Abstract
Osteochondral (OC) defects are prevalent among young adults and are notorious for being unable to heal. Although they are traumatic in nature, they often develop silently. Detection of many OC defects is challenging, despite the criticality of early care. Current repair approaches face limitations and cannot provide regenerative or long-standing solution. Clinicians and researchers are working together in order to develop approaches that can regenerate the damaged tissues and protect the joint from developing osteoarthritis. The current concepts of tissue engineering and regenerative medicine, which have brought many promising applications to OC management, are overviewed herein. We will also review the types of stem cells that aim to provide sustainable cell sources overcoming the limitation of autologous chondrocyte-based applications. The various scaffolding materials that can be used as extracellular matrix mimetic and having functional properties similar to the OC unit are also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Osteochondral defects
- Osteochondral tissue engineering
- Regenerative medicine
- Chondrocytes
- Stem cell therapy
- iPS cells
- Scaffold design
- and Bilayered scaffolds
1 Introduction
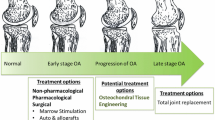
OC lesions are notorious for being unable to heal. They mostly affect the knee and the ankle. Usually the defects appear when the joint is used extensively, as caused by repetitive strain or direct trauma to the articulation, typical for sports activity. Genetic predisposition could be another factor responsible for OC damage [1]. Comorbidities of OC defects include joint malalignment, meniscal tear and ligamentous laxity [2]. In many cases these concomitant pathologies occur before the cartilage lesion, and known as contributors of the lesion development. Although the exact aetiology of OC defects has not yet been fully elucidated, researchers agree on two distinct phenotypes, manifested in degenerative lesions or traumatic focal defects [3, 4]. In either case, OC damages put the joint in a great risk of developing osteoarthritis; therefore, early recognition of the presence of any lesion or damage to any joint is of paramount importance [5, 6], especially because OC damages are more prevalent among adolescents and the physically more active younger population [7, 8]. Articular cartilage defects can be of many sizes and shapes and vary in depths. In most severe cases, the defects reach down to the subchondral bone. Their diagnosis is challenging, as many lesions can exist without any symptoms, and their presence is often missed in the early stages [9, 10]. Unfortunately, even the silent lesions usually progress from partial to focal defects if they are left untreated (Fig. 1.1). Having OC lesions can often cause symptoms such as discomfort, tenderness or, in more severe cases, pain, swelling of the knee and limitation in motion of the patient [11].
To establish consistency and aid communication among physicians, there were several grading scales developed, which describe the degree, severity and sometimes the location of the cartilage lesions. The most frequently used classification system was invented by Outerbridge, which was named after him, called the Outerbridge scaling system (Table 1.1) [12]. This system divides the lesions into four categories, but it does not characterize the depth of the lesion. Another widely used scaling system was developed by the International Cartilage Repair Society (ICRS) , which also has four grades, complemented with a zero state marking the normal state of the cartilage. The advantage of the ICRS scaling over the Outerbridge scaling is that it describes the lesions based on their extent as well as depth (Table 1.2) [4, 13, 14].
For the management of OC lesions, there are several conservative and operative techniques, which are applied according to the extent of damage, age of the patient, doctor’s opinion and some other factors. These approaches have a common goal of providing pain relief, repairing the damaged tissues and improving joint functionality [15]. The conservative management of cartilage lesions includes medications to fight pain and inflammation, drilling techniques, abrasion, microfracture as well as the transplantation of OC allografts and autologous chondrocyte implantation with or without matrix [16]. Despite their continuing use in the clinics, none of the mentioned approaches brought long-term solutions so far and could not fully regenerate the OC unit. In fact, according to prospective and retrospective studies, around 60% of knee arthroplasties performed were a result of previous cartilage lesions in the joint [17]. Knee arthroplasty is the most common clinical approach used to restore the joint function. Annually there are 700,000 arthroplasties performed in the USA, and it is estimated that this number will further increase, as much as reaching 3.48 million by the year 2030 [17]. It was indicated that 5% of all knee illnesses in the general population originated from previous cartilage injuries, while 61% of the cases had OC damages, among which 19% could be specified as focal OC injury [18]. It is therefore leaving no doubt that the burden of OC injuries on today’s healthcare and society is huge and its management requires the development of more effective techniques.
In this chapter, we will be briefly discussing the basics of current approaches to diagnose and treat OC defects, but our main focus will be on the future therapeutic regimens of OC lesion management with special focus on the knee joint. We will describe how the advancement of tissue engineering and regenerative medicine (TERM) brought new aspects to articular cartilage healing and will demonstrate the main components of these approaches, namely, the cell sources and matrices used in TERM .
2 Knee Joint
The largest and most complex joint of the body is the knee. It is a modified hinge joint, which actually consists of two articulations, namely, the patellofemoral and tibiofemoral [19, 20]. The knee is subjected to a great load while walking, jumping, running or performing other activities [21]. It provides stability via a combination of ligaments, tendons, synovial capsule and muscular components [22]. The osseous part is composed of the tibia, fibula, femur and patella. The surfaces of the joints are covered by articular cartilage, which allows frictionless movement [23, 24]. For extra cushioning, there is a pair of moon-shaped fibrocartilage tissues, called the menisci, between the femoral condyles and tibial plateau. Both the AC and the menisci have important shock-absorbing function in the joint [25].
2.1 Articular Cartilage
Articular cartilage is a type of hyaline cartilage which covers the joint surface providing gliding to it. It is a thin layer of tissue ranging from 3 to 7 mm of thickness at the various anatomical sites [25, 26]. The AC has a white, glassy appearance and lacks both vascularization and innervation in its healthy state. Despite its simple appearance, articular cartilage is a complex and highly specialized tissue. It consists of four layers, each of which bears with a unique structure. The main macromolecular components of the cartilage are the collagen and proteoglycan molecules, which create distinctive patterns at the different zones of the tissue and thus also define functional differences among the layers [27, 28]. Aggrecan is the most abundantly found proteoglycan molecule of the articular cartilage, while among collagen molecules, type II collagen is the most typical [29, 30]. The only cell type found in the cartilage, are the chondrocytes , which possess different properties at the different layers of the tissue. Accordingly, the (i) uppermost layer called the superficial or tangential zone has rather flattened cells localized densely. Both the cells and collagen fibrils lie parallel to the surface herein. This layer has lesser amounts of proteoglycan molecules, among which biglycan and decorin are the most representative [31, 32]. The superficial layer makes up 10–20% of the whole cartilage. The chondrocytes found in this layer also produce a protein called lubricin, which is a mucous glycoprotein. Lubricin serves as a lubricant on the cartilage surface and together with the superficial zone protein creates a film which equips the cartilage with the mentioned low-friction properties [24, 33]. This layer is also rich in fibronectin and water. The (ii) intermediate zone builds up 40–60% of the total volume of the cartilage. The cells are localized less dense, have a spheroid morphology and are larger as compared to the upper layer [34]. The extracellular matrix is abundant in proteoglycan and thick collagen fibrils. The organization of the fibrils show less structure [35, 36]. The (iii) deep or radial zone of the articular cartilage makes up 30% of the total tissue volume. This layer has the least amount of cells and water, but the most of the proteoglycan molecules and the largest collagen fibrils [37, 38]. The chondrocytes are stack in columns and have spherical shapes. Both the collagen molecules and chondrocytes lie perpendicular to the cartilage surface. The (iv) calcified cartilage is located beneath the radial zone, also called the basal layer [25, 39, 40]. It is separated from the radial zone by the tidemark, which is narrow layer, metabolically active for the calcification. The tidemark is an important interface between the soft and calcified cartilage. It also has a crucial role in damping the loads. The calcified cartilage creates a connection between the hyaline cartilage and subchondral bone [41, 42]. This layer has a sparse amount of chondrocytes , which are smaller in size and have a hypertrophic phenotype. These chondrocytes are metabolically less active and are completely embedded in the extracellular matrix. Most typical collagen in this zone is the collagen type X [43, 44]. Collagen type X is believed to have an important role in mechanical load transmission [31, 45]. Any structural change to the articular cartilage as a result of either biomechanical or biochemical changes will ultimately result in poor mechanical properties and cartilage degeneration.
2.2 Subchondral Bone
The subchondral bone is located underneath the calcified cartilage. Together they compose the OC unit. The AC acts in concert with the subchondral bone to absorb the mechanical load in the joint [46]. The subchondral bone consists of two anatomical entities, the subchondral plateau and the subchondral spongiosa [41]. The subchondral bone is richly perforated by veins and arteries as well as by nerves. The subchondral bone is an important exchange interface for the AC. It has been demonstrated that the AC and the subchondral bone have an intense crosstalk, especially during the onset of joint degeneration [40, 47, 48].
3 Limitations of Current Clinical Approaches
There are several major limitations to effective treatment of OCDs, and their diagnosis is also extremely challenging in many cases. Often subtle injuries show little or no dysfunction, which results in their delayed detection. But even if diagnosis is performed accurately, due to the low intrinsic capacity of the cartilage, conservative care is often unable to provide a long-term solution. Many of the current techniques focus on symptom management and functional improvement of the joint [7, 49]. Common treatment strategies are the use of non-steroidal anti-inflammatory drug (NSAID) agents , which are often used together with cast immobilization, or in cases where surgical intervention is required microfracture, drilling techniques and the use of allografts or autologous chondrocyte transplants are the routined applications [16].
3.1 Diagnosis of OC Lesions
Patients with acute joint injury usually suffer from pain, swelling and joint effusion, which appear as the consequence of the influx of inflammatory mediators. They usually are also limited in motion and have the joint inflexible in many cases or report mechanical symptoms such as clicking and locking of the knee. OC injury often goes together with the damage of the menisci and the ligaments or results in loose bodies in the joint cavity. In many cases on the other hand, OC lesions can go unnoticed, having no symptoms, and thus being challenging to diagnose [28].
Currently, the diagnosis of OC lesions relies on patient medical history, patient self-assessment, symptomatic findings, furthermore radiographic and magnetic resonance imaging, as well as arthroscopy [50]. Plain radiography is usually considered to be the gold standard as it is largely available and cost-sensitive [51, 52]. However, radiographic images may not depict the presence of small superficial cracks but can only identify more prominent lesions and loose bodies in the joint cavity. Magnetic resonance imaging (MRI) is a more precise method allowing higher resolution and thus providing more information but is also more costly [9, 53]. MRI often goes together with the application of X-ray computed tomography (CT) [54]. Both MRI and CT can use contrast agents to enhance tissue differentiation and enable better evaluation. Contrast agents are especially important for the visualization of cartilage and other soft tissue components in the joint [54, 55]. There is a large variety of contrast agents available. The use of metal chelates, i.e. gadolinium-based agents, is common for MRI application, while for CT iodine-based agents such as sodium iodide and sodium diatrizoate hydrate are the most established [56,57,58,59,60]. Arthroscopy is an intra-articular method used to visualize and examine the cartilage pathology. Arthroscopy is performed by orthopaedic surgeons, and during the procedure a small camera is led to the joint cavity via a small incision, which can accurately locate and classify the damage [61, 62]. Given its in situ nature, arthroscopy is a very precise technique, but consequently it is also more invasive compared to other imaging approaches. However, arthroscopy is not only used for diagnosis but also for the therapy of the OC tissue which we will introduce in the next section [61, 62].
Taken together all possibilities for diagnosis, unfortunately it is still a hurdle to detect less pronounced cartilage damages. Early diagnosis and accurate evaluation of articular lesions should be immediate, as all defects can eventually progress to focal lesions.
3.2 Conservative and Surgical Care
OC injuries are traumatic in nature. When patients present with OC injury without any severe symptoms, typically ICRS grades I and II (Table 1.2), they are usually advised to rest, and often cast is used to immobilize the joint. The treatment regimen also includes the use of NSAIDs. The goal is to help the potential oedema resolve and avoid the development of any necrosis [49, 63]. In grades III and IV, the injury is accompanied by pain and larger damage to the joint; therefore, usually a surgery is imperative. Current surgical concepts for the restoration of articular cartilage structure and function mostly include approaches such as arthroscopic lavage and debridement, drilling, microfracture and the implantation of OC allografts and autologous chondrocytes [64,65,66,67].
Arthroscopic lavage and debridement involves the incision of the joint and insertion of a small camera, called the arthroscope, for the evaluation of damage as mentioned before. When the location and degree of defect are assessed, it is washed thoroughly to remove all loose bodies and damaged OC tissue from the cavity [68, 69]. Microfracture and drilling are marrow stimulation techniques and are used in an attempt to initiate cartilage regeneration [70, 71]. After perforating the subchondral bone, the blood is drained into the joint covering the cartilage surface and lesions with stem cells and bioactive molecules, such as growth factors which are believed to facilitate the cartilage repair capacity. The drilling holes are in close proximity to each other, approximately 3–4 mm apart [72, 73]. The ultimate goal is to reproduce biomechanically functioning cartilage. However, this procedure mostly leads to the formation of fibrocartilage only, which has knowingly reduced capacity to bear loading. OC graft implantation is used when there are larger defects present with size around 2 cm2 [66, 74]. Initially, autografts were harvested from the same patient’s intact joint region, usually from the patellofemoral site. However, due to anatomical misalignment, this procedure often failed. Advancement was brought by the multiple use of cylindrical shape osseous grafts, a procedure called mosaicplasty, developed by Hangody and Bobić [75, 76]. Further popularity of the usage of graphs came when standardization of tissue and cell storage was established by the American Association of Tissue Bank and US Food and Drug Administration in 1998. Clear guidance on how long cells and tissues stay viable in refrigerated condition allowed commercialization and wider use of OC allografts [66]. While immunological intolerance is relatively rare, the lack of tissue incorporation and a possibility to disease transmission remain a relative complication to this procedure.
4 The Future of OC Repair: Advanced Therapies
The future of OC lesion repair has been taken over by the emerging approaches of TERM strategies, where the focus is on replacing and regenerating the damaged tissues, instead of restoring the function only [77, 78]. The number of TERM techniques has been substantially increasing over the past years, resulting in a wide range of procedures to tackle tissue regeneration. Given the prevalence and low chances of healing on its own, OC lesions are prime candidates for stem cell therapies and tissue engineering approaches.
4.1 Cell Sources
The only cell type which has the perfect capacity to regrow the native cartilage is the chondrocytes. However, continuous supply of fresh chondrocytes is hard, if not impossible, to maintain. Researchers and clinical experts are constantly looking for other more optimal cell sources and feasible strategies to repair OC lesions. Here we will list and describe the most promising cell candidates for cartilage regeneration.
4.1.1 Chondrocytes
Regeneration of OCDs requires the application of chondrocytes and osteoblastic cells. Autologous chondrocytes were for long the commonest choice for conservative and advanced approaches of cartilage therapy [61]. Autologous chondrocytes are the patient’s own cells harvested from a nondamaged area of the joint. The derivation of biopsies from a healthy area of the cartilage can potentially initiate secondary osteoarthritis, which is a disadvantage of this technique [79]. Also, not all patients have enough healthy areas of the joint. Owing a restricted size of biopsies, the acquired cell number is limited. Therefore, to achieve sufficient amount of cells, the chondrocytes must first be expanded in vitro. This is a risk factor as the chondrocytes can lose their phenotype easily when not in their native environment [80, 81]. Once they bear with reduced chondrogenic capacity, their clinical use and therapeutic outcome are impeded [82]. Therefore, there had been various approaches developed to prevent dedifferentiation during ex vivo expansion, including the use of growth factors in the culture media, providing dynamic hydrostatic pressure to the cells while in culture and limiting the levels of oxygen in the environment [83, 84]. Another crucial factor is the amount of time of expansion; the shorter time the chondrocytes spend in culture, the better the chances are to preserve their phenotype. However, these cells have a very low proliferation rate, and consequently the shorter the time, the smaller cell number is being achieved. Ergo, donor site morbidity, cell dedifferentiation and limitations in cell number are the most critical detriment of the use of autologous chondrocytes [82].
To overcome obstacles given by the limited availability of autologous chondrocytes , non-autologous chondrocytes had also been considered and explored as a potential cell source to manage OC defects [85]. The use of allogeneic or xenogeneic chondrocytes has the advantage of acquiring larger cell populations while avoiding damaging the healthy sites of the joint and thus the development of secondary osteoarthritis. One could even obtain cells from younger populations with better chondrogenic capacity [86]. However, this technique also has drawbacks, namely, immunological intolerance and the possibility of disease transmission.
4.1.2 Stem Cells
Stem cells had been widely explored to overcome the limited supply of primary cells. Continuous cell sources could be provided by using off-the-shelf stem cell techniques. Most research involves the application of mesenchymal stem cells (MSCs), but embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) had also been considered as ideal candidates for OC regeneration (Table 1.3).
Mesenchymal stem cells (MSCs) are multipotent cells which can differentiate into osteoblast or chondroblast. They can be originated from various sources, such as the bone marrow, adipose tissue, synovium, infrapatellar fat pad, muscle, dermis, blood or the umbilical cord [87]. MSCs are defined by a minimal prerequisite established by the International Society for Cellular Therapy . This includes the expression of surface markers CD105, CD73 and CD90 and the exclusion of surface molecules CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR in standard culture conditions. They also have to be plastic adherent and capable of differentiating into chondroblasts, osteoblasts and adipocytes in vitro [88].
Chondrogenic differentiation of MSCs is induced via the application of growth factors and by the manipulation of their culture environment. It is usually divided into three stages starting with (i) cell condensation and the expression of adhesion molecules, such as N-cadherin and tenascin-C, which facilitate the cell-cell interaction. This is followed by (ii) the activation of transcription mediators including bone morphogenetic proteins (BMPs) , Sox-9 and FGF signalling pathways. The last step is (iii) ECM deposition and pre-chordial cell formation which is continued by full chondrocyte formation [89]. MSCs isolated from different sources may differ in their differentiation potentials [90].
Synovium-derived MSCs (SMSCs) were demonstrated to have excellent chondrogenic potential, greater than the MSCs isolated from the donor-matched bone marrow, periosteum, muscle and adipose tissue [91]. Furthermore, SMSCs are closely related and share many characteristics with the chondrocytes. AC and the synovium originate from the same pool of precursor cells and have a similar gene expression profiles, i.e. superficial zone protein, collagen type II and aggrecan are all expressed by both the chondrocytes and SMSCs [92, 93]. SMSCs also have a relatively high rate of proliferation as compared to other MSCs. Furthermore, they were shown to deposit greater amount of extracellular matrix, as compared to BMSCs. This is especially important since ECM deposition is believed to delay cell senescence and dedifferentiation [94, 95]. Additionally, SMSCs are able to form hyaline cartilage in vitro which holds extreme value for OC regeneration [96]. SMSCs are harvested via arthroscopy or from the synovial fluid. Unfortunately, the yield of cells is relatively low, having only around 14 cells per mL of synovial fluid, which have the capacity to form CFU-F colonies [97].
MSCs of other origin, such as bone marrow-derived stem cells (BMSCs) or adipose-derived stem cells (ADSCs), can also differentiate into chondrocytes or osteoblasts. Despite these cells having less chondrogenic potential, they bear with other advantages making them desirable for the OC tissue repair. In particular, ADSCs are routinely available from excised fat or lipoaspiration, which yields on average around 400,000 cells per mL, and thus provide the sufficient amount of cells easily [90]. Thorough molecular analysis had been performed to characterize these cells, and their trilineage mesodermal potential has been confirmed by several studies [98]. Different induction conditions had been identified where adipogenic, osteogenic and chondrogenic differentiation can be stimulated [99,100,101]. These include the presence of growth factors, hormones and mechanical stimuli, as well as 3D scaffold cultivation using scaffolding materials with different composition. Studies using alginate scaffolds demonstrated a superficial chondrogenic capacity of chondrocytes differentiated from ADSCs as compared to normal chondrocytes after 21 days of in vitro culture in alginate scaffolds [102]. On the other hand, when using hyaluronic acid scaffolds, collagen type II expression was similar in both ADSC and chondrocyte cultures and was lower in BMSC cultures. Numerous papers had been discussing whether BMSCs or ADSCs have stronger ability to differentiate into the chondrogenic lineage. Most of them agree that BMSCs possess more chondrogenic potential. Interestingly, it was also shown that although ADSCs and BMSCs have similar surface receptor profile, they may require different induction conditions [103]. For instance, stimulating BMSCs and ADSCs by transforming growth factor-β results in different chondrogenic profile as measured by chondrogenic marker expression, such as aggrecan and collagen type II. It was pointed out that while bone morphogenetic protein-6 (BMP-6) had successfully resulted in increased expression of aggrecan by ADSCs, BMSCs required stimuli provided by TGF-β to achieve the same [104]. Unfortunately, BMSCs yield significantly less cells upon harvest as compared to ADSCs, and they are harder to obtain involving a more invasive procedure, called bone marrow aspiration. It was shown that 1 mL marrow yields between 100 and 1000 cells.
ESCs and iPSCs have also tremendous potential as a cell source for the manufacture of OC therapy. The advantage of ESCs and iPSCs over adult stem cells is that they can be expanded indefinitely without undergoing senescence. Blastocysts which are unsuitable for in vitro fertilization are used to acquire ESCs. ESCs are the inner cell mast of blastocyst. Consequently, scientific use of ESCs is ethically unaccepted in many countries despite the immense interest and potential of their application. There are four main categories of methodologies to generate chondrogenic cells from ESCs, namely, (i) targeted differentiation, (ii) differentiation of ESCs via their coculture with mature chondrocytes, (iii) embryoid body formation with stimuli of growth factors and (iv) spontaneous differentiation into MSCs and subsequent chondrogenic differentiation [105]. Besides ethical concerns, teratoma formation is another problem encountered in the application of ESCs in regenerative therapy.
iPS cells are not burdened by ethical issues and were proven to be safe and stabile when used for cartilage formation [106]. Since their first introduction in 2006, iPSCs were widely explored for various applications [107,108,109]. Originally, they were generated in a mouse model using fibroblasts. Four factors were manipulated, namely, two transcriptional factors, the octamer-binding transcription factors 3 and 4 (Oct3/4) and the Kruppel-like factor 4 (Klf4) , and two tumour-related genes, the v-myc avian myelocytomatosis viral oncogene homolog (c-Myc) and Sox-2 [107]. Successful chondrogenic differentiation of iPSCs derived from various sources, such as fibroblast-like cells, neural stem cells and human osteoarthritic chondrocytes, was also demonstrated [110, 111]. Generation of iPSCs and subsequent differentiation into chondrogenic lineage from murine neural cells were achieved by the simultaneous overexpression of Oct4 and Klf4. Another approach, using somatic cells, had successfully generated iPSCs via a reprogramming approach using c-Myc, Klf4 and Sox-9 which were later able to differentiate into the chondrogenic lineage [112, 113]. Interestingly, even the reprogrammed cells show differences in their chondrogenic potential. Namely, iPSCs originating from chondrocytes show superior properties than iPSCs generated from neural or fibroblast-like cells [97]. iPSCs have tremendous potential for regenerative approaches. It is possible to establish allogeneic iPSC libraries, based on HLA phenotypes. These cells could be induced to differentiate into chondrogenic or osteogenic phenotype and could serve as off-the-shelf solution to heal OC lesions providing a truly advanced solution [89].
4.2 Biomaterials
Apart from the cell and molecular component, another pillar of the TERM approaches is the carrier. Although cells might be administered without scaffolding material, they often require support provided by a 3D structure to survive and keep their phenotype. Scaffolds need to be compatible with the cells as well as mechanically matching the environment of implantation. To design appropriate scaffolds for the OC unit, one must understand the biology of both the cartilage and the subchondral bone; therefore, it is particularly challenging. The scaffolds should integrate to their environment by being biocompatible and slowly degrade as the cells rebuild the tissue and thus also have to be biodegradable. There have been a myriad of materials proposed already originating from both natural and synthetic polymers [114]. The usage of inorganic materials, such as glasses and ceramics and metallic materials, had also been introduced. In this section we will review the advantages and disadvantages of these different materials.
4.2.1 Natural Polymer-Based Materials
Natural polymers are derived from the nature; therefore, they bear with high biocompatibility properties which is an advantage over the synthetic polymers. They are of animal or vegetal origin, or obtained from algae, and can be synthesized as well [115,116,117,118]. They can also aid cells in their tissue interaction, synthesis and development due to molecular domains present on their structures. Collagen polymers, for instance, contain ligands, which promote cell adhesion and matrix deposition [119, 120]. Collagen-based scaffolds showed improvement when regenerating cartilage; however, they also bear with relatively weak mechanical properties. Since both the cartilage and bone contain large amounts of collagen molecules, collagen-based scaffold seems to be an ideal candidate for OC regeneration. Current commercially available applications of collagen include Zyderm, CaReS (Arthro-Kinetics, Essingen, Germany) and Carticel (Genzyme Inc., Cambridge, UK) [121, 122]. Another favoured natural polymer is the alginate. Alginate is a linear, unbranched copolymer of L-glucuronic and D-mannuronic acid [123]. Ca-alginate porous scaffolds combined with gelatine were able to differentiate MSCs into both chondrogenic and osteogenic lineages [124]. Alginate-based 3D scaffolds were shown to have the capacity to redifferentiate chondrocytes [125]. Among the cationic polymers, chitosan is the most popular. Based on the source and method of preparation, various chitosan materials can be attained, which also bear with different properties. Successful subchondral bone and cartilage generation had been achieved by chitosan matrices [126]. Chitosan polymers have structural similarities with two major cartilage molecules, namely, hyaluronic acid and glycosaminoglycan molecules; thus, it is also prepared as a blend [127]. Silk polymers obtained from silkworm cocoon or spider silk are also widely explored for the use of TERM approaches [128]. They can be combined with synthetic polymers, which are used to improve their mechanical stability and tailorability/processability. For instance, when silk was prepared in a combination as silk fibroin/nano-CaP to obtain bilayered scaffolds , it showed superior mechanical properties compared to silk-only scaffolds [129].
Natural matrices have the advantage of being biocompatible and biodegradable and having more similarity to biological macromolecules; therefore, the native environment can recognize it and process metabolically. However, natural matrices have the danger of transition hazardous agents and have purification issues; therefore, their use on the clinics is hampered. They also bear with weaker mechanical properties as compared to synthetic polymers.
4.2.2 Synthetic Polymer-Based Materials
As mentioned before, the main advantage of using synthetic polymers is owing complete control over their structure and having better mechanical properties. Synthetic polymers also do not have issues with disease transmission or limitation in polymer supply. However, biocompatibility can become a major issue when using matrices fabricated from synthetic polymers. The most commonly used polymers for cartilage and bone engineering are poly(ethylene glycol) (PEG) and its derivatives poly(L-lactic acid) (PLLA) and poly(lactide-co-glycolide) (PLGA). These synthetic biodegradable polymers are often used as a blend of each other or combined with natural polymers to promote biological integration [78, 130, 131]. Despite their easy manufacture and large availability, synthetic polymers must be explored via the combination of natural polymers to enable better biocompatibility and orchestrate cellular events in vivo, e.g. cell adhesion, proliferation and matrix deposition. Accordingly, several studies demonstrated chondrogenic differentiation of PLLA in combination with polymers of natural origin, such as chitosan and silk, proving their potential for OC regeneration [132, 133]. Besides the lack of cell recognition sites, synthetic materials also tend to degrade easily once implanted promoting the development of inflammation. Thus, despite their advantages, synthetic polymer-based scaffolds are only ideal to be used as blend with natural matrices.
4.2.3 Other Materials (Ceramics, Glasses, Metallic Materials)
Ceramics , such as calcium phosphate ceramics and hydroxyapatite, are favoured because of their biocompatibility and osteoconductive and osteoinductive nature [85]. They were shown to promote the production of bone-like apatite when implanted, therefore being able to integrate better and induce bone regeneration [134, 135]. As it was recently summarized and demonstrated, 3D–printed Ca ceramics have tremendous potential to establish patient-specific bone grafting application; however, poor mechanical stability must be first addressed and solved [136]. Metallic materials include the application of stainless steel, titanium, titanium alloys and cobalt-based alloys. The main advantage of these materials is the structural architecture and mechanical property similar to the native grafts. Porous titanium bases were recently fabricated in combination with hydrogels for OC regeneration [137]. These biphasic scaffolds had the advantage of bearing with mechanical stability at the bone phase and having viable cell-seeded hydrogel integrated on top to support cartilage regeneration [137]. The major disadvantage of metallic materials is the lack of degeneration after implantation.
5 Final Remarks
There is no doubt that regeneration of OC damages is of major importance. Considering the poor healing capacity of the OC unit, so far only repair techniques had been applied, which fail to provide long-term solutions and full regeneration of the joints. Due to lack of regeneration, the danger of developing osteoarthritis is high in joints affected by OC injury. Orthopaedic surgeons and researchers are working on solutions, which can heal the damaged area by introducing cells, bioactive molecules and support matrices to the defect site. Whereas, in case of larger injuries, the application of in vitro engineered allografts could bring solutions, TERM approaches have the potential to overcome several limitations of the existing applications, such as insufficient cell number or cell differentiation, as well as can tackle the issue of lack of donors for OC grafts. TERM approaches hold great promise but also face many challenges. Finding the appropriate cell source, and establishing the environment to these cells, in which they are able to keep their phenotype or conversely differentiate into the desired phenotype, is extremely difficult, especially if we consider off-the-shelf solutions. Moreover, these cells usually need 3D support in vitro or when implanted. This is provided by scaffolding materials, which come in many shapes and forms. Finding the appropriate combination which then will facilitate tissue formation is hard. Furthermore, these materials have to be able to integrate into the native tissue and withstand the mechanical forces therein. This is especially difficult in the knee articulation, which is the largest joint in our body experiencing mechanical load over 20 MPa. Considering that the OC unit is composed of both the articular cartilage and subchondral bone, biphasic scaffolds obtained by the combination of materials and cells show promise for OC regeneration. Many routes had been proposed for material fabrication. Novel strategies using the combination of bioceramics and polymeric materials or the blend of natural and synthetic materials are considered as optimal candidates. Among the cells, MSCs including SMSCs, BMSCs and ADSCs as well iPSCs are the favoured ones due to their large availability and chondrogenic potential. The design of strategies for OC regeneration is open for further development and requires the combination of multidisciplinary approaches to reach its goal. Nevertheless, if cell and molecular biology, material science and orthopaedics are effectively brought together, chances are that we will be able to reach clinics with excellent and sustainable regenerative approaches for the OC unit.
References
Ding C, Cicuttini F, Scott F et al (2005) The genetic contribution and relevance of knee cartilage defects: case-control and sib-pair studies. J Rheumatol 32:1937–1942
Hjelle K, Solheim E, Strand T et al (2002) Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18:730–734. https://doi.org/10.1053/jars.2002.32839
Widuchowski W, Widuchowski J, Trzaska T (2007) Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14:177–182. https://doi.org/10.1016/j.knee.2007.02.001
Brittberg M, Lindahl A, Nilsson A et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895. https://doi.org/10.1056/NEJM199410063311401
Kocher MS, Tucker R, Ganley TJ, Flynn JM (2006) Management of osteochondritis dissecans of the knee. Am J Sports Med 34:1181–1191. https://doi.org/10.1177/0363546506290127
Espregueira-Mendes J, Pereira H, Sevivas N et al (2012) Assessment of rotatory laxity in anterior cruciate ligament-deficient knees using magnetic resonance imaging with Porto-knee testing device. Knee Surg Sports Traumatol Arthrosc 20:671–678. https://doi.org/10.1007/s00167-012-1914-9
Lee JE, Ryu KN, Park JS et al (2014) Osteochondral lesion of the bilateral femoral heads in a young athletic patient. Korean J Radiol 15(6):792. https://doi.org/10.3348/kjr.2014.15.6.792
Cahill (1995) Osteochondritis dissecans of the knee: treatment of juvenile and adult forms. J Am Acad Orthop Surg 3:237–247
Eckstein F, Cicuttini F, Raynauld J-P et al (2006) Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage 14 Suppl A:A46–A75. https://doi.org/10.1016/j.joca.2006.02.026
Ryd L, Brittberg M, Eriksson K et al (2015) Pre-osteoarthritis: definition and diagnosis of an elusive clinical entity. Cartilage 6:156. https://doi.org/10.1177/1947603515586048
Durur-Subasi I, Durur-Karakaya A, Yildirim OS (2015) Osteochondral lesions of major joints. Eurasian J Med 47:138–144. https://doi.org/10.5152/eurasianjmed.2015.50
Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Jt Surg 43:752–757
da Cunha Cavalcanti FM, Doca D, Cohen M, Ferretti M (2012) Updating on diagnosis and treatment of chondral lesion of the knee. Rev Bras Ortop 47:12–20. https://doi.org/10.1590/S0102-36162012000100001
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A(Suppl 2):58–69
Magnussen RA, Dunn WR, Carey JL, Spindler KP (2008) Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res 466:952–962. https://doi.org/10.1007/s11999-007-0097-z
Seo S-S, Kim C-W, Jung D-W (2011) Management of focal chondral lesion in the knee joint. Knee Surg Relat Res 23:185–196. https://doi.org/10.5792/ksrr.2011.23.4.185
Kurtz S, Ong K, Lau E et al (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785. https://doi.org/10.2106/JBJS.F.00222
Kim J, Nakamura N, Brittberg M (2014) Techniques in cartilage repair surgery. https://doi.org/10.1007/978-3-642-41921-8
Last RJ (1948) Some anatomical details of the knee joint. J Bone Joint Surg Br 30B:683–688
Akizuki S, Mow VC, Müller F et al (1986) Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res 4:379–392. https://doi.org/10.1002/jor.1100040401
Hasler EM, Herzog W, Wu JZ et al (1999) Articular cartilage biomechanics: theoretical models, material properties, and biosynthetic response. Crit Rev Biomed Eng 27:415–488
Ralphs JR, Benjamin M (1994) The joint capsule: structure, composition, ageing and disease. J Anat 184:503–509
Wilson R, Diseberg AF, Gordon L et al (2010) Comprehensive profiling of cartilage extracellular matrix formation and maturation using sequential extraction and label-free quantitative proteomics. Mol Cell Proteomics 9:1296–1313. https://doi.org/10.1074/mcp.M000014-MCP201
Fujie H, Nakamura N (2013) Frictional properties of articular cartilage-like tissues repaired with a mesenchymal stem cell-based tissue engineered construct. Conf Proc IEEE Eng Med Biol Soc. 2013:401–404
Thambyah A, Nather A, Goh J (2006) Mechanical properties of articular cartilage covered by the meniscus. Osteoarthr Cartil 14:580–588. https://doi.org/10.1016/j.joca.2006.01.015
Goldring MB (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis 4:269–285. https://doi.org/10.1177/1759720X12448454
Kheir E, Shaw D (2009) Hyaline articular cartilage. Orthop Trauma 23:450–455. https://doi.org/10.1016/j.mporth.2009.01.003
Bhosale AM, Richardson JB (2008) Articular cartilage: structure, injuries and review of management. Br Med Bull 87:77–95. https://doi.org/10.1093/bmb/ldn025
Kiani CH, Chen LI, Wu YJ et al (2002) Structure and function of aggrecan 12:19–32
Li J, Anemaet W, M a D et al (2011) Knockout of ADAMTS5 does not eliminate cartilage aggrecanase activity but abrogates joint fibrosis and promotes cartilage aggrecan deposition in murine osteoarthritis models. J Orthop Res 29:516–522. https://doi.org/10.1002/jor.21215
Lu XL, Mow VC (2008) Biomechanics of articular cartilage and determination of material properties. Med Sci Sports Exerc 40:193–199. https://doi.org/10.1249/mss.0b013e31815cb1fc
Bock HC, Michaeli P, Bode C et al (2001) The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthr Cartil 9:654–663. https://doi.org/10.1053/joca.2001.0420
Jay GD, Tantravahi U, Britt DE et al (2001) Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res 19:677–687. https://doi.org/10.1016/S0736-0266(00)00040-1
Sophia Fox AJ, Bedi A, Rodeo SA (2009) The basic science of articular cartilage: structure, composition, and function. Sports Health 1:461–468. https://doi.org/10.1177/1941738109350438
Eyre DR, Weis MA, Wu JJ (2006) Articular cartilage collagen: an irreplaceable framework? Eur Cells Mater 12:57–63. https://doi.org/10.22203/eCM.v012a07
Eyre D (2002) Collagen of articular cartilage. Arthritis Res 4:30–35
Bayliss MT, Venn M, Maroudas A, Ali SY (1983) Structure of proteoglycans from different layers of human articular cartilage. Biochem J 209:387–400
Zhytkova MA, Chizhik SA, Wierzcholski K et al (2010) Properties of cartilage on micro- and nanolevel. Adv Tribol. https://doi.org/10.1155/2010/243150
Redler I, Mow VC, Zimny ML, Mansell J (1975) The ultrastructure and biomechanical significance of the tidemark of articular cartilage. Clin Orthop Relat Res:357–362
Lories RJ, Luyten FP (2011) The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 7:43–49. https://doi.org/10.1038/nrrheum.2010.197
Duncan H, Jundt J, Riddle JM et al (1987) The tibial subchondral plate. A scanning electron microscopic study. J Bone Joint Surg Am 69:1212–1220
Lyons TJ, Stoddart RW, McClure SF, McClure J (2005) The tidemark of the chondro-osseous junction of the normal human knee joint. J Mol Histol 36:207–215. https://doi.org/10.1007/s10735-005-3283-x
Eyre DR, Wu JJ (1995) Collagen structure and cartilage matrix integrity. J Rheumatol Suppl 43:82–85
Buckwalter JA, Mankin HJ (1998) Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect 47:477–486
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci 1192:230–237. https://doi.org/10.1111/j.1749-6632.2009.05240.x
Obeid EM, Adams MA, Newman JH (1994) Mechanical properties of articular cartilage in knees with unicompartmental osteoarthritis. J Bone Joint Surg Br 76:315–319
Pan J, Wang B, Li W et al (2012) Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone 51:212–217. https://doi.org/10.1016/j.bone.2011.11.030
Sharma AR, Jagga S, Lee S-S, Nam J-S (2013) Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci 14:19805–19830. https://doi.org/10.3390/ijms141019805
Bentley G, Bhamra JS, Gikas PD et al (2013) Repair of osteochondral defects in joints-how to achieve success. Injury 44:S3–S10. https://doi.org/10.1016/S0020-1383(13)70003-2
Oei EHG, Van Tiel J, Robinson WH, Gold GE (2014) Quantitative radiologic imaging techniques for articular cartilage composition: toward early diagnosis and development of disease-modifying therapeutics for osteoarthritis. Arthritis Care Res 66:1129–1141. https://doi.org/10.1002/acr.22316
Wenham CYJ, Conaghan PG (2009) Imaging the painful osteoarthritic knee joint: what have we learned? Nat Clin Pract Rheumatol 5:149–158. https://doi.org/10.1038/ncprheum1023
Braun HJ, Gold GE (2012) Diagnosis of osteoarthritis: imaging. Bone 51:278–288. https://doi.org/10.1016/j.bone.2011.11.019
Van Tiel J, Reijman M, Bos PK et al (2013) Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) shows no change in cartilage structural composition after viscosupplementation in patients with early-stage knee osteoarthritis. PLoS One 8:e79785. https://doi.org/10.1371/journal.pone.0079785
Lusic H, Grinstaff MW (2013) X-ray-computed tomography contrast agents. Chem Rev 113:1641–1666. https://doi.org/10.1021/cr200358s
Shafieyan Y, Khosravi N, Moeini M, Quinn TM (2014) Diffusion of MRI and CT contrast agents in articular cartilage under static compression. Biophys J 107:485–492. https://doi.org/10.1016/j.bpj.2014.04.041
Lee N, Hyeon T (2012) Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev 41:2575–2589. https://doi.org/10.1039/c1cs15248c
Zhu D, Liu F, Ma L et al (2013) Nanoparticle-based systems for T1-weighted magnetic resonance imaging contrast agents. Int J Mol Sci 14:10591–10607. https://doi.org/10.3390/ijms140510591
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB (1999) Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev 99:2293–2352
Wiewiorski M, Miska M, Kretzschmar M et al (2013) Delayed gadolinium-enhanced MRI of cartilage of the ankle joint: results after autologous matrix-induced chondrogenesis (AMIC)-aided reconstruction of osteochondral lesions of the talus. Clin Radiol 68:1031–1038. https://doi.org/10.1016/j.crad.2013.04.016
Freedman JD, Lusic H, Wiewiorski M et al (2015) A cationic gadolinium contrast agent for magnetic resonance imaging of cartilage. Chem Commun (Camb) 51:11166–11169. https://doi.org/10.1039/c5cc03354c
Filardo G, Kon E, Di Martino A et al (2012) Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 20:1704–1713. https://doi.org/10.1007/s00167-011-1732-5
Piontek T, Ciemniewska-Gorzela K, Szulc A et al (2012) All-arthroscopic AMIC procedure for repair of cartilage defects of the knee. Knee Surgery, Sport Traumatol Arthrosc 20:922–925. https://doi.org/10.1007/s00167-011-1657-z
Rodrigues MT, Gomes ME, Reis RL (2011) Current strategies for osteochondral regeneration: from stem cells to pre-clinical approaches. Curr Opin Biotechnol 22:726–733. https://doi.org/10.1016/j.copbio.2011.04.006
Karkabi S, Rosenberg N (2015) Arthroscopic debridement with lavage and arthroscopic lavage only as the treatment of symptomatic osteoarthritic knee. Open J Clin Diagnostics 05:68–73. https://doi.org/10.4236/ojcd.2015.52013
Steadman JR, Rodkey WG, Briggs KK (2010) Microfracture: its history and experience of the developing surgeon. Cartilage 1:78–86. https://doi.org/10.1177/1947603510365533
Torrie AM, Kesler WW, Elkin J, Gallo RA (2015) Osteochondral allograft. Curr Rev Musculoskelet Med 8:413–422. https://doi.org/10.1007/s12178-015-9298-3
Espregueira-Mendes J, Pereira H, Sevivas N et al (2012) Osteochondral transplantation using autografts from the upper tibio-fibular joint for the treatment of knee cartilage lesions. Knee Surg Sports Traumatol Arthrosc 20:1136–1142. https://doi.org/10.1007/s00167-012-1910-0
Health Quality Ontario (2005) Arthroscopic lavage and debridement for osteoarthritis of the knee: an evidence-based analysis. Ont Health Technol Assess Ser 5:1–37
Segal NA, Buckwalter JA, Amendola A (2006) Other surgical techniques for osteoarthritis. Best Pract Res Clin Rheumatol 20:155–176. https://doi.org/10.1016/j.berh.2005.09.009
Chen H, Sun J, Hoemann CD et al (2009) Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res 27:1432–1438. https://doi.org/10.1002/jor.20905
Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ (1999) The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade 28:26. https://doi.org/10.1007/s001320050318
Steadman JR, Rodkey WG, Singleton SB, Briggs KK (1997) Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop 7:300–304. https://doi.org/10.1016/S1048-6666(97)80033-X
Steadman JR, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391:S362–S369
Gadjanski I, Vunjak-Novakovic G (2015) Challenges in engineering osteochondral tissue grafts with hierarchical structures. Expert Opin Biol Ther 15:1583–1599. https://doi.org/10.1517/14712598.2015.1070825
Hangody L, Kish G, Kárpáti Z et al (1997) Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. Knee Surgery, Sport Traumatol Arthrosc 5:262–267. https://doi.org/10.1007/s001670050061
Bobić V (1996) Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg Sports Traumatol Arthrosc 3(4):262
Makris EA, Gomoll AH, Malizos KN et al (2014) Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. https://doi.org/10.1038/nrrheum.2014.157
Nooeaid P, Salih V, Beier JP, Boccaccini AR (2012) Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med 16:2247–2270. https://doi.org/10.1111/j.1582-4934.2012.01571.x
Foldager CB (2013) Advances in autologous chondrocyte implantation and related techniques for cartilage repair. Dan Med J 60:B4600
Diaz-romero J, Nesic D, Grogan SP et al (2008) Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor. J Cell Physiol 214(1):75–83. https://doi.org/10.1002/JCP
Tseng A, Pomerantseva I, Cronce MJ et al (2014) Extensively expanded auricular chondrocytes form neocartilage in vivo. Cartilage 5:241–251. https://doi.org/10.1177/1947603514546740
Ma B, Leijten JCH, Wu L et al (2013) Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthr Cartil 21:599–603. https://doi.org/10.1016/j.joca.2013.01.014
Leijten JCH, Georgi N, Wu L et al (2013) Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng Part B Rev 19:31–40. https://doi.org/10.1089/ten.teb.2012.0273
Hubka KM, Dahlin RL, Meretoja VV et al (2014) Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng Part B Rev 20:641–654. https://doi.org/10.1089/ten.TEB.2014.0034
Panseri S, Russo A, Cunha C et al (2012) Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc 20:1182–1191. https://doi.org/10.1007/s00167-011-1655-1
Hunziker EB (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil 10:432–463. https://doi.org/10.1053/joca.2002.0801
Caplan a I, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7:259–264
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Wang M, Yuan Z, Ma N et al (2017) Advances and prospects in stem cells for cartilage regeneration. Stem Cells Int 2017:1–16. https://doi.org/10.1155/2017/4130607
Bourin P, Bunnell BA, Casteilla L et al (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52:2521–2529. https://doi.org/10.1002/art.21212
Schumacher BL, Hughes CE, Kuettner KE et al (1999) Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res 17:110–120. https://doi.org/10.1002/jor.1100170117
Zhou C, Zheng H, Seol D et al (2014) Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res 32:981–988. https://doi.org/10.1002/jor.22641
Pei M, Li JT, Shoukry M, Zhang Y (2011) A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater 22:333–343. discussion 343
Pei M, He F (2012) Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol 227:2163–2174. https://doi.org/10.1002/jcp.22950
Ando W, Tateishi K, Katakai D et al (2008) In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A 14:2041–2049. https://doi.org/10.1089/ten.tea.2008.0015
Vonk LA, de Windt TS, Slaper-Cortenbach ICM, Saris DBF (2015) Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: a concise review. Stem Cell Res Ther 6:94. https://doi.org/10.1186/s13287-015-0086-1
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. https://doi.org/10.1091/mbc.E02-02-0105
Puetzer JL, Petitte JN, Loboa EG (2010) Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 16:435–444. https://doi.org/10.1089/ten.TEB.2009.0705
Mehlhorn AT, Niemeyer P, Kaschte K et al (2007) Differential effects of BMP-2 and TGF-beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif 40:809–823. https://doi.org/10.1111/j.1365-2184.2007.00473.x
Schelbergen RF, van Dalen S, ter Huurne M et al (2014) Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthr Cartil 22:1158–1166. https://doi.org/10.1016/j.joca.2014.05.022
Mardani M, Hashemibeni B, Ansar MM et al (2013) Comparison between chondrogenic markers of differentiated chondrocytes from adipose derived stem cells and articular chondrocytes in vitro. Iran J Basic Med Sci 16:763–773
Hildner F, Albrecht C, Gabriel C et al (2011) State of the art and future perspectives of articular cartilage regeneration : a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med 5:36–51. https://doi.org/10.1002/term.386
Diekmann B, Rowland C, DP L (2010) Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A 16:523–533
RA O (2012) Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 93:389–400. https://doi.org/10.1111/j.1365-2613.2012.00837.x
Kobayashi Y, Okada Y, Itakura G et al (2012) Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One 7:e52787. https://doi.org/10.1371/journal.pone.0052787
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Singh VK, Kalsan M, Kumar N et al (2015) Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol 3:2. https://doi.org/10.3389/fcell.2015.00002
Medvedev SP, Grigor’eva EV, Shevchenko AI et al (2011) Human induced pluripotent stem cells derived from fetal neural stem cells successfully undergo directed differentiation into cartilage. Stem Cells Dev 20:1099–1112. https://doi.org/10.1089/scd.2010.0249
Wei Y, Zeng W, Wan R et al (2012) Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cell Mater 23:1–12
Hiramatsu K, Sasagawa S, Outani H et al (2011) Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest 121:640–657. https://doi.org/10.1172/JCI44605
Outani H, Okada M, Yamashita A et al (2013) Direct induction of chondrogenic cells from human dermal fibroblast culture by defined factors. PLoS One 8:e77365. https://doi.org/10.1371/journal.pone.0077365
Salgado AJ, Oliveira JM, Martins A et al (2013) Tissue engineering and regenerative medicine: past, present, and future. Int Rev Neurobiol. https://doi.org/10.1016/B978-0-12-410499-0.00001-0
Mano JF, Silva GA, Azevedo HS et al (2007) Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 4:999–1030. https://doi.org/10.1098/rsif.2007.0220
Kobayashi S, Fujikawa S-i, Ohmae M (2003) Enzymatic synthesis of chondroitin and its derivatives catalyzed by hyaluronidase. J. Am. Chem. Soc. 125(47):14357–14369. https://doi.org/10.1021/JA036584X
Silva TH, Alves A, Popa EG et al (2012) Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2:278–289. https://doi.org/10.4161/biom.22947
Yan LP, Oliveira JM, Oliveira AL, Reis RL (2013) Silk fibroin/nano-CaP bilayered scaffolds for osteochondral tissue engineering. Key Eng Mater 587:245–248. https://doi.org/10.4028/www.scientific.net/KEM.587.245
Yang C, Hillas PJ, Julio AB et al (2004) The application of recombinant human collagen in tissue engineering. BioDrugs 18:103–119
Rutgers M, Saris DB, Vonk LA et al (2013) Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng Part A 19:59–65. https://doi.org/10.1089/ten.TEA.2011.0416
Burdick JA, Mauck RL (2010) Biomaterials for tissue engineering applications: a review of the past and future trends. Springer Science & Business Media, Austria
Parenteau-Bareil R, Gauvin R, Berthod F (2010) Collagen-based biomaterials for tissue engineering applications. Materials (Basel) 3:1863–1887. https://doi.org/10.3390/ma3031863
Grant GT, Morris ER, Rees DA et al (1973) Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 32:195–198. https://doi.org/10.1016/0014-5793(73)80770-7
Petrenko YA, Ivanov RV, Petrenko AY, Lozinsky VI (2011) Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. J Mater Sci Mater Med 22:1529–1540. https://doi.org/10.1007/s10856-011-4323-6
Bonaventure J, Kadhom N, Cohen-Solal L et al (1994) Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res 212:97–104. https://doi.org/10.1006/excr.1994.1123
Abarrategi A, Lópiz-Morales Y, Ramos V et al (2010) Chitosan scaffolds for osteochondral tissue regeneration. J Biomed Mater Res Part A 95A:1132–1141. https://doi.org/10.1002/jbm.a.32912
Deng Y, Ren J, Chen G et al (2017) Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci Rep 7:2699. https://doi.org/10.1038/s41598-017-02962-z
Yan L-P, Oliveira JM, Oliveira AL, Reis RL (2015) In vitro evaluation of the biological performance of macro/micro-porous silk fibroin and silk-nano calcium phosphate scaffolds. J Biomed Mater Res B Appl Biomater 103:888–898. https://doi.org/10.1002/jbm.b.33267
Yan L-P, Silva-Correia J, Oliveira MB et al (2015) Bilayered silk/silk-nanoCaP scaffolds for osteochondral tissue engineering: in vitro and in vivo assessment of biological performance. Acta Biomater 12:227–241. https://doi.org/10.1016/j.actbio.2014.10.021
Ondrésik M, Azevedo Maia FR, da Silva Morais A et al (2017) Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng 114:717–739. https://doi.org/10.1002/bit.26182
Antunes JC, Oliveira JM, Reis RL et al (2010) Novel poly(L-lactic acid)/hyaluronic acid macroporous hybrid scaffolds: characterization and assessment of cytotoxicity. J Biomed Mater Res A 94:856–869. https://doi.org/10.1002/jbm.a.32753
Wright LD, McKeon-Fischer KD, Cui Z et al (2014) PDLA/PLLA and PDLA/PCL nanofibers with a chitosan-based hydrogel in composite scaffolds for tissue engineered cartilage. J Tissue Eng Regen Med 8:946–954. https://doi.org/10.1002/term.1591
Liu W, Li Z, Zheng L et al (2016) Electrospun fibrous silk fibroin/poly(L-lactic acid) scaffold for cartilage tissue engineering. Tissue Eng Regen Med 13:516–526. https://doi.org/10.1007/s13770-016-9099-9
Miljkovic ND, Cooper GM, Marra KG (2008) Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthr Cartil 16:1121–1130. https://doi.org/10.1016/j.joca.2008.03.003
Samavedi S, Whittington AR, Goldstein AS (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9:8037–8045. https://doi.org/10.1016/j.actbio.2013.06.014
Trombetta R, Inzana JA, Schwarz EM et al (2017) 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann Biomed Eng 45:23–44. https://doi.org/10.1007/s10439-016-1678-3
Nover AB, Lee SL, Georgescu MS et al (2015) Porous titanium bases for osteochondral tissue engineering. Acta Biomater 27:286–293. https://doi.org/10.1016/j.actbio.2015.08.045
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ondrésik, M., Oliveira, J.M., Reis, R.L. (2018). Advances for Treatment of Knee OC Defects. In: Oliveira, J., Pina, S., Reis, R., San Roman, J. (eds) Osteochondral Tissue Engineering. Advances in Experimental Medicine and Biology, vol 1059. Springer, Cham. https://doi.org/10.1007/978-3-319-76735-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-76735-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76734-5
Online ISBN: 978-3-319-76735-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)