Abstract
For many years, the avascular nature of cartilage tissue has posed a clinical challenge for replacement, repair, and reconstruction of damaged cartilage within the human body. Injuries to cartilage and osteochondral tissues can be due to osteoarthritis, sports, aggressive cancers, and repetitive stresses and inflammation on wearing tissue. Due to its limited capacity for regeneration or repair, there is a need for suitable material systems which can recapitulate the function of the native osteochondral tissue physically, mechanically, histologically, and biologically. Tissue engineering (TE) approaches take advantage of principles of biomedical engineering, clinical medicine, and cell biology to formulate, functionalize, and apply biomaterial scaffolds to aid in the regeneration and repair of tissues. Nanomaterial science has introduced new methods for improving and fortifying TE scaffolds, and lies on the forefront of cutting-edge TE strategies. These nanomaterials enable unique properties directly correlated to their sub-micron dimensionality including structural and cellular advantages. Examples include electrospun nanofibers and emulsion nanoparticles which provide nanoscale features for biomaterials, more closely replicating the 3D extracellular matrix, providing better cell adhesion, integration, interaction, and signaling. This chapter aims to provide a detailed overview of osteochondral regeneration and repair using TE strategies with a focus on nanomaterials and nanocomposites.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Theterm “osteochondral” is derived from the roots “osteo” meaning bone, and “chondro,” which refers to cartilage. Thus, osteochondral tissue is tissue composed of or related to bone and cartilage. Osteochondral tissue is found primarily in joints throughout the body, specifically at the smooth end of bones and the articular cartilage that cover them [1]. Injuries to osteochondral tissue are common in the field of orthopedics, and can result in pain and swelling, as well as instability of the joint. Current treatments include tissue transplantation, allografts, as well as the delivery of bioactive agents. However, each treatment carries a number of drawbacks. For example, “autografts and allografts are often associated with limited availability and risks of immunogenicity, respectively” [2]. The aim of tissue engineering is to repair and regenerate tissue, as well as to provide a viable tissue substitute. In practice, the discipline uses one or more of three key components: three-dimensional (3D) scaffolds, healthy harvested cells, and biologically active factors [2]. Any biomaterial used for tissue engineering must meet a number of requirements, including but not limited to biocompatibility, biodegradability, and bioactivity. Composites are often used in order to synthesize the beneficial properties of multiple constituents, and recent advances in nanotechnology have demonstrated the importance of nanoscale structural properties in signaling for cellular regeneration [3]. This chapter outlines the background and clinical relevance of nanomaterials for osteochondral regeneration, as well current tissue engineering techniques and the challenges the discipline faces.
2 Background and Clinical Relevance
2.1 Cartilage Tissue Biology
Cartilage is a smooth , elastic tissue found throughout the body. In addition to providing support to various structures in the body such as the rib cage, ear and nose, cartilage acts as a rubber-like padding between bones to minimize friction and provide protection at the joints. Cartilage is classified into three different types : fibrocartilage, elastic cartilage, and hyaline cartilage. Each type of cartilage differs based on the amount of collagen and proteoglycans, two proteins that make up much of the structure of cartilage.
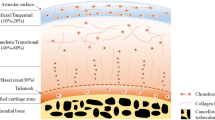
There are three types of joints in the body: fibrous, cartilaginous and synovial. Out of these three types, only synovial joints allow for a large degree of motion. This type of joint is covered by the thin, dense, translucent connective tissue known as hyaline cartilage. As it covers the articulating surfaces of bone, this type of connective tissue is also referred to as articular cartilage. The articular cartilage can be considered a “soft tissue composed primarily of a large extracellular matrix with a population of chondrocytes distributed throughout the tissue” [4]. Extracellular matrix (ECM) is composed mainly of tightly wound collagen fibers, which lend the matrix a high tensile strength. A proteoglycan–water gel is also distributed throughout the collagen framework, which allows the hyaline cartilage to withstand compressive forces by attracting and trapping large amounts of water. This structure allows it to perform well as a load-bearing material to support joint movement, showing a low coefficient of friction as well as high wear strength [5]. Figure 4.1 displays the physical structure of cartilage tissue .
It should be noted that the composition and cellular organization of human adult articular cartilage vary depending on the regions of the matrix investigated, with different matrix proteins found in superficial and deep layers. These differences are both qualitative and quantitative. The interterritorial region of the matrix contains a collagen network composed of collagens II, IX, and XI while the pericellular matrix contains collagen VI, fibromodulin, and matrilin 3, but is deficient in or completely lacks type II collagen. The morphology of chondrocytes, the cells responsible for secreting the matrix or cartilage, differs as well, from more flattened nearer to the surface and rounder at the deeper zones [4].
2.2 Cartilage Development
The formation of cartilaginous tissue occurs through a process known as chondrogenesis , and takes place as early as during fetal development. Here, it is a precursor for the process known as endochondral ossification in which “hypertrophic cartilage is replaced by bone,” thus giving way to the early skeleton [4]. Chondrogenesis depends “upon signals initiated by cell–cell and cell–matrix interactions and is associated with increased cell adhesion and formation of gap junctions and changes in the cytoskeletal architecture” [6].
The process of chondrogenesis begins with the recruitment, proliferation and condensation of mesenchymal cells. In craniofacial bones, mesenchymal stem cells are recruited from neural crest cells of the neural ectoderm, whereas they are recruited from the sclerotome of the paraxial mesoderm and the somatopleure of the lateral plate mesoderm in axial and appendicular skeleton, respectively [6]. After condensation, these cells differentiate into chondroblasts, which synthesize the cartilage ECM and fibers. As the matrix grows, the chondroblasts mature into chondrocytes. In limb development, the chondrocytes either produce cartilage at the ends of bones or proliferate and undergo terminal differentiation “to hypertrophy, and apoptosis to permit endochondral ossification” [4]. Whether or not a chondrocyte goes down a certain path is determined by positive and negative signaling factors, such as Sox9 and Runx2 [6].
It has been noted that mechanical factors influence the development, maintenance and degradation. Carter et al. report that different types of stresses can either inhibit or promote bone growth, or ossification. Specifically, intermittent hydrostatic compression stress is shown to inhibit ossification, and as a result maintains the cartilage phenotype. On the other hand, intermittent nondestructive octahedral shear stress, resulting in mild tensile stress, promotes ossification and bone growth (The differences in hydrostatic compression stress and octahedral shear stress are illustrated in Fig. 4.2). This trend is supported by the findings that “tensile strain of chondrocytes increases cell proliferation, maturation, and hypertrophy” while “intermittent hydrostatic pressure has been shown to up-regulate aggrecan and collagen II, while inhibiting proinflammatory mediators in chondrocytes” [7].
2.3 Cartilage Disease and Injury
Articular cartilage is mainly loaded in compression. Therefore, its defects are often related to trauma-induced injuries, but problems can also arise from pediatric growth plate disorders and congenital defects. Injuries to the tissue are fairly common; it has been reported that over 900,000 Americans suffer from articular cartilage injuries each year [8]. Once injured, self-recovery is generally poor due to the lack of blood flow to the area.
Cartilage diseases include such disorders as osteoarthritis, costochondritis, herniation of intervertebral discs, achondroplasia, and relapsing polychondritis. These diseases can be a result of a number of factors, such as the failure of chondrocytes within the cartilage failing to proliferate or the inflammation of cartilage in key areas of the body, but the focus of tissue engineering is to alleviate conditions that result specifically from cartilage degeneration.
Osteoarthritis is an example of a very common disorder resulting from the deterioration of cartilage, with knee arthritis affecting an estimated 6% of adults over the age of 30, and hip arthritis affecting around 3% of the same demographic [9]. As increased functional loading in healthy joints by moderate exercise leads to an increase in articular cartilage thickness, it makes sense that the disease would be more prominent in those with a more sedentary lifestyle, such as the elderly [7]. In fact, the disease is the most common chronic condition affecting patients over the age of 70. Wood et al. characterize the disease as “damage to hyaline articular cartilage [which]… involves the whole joint and has subsequent changes to the subchondral surface involving bone remodeling” [9]. Refer to Fig. 4.3 for a visual representation of the breakdown in hyaline cartilage that takes place in osteoarthritis.
2.4 Current Treatments
Most current treatments for osteochondral diseases such as osteoarthritis are symptomatic, and attempt to regulate pain and improve mobility. Such treatments include self-care to prevent or reduce risks of disease and anti-inflammatory medication to reduce pain, as well as physical therapy. In some cases, the entire joint may be replaced by surgery. However, none of these measures address the root cause of cartilage degeneration in the joint. Tissue engineering offers the exciting prospect to repair or regenerate tissues, as well as providing alternative substitutes for the lost tissue. This chapter provides an outline for some of the tissue engineering approaches and objectives for osteochondral tissue being researched today.
3 Tissue Engineering and Regenerative Medicine
3.1 Tissue Engineering Approaches and Objectives
The approaches to osteochondral tissue regeneration follow from the general tissue engineering objective to repair and restore the function of defect tissues caused by disease or injury. Tissue engineering approaches accomplish successful tissue repair when the resulting material is fully integrated with surrounding tissues and replicates the functionality of the native tissue, while showing no adverse effects. Biomaterial scaffold designs are a central feature in tissue engineering. Specifically, polymeric biomaterial scaffolds are used extensively. The approach to polymeric scaffold design is dependent upon the intended function of the scaffold. Scaffolds are typically used either as structural space fillers that promote tissue development or as delivery vehicles of therapeutic cell treatments. Biomaterials, cells, and bioreactors are the three components that are considered for use in osteochondral tissue engineering design. Combinations of biomaterials with cells and/or bioreactors constitute a strategy that has been extensively studied for tissue engineering applications. Biomaterial scaffolds can provide three-dimensional structural support or morphology and help transport and control delivery or cellular treatments or bioreactor molecules. The biomaterial scaffold , cells, and bioreactors can be used in tissue engineering designs in different combinations to promote tissue regeneration and integration to repair tissue defects with viable tissue substitutes. Understanding the architectural and molecular composition of cartilage as well as the cellular and biochemical interactions characteristic to both the development and function of the native tissue is imperative to engineer a material that matches the physiological, biomechanical, and biochemical signaling properties of the native tissue [10]. Biomaterial selection for scaffold design considers the biocompatibility, mechanical properties, biodegradability, three-dimensional architecture, and bioactivity of the material related to the native tissue [10]. Cartilage has a hierarchical structure with features that can be seen starting at its microstructure and continues down to the nanoscale. This hierarchical architecture inspires scaffold design that incorporates features starting at the nanoscale. Nanomaterials are used to replicate nanolevel features of native cartilage. Cellular and bioreactor components are subsequently added to scaffold materials to enhance their bioactivity in ways relevant to the capacity to regenerate cartilage tissue by facilitating the replication of developmental processes of cartilage formation. Another tissue engineering approach to mimic the native hierarchical structure of cartilage tissue is to create gradient scaffolds with layers representing the layered physiology of articular cartilage at the interface between cartilage and bone (Fig. 4.3).
3.2 Biomaterials
The three main classes of biomaterials are ceramics, metals, and polymers. Materials within these three classes can be classified as either natural biomaterials or synthetic biomaterials. Natural biomaterials are those derived from either an animal or plant source while synthetic biomaterials are synthesized in a lab [11].
Biomaterials can be further classified as biodegradable or nonbiodegradable. Biodegradability is an attractive feature for tissue engineering and regenerative purposes because it gives a material the capacity to initially function as a structural support then gradually degrade away as the new tissue moves in. A degradation-regeneration approach to tissue repair represents an ideal of tissue engineering to regenerate a fully integrated replication of native tissue. Tissue engineering methods that use biodegradable scaffold material eliminate the problem of long-term durability through the lifetime of the implant that must be considered for nondegradable implants. For biodegradable scaffolds, the implant material should ideally have a tunable degradation rate to ensure that the degradation and resorption of the implant material are compatible with the rate of new tissue generation [11].
Replicating the mechanical properties is particularly important in osteochondral applications in order for the engineered tissue to mimic and restore the functionality of the native tissue. Osteochondral tissues act as support structures in the body and, therefore, scaffold materials intended to function as structural space fillers for osteochondral repairs must support mechanical loads while the new tissue development occurs. Polymer biomaterials are used in osteochondral tissue engineering applications to create both rigid scaffold structures as well as hydrogel scaffolds. The rigid polymers are used in applications where a three-dimensional structural support is a priority while hydrogel scaffolds are more ideal as cell carrier systems. Although polymeric scaffolds are the foundation of most tissue engineering methods, they do not always exhibit entirely ideal properties for their intended applications when used independently. Composite biomaterials are created for osteochondral tissue engineering applications to improve the scaffold properties to more closely match its intended function and to promote effective tissue repair. Composite biomaterials are also used in efforts to mimic the heterogeneous, hierarchical composition of native cartilage. Creating multilayered scaffolds is an approach incorporating composite biomaterials intended to regenerate both cartilage and subchondral bone tissue at the osteochondral interface.
3.2.1 Natural Biomaterials
Some advantages of natural biomaterials for tissue engineering applications are their bioactivity, biocompatibility, and biodegradability. Natural biomaterials are typically used in the form of polymer hydrogels. Immunogenic incompatibility is a concern when using scaffolds made from natural material, however. Common natural biomaterials used for osteochondral tissue engineering include alginate, chitosan, collagen, fibrin, and hyaluronan [12].
Collagen and hyaluronic acid are both essential components of the ECMError! Bookmark not defined. of native cartilage. Their natural derivation from mammalian tissue allows for the polymers to be recognized by cells, facilitating cell attachment and triggering ECM production unlike plant derived polymers [11]. Experimental results support collagen’s potential as a biomaterial useful in tissue engineering applications. An in vivo study using stem cell-seeded type II collagen scaffolds implanted into rabbits for articular cartilage repair showed results of chondrocyte-like cells and extracellular molecules found in the newly formed tissue and with no signs of inflammation after 8 weeks [13]. Chondrocytes and extracellular synthesis are characteristic to cartilage formation and markers of cartilage regeneration potential. Collagen scaffolds the most extensively used material in clinical applications [14]. Natural biomaterials, in general, have nonideal mechanical properties for the load-bearing functions that are characteristic to osteochondral tissues and therefore are used in combinations with other biomaterials. Collagen is an example of a material that is mechanically weaker than native cartilage but shows improved mechanical properties with the addition of other materials to create a composite. Chitosan is a biodegradable natural polymer that shows potential for cartilage tissue engineering. A chitosan-pluronic hydrogel injected for cartilage regeneration yielded a proliferation of chondrocytes and synthesis of GAGs [15]. Another cell-seeded chitosan hydrogel was tested in vivo and was found to fill cartilage defects completely 24 weeks after transplantation [16]. In addition to collagen, hyaluronan and fibrin have also been used clinically for cartilage reconstruction [17, 18]. A list of natural biomaterials and their applications can be found in Table 4.1.
3.2.2 Synthetic Biomaterials
Synthetic biomaterials allow for a higher degree of variability due to the opportunity to control some of their properties via processing methods. Controlling the composition and structure is a method used to obtain particular mechanical properties. Synthetic materials used in cartilage tissue engineering are primarily polymers. Synthetic polymer hydrogels are used for their high potential to entrap cells and provide biological stimuli for their migration, proliferation, and differentiation by providing a hydrated environment that facilitates diffusion [19]. Although synthetic polymers lack the bioactive capacity to integrate with surrounding host tissue, they can be functionalized with bioactive molecules. Functionalization of synthetic polymer scaffolds gives the material the ability of cellular interaction to facilitate cell attachment and stimulate matrix production and, therefore, greater potential to modulate cartilage regeneration.
The most common synthetic polymers used in osteochondral tissue engineering applications include poly(ethylene glycol) (PEG), polylactide (PLA) and its derivatives poly(L-lactide) (PLLA) and poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone) (PCL), poly(vinyl alcohol) (PVA), and polyglycolide (PGA). PEG in the form of both a hydrogel and rigid scaffold has been seeded with chondrocytes and proved to support their attachment, viability, proliferation and production of ECM [20]. PLGA scaffolds seeded with MSCs yielded hyaline-like smooth tissue after 12 weeks of implantation into the defect site within rabbit knees [21]. PGA seeded scaffolds show instances of higher expression of the cartilage specific protein, aggrecan, and collagen II when compared with PLGA seeded scaffolds [22]. Polymer scaffolds alone may still lack ideal mechanical strength and therefore composite material strategies are also used for synthetic biomaterials to give the scaffold its required characteristics. Synthetic biomaterials used in osteochondral tissue engineering application are listed in Table 4.2.
3.3 Nanomaterials and Nanocomposites
Nanobiomaterials can be introduced to create nanocomposite materials with a nanostructure engineered to mimic the nanoscale level of the hierarchical composition of native cartilage. Adding nanoscale elements into the design improves the functional ability of the material to more closely resemble native tissue behavior with respect to both mechanical properties and biochemical activity.
3.3.1 Nanomaterial Strategies
Adding nanomaterials such as nanoparticles (NPs) or nanotubes create nanostructured composition of biomaterial scaffolds. Ceramic NPs help improve the mechanical strength of polymer biomaterials [23, 24]. Adding hydroxyapatite nanoparticles to PVA hydrogels improves their mechanical properties and also creates a bioactive nanocomposite from the synthetic polymer that is not bioactive on its own [25]. NPs are also added for nanosurface modifications. Both metal and ceramic NPs can be used in combination with polymer scaffolds in osteochondral tissue engineering. The NPs are added to scaffolds through chemical treatments. The type and quantity of NPs added to the scaffold can control specific nanosurface properties such as surface area, roughness, and electrical charge.
Another nanomaterial used in tissue engineering is carbon nanotubes (CNTs) . Similar to NPs , adding CNTs can alter mechanical and electrical properties of a material as well as influence cellular behavior by increasing the surface area within the scaffold giving it a higher affinity for cellular attachment. Nanoscale surface modifications can be made with physical and chemical treatment methods. Controlling the nanoscale porosity and roughness at the surface can influence scaffolds to promote cellular activity associated with cartilage regeneration. Nanoembossing of both polyurethane (PU) and PCL scaffolds creates highly porous surface with nanoscale surface roughness. The modified surfaces yield increases in chondrocyte numbers, intracellular protein production, and collagen secretion by chondrocytes when compared with the smooth surface scaffolds [26].
Nanostructured fibrous scaffolds are another method of introducing a nanoscale dimension to a material. Nanofiber scaffolds are created by electrospinning a polymer solution or through thermally induced polymer separation (TIPS) techniques . Nanofibers resemble the collagen fibrils of native cartilage. An additional osteochondral tissue engineering strategy using nanostructured scaffold designs incorporates nanocomposite materials in a layered orientation to create a multiphase construct. A layered nanocomposite design includes biomimetic nanoscale properties while also replicating the heterogeneous architecture of articular cartilage that is found as you move up from the subchondral bone to the articulating surface. Multilayered scaffolds with a gradient of nanoscale features create the most structurally similar 3D scaffold replication of native articular cartilage tissue. The layered composition of cartilage at the osteochondral junction is displayed in Fig. 4.4.
Multilayer scaffolds show potential for tissue repair at the osteochondral junction where both tissue types must be repaired. In a recent study, by Castro et al. [10] a biphasic, layered, nanocomposite scaffold including both nanocomponents and a microstructure yielded results supporting this tissue engineering design approach and its feasibility for cartilage and bone repair. The high impact polystyrene(HIPS) mold of the scaffold is composed of an osseous layer characterized by a 40% in-fill density and a cartilage layer with a 0% in-fill density. The in-fill density controls the pore density of the respective layers that is representative of their natural composition . A cross-linked poly(ethylene glycol)-diacrylate (PEG-DA): PEG hydrogel is used as the bulk matrix material. Nanostructured hydroxyapatite nanoparticles (nHAs) are added to the osseous layer of the scaffolds and growth factors are added to the cartilage layer. The scaffold is seeded with stem cells and cultured in stem cell media. Scaffolds treated with nHA and the growth factor display higher levels of GAG (a biochemical marker for stem-cell chromogenic differentiation), the presence of proteins indicating type II collagen synthesis, and higher levels of calcium deposition.
The findings support that the addition of nHAs in physiologically relevant concentrations promotes cell adhesion and proliferation. The results demonstrate that the scaffold design provides 3D structural support for cellular attachment and effectively facilitates osteochondral tissue regeneration by incorporating interconnected microchannels, a controlled porosity, nHA nanoparticles, and controlled bioactive factor delivery. This study clearly illustrates how cellular activity that influences tissue formation can be influenced by scaffold geometry and optimizes tissue regeneration and integration.
3.3.2 Advantages of Nanomaterials and Nanocomposites
Scaffolds must provide support for cellular activity and suitable mechanical properties. Nanocomposites enhance the structural and mechanical properties and influence cellular activity. A more biomimetic structure improves mechanical characteristics of engineered scaffold materials. Mechanical properties of nanocomposite scaffolds match the properties of native cartilage more closely than scaffolds without nanofeatures. The addition of nanoparticles to hydrogels has resulted in native-like mechanical properties for the scaffold. The interconnection between structure and function is especially relevant for the ECM of cartilage tissue. Adding nanoscale features gives scaffolds the ability to stimulate cellular interaction that induces and promotes tissue regeneration. Controlled porosity can facilitate cellular infiltration and migration as well as nutrient flow within the scaffold. Cellular migration promotes tissue integration. Surface roughness and nanofeatures increase the surface area within the scaffold and give it a higher probability for cell attachments. Cell adhesion leads to increased cell proliferation and differentiation and thus, tissue generation. Therefore, nanostructured scaffolds also allow for a more controlled release of bioreactor elements to more closely replicate the dynamic kinematics of biochemical activity in native cartilage development. Nanomaterials and nanocomposites give engineered tissues a more biomimetic structural composition that promotes the restoration of native tissue functionality.
4 Stem Cell Strategies
Stem cells are capable of differentiating into various other types of cell types found throughout the body. As this multilineage, differential potential is what provides the means to recreate and rebuild tissue, stem cells are a fundamental pillar in tissue engineering.
There are various types of stem cells found in the adult body. The first type of cells is mesenchymal stem cells (MSCs ). These types of stem cells are most commonly found in bone marrow, and are among the most popular type of cell used in tissue engineering due to their multipotency. The other types of stem cells are hematopoietic stem cells (HSCs) and skin or epidermal stem cells, found in bone marrow and the epidermis respectively. In regards to osteochondral tissue, mesenchymal stem cells are the stem cells of choice, considering that they are precursors to the chondrocytes.
Mesenchymal stem cells are capable of giving rise to chondrocytes when maintained in a 3D structure and treated with growth factors of the transforming growth factors-β (TGF-β) family. Studies have shown that TGF-β can induce in vitro chondrogenesis of mesenchymal stem cells when maintained in aggregates and pellets, as well as when seeded onto nanofibrous scaffolds when treated with proper growth factors. It should be noted that the material used to create the nanofibrous scaffolds has an effect on the tendency of MSCs to initiate chondrogenesis. In the previously discussed study, the synthetic biodegradable polymer poly(ε-caprolactone) (PCL) was used [27]. Therefore, when utilizing stem cells to achieve tissue regeneration, one must consider the extracellular environment, growth factor interaction, and the material and structure of the scaffold onto which the cells are seeded.
5 Growth Factors
Growth factors , mentioned several times throughout this chapter, are naturally occurring substances, such as proteins or hormones, which are capable of stimulating cellular growth. In terms of osteochondral tissue engineering, growth factors are capable of providing more suitable culture conditions to tissue constructs by supporting chondrogenesis. There are many different types of growth factors available for use by researchers and engineers for a variety of different purposes. In addition to inducing differentiation in stem cells, certain growth factors have been shown to influence the physical properties of engineered cartilage. Factors such as bone morphogenetic protein-2 (BMP-2 ) , IGF-1 , and the previously mentioned TGF-β have been shown capable of increasing compressive and tensile properties of engineered cartilage tissues [Elder]. Proliferation of chondrocytes has been increased through the addition of growth factors such as TGF-β , fibroblast growth factor 2 (FGF-2) and platelet–derived growth factor BB (PDGF-BB) [28]. Other studies have shown that when insulin or IGF-1 was added, rabbit auricular chondrocytes showed “increased deposition of cartilaginous ECM, improved mechanical properties, and thicknesses comparable to native auricular cartilage after 4 weeks of growth” [29]. These and other studies show that growth factors play a vital role in influencing the effectiveness of stem cells in regenerating tissue.
6 Clinical Relevance
While the medical implications for successful tissue engineering are extensive throughout the body, the potential for improvement in osteochondral diseases alone merits a separate discourse. As mentioned earlier in the chapter, damages to articular cartilage alone affect hundreds of thousands of Americans every year. Osteoarthritis alone affects a significant number of the population. Not only is the osteochondral disease the most frequently cited cause of difficulty in walking, but the condition has a significant impact on the economy as well: absence from work and early retirement relating to the disease exceed 2% of the gross domestic product [9].
Current treatments for osteochondral disease such as osteoarthritis are palliative, and “on the basis of medical evidence … do not change the course of the disease”. Surgical treatments aim to completely replace the entire joint, and while they provide long-term relief for pain, they do not promote regeneration of tissue and are risky to implement in some elderly patients [30].
Successfully incorporating effective tissue engineering solutions into a clinical setting could provide a greater degree of recovery to a wider pool of patients suffering from osteochondral disease than currently available solutions.
7 Challenges for OC Tissue Engineering
Several problems face researchers and engineers working to advance the field of osteochondral tissue engineering. These problems include but are not limited to biocompatibility regarding immune response, ethical challenges, and current scientific limitations.
7.1 Biocompatibility and Immune Response
In any field dealing with the body, biocompatibility is a primary concern. When a foreign body is introduced to an organism, that organism’s immune system will identify it and attempt to protect the surrounding tissue and organs. This can result in inflammation as well as breakdown of the implant. Such a response is associated with allografts, the transplantation of tissue, usually bone, from one person to another. If the body does not recognize the transplant as its own, it will attempt to reject it, resulting in an unsuccessful transplant. Therefore, it is of utmost importance to consider biocompatibility when designing implantable materials. The types of materials chosen to develop the implant and the inclusion of certain bioactive factors play an important role in this aspect.
7.2 Ethical Issues
When discussing the use of new technologies on living organisms, it is important to address the ethics involved that come along with it. For instance, stem cells are an important factor in any field of tissue engineering. Nanocomposites may be seeded with stem cells and growth factors in order to induce differentiation into cells that will promote tissue regeneration. However, the use of certain types of stem cells can be a controversial issue depending on the source. Although embryonic stem cells can easily differentiate into many different types of cells, some question the ethics of cell retrieval from undeveloped embryonic tissue. As such, most researchers use stem cells derived from different sources, including adipose-derived stem cells and human mesenchymal stem cells. These cells are also capable of differentiating into various other types of cells, and since they can be retrieved from adult tissue, their use avoids ethical scrutiny .
7.3 Current Scientific Challenges
Perhaps the most obvious challenge is successfully integrating multidisciplinary techniques to accomplish a wide range of problems. There are currently issues that researchers and engineers do not have answers for yet. For instance, in just osteochondral tissue engineering, there is a major challenge to overcome the inability of “resident chondrocytes to lay down a new matrix with the same properties as it had when it was formed during development” [4]. This problem is seen at the more macro level when considering larger tissue engineering endeavors. Something as grand as complete limb regeneration, an ultimate goal of musculoskeletal tissue engineering, requires the “simultaneous formation of multiple types of tissues and the functional assembly of these tissues into complex organ systems” [2]. However, such multiscale organization is rarely reestablished after surgery, and it is even more difficult to restore functionality similar to the original tissue or organ to affect long term clinical outcome.
Despite these challenges , advances in tissue engineering are made every day, and it remains one of the most promising approaches for tissue and organ recovery. It is possible to envision a future where regenerative tissue engineering will continue to improve with new strategies and technologies that will ultimately push tissue engineering beyond individual tissue repair and be capable to address more complex tissue systems, organs, and limbs.
References
Liu H, Liu H (2016) Nanocomposites for musculoskeletal tissue regeneration (Woodhead Publishing Series in Biomaterials). Elsevier Science & Technology, p. 406
Roshan J, Cato LT (2014) Musculoskeletal regenerative engineering: biomaterials, structures, and small molecules. Hindawi Publishing Corporation. p. 12
Zhang L, Webster TJ (2009) Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4:66–80
Goldring MB (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Therap Adv Musculoskeletal Dis 4:269–285
Gloria A, De Santis R, Ambrosio L (2010) Polymer-based composite scaffolds for tissue engineering. J Appl Biomater Biomech 8:57–67
Goldring MB, Tsuchimochi K, Ijiri K (2006) The control of Chondrogenesis. J Cell Biochem 97:11
Carter DR, BeauprÈ GS, Wong M, Smith RL, Andriacchi TP, Schurman DJ. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res 427, pp. 69–77, 2004
Cheung H-Y, Lau K-T, Lu T-P, Hui D (2006) A critical review on polymer-based bio-engineered materials for scaffold development. 38: 291–300
Wood AM, Brock TM, Heil K, Holmes R, Weusten A (2013) A review on the management of hip and knee osteoarthritis. Int J Chronic Dis. 2013(845015): 10
Castro NJ, Patel R, Zhang LG (2015) Design of a novel 3D printed bioactive nanocomposite scaffold for improved osteochondral regeneration. Cell Mol Bioeng 8(3):416–432
Bernhard JC, Vunjak-Novakovic G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res Ther. 2016; 7(1): 56
Duarte Campos DF, Drescher W, Rath B, Tingart M, Fischer H (Jul 2012) Supporting biomaterials for articular cartilage repair. Cartilage 3(3):205–221
Chen WC, Yao CL, Wei YH, Chu IM (2011) Evaluating osteochondral defect repair potential of autologous rabbit bone marrow cells on type II collagen scaffold. Cytotechnology 63(1):13–23
Steinwachs M (2009) New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy 25(2):208–211
Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD (2009) Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater 5(6):1956–1965
Hao T et al (2010) The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthr Cartil 18(2):257–265
Manfredini M, Zerbinati F, Gildone A, Faccini R (2007) Autologous chondrocyte implantation: a comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop Belg 73(2):207–218
Kim MK, Choi SW, Kim SR, Oh IS, Won MH (2010) autologous chondrocyte implantation in the knee using fibrin. Knee Surg Sports Traumatol Arthrosc 18(4):528–534
Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ (Aug 2007) Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng 13(8):1905–1925
Woodfield TB, Van Blitterswijk CA, De Wijn J, Sims TJ, Hollander AP, Riesle J (2005) Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Engl. 11(9–10):1297–1231
Uematsu K et al (Jul 2005) Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 26(20):4273–4279
Zwingmann J, Mehlhorn AT, Südkamp N, Stark B, Dauner M, Schmal H (Sep 2007) Chondrogenic differentiation of human articular chondrocytes differs in biodegradable PGA/PLA scaffolds. Tissue Eng 13(9):2335–2343
Hule RA, Pochan DJ (2007) Polymer nanocomposites for biomedical applications. MRS Bulletin 32(4):354–358
Winey KI, Vaia RA (2007) Polymer nanocomposites. MRS Bulletin 32(4):314–322
Pan Y, Xiong D, Gao F (2008) Viscoelastic behavior of nano-hydroxyapatite reinforced poly(vinyl alcohol) gel biocomposites as an articular cartilage. J Mater Sci Mater Med 19(5):1963–1969
Balasundaram G, Storey DM, Webster TJ (2014) Novel nano-rough polymers for cartilage tissue engineering. Int J Nanomedicine 9:1845–1853
Lia W-J et al (2005) A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26(6):10
Francioli SE et al (2007) Tissue Engine 13:7
Rosa RG, Joazeiro PP, Bianco J, Kunz M, Weber JF, Waldman SD (2014) Growth factor stimulation improves the structure and properties of scaffold-free engineered auricular cartilage constructs. PLoS One 9:e105170
Schurman DJ, Smith RL (2004) Osteoarthritis: current treatment and future prospects for surgical, medical, and biologic intervention. PHD Clin Orthop Relat Res. 247:6
Acknowledgements
Authors acknowledge funding support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (award number: R01EB020640), the Connecticut Regenerative Medicine Research Fund (grant Number: 15-RMB-UCHC-08) in support of this work. Ohan S. Manoukian acknowledges the National Science Foundation Graduate Research Fellowship under Grant No. (DGE-1747453).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Manoukian, O.S., Dieck, C., Milne, T., Dealy, C.N., Rudraiah, S., Kumbar, S.G. (2018). Nanomaterials/Nanocomposites for Osteochondral Tissue. In: Oliveira, J., Pina, S., Reis, R., San Roman, J. (eds) Osteochondral Tissue Engineering. Advances in Experimental Medicine and Biology, vol 1058. Springer, Cham. https://doi.org/10.1007/978-3-319-76711-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-76711-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76710-9
Online ISBN: 978-3-319-76711-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)