.1088/0957-4484/27/41/414001
Pina S, Oliveira JM, Reis RL (2015) Natural-based nanocomposites for bone tissue engineering and regenerative medicine: a review. Adv Mater 27:1143–1169. https://doi.org/10.1002/adma.201403354
Verma S, Domb AJ, Kumar N (2011) Nanomaterials for regenerative medicine. Nanomedicine (Lond) 6:157–181. https://doi.org/10.2217/nnm.10.146
Mellor LF, Mohiti-Asli M, Williams J et al (2015) Extracellular calcium modulates Chondrogenic and osteogenic differentiation of human adipose-derived stem cells: A novel approach for osteochondral tissue engineering using a single stem cell source. Tissue Eng Part A 21:2323. https://doi.org/10.1089/ten.tea.2014.0572
Fan J, Tan Y, Jie L et al (2013) Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res Ther 4:44. https://doi.org/10.1186/scrt191
Lalande C, Miraux S, Derkaoui SM et al (2011) Magnetic resonance imaging tracking of human adipose derived stromal cells within three-dimensional scaffolds for bone tissue engineering. Eur Cell Mater 21:341–354
Meir R, Motiei M, Popovtzer R (2014) Gold nanoparticles for in vivo cell tracking. Nanomedicine 9:2059–2069. https://doi.org/10.2217/nnm.14.129
Shen Y, Shao Y, He H et al (2013) Gadolinium3+−doped mesoporous silica nanoparticles as a potential magnetic resonance tracer for monitoring the migration of stem cells in vivo. Int J Nanomedicine 8:119–127. https://doi.org/10.2147/IJN.S38213
Wegner KD, Hildebrandt N (2015) Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev 44:4792. https://doi.org/10.1039/C4CS00532E
Shin T-H, Choi Y, Kim S, Cheon J (2015) Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem Soc Rev 44:4501. https://doi.org/10.1039/C4CS00345D
Colombo M, Carregal-Romero S, Casula MF et al (2012) Biological applications of magnetic nanoparticles. Chem Soc Rev 41:4306
Pandey RK, Prajapati VK (2018) Molecular and immunological toxic effects of nanoparticles. Int J Biol Macromol 107(Pt A):1278–1293
Su JY, Chen SH, Chen YP, Chen WC (2017) Evaluation of magnetic nanoparticle-labeled chondrocytes cultivated on a type II collagen–chitosan/poly(lactic-co-glycolic) acid biphasic scaffold. Int J Mol Sci 18. https://doi.org/10.3390/ijms18010087
Monteiro N, Martins A, Reis RL, Neves NM (2015) Nanoparticle-based bioactive agent release systems for bone and cartilage tissue engineering. Regen Ther 1:109. https://doi.org/10.1016/j.reth.2015.05.004
Zhang W, Yang G, Wang X et al (2017) Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv Mater 29:1703795. https://doi.org/10.1002/adma.201703795
Lee Y-H, Wu H-C, Yeh C-W et al (2017) Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater 63:210–226. https://doi.org/10.1016/J.ACTBIO.2017.09.008
Yoo HS, Kim TG, Park TG (2009) Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev 61:1033–1042. https://doi.org/10.1016/j.addr.2009.07.007
Rao JP, Geckeler KE (2011) Polymer nanoparticles: Preparation techniques and size-control parameters. Prog Polym Sci 36:887–913. https://doi.org/10.1016/j.progpolymsci.2011.01.001
Wang C, Hou W, Guo X et al (2017) Two-phase electrospinning to incorporate growth factors loaded chitosan nanoparticles into electrospun fibrous scaffolds for bioactivity retention and cartilage regeneration. Mater Sci Eng C 79:507. https://doi.org/10.1016/j.msec.2017.05.075
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Oliveira, I., Vieira, S., Oliveira, J.M., Reis, R.L. (2018). Nanoparticles-Based Systems for Osteochondral Tissue Engineering. In: Oliveira, J., Pina, S., Reis, R., San Roman, J. (eds) Osteochondral Tissue Engineering. Advances in Experimental Medicine and Biology, vol 1059. Springer, Cham. https://doi.org/10.1007/978-3-319-76735-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-76735-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76734-5
Online ISBN: 978-3-319-76735-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)