Abstract
Sino-nasal sarcoidosis is an infrequent but serious complication of sarcoidosis. Sarcoidosis patients with sino-nasal involvement usually have chronic disease. Involvement of the paranasal sinuses is usually detected by imaging, which can demonstrate destruction of tissue and/or replacement with granulomatous inflammation. Although the diagnosis may be suggested by visualization, biopsy confirmation is highly supportive of the diagnosis. In some cases, local corticosteroids may be sufficient to control inflammation. However, many patients require systemic therapy. While glucocorticoids are first-line therapy, steroid-sparing agents such as methotrexate are often employed to minimize toxicity. In some cases, third-line agents, including the antitumor necrosis factor monoclonal antibodies such as infliximab, are needed to control the inflammation. Recurrent infections may complicate the management of sino-nasal disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Case Presentation 1
DW was a 41-year-old black female when she developed eye pain and was found to have anterior uveitis. As part of her evaluation, she had a chest X-ray which demonstrated bilateral hilar adenopathy and diffuse lung filtrates. She was diagnosed as having sarcoidosis on clinical grounds. She was not dyspneic and denied cough. Her iritis was treated with topical steroids and within 6 months she was tapered off steroid drops.
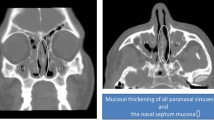
She then started having recurrent sinus symptoms . Initially this was felt to be allergic rhinitis and she was treated with intranasal steroids. Three years later, she developed severe pain behind and below her right eye. She underwent an MRI and was found to have complete opacification of her right maxillary sinus (Fig. 13.1a, b). She was seen by an ophthalmologist, who diagnosed her as having dacrocystitis, with compression of her nasolacrimal duct. She first had surgical intervention to clear her ethmoid and maxillary sinuses. Pathologic examination of the surgical specimen revealed non-caseating granulomas. After recovery, a nasolacrimal stent was placed by her ophthalmologist. Confirmation of placement of the stent beyond the inflammation was made by the otolaryngologist in the operating room.
Postoperatively the patient was placed on prednisone 40 mg a day. Over the next 6 months, attempts to reduce the dose below 20 mg a day led to recurrence of pain and bleeding from her sinuses. She was started on methotrexate and after 6 months she was able to reduce her prednisone to 10 mg a day.
Two years later, she began having recurrent sinus infections. These usually responded to antibiotics and prolonged courses of high-dose prednisone. She developed macular papular lesions on her cheeks and nasal alae. She also had new papular lesions on her arms. A biopsy of one of the arm lesions again found granulomas consistent with sarcoidosis.
She was felt to have refractory sarcoidosis with sinus disease and lupus pernio . The antitumor necrosis factor (TNF) antibody infliximab was initiated. The patient received 5 mg/kg initially, then 2 weeks later, and then once a month. She has done well on the combination of infliximab, methotrexate, and low-dose prednisone. She has been maintained on 5 mg-a-day prednisone and has not required increased prednisone for more than a year.
Case Presentation 2
MC is a black female who at the age of 39 was complaining of sinus congestion and a sore on the roof of her mouth. She was referred to an otolaryngologist because of refractory sinus disease. He noted on exam that her hard palate was perforated. She denied cocaine or other drug use. A sinus biopsy found non-caseating granulomas. Her antineutrophil cytoplasmic antibody (ANCA) test was repeatedly negative. She was referred to our sarcoidosis clinic. On exam, she was noted to have extensive purplish, raised lesions of the entire nose. She also had lesions on both cheeks and was diagnosed as having lupus pernio. Her chest X-ray demonstrated upper lobe fibrosis consistent with Scadding stage 4 sarcoidosis. She was initially treated with prednisone and methotrexate with only modest response.
Because of insurance issues, she was not seen for 3 years. She was referred back to clinic because of respiratory distress . She had been taken off her methotrexate for unclear reasons and was on only 5 mg-a-day prednisone when she had developed acute stridor. She was found to have recurrence of pan sinusitis (Fig. 13.2a) as well as laryngeal infiltration and upper airway narrowing (Fig. 13.2b) by what proved to be her sarcoidosis. She was only marginally better on 40 mg prednisone.
She was then started on adalimumab since her insurance would not cover infliximab. After 3 months, she started improving and eventually she was weaned to 10 mg-a-day prednisone and maintained on adalimumab 40 mg weekly.
After 2 years, she developed left hip and upper leg pain. On MRI , she was found to have an infiltrative lesion. A percutaneous needle aspirate of the bone lesion was consistent with a low-grade liposarcoma. She had surgical resection of the lesion. Because of concerns about potential carcinogenicity of adalimumab, the drug was discontinued.
Over the next 2 years, she has had no evidence of recurrence of her tumor. However, her sinus disease and facial lesions returned within 3 months of stopping the adalimumab . She was initially controlled with prednisone alone. However, as she gained more weight with the prednisone, she asked for another steroid-sparing regimen. She was begun on repository corticotrophin injection (RCI) 40 units twice. For the past year, she has been maintained on RCI alone, that with no oral prednisone.
Comments on two cases: Both of these cases had sinus disease and lupus pernio . This skin lesion is highly specific for sarcoidosis. It is also commonly associated with sinus sarcoidosis. In some series of lupus pernio , around half of the patients had symptomatic sarcoidosis of the sinus [1, 2]. Dacrocystis is another condition associated with sinus sarcoidosis. In sarcoidosis patients with dacrocystitis, stenting alone is unlikely to work. That is because there is a high risk for granulomatous reaction to the stent. In case one, immunosuppressive therapy was able to control the disease. Both of these cases had refractory disease requiring third-line therapy. Anti-TNF antibodies can be quite effective in chronic conditions, including lupus pernio [3]. Unfortunately, the second case developed a liposarcoma while receiving were adalimumab. The patient is currently stable on another third-line treatment , RCI.
General Discussion
Sino-nasal sarcoidosis is one of the many manifestations of sarcoidosis of the upper respiratory tract (SURT) [4, 5]. Nasal and sino-nasal disease is the most common manifestation of SURT , but the larynx, oral cavity, and tongue can also be affected [5, 6]. Sinus symptoms are common in sarcoidosis patients. In one prospective study, nearly 40% of patients complained of nasal symptoms that had lasted for more than 3 weeks [7]. Half of these patients continued to have symptoms despite nasal steroids and short-course antibiotic therapy. However, biopsy confirmation of sino-nasal sarcoidosis was made in only 4% of all patients in the study.
Etiology and Epidemiology of Sarcoidosis
Sarcoidosis is a granulomatous disease of unknown etiology [8]. The defining feature of sarcoidosis is the granuloma. One proposed model of sarcoidosis is exposure to an antigen, usually through inhalation [9]. This antigen activates several cells including macrophages and dendritic cells through the Toll-like receptor-2 (TLR-2). The dendritic cell transports the antigen across the epithelium to the lymph node, where it is processed with differentiation and clonal expression of T helper cells (Th1 and Th17). The antigen also stimulates macrophages to release tumor necrosis factor (TNF). TNF crosses the epithelial layer where it activates tissue macrophages and natural killer (NK) cells. Stimulated NK cells release interferon gamma (IFN-γ) which upregulates the tissue macrophages. The activated macrophages and clonal Th1/Th17 cells form the core of the granuloma. Other cells in the granuloma include T regulatory cells (Treg) and B cells (B cells). Key cytokines involved in the granuloma formation include MCP-1, CCL20, and CXCL10.
For most sarcoidosis patients, the granuloma resolves over the first few years. However, persistence of granulomas leads to chronic disease. Several features have been associated with persistent granulomas. The most important may be the upregulation of Th17.1 cells [10, 11], programmed death cells (PD-1) [12], and Treg cells [13]. Certain cytokines have been associated with chronic disease, including CXCL9 [14] and interleukin 8 (IL-8) [15, 16]. Persistent production of TNF by alveolar macrophages has also been found in patients with chronic sarcoidosis [17]. These observations have led to treatment strategies focused on these potential targets .
The antigen which stimulates the inflammatory response of sarcoidosis remains unknown. Several potential ligands for TL2-R have been studied. Antibodies for mycobacterial proteins mKatG [18], ESAT-6 [19], and M. tuberculosis heat-shock proteins (Mtb-hsp) [20] have been reported in a significant number of sarcoidosis patients, mostly from North America. However, no studies to date have been able to identify mycobacteria that are causing the antibody reaction. Studies from Japan and China have found evidence for propionibacterium including P. acnes in over half of the cases they studied [21, 22]. Inhaled particles have also been reported to cause a sarcoidosis-like reaction. Some first responders to the World Trade Center attack developed a sarcoidosis-like reaction, including multi-organ disease [23]. These observations support the hypothesis that sarcoidosis is due to multiple antigens. What makes sarcoidosis sarcoidosis is the reaction to the antigen(s) (Fig. 13.3).
Inhaled antigen comes into contact with cells at the epithelial layer. Activation of both macrophages and dendritic cells occurs through the Toll-like receptor-2 (TLR-2). The dendritic cell transports the antigen across the epithelium to the lymph node, where it is processed and differentiation and clonal expression of T helper cells (Th1 and Th17) occur. The antigen also stimulates macrophages on the surface of the epithelium and leads to the release of tumor necrosis factor (TNF). TNF crosses epithelial layer where it activates tissue macrophages and natural killer (NK) cells. NK releases interferon-gamma (IFN-γ) which upregulates the tissue macrophages. The activated macrophages and clonal Th1/Th17 cells form the core of the granuloma. Other cells in the granuloma include T regulatory cells (Treg) and B cells (B cells). Key cytokines involved in the granuloma formation include MCP-1, CCL20, and CXCL10
Sarcoidosis is a worldwide disease. Table 13.1 summarizes the estimated incidence and prevalence of sarcoidosis for some countries across the world [24,25,26]. In the United Sates, several studies have noted that the disease is more frequent in African-American women [25, 27, 28]. Figure 13.4 demonstrates the prevalence rate per 100,000 population for African-American and Caucasian women and men in one recent study of over thirty-two million Americans [25]. In this study, sarcoidosis was more frequently observed in women than men for all races studied, including Asian and Hispanics.
Prevalence rate for sarcoidosis in the United States for African-Americans and Caucasians. Higher rate observed for female versus male for both races [25]
Clinical Presentation and Diagnosis of Sino-Nasal Sarcoidosis
Nasal congestion is the most common feature in patients with sino-nasal sarcoidosis [5, 29, 30]. In a prospective study of 159 sarcoidosis patients, 60 (38%) had nasal symptoms (usually congestion) for at least 3 weeks [7]. Twenty-seven still had symptoms after 3 weeks of nasal steroids and oral antibiotics. Of these, six were found to have biopsy-confirmed sino-nasal sarcoidosis. Epistaxis was noted in 10–30% of cases [5, 29, 30]. In one series of 12 cases of biopsy -confirmed sino-nasal sarcoidosis, anosmia was noted in five, crusting in eight, and polyps in four cases [29]. Other areas in the upper airway can be involved in sino-nasal sarcoidosis. These include the larynx, oral cavity, and tongue [5, 6].
At the University of Cincinnati Sarcoidosis clinic, we have seen 2000 patients with sarcoidosis in the past 6 years. Of these, 64 (3.2%) had sino-nasal sarcoidosis. This was the most common manifestation of SURT in our clinic, with an additional 39 patients having upper airway or parotid involvement without documented sino-nasal disease. Table 13.2 summarizes the clinical features and the frequency of other organ involvement, using standard organ involvement criteria [31]. Patients with sino-nasal involvement were younger at the time of diagnosis of sarcoidosis than those without sino-nasal involvement. There was no difference in the race or gender for those with or without sino-nasal involvement. Lung and eye involvement were reported with equal frequency in both groups. Skin involvement was more common in those with sino-nasal disease, often on the face (Fig. 13.5a). In this group, 20% of patients with sino-nasal disease had lupus pernio (Fig. 13.5b).
Diagnosis of Sino-Nasal Sarcoidosis
Many patients with documented sino-nasal sarcoidosis have been diagnosed prior to the diagnosis of sino-nasal involvement. Sarcoidosis patients present with a wide range of symptoms, including no symptoms at all. In up to a third of cases, patients are detected based on an abnormal chest X-ray or laboratory test [32]. Symptoms from sarcoidosis depend on what organ is affected. Table 13.3 summarizes the symptoms, physical findings, and laboratory tests for various manifestations of the disease. Criteria have been established for identifying various organ involvement [33].
Criteria have also been developed to define sino-nasal involvement in sarcoidosis [33]. A patient with known sarcoidosis elsewhere who has granulomatous changes on direct fiber-optic nasal endoscopy or imaging studies (Fig. 13.6) is felt to have at least probable sino-nasal sarcoidosis. Patients with chronic sinusitis are felt to have at least possible sino-nasal sarcoidosis. Patients with a positive sinus or nasal biopsy demonstrating non-caseating granulomas are highly probable to have sino-nasal sarcoidosis.
Patients with sino-nasal sarcoidosis can have a range of symptoms , including no specific complaints [5, 30, 34], although nasal congestion and rhinorrhea are reported in most cases. Crusting and/or epistaxis occur in about a quarter of patients. Anosmia, purulent rhinorrhea, and facial pain can also occur. Local examination will often demonstrate hypertrophy and/or a purplish hue due to the granulomatous inflammation. Figure 13.6 shows an endoscopic view of a patient with sino-nasal sarcoidosis.
Figure 13.7 shows our approach to evaluation of patients with possible sino-nasal disease [7]. Patients with nasal congestion or other symptoms are treated with nasal steroids and/or antibiotics. If symptoms persist for more than 3 weeks, a CT scan is performed. If the scan is suggestive of sinus disease, the patient is considered for referral to an otolaryngologist for possible endoscopy and biopsy. If the CT scan is normal, the patient may receive a longer course of therapy. If the patient is still requiring therapy after 2 months, they are referred for evaluation.
University of Cincinnati Sarcoidosis Clinic approach to evaluation of patients with possible sino-nasal disease [7]. *Treatment with nasal steroids and/or oral antibiotics
CT scanning is the most common imaging modality to detect sino-nasal disease, with abnormalities seen in most cases [30, 34]. Mucosal hypertrophy and/or opacification occurs in almost all cases of sino-nasal involvement. Turbinate or septal nodularity is present in about a third of cases (see Fig. 13.6), and bone lesions including erosions and osteoneogenesis is present in over a third of cases [30, 34].
There are some additional features that heighten the likelihood of sino-nasal sarcoidosis. Lupus pernio are papular lesions on the cheeks and nose, especially the nares [35]. In general, lupus pernio is seen in less than 2% of all sarcoidosis patients [36]. It is more frequent in patients of African descent, but can be seen in Caucasians [2, 3, 35]. There is strong association between lupus pernio and sino-nasal disease [1, 2].
Another important association is dacrocystitis . The drainage of the tear duct into the sinus can be blocked and lead to significant morbidity. This can be treated surgically with a dacryocystorhinostomy, although recurrent obstruction may occur [37]. Patients with dacrocystitis often have adnexal lesions [38]. In these patients, a CT scan may demonstrate lacrimal gland involvement as well as significant sinus disease (Fig. 13.8).
Table 13.4 lists the differential diagnosis of granulomatous sinus disease . In addition to routine pathologic examination, special stains should be performed to look for evidence of lymphoma or infection. In addition, testing for antineutrophil cytoplasmic antibody (ANCA ) should be performed. Further characterization of ANCA should be done to distinguish between cytoplasmic (c-ANCA) and perinuclear (p-ANCA). Systemic granulomatosis disease with polyangiitis (GPA), formerly known as Wegener’s disease, is strongly associated with a positive c-ANCA. On the other hand, a positive p-ANCA test has been reported in various inflammatory diseases, including Churg-Strauss.
The serum angiotensin-converting enzyme (ACE) has limited sensitivity and specificity in part because of genetic polymorphisms of the ACE enzyme [39] and because of the effect of corticosteroid therapy [40] on levels. However, a significantly elevated ACE level can be helpful in confirming the diagnosis of sarcoidosis. Over half of patients with active sarcoidosis will have an ACE level greater than 20% of the upper limit of normal. Patients with an ACE level that high have a greater than 90% chance of having sarcoidosis [41].
Management
The management of sino-nasal disease is usually a stepwise process. Figure 13.9 is the approach we employ in management of patients at our clinic. Initial therapy is topical, with use of nasal corticosteroids to control inflammation. If that is unsuccessful, we will use oral corticosteroids, usually prednisone.
A stepwise approach to management of sino-nasal sarcoidosis. Treatment is for the underlying sarcoidosis (top half of the flowchart) as well as management of any infection. Prednisone is the most commonly used oral corticosteroid in our clinic. Surgical management of residual scarring is usually reserved until after inflammation is controlled
In general, oral corticosteroid therapy for sarcoidosis is a long-standing intervention. Short-course treatments of up to 3 weeks are reserved for acute events [42, 43]. The rationale for long-term treatment with corticosteroids is because of the high rate of relapse of sarcoidosis when treatment is withdrawn [5, 30]. Once systemic therapy is initiated for sarcoidosis, about half of patients will require systemic therapy for more than 2 years [44, 45]. Some features, such as lupus pernio, are associated with the need for systemic treatment for 5 years or longer [46]. The goal with oral corticosteroid therapy is to reduce the patient to the lowest possible dose that maintains a clinical remission [47]. For most sarcoidosis patients, a maintenance dose of prednisone of less than 10 mg daily or its equivalent is associated with minimal adverse effects and is generally well tolerated [48].
For those patients unable to tolerate maintenance-dose prednisone or who have progressive disease despite corticosteroid therapy, antimetabolite therapy is a steroid-sparing alternative. Methotrexate is the most widely studied and used treatment as a second-line therapy for sarcoidosis [25, 49]. Table 13.5 summarizes several reported series as well as our own experience with various systemic therapies to treat sino-nasal sarcoidosis. For most studies, methotrexate was the most widely used steroid-sparing agent. Table 13.6 compares the various systemic treatments for sarcoidosis, including dosage and toxicity. Specific recommendations have been made for administrating and monitoring methotrexate therapy in sarcoidosis patients [50]. Azathioprine and leflunomide have been used less frequently to treat sarcoidosis. However, these drugs appear to be about as effective as methotrexate [51,52,53]. Mycophenolate has recently been reported as an effective steroid-sparing agent in sarcoidosis [54, 55]. All four of these agents seem to work about two-thirds of the time as steroid sparing. The use of an individual agent depends on the experience of the clinician and potential or real toxicity for the individual patient.
The antimalarial drugs hydroxychloroquine and chloroquine appear to be most effective for treatment of cutaneous disease [56]. They are not as effective for more aggressive forms of sarcoidosis, such as lupus pernio [3]. For sino-nasal sarcoidosis, they are often used as an adjunct to other treatments (see Table 13.5). Their toxicity is relatively low, although patients need to undergo routine ocular screening [57].
Monoclonal antibodies to tumor necrosis factor (anti-TNF) have changed the outcome of many patients with chronic sarcoidosis. Infliximab, a chimeric monoclonal antibody, has been the most widely used anti-TNF drug . In advanced pulmonary sarcoidosis, it was found to be significantly better than placebo treatment [58]. It was also found to be more likely to induce complete resolution of lupus pernio than any other drug combination [3]. Adalimumab has also been reported as effective in treating sarcoidosis, including lupus pernio [59, 60]. Not all anti-TNF agents are equally effective in treating sarcoidosis. Adalimumab appears to be less potent than infliximab [61]. A recent randomized trial failed to demonstrate a benefit of golimumab versus placebo [62]. Moreover, these drugs are associated with significant potential toxicity. Guidelines have been developed for use of these drugs in patients with sarcoidosis [63].
Rituximab is a monoclonal antibody against B cells. While originally developed to treat lymphoma, it has been found to have significant immunomodulatory effects. The drug has been reported as effective in refractory pulmonary [64] and ocular [65] disease.
Repository corticotrophin injections (RCI) have recently been reported as effective in treating advanced sarcoidosis [66]. While an old therapy, it had been abandoned for many years in routine management of sarcoidosis because of cost and question of mechanism of action. The drug stimulated the melanocortin receptors (MCR), including MCR-2. The MCR-2 is on the adrenal gland and stimulation leads to release of cortisol. However, there are several other MCRs , including some that regulate the immune system. Stimulation of these other MCR is felt to have benefit beyond just steroid effect of the drug [67]. Repository corticotrophin injection has been used in cases of refractory sarcoidosis who have failed conventional therapy and/or developed significant toxicity to various treatments [68].
Most patients with sarcoidosis can be managed medically [34]. As noted in Table 13.5, surgical intervention has been used in the management of sino-nasal sarcoidosis [29, 69]. While some cases may respond to surgery [69], relapses after surgery are common [7, 29, 30]. Endoscopic surgery may be effective in controlling symptoms [70], but it can be very difficult to control healing and prevent scarring. The risk for relapse can be reduced by aggressive use of immunosuppressive agents. However, immunosuppression will only reduce inflammation and has no impact on scar tissue. Once scarring occurs, surgery may prove effective in removing obstruction. Even extensive reconstruction surgery has been successfully performed when inflammation has been controlled [71].
Lawson et al. have proposed the classification of sino-nasal sarcoidosis as atrophic, hypertrophic, destructive, and nasal enlargement [72]. They reported good results with surgery only for the subgroup of patients with architectural changes. While these recommendations seem reasonable, they were based on a retrospective review of a limited number of cases and need to be confirmed prospectively.
For the therapy of sino-nasal sarcoidosis, one has to also consider infection. Abnormal sinus architecture from sarcoidosis represents the same challenge for the clinician as any other condition which affects the sinuses. Antibiotic regimens often progress in a stepwise fashion as depicted on the bottom half of Fig. 13.9 [73]. Cultures may provide evidence to support targeted therapy, especially for aspergillosis and atypical mycobacteria. Figure 13.10 demonstrates the CT scan of a patient with chronic ocular, pulmonary, and sinus sarcoidosis treated at our institution. While on infliximab and methotrexate therapy, she had developed a chronic sinus infection. Cultures of her left maxillary sinus grew M. avium. Her sinus symptoms responded well to withdrawal of infliximab and anti-mycobacterial therapy. However, she had to be placed back on prednisone 20 mg to control her ocular disease.
Axial CT scan of head of a 70-year-old white female with chronic ocular, pulmonary, and sinus sarcoidosis for more than 12 years. Patient had developed headache and fever while on maintenance therapy of methotrexate 10 mg a week and infliximab 5 mg/kg once a month. Her CT scan demonstrated fluid collection in left maxillary sinus. Biopsy of sinus showed highly cellular inflammation with numerous acid-fast bacilli seen on special staining. Culture grew M. avium complex (MAC). Her infliximab was discontinued and she was placed on anti-mycobacterial therapy. While her sinus symptoms resolved, her optic neuritis flared and prednisone was reinstituted. After 1 year of anti-mycobacterial therapy, she is stable and without evidence of mycobacterial infection. However, she remains on 20 mg prednisone daily with 10 mg-a-week methotrexate
In addition to targeted antibiotic therapy, nasal rinses with broad-spectrum antibiotics can be utilized. Gentamicin is commonly used, since topical application usually does not lead to toxicity. However, systemic absorption can still occur [74] and toxicity should be assessed for those on chronic therapy. Prolonged use of systemic antibiotics has shown benefit in some patients [75]. However, these antibiotic regimens have not been studied in sino-nasal sarcoidosis.
Conclusion
While sino-nasal sarcoidosis is an unusual form of sarcoidosis, it often leads to chronic disease. For some patients, local therapy may be sufficient. Systemic therapy follows a stepwise approach, with prednisone or similar oral corticosteroid the initial drug of choice. However, because of the need for long-term therapy , steroid-sparing alternatives should be considered early in the management of advanced sino-nasal disease. Antimetabolites are effective steroid-sparing agents. Newer modalities, including anti-TNF antibodies , have proved effective in treating refractory cases. Surgery is effective in addressing architectural changes due to scarring. It is most effective in patients in whom inflammation is controlled by immunosuppression therapy.
References
Baughman RP, Judson MA, Teirstein AS, Moller DR, Lower EE. Thalidomide for chronic sarcoidosis. Chest. 2002;122:227–32.
Spiteri MA, Matthey F, Gordon T, Carstairs LS, James DG. Lupus pernio: a clinico-radiological study of thirty-five cases. Br J Dermatol. 1985;112(3):315–22.
Stagaki E, Mountford WK, Lackland DT, Judson MA. The treatment of lupus pernio: results of 116 treatment courses in 54 patients. Chest. 2009;135(2):468–76.
Baughman RP, Lower EE, Tami T. Upper airway. 4: sarcoidosis of the upper respiratory tract (SURT). Thorax. 2010;65(2):181–6.
Panselinas E, Halstead L, Schlosser RJ, Judson MA. Clinical manifestations, radiographic findings, treatment options, and outcome in sarcoidosis patients with upper respiratory tract involvement. South Med J. 2010;103(9):870–5.
Bouaziz A, Le SJ, Chapelon-Abric C, Varron L, Khenifer S, Gleizal A, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105(8):755–67.
Zeitlin JF, Tami TA, Baughman R, Winget D. Nasal and sinus manifestations of sarcoidosis. Am J Rhinol. 2000;14(3):157–61.
Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(Sep):149–73.
Broos CE, van Nimwegen M, Hoogsteden HC, Hendriks RW, Kool M, van den Blink B. Granuloma formation in pulmonary sarcoidosis. Front Immunol. 2013;4:437. https://doi.org/10.3389/fimmu.2013.00437.:437.
Broos CE, van NM, In ’t Veen JC, Hoogsteden HC, Hendriks RW, van den Blink B, et al. Decreased cytotoxic T-lymphocyte antigen 4 expression on regulatory T cells and Th17 cells in sarcoidosis: double trouble? Am J Respir Crit Care Med. 2015;192(6):763–5.
Ramstein J, Broos CE, Simpson LJ, Ansel KM, Sun SA, Ho ME, et al. IFN-gamma-producing T-helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-helper type 1 cells. Am J Respir Crit Care Med. 2016;193(11):1281–91.
Celada LJ, Rotsinger JE, Young A, Shaginurova G, Shelton D, Hawkins C, et al. Programmed death-1 inhibition of phosphatidylinositol 3-kinase/AKT/mechanistic target of rapamycin signaling impairs sarcoidosis CD4+ T cell proliferation. Am J Respir Cell Mol Biol. 2017;56(1):74–82.
Prasse A, Zissel G, Lutzen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182(4):540–8.
Su R, Li MM, Bhakta NR, Solberg OD, Darnell EP, Ramstein J, et al. Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. Eur Respir J. 2014;44(4):985–93.
Baughman RP, Keeton D, Lower EE. Relationship between interleukin-8 and neutrophils in the BAL fluid of sarcoidosis. Sarcoidosis. 1994;11:S217–20.
Loza MJ, Brodmerkel C, du Bois RM, Judson MA, Costabel U, Drent M, et al. Inflammatory profile and response to anti-TNF therapy in patients with chronic pulmonary sarcoidosis. Clin Vaccine Immunol. 2011;18:931–9.
Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Müller-Quernheim J. Sarcoidosis: TNF-alpha release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med. 1997;156(5):1586–92.
Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201(5):755–67.
Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75(1):527–30.
Dubaniewicz A, Trzonkowski P, Dubaniewicz-Wybieralska M, Dubaniewicz A, Singh M, Myśliwski A. Mycobacterial heat shock protein-induced blood T lymphocytes subsets and cytokine pattern: comparison of sarcoidosis with tuberculosis and healthy controls. Respirology. 2007;12(3):346–54.
Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40(Jan):198–204.
Zhou Y, Wei YR, Zhang Y, Du SS, Baughman RP, Li HP. Real-time quantitative reverse transcription-polymerase chain reaction to detect propionibacterial ribosomal RNA in the lymph nodes of Chinese patients with sarcoidosis. Clin Exp Immunol. 2015;181:511–7.
Izbicki G, Chavko R, Banauch GI, Weiden MD, Berger KI, Aldrich TK, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131(5):1414–23.
Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J. 2013;41(3):621–6.
Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc. 2016;13(8):1244–52.
Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J. 2016;48(6):1690–9.
Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a five year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–41.
Dumas O, Abramovitz L, Wiley AS, Cozier YC, Camargo CA Jr. Epidemiology of sarcoidosis in a prospective cohort study of U.S. women. Ann Am Thorac Soc. 2016;13(1):67–71.
Kirsten AM, Watz H, Kirsten D. Sarcoidosis with involvement of the paranasal sinuses - a retrospective analysis of 12 biopsy-proven cases. BMC Pulm Med. 2013;13:59. https://doi.org/10.1186/1471-2466-13-59.:59-13.
Aubart FC, Ouayoun M, Brauner M, Attali P, Kambouchner M, Valeyre D, et al. Sinonasal involvement in sarcoidosis: a case-control study of 20 patients. Medicine (Baltimore). 2006;85(6):365–71.
Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H Jr. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86.
Pietinalho A, Ohmichi M, Hiraga Y, Löfroos AB, Selroos O. The mode of presentation of sarcoidosis in Finland and Hokkaido, Japan. A comparative analysis of 571 Finnish and 686 Japanese patients. Sarcoidosis. 1996;13:159–66.
Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):19–27.
Aloulah M, Manes RP, Ng YH, Fitzgerald JE, Glazer CS, Ryan MW, et al. Sinonasal manifestations of sarcoidosis: a single institution experience with 38 cases. Int Forum Allergy Rhinol. 2013;3(7):567–72.
Baughman RP, Judson MA, Teirstein A, Lower EE, Lo K, Schlenker-Herceg R, et al. Chronic facial sarcoidosis including lupus pernio: clinical description and proposed scoring systems. Am J Clin Dermatol. 2008;9(3):155–61.
Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;208:525–33.
Garcia GH, Harris GJ. Sarcoid inflammation and obstruction of the nasolacrimal system. Arch Ophthalmol. 2000;118(5):719–20.
Demirci H, Christianson MD. Orbital and adnexal involvement in sarcoidosis: analysis of clinical features and systemic disease in 30 cases. Am J Ophthalmol. 2011;151(6):1074–80.
Tomita H, Ina Y, Sugiura Y, Sato S, Kawaguchi H, Morishita M, et al. Polymorphism in the angiotensin-converting enzyme (ACE) gene and sarcoidosis. Am J Respir Crit Care Med. 1997;156(1):255–9.
Baughman RP, Ploysongsang Y, Roberts RD, Srivastava L. Effects of sarcoid and steroids on angiotensin-converting enzyme. Am Rev Respir Dis. 1983;128:631–3.
Lieberman J, Nosal A, Schlessner A, Sastre-Foken A. Serum angiotensin-converting enzyme for diagnosis and therapeutic evaluation of sarcoidosis. Am Rev Respir Dis. 1979;120(2):329–35.
Baughman RP, Lower EE. Frequency of acute worsening events in fibrotic pulmonary sarcoidosis patients. Respir Med. 2013;107:2009–13.
McKinzie BP, Bullington WM, Mazur JE, Judson MA. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am J Med Sci. 2010;339(1):1–4.
Gottlieb JE, Israel HL, Steiner RM, Triolo J, Patrick H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest. 1997;111(3):623–31.
Baughman RP, Judson MA, Teirstein A, Yeager H, Rossman M, Knatterud GL, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM. 2006;99(5):307–15.
Baughman RP, Lower EE. Features of sarcoidosis associated with chronic disease. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(4):275–81.
Johns CJ, Michele TM. The clinical management of sarcoidosis: a 50-year experience at the Johns Hopkins hospital. Medicine. 1999;78:65–111.
Judson MA, Chaudhry H, Louis A, Lee K, Yucel R. The effect of corticosteroids on quality of life in a sarcoidosis clinic: the results of a propensity analysis. Respir Med. 2015;109(4):526–31.
Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010;104(5):717–23.
Cremers JP, Drent M, Bast A, Shigemitsu H, Baughman RP, Valeyre D, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. 2013;19:545–61.
Vorselaars AD, Wuyts WA, Vorselaars VM, Zanen P, Deneer VHM, Veltkamp M, et al. Methotrexate versus azathioprine in second line therapy of sarcoidosis. Chest. 2013;144:805–12.
Sahoo DH, Bandyopadhyay D, Xu M, Pearson K, Parambil JG, Lazar CA, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145–50.
Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:43–8.
Hamzeh N, Voelker A, Forssen A, Gottschall EB, Rose C, Mroz P, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med. 2014;108:1663–9.
Brill AK, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration. 2013;86:376–83.
Jones E, Callen JP. Hydroxychloroquine is effective therapy for control of cutaneous sarcoidal granulomas. J Am Acad Dermatol. 1990;23(3 Pt 1):487–9.
Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–22.
Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802.
Sweiss NJ, Noth I, Mirsaeidi M, Zhang W, Naureckas ET, Hogarth DK, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):46–54.
Judson MA. Successful treatment of lupus pernio with adalimumab. Arch Dermatol. 2011;147(11):1332–3.
Baughman RP. Tumor necrosis factor inhibition in treating sarcoidosis: the American experience. Revista Portuguesa de Pneumonologia. 2007;13:S47–50.
Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–307.
Drent M, Cremers JP, Jansen TL, Baughman RP. Practical eminence and experience-based recommendations for use of TNF-alpha inhibitors in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(2):91–107.
Sweiss NJ, Lower EE, Mirsaeidi M, Dudek S, Garcia JG, Perkins D, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J. 2014;43(5):1525–8.
Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol. 2012;6:1613–8.
Baughman RP, Barney JB, O'Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72.
Berkovich R, Agius MA. Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther Adv Neurol Disord. 2014;7(2):83–96.
Zhou Y, Lower EE, Li H, Baughman RP. Sarcoidosis patient with lupus pernio and infliximab-induced myositis: response to Acthar gel. Respir Med Case Rep. 2015;17:5–7.
Gulati S, Krossnes B, Olofsson J, Danielsen A. Sinonasal involvement in sarcoidosis: a report of seven cases and review of literature. Eur Arch Otorhinolaryngol. 2012;269(3):891–6.
Kay DJ, Har-El G. The role of endoscopic sinus surgery in chronic sinonasal sarcoidosis. Am J Rhinol. 2001;15(4):249–54.
Gurkov R, Berghaus A. Nasal reconstruction in advanced sinunasal sarcoidosis. Rhinology. 2009;47(3):327–9.
Lawson W, Jiang N, Cheng J. Sinonasal sarcoidosis: a new system of classification acting as a guide to diagnosis and treatment. Am J Rhinol Allergy. 2014;28(4):317–22.
Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015;314(9):926–39.
Whatley WS, Chandra RK, MacDonald CB. Systemic absorption of gentamicin nasal irrigations. Am J Rhinol. 2006;20(3):251–4.
Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AG, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011994. https://doi.org/10.1002/14651858.CD011994.pub2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Baughman, R.P., Seiden, A., Lower, E.E. (2018). Sino-Nasal Sarcoidosis. In: Bernstein, J. (eds) Rhinitis and Related Upper Respiratory Conditions. Springer, Cham. https://doi.org/10.1007/978-3-319-75370-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-75370-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75369-0
Online ISBN: 978-3-319-75370-6
eBook Packages: MedicineMedicine (R0)