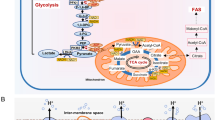
Abstract
Metabolism is highly coordinated component of the cellular activity that involves sequential chemical transformations, within a so-called metabolic network. Through these coordinated actions, living organisms acquire energy and biosynthetic precursors to maintain cellular homeostasis and function. Metabolism relies on the breaking down of macromolecules to produce energy [catabolism] and/or intermediary metabolites that are then used to construct essential building blocks for macromolecule production [anabolism]. Overall, these metabolic processes are controlled by cellular energy status: when the energy released from catabolic processes exceeds the cellular demands the storage of metabolites in the form of lipids and glycogen takes place. These phenomena have been vastly associated with the genesis of metabolic disorders, such as obesity. In recent years, we have assisted to a rediscovery of metabolism through the identification of metabolic intermediaries that act as key players on differentiation, proliferation, and function of immune cells. This recent acknowledgement of the impact of metabolism in the overall immune response originated the ground-breaking field of immunometabolism. Here, we will provide a holistic view of metabolism highlighting the biochemical principles underlying its regulation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Key Concepts on the Regulation of Cellular Metabolism
Metabolism (or the RoadMap of the metabolic reactions) relies on energy interconversion within the adenylate system [composed by adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP)] and/or oxidation–reduction reactions, where the electrons are transiently stored [mainly within the nicotinamide adenine dinucleotide (NAD) and/or flavin adenine dinucleotide (FAD) systems, as well as nicotinamide adenine dinucleotide phosphate (NADP)] to govern the overall cellular metabolic fluxes. Despite few exceptions, these are the main players that dictate the dynamic flow of metabolites between the distinct metabolic pathways. These metabolic interconnectors are constantly linking and modulating specific pathways to maintain their balance. Although frequently forgotten, the Le Chatelier’s principle is inevitably respected, and it is imperative for the maintenance of cellular homeostasis. The flow of metabolites between pathways relies on a sequence of reactions that are maintained in a state of dynamic equilibrium. For instance, it is known that the glycolytic flux is highly dependent on NAD+ availability within the carbohydrate metabolism. Consequently, an increased rate of this pathway has to rely on increased rate of NAD+ regeneration. This is commonly achieved through the utilization of pyruvate as final electron acceptor resulting in the production of lactate, in a process named lactic fermentation. Such process occurs due to the fact that the rate for aerobic respiration is unable to sustain the velocity of NAD+ replenish to fulfill the high demand of this intermediate for glycolysis to accomplish the ATP requests. Although this process occurs mainly under anaerobic conditions, an impressive amount of recent data shows that oxygen is only an additional player in this crosstalk (Cheng et al. 2014; Déry et al. 2005; Jiang 2017; Jones and Bianchi 2015; Lunt and Vander Heiden 2011; Romero-Garcia et al. 2016).
Several factors strongly impact the metabolic route followed by a metabolite, namely: (1) the affinity of the enzyme for the substrate within a metabolic branching point, (2) the amount of enzyme (translated by the maximum velocity of the reaction), and (3) their post-translation activation/inactivation either by covalent modifications or by allosteric regulators. Having these concepts in consideration, to define a metabolic RoadMap for a certain substrate, it is essential to keep in mind that several metabolites have to cross cellular membranes and, for those that do not freely diffuse across the membranes, selective transporters are needed. For instances, NAD+ is unable to cross membranes and, therefore, this molecule is stored in different cellular compartments, with the cytosol and the mitochondria representing the main NAD+ pools (reviewed in Chap. 4). Therefore, the transport-mediated flux and counter flux of oxidized/reduced metabolites through cellular membranes (e.g., malate/aspartate shuttle) allow the maintenance of redox balance (NAD+/NADH) in cellular compartments and are responsible for driving metabolic flow within specific pathways.
The overarching role of metabolism in cellular function has driven the scientific community to open access to detailed data regarding metabolites, cofactors, and enzymes involved in each metabolic process for different cellular condition/stimuli/insult. However, the dynamic interplay between several metabolic nodes that regulate cellular function and metabolism cannot be overlooked when analyzing these data at the cellular functionality level. As such, the main key to integrate metabolism toward a better understanding of physiology and pathology is to comprehend the intricate connections between all of the metabolic pathways and their main sites of regulation.
2 An Introduction to Glycolysis
Glycolysis was the first identified and better studied metabolic pathway, which accounted for the contributions of several renowned scientists, among them was Eduard Buchner, Otto Warburg, and Hans von Euler-Chelpin. The development of molecular biology and biochemistry techniques allowed the purification of glycolytic enzymes and the discovery of important cofactors, such as NAD+, which boosted the knowledge on this vital pathway. Glycolysis is a cytosolic pathway virtually present in all cells. During this process, one molecule of glucose is enzymatically broken in two molecules of pyruvate, with the consequent release of free energy in the form of two molecules of ATP and two NADH. The cellular rate for glucose metabolism depends mainly on the major cellular metabolic fluxes, as a cellular requirement of ATP or building blocks for the anabolic reactions.
2.1 Glucose Uptake
The first step on the metabolism of glucose is its entrance in the cell. Due to its biochemical properties, this molecule does not freely diffuse through cellular membranes. The majority of mammalian cells import glucose by facilitative diffusion, through the action of glucose transporters (GLUT) that belong to the (SLC2A) family of membrane transport proteins. In some cellular tissues, this transport defines the rate-determining step in glucose metabolism (Mueckler and Thorens 2013; Thorens and Mueckler 2010). The most studied members of this family range from GLUT1 to GLUT4, which display distinct kinetic properties and regulatory mechanisms. GLUT1, which is encoded by the gene SLC2A1, was one of the first membrane transporters to be purified and it is the most studied glucose transporter. Along with GLUT3, it mediates the basal and constant glucose uptake in several tissues, namely the brain, which is dependent on glucose catabolism. On the other hand, GLUT2 has a very low affinity for glucose, which implies that glucose uptake occurs only when high concentration of this metabolite is available. The SLC2A4 gene encodes for GLUT4, which is mainly expressed in adipocytes, skeletal muscle, and cardiomyocytes. An interesting trait of this transporter is that, under basal conditions, GLUT4 resides in intracellular vesicles. However, upon insulin signaling, these intracellular vesicles containing transporters are fused to the plasma membrane exposing GLUT4 to the extracellular compartment, thus increasing glucose uptake and metabolism.
2.2 Glycolytic Process
The glycolytic process leads to the degradation of glucose [six-carbon (C6) molecule] in two pyruvates [three-carbon (C3) molecule]. Pyruvate may then follow different metabolic pathways (Fig. 1.1). The ten enzymatic steps that govern glycolysis occur in the cytosol, and some of them are tightly regulated to maintain an adequate glycolytic flux (Berg et al. 2002a, b). The first step relies on the phosphorylation of glucose by the rate-limiting enzyme hexokinase, which yields glucose-6-phosphate. The quick phosphorylation of intracellular glucose prevents its cellular efflux as well as the enzymatic conservation of high-energy bonds through the formation of phosphate esters. Additionally, phosphoryl groups destabilize glucose and facilitate its consequent metabolism. Hexokinase is regulated through a negative feedback loop by glucose-6-phosphate that when in high intracellular quantity signals for excess of cellular energy. Glucose-6-phosphate is then reversibly isomerized to fructose-6-phosphate, which can be further phosphorylated by phosphofructokinase (PFK), at the cost of one ATP molecule, to fructose-1,6-bisfosfate. The irreversible step mediated by PFK is a very important pacemaker of glycolysis being allosterically positively regulated by AMP and negatively by ATP, fructose-2,6-phosphate, and citrate. This balance points out the importance of the ATP/AMP ratio for the rate of glycolysis, as well as its regulation to channel carbon molecules through the oxidative branch of the pentose phosphate pathway (PPP) for maintaining the NADPH pool for fatty acid synthesis. Ultimately, FK is also capable of indirectly inhibiting hexokinase. If PFK is inactive, fructose-6-phosphate and glucose-6-phosphate accumulates, thus inhibiting hexokinase. However, this dynamic may be variable depending on the final destination of glucose-6-phosphate. Although the latter is an important intermediate, since it can modulate the glycolytic flux, it may also be shuttled to feed glycogenesis or the pentose phosphate pathway. The other irreversible step of glycolysis is mediated by pyruvate kinase where phosphoenolpyruvate is converted into the end glycolytic product, pyruvate. In fact, pyruvate kinase isoforms have been suggested to support divergent energetic and biosynthetic output and thus fulfilling differential requirements of cellular metabolism. As such, this catalytic activity plays a crucial role in energy metabolism and has recently been associated with cell proliferation and tumor growth (Israelsen and Vander Heiden 2015; Yang and Lu 2013).
Although glucose represents a high-energy substrate, only a small portion of free energy is released during glycolysis: most of it remains in the end product, pyruvate, that needs to be further catabolized.
2.3 The Fate of Glycolysis End Products: Pyruvate and NADH
In mammalian organisms, the end product of glycolysis, pyruvate, may then be further metabolized via two major catabolic pathways. In one hand, pyruvate may be oxidized in the mitochondria to yield acetyl-Coenzyme A, which will sustain the Tricarboxylic Acid (TCA) cycle (also named citric acid cycle or Krebs cycle). On the other, pyruvate may be reduced to lactate. Furthermore, in certain invertebrates, protists, and microorganisms, as the brewer’s yeast, pyruvate may be redirected to the production of ethanol and carbon dioxide, in a process known ethanol fermentation (Boulton 1996).
To maintain glycolysis in perpetuation, cytosolic redox balance (NAD+/NADH) has to be attained. One way to regenerate the cytosolic NAD+ is through shuttles to the mitochondria (discussed below in this chapter) or by fermentation through the action of lactate dehydrogenase. In this process, pyruvate is reduced to lactate and the NAD+ pool is reestablished. This process was thought to occur mainly at low oxygen tension (such as in hypoxic environments), but recent data show that it also occurs under normoxic conditions in specific cell types and within specific differential activations. Although the fermentation of pyruvate restores NAD+ efficiently, only a small fraction of the glucose’s free energy is released in this process. The yield of ATP per glucose consumed is lower under fermentative conditions when compared with respiration. However, the amount of ATP per time unit (velocity) is higher under fermentative conditions.
The mitochondrial oxidative decarboxylation of pyruvate by the pyruvate dehydrogenase complex, which is an irreversible reaction, produces acetyl-CoA, the carbon substrate of the TCA cycle (Berg et al. 2002a). Furthermore, pyruvate may also be shifted towards anabolism. Pyruvate may be transaminated into alanine, thus providing a direct link between amino acid and carbohydrate metabolisms. Also, pyruvate may also be carboxylated into oxaloacetate in the mitochondria, which yields a precursor for gluconeogenesis or for amino acid synthesis (aspartate).
2.4 The Pentose Phosphate Pathway, the Link to Glycolysis and Cellular Detoxification
The pentose phosphate pathway (PPP), also known as the hexose monophosphate shunt or phosphogluconate pathway, is a major glycolytic-divergent pathway that shifts metabolic reactions towards the production of precursors for nucleotide and amino acid synthesis as well as the mostly studied product, the reducing co-factor NADPH (Berg et al. 2002c) (Fig. 1.2). This pathway functions to support cell proliferation and survival (Buchakjian and Kornbluth 2010; Patra and Hay 2014). The reducing equivalent NADPH is a cofactor for the biosynthesis of fatty acids and sterols, and it also has a vital role in countering oxidative stress. The PPP comprises an oxidative and a non-oxidative branch. The rate limiting step of the oxidative branch is mediated by glucose-6-phosphate dehydrogenase (G6PD) that through the oxidative decarboxylation of glucose-6-phosphate, produces two molecules of NADPH and one ribose-5-phosphate. As expected, this branch relies on the availability of NADP+, as the final electron acceptor, while excess of NADPH is a negative regulator. The non-oxidative branch includes several reversible reactions that interchange carbons from/to three-carbon and six-carbon molecules to/from five-carbon molecules, depending on the cellular concentration of NADPH and ribose-5-phosphate, being rate limited by transketolase and transaldolase.
The fine balance between the PPP and glycolysis is tightly regulated. Whereas ribose-5-phosphate can be produced for nucleotide synthesis, NADPH production mainly supports fatty acid synthesis and has an important action in counteracting oxidative stress. For the latter, six molecules of ribose-5-phosphate are shifted towards production of two molecules of glyceraldehyde-3-phosphate and four molecules of fructose-6-phosphate, thus fuelling glycolysis. The maintenance of redox homeostasis is a vital requisite for cell survival, particularly in aerobic organisms. Reactive oxygen species (ROS) are continuously produced by the partial reduction of molecular oxygen, which release free radicals such as the superoxide anion or the hydroxyl radical. The exacerbated production of these molecules and/or decreased elimination by cellular detoxification systems originates molecular damage in several macromolecules, resulting in the development of mutations and eventually cell death (Dan Dunn et al. 2015; Ott et al. 2007). The steady-state control of oxidative stress is maintained by the action of several detoxification systems, which include glutathione reductase. This enzyme reduces oxidized glutathione (a tripeptide with a free sulfhydryl group) with the concerted action of NADPH, as electron donor.
When taken together, these data show that PPP is essential not only in the coordination of cellular metabolism (catabolism/anabolism) but also for fulfilling intermediaries that are needed for cellular defense and detoxification.
2.5 Gluconeogenesis
Gluconeogenesis is the anabolic process by which glucose is synthesized, occurring mainly at the liver and kidney. The noncarbohydrate biosynthetic precursors for this pathway are essentially lactate, amino acids, and glycerol, whose availability is responsible for determining the rate of gluconeogenesis. Although glycolysis and gluconeogenesis are not exactly the reverse of each other, both pathways share several enzymes and are reciprocally regulated within the same cell.
The precursors of this pathway are molecules with at least three carbons that may enter directly in the TCA or glycolytic intermediates, such as dihydroxyacetone phosphate. The major differences between gluconeogenesis and glycolysis are at: (1) the conversion of pyruvate to phosphoenolpyruvate (mediated by two enzymatic steps), (2) the hydrolysis of fructose-1-6-biphosphate in fructose 6-phosphate, and (3) the hydrolysis of glucose-6-phosphate in glucose. The first step of gluconeogenesis takes place in the mitochondria and consists in the carboxylation of pyruvate to oxaloacetate, at the expense of one ATP molecule by pyruvate carboxylase. Oxaloacetate can then be cleaved into phosphoenolpyruvate, by phosphoenolpyruvate carboxykinase (PEPCK) that is transported to the cytosol to follow the reversible glycolytic reversible steps until the formation of 1,3-bisphosphoglyerate (discussed below) fueling the reaction mediated by glyceraldehyde 3-phosphate dehydrogenase. However, this reaction requires reducing equivalents at the level of NADH. As such, translocation of reducing equivalents from the mitochondria (where β-oxidation of fatty acid is taking place leading to an excess of this cofactors) area is essential for gluconeogenesis. In this sense, mitochondrial oxaloacetate may be reduced to malate using the excess of mitochondrial NADH, which is then transported to the cytosol. Once in cytosolic, malate is re-oxidized to oxaloacetate, which, by the action of phosphoenolpyruvate carboxykinase, is simultaneously decarboxylated and phosphorylated to generate phosphoenolpyruvate at the expense of energy. Following these reactions, phosphoenolpyruvate is reversely converted in the upstream intermediates of the glycolytic pathway until the irreversible conversion of fructose-1-6-biphosphate into fructose-6-phosphate. The phosphate ester at C1 is hydrolyzed by fructose-1-6-biphosphatase, thus yielding fructose 6-phosphate. After isomerization by phosphoglucose isomerase, the obtained glucose-6-phosphate is cleaved by endoplasmic reticulum-bounded glucose-6-phosphatase, which originates glucose that is secreted to maintain the serum glucose levels. The ratio of mitochondrial transport of malate or phosphoenolpyruvate, or other source, to cytosol depends mainly on the availability of cytosolic NADH.
This pathway is energetically expensive, being, therefore, replenished via fatty acid β-oxidation. Indeed, fatty acids are rapidly mobilized by the hydrolytic action of lipases from triglycerides. The fatty acids products follow β-oxidation, whereas glycerol produced is mainly used to replenish gluconeogenic precursors.
3 The Tricarboxylic Acid Cycle and Oxidative Phosphorylation
The TCA cycle is the universal aerobic pathway for the complete oxidation of several macromolecules, including glucose, fatty acids, and some amino acids (Akram 2014; Citric and Cycle 2010; Kornberg 2000) (Fig. 1.3). In summary, the organic carbon fuels are oxidized to acetyl-CoA, which is fed to TCA and completely oxidized to H2O and CO2. The released energy is conserved in the form of NADH and FADH2, as well as GTP. The reduced molecules are highly energetic being used as substrates for the electron transport chain (ETC), which culminates with the reduction of molecular oxygen to H2O and the buildup of a proton gradient along the internal mitochondrial membrane (Fig. 1.4). The energy of this electrochemical proton gradient is further used by ATP synthase for ATP production, in a process usually called oxidative phosphorylation. The respiratory control guarantees that the rate of the TCA cycle matches the cellular ATP utilization levels. These processes take place in the mitochondria, which are the cellular powerhouses responsible for the generation of energy in eukaryotic cells. While the TCA cycle occurs in the mitochondrial matrix, the ETC is localized within the mitochondrial inner membrane. Although the combination of these processes may result in the complete oxidative catabolism of organic carbon fuels, the TCA cycle is also a pathway used for the building blocks that will feed the de novo synthesis of several macromolecules (therefore designated an amphibolic pathway).
3.1 The Molecular Mechanisms Underlying the TCA Cycle
A mitochondrial complex named pyruvate dehydrogenase complex (PDH) is responsible for the decarboxylation of pyruvate in acetyl-CoA committing pyruvate carbons to be either converted into CO2 or shifted to de novo lipid synthesis, via export of mitochondrial citrate. Therefore, the PDH is highly regulated by both energy and redox state. High concentrations of NADH, acetyl-CoA, and ATP indicate high levels of energy and biosynthetic precursors, which function as negative regulators of PDH activity, via activation of pyruvate dehydrogenase kinase (PDHK). PDHK regulates negatively PDH activity through phosphorylation. On the other hand, increased concentration of pyruvate signals availability of precursors for replenishment of the TCA cycle, consequently negatively modulating PDHK activity, increasing the flux through PDH. Therefore, it is clear that the TCA cycle rate is dependent and adjusted to the cellular concentrations of acetyl-CoA, ATP, and NADH, allowing a translation between biosynthesis and catabolism.
The first step of the TCA cycle is the condensation of acetyl-CoA with oxaloacetate by citrate synthase, which yields the six-carbon molecule citrate. The first primary control point in the TCA cycle occurs in the decarboxylation of isocitrate in α-ketoglutarate. NADH and ATP inhibit this reaction, which under certain circumstances (excess of pyruvate and excess of acetyl-CoA) may culminate in accumulation of citrate triggering lipid synthesis. The allosteric inhibition of α-ketoglutarate dehydrogenase, by succinyl-CoA and NADH, hampers its activity to convert α-ketoglutarate into succinyl-CoA. Once again, the inhibition of this step by reducing equivalents or end products may provide the metabolic intermediates for amino acid synthesis, via glutamate dehydrogenase. Succinyl-CoA is further converted into succinate, with the production of GTP, and then the carbon skeleton is oxidized by succinate dehydrogenase (complex II of the ETC) to form fumarate, which is again oxidized to malate. This final intermediate is the able to regenerate oxaloacetate, thus closing the cycle.
The result of one cycle turn of the TCA cycle is: three molecules of NADH, one of FADH2, one of GTP, and two of CO2. Each highly energetic reducing equivalent produced contains a pair of electrons that are substrates of the ETC.
3.2 TCA Cycle Intermediates Are Important Biosynthetic Precursors
TCA cycle is an amphibolic pathway that generates several biosynthetic precursors for de novo synthesis of macromolecules are produced. Oxaloacetate and α-ketoglutarate are precursors for aspartate and glutamate synthesis, which in turn may originate other amino acids, such as asparagine, arginine, proline, and glutamine. Furthermore, these intermediates can also contribute to the synthesis of pyrimidines and purines, which are the main building blocks of nucleic acids (Lane and Fan 2015). Oxaloacetate may also be converted into phosphoenolpyruvate, by phosphoenolpyruvate carboxykinase, thus completing the first step of gluconeogenesis. Succinyl-CoA withdrawal is responsible for the central metabolism that originates the synthesis of porphyrin rings, essential for hemoglobin production (Bonkovsky et al. 2013). Citrate production may have a vital role in de novo fatty acid synthesis. However, when these intermediates are withdrawn from the TCA cycle, the rate of the cycle is reduced and only their replenishment will allow the continuation of the oxidative capacity of this cycle. This exquisite and dynamic balance is mainly assured by pyruvate carboxylase that produces oxaloacetate via carboxylation of pyruvate, which guarantees the maintenance of homeostatic levels of TCA intermediates.
3.3 Oxidative Phosphorylation
Oxidative phosphorylation is the process where electrons from NADH or FADH2 are transferred to the final electron acceptor oxygen, which is reduced to water originating a transmembrane electrochemical proton gradient (ΔΨm) (Fig. 1.4). The latter is further dissipated towards ATP formation through the ATP synthase (Balaban 1990; Hansford 2002; Nath and Villadsen 2015). This process is the major source of energy in aerobic organisms, resulting in the generation of 26 from the 30 molecules of ATP. These are produced upon complete oxidation of one molecule of glucose in water and carbon dioxide contrasting with those two from lactic acid fermentation. Oxidative phosphorylation takes place in the mitochondria, specifically in the inner membrane. This membrane is folded into cristae, and it is embedded with the mitochondrial respiratory complexes, which are responsible for the electron flux along respiration, as well as the proton extrusion from the mitochondrial matrix. During cellular respiration, electrons from NADH are transferred to the final electron acceptor through three major respiratory complexes: NADH-Q-oxidoreductase (complex I), Q-cytochrome c oxido-reductase (complex III), and cytochrome c oxidase (complex IV). On the other hand, the ETC II (complex II) is the succinate dehydrogenase that oxidizes succinate into fumarate linking directly the ETC and the TCA. Moreover, two mobile electron carriers, ubiquinone and cytochrome C, are responsible for the elaborated electron transport process from complex I and II to the complex III and from complex III to complex IV, respectively. As such, the final step of the ETC is the oxidation of the reduced cytochrome c by cytochrome oxidoreductase with the consequent reduction of oxygen to water. ATP production from ADP is achieved when protons flow back into the mitochondrial matrix, through the fifth complex, ATP synthase (or F1F0 ATPase). The F1 subunit is responsible for the ATPase activity, while the F0 one contains the proton channel that allows the reverse flux of H+, pumping three protons back to mitochondria, resulting in the synthesis of one molecule of ATP. However, because of the electronegativity of the ATP/ADP translocator, the overall proton leakage per an ATP molecule is the equivalent to four protons. The rate of oxidative phosphorylation is dictated by the cellular energy flux, and it depends on the availability of a high-energy source of electrons (such as NADH and FADH2), oxygen, ADP, and inorganic phosphate. However, intracellular ADP content is the most important and regulatory mechanism regarding the oxidative process.
Overall, the electron-motive force is coupled to the proton pumping activity of complexes I, III, and IV, which generates the mitochondrial proton motive force ΔΨm across the inner-mitochondrial membrane. The dissipation of energy of ΔΨm from the backflow of protons from the mitochondrial intermembrane space to the matrix is further used by ATP synthase to synthesize ATP from ADP and phosphate (Senior et al. 2002).
3.4 NAD+ Regeneration and Shuttles Across Mitochondrial Membranes
Although the mitochondrial membrane remains impermeable to most molecules, the exchange of certain molecules between the cytosol and the mitochondria (and vice versa) is mediated by transport systems essential for metabolic homeostasis. Some of these systems are used to translocate reducing equivalents from the cytosol to the mitochondrial matrix or vice versa (Fig. 1.5). In some cellular tissues, glucose is completely oxidized to CO2, which requires a substantial transfer of reducing equivalents from cytosolic NADH to the mitochondria. As such, during glycolysis, the oxidation of glyceraldehyde-3-phosphate by glyceraldehyde-3-phosphate dehydrogenase originates NADH that needs to be oxidized to NAD+ for endowing glycolysis. However, cytosolic NADH cannot freely diffuse through membranes. In accordance with this, the cytosolic and mitochondrial pools of NADH are conserved and independent as a whole. Therefore, the electrons are transferred from these reduced molecules to several oxidized carbon compounds that can be shuttled to the mitochondria and vice versa. The glycerol-3-phosphate shuttle ensures the introduction of electrons from NADH into the ETC, thus allowing the regeneration of cytosolic NAD+ and consequently the maintenance of the glycolytic flux. Firstly, a pair of electrons is transferred from cytosolic NADH to dihydroxyacetone phosphate, thus originating glycerol-3-phosphate. This reaction is catalyzed by the soluble isoform of glycerol-3-phosphate dehydrogenase. Glycerol-3-phosphate is then reoxidized to dihydroxyacetone phosphate in the mitochondrial intermembrane space by the mitochondrial inner-membrane-bounded glycerol-3-phosphate dehydrogenase. This reaction yields FADH2 that is further oxidized by ubiquinone responsible for transferring the electrons to the mitochondrial complex III. The oxidative/reductive state of each compartment cytosol versus inner-mitochondrial membrane is the driving force for this electric metabolic flux.
The malate-aspartate shuttle offers a distinctive way of regenerating NAD+ with the simultaneous exchange of intermediates between the cytosol and the mitochondria. This shuttle is composed by two sets of enzymes, the malate dehydrogenase and the aspartate aminotransferase, each of which with mitochondrial and cytosolic localization, and two mitochondrial carriers, the α-ketoglutarate/malate and the aspartate/glutamate, with an antiport mechanism. As such, upon glycolysis, the excess of cytosolic NADH forces the equilibrium of cytosolic malate dehydrogenase to be shifted from oxaloacetate to malate, which allows the regeneration of NAD+. Malate enters the mitochondria (with an export of mitochondrial α-ketoglutarate), and it is oxidized by malate dehydrogenase to oxaloacetate and NADH. This NADH is a substrate of complex I of the electron transport chain. Mitochondrial oxaloacetate transamination with glutamate gives rise to aspartate and α-ketoglutarate (used for the entrance of malate in the mitochondria). In the cytosol, aspartate transamination with in α-ketoglutarate gives rise to oxaloacetate and glutamate. The antiport aspartate/glutamate shuttles this cytosolic glutamate into the mitochondria with the counter flux of mitochondrial aspartate. As described above (Sect. 1.2.3), NAD+ can also be regenerated through fermentation of pyruvate to lactate by the lactate dehydrogenase. Similar to NAD+ and NADH, the mitochondrial membrane is also impermeable to ADP and ATP. Therefore, the carrier ATP-ADP translocase couples the flow of ADP and ATP through the mitochondrial membrane. This means that the mitochondrial efflux of ATP is accompanied by the influx of ADP.
Out of the scope of this chapter are other mitochondrial transporters that play crucial roles ensuring a correct functioning of cellular respiration.
4 Glycogen Metabolism
Glycogen is a multibranched polymer of glucose residues, which may quickly be degraded to supply glucose. Most of the residues are sequentially linked by α-1,4-glycosidic bonds, although branched chains are connected through α-1,6-glycosidic links. This energetic reservoir is capable of yielding large quantities of glucose, and consequently high amounts of energy upon catabolism. Contrary to what happens with fatty acids, glycogen-derived glucose may be used for fermentation, thus allowing the establishment of high glycolytic fluxes. The excess of serum glucose triggers insulin-driven regulatory processes that culminate in increased glucose uptake and consequent glycogen synthesis. The skeletal muscle and the liver are the main sites for glycogen storage, which is accumulated in the form of cytoplasmic granules. Although the muscle tissue uses glycogen for catabolism under intensive exercise for fulfilling high-energy requirements, the liver uses this reservoir to maintain glucose serum levels.
Overall, the net of cellular glycogen deposition depends on the coordinated suppression of glycogenolytic flux (glycogenolysis) and the stimulation of glycogen synthetic flux (glycogenesis). In spite of its importance, in the field of immunometabolism the knowledge of the effects of glycogen metabolism on immune (dys)function are scarce, and further studies are required to understand how the modulation of these processes impact cell response toward challenges.
4.1 Glycogenolysis
Glycogen degradation or glycogenolysis relies on the concerted activity of the following enzymes: (1) glycogen phosphorylase that degrades α-1,4-glycosidic bonds of glycogen with the release of glucose-1-phosphate; (2) a glycogen-debranching enzyme with a bifunctional activity, with transferase and α-1,6-glucosidase activity, that remodels glycogen within the boundaries of α-1,6-glycosidic bonds for further catabolism; and (3) phosphoglucomutase that converts the released intermediate (glucose-1-phosphate) in glucose-6-phosphate.
The key enzyme for glycogen breakdown by orthophosphate is glycogen phosphorylase. This phosphorolysis cleaves glycogen to yield a glucose-1-phosphate, in a bioenergetically advantageous reaction. Glucose-1-phosphate is readily converted into glucose-6-phosphate, the first intermediate of the glycolytic pathway or of gluconeogenic pathway in the liver. Produced glucose-6-phosphate has three possible outcomes: (1) glycolytic pathway yielding pyruvate; (2) enter the PPP yielding riboses and NADPH; or (3) re-converted into glucose through the last step of gluconeogenesis and released in the bloodstream (a process that takes place mainly in the liver).
As mentioned above, glycogen phosphorylase is responsible for cleaving α-1,4-glycosidic bonds and releasing glucose-1-phosphate. However, this enzyme is unable to recognize glycogen as substrate within a branch point with four glycosidic residues away from a branch point (after a α-1,6-glycosidic bond). Thus, to be further processed, this branch point of glycogen needs to be remodeled. As such, glycogen-debranching enzyme shifts three glycosidic residues from an outer branch to the linear chain of α-1,4-glycosidic substrate for action of phosphorylase. This reaction leaves an α-1,6-linked residue of glucose to an α-1,4-glycosidic linked chain that is released by a free molecule of glucose, by the action of α-1,6-glucosidase activity of the debranching enzyme. In the muscle, this glucose is prone to be phosphorylated by hexokinase, thus entering the conventional glycolytic pathway.
4.2 Glycogenesis
Glycogen is synthesized in a process that requires glucose-1-phosphate activation by uridine triphosphate (UTP) mediated by uridine diphosphate (UDP)-glucose pyrophosphorylase. This originates the ultimate glucose donor UDP-glucose. The initial priming is performed by the self-glucosylating protein glycogenin, which catalyzes the assembly of approximately the first ten glucose residues within a α-1,4-glycosidic linkage. The efficiency of transfer activity decreases pasting this length. This protein uses UDP-glucose for the polymerization of the initial glucose molecules. After junction of at least a minimal of four residues of glucose, glycogen synthase may begin the elongation steps. Using UDP-glucose, this enzyme is capable of adding these units to the nonreducing terminal residues of the growing glycogen molecule. However, since glycogen synthase is also capable of creating α-1,4-glycosidic linkages, another enzyme is required to form the α-1,6-glycosidic bonds that create the branched structure. This branching activity is catalyzed by a transferase that shifts a block of usually seven residues from a chain of at least eleven residues long to a more interior site to create an α-1,6-glycosidic branch point. This ramification process increases glycogen solubility, as well as the rate of synthesis versus degradation.
5 Fatty Acid Metabolism: The Balance Between Synthesis and Oxidation
Fatty acids are composed of long hydrocarbon chains and a terminal carboxylate group. These macromolecules have mainly four major biological roles as the building blocks for: (1) phospholipids and glycolipid synthesis, which are vital components of cellular membranes; (2) posttranslational modifications of proteins, as lipoprotein; and (3) the synthesis of the neutral lipids, triglycerides for energy storage.
In terms of metabolism, fatty acids can be synthesized or degraded, and these processes are not simply the reverse of each other and as such the fatty acid synthesis is not a reversal of the degradative pathway.
5.1 Fatty Acid β-Oxidation
Fatty acids are mainly stored in the form of triglycerides, which are constituted by three fatty acids and a glycerol molecule (Houten and Wanders 2010). Through the activity of a lipase, glycerol and the free fatty acids are released from the triglycerides. Following release of fatty acids, those with twelve or fewer carbons enter freely in the mitochondria where they are activated to acyl-CoA. Those with more than 14 and lower than 20 carbon are activated in the cytosol, which consists of the attachment of a CoA group to the fatty acid carboxyl group, in a process mediated by acyl-CoA synthase. After activation, the carnitinepalmitoyl transferase (CPT) I promotes an exchange of the CoA group of the acyl with a carnitine, giving rise to an acyl-carnitine. The acyl-carnitine is consequently transported into the mitochondrial matrix by the carnitine/acylcarnitine antiport system (translocase) (Fig. 1.6). Once inside the matrix, the CoA group is conjugated with the acyl group, through the activity of CPT II, giving rise to carnitine that fuels translocase for recycling in the cytosol. Inside the mitochondrial matrix, fatty acids are committed to β-oxidation. Overall, each round of oxidation consists of four reactions (oxidation, hydration, oxidation, and thiolysis) and culminates in the shortening of the acyl chain by two carbons, as well as the generation of FADH2, NADH, and acetyl-CoA (Fig. 1.7). The first step consists of the oxidation of acyl-CoA by inner-mitochondrial membrane-bounded complex acyl-CoA dehydrogenase, which yields a trans-enoyl-CoA and one molecule of FADH2. As mentioned above, this high-energy molecule immediately transfers the electrons to the mitochondrial respiratory chain, at the level of ubiquinone to produce ubiquinol. The acyl-CoA dehydrogenase displays length specificity. Acyl chains with 12–18 carbon atoms are oxidized by the long-chain enzyme while medium-chain fatty acids (four to fourteen carbon atoms) are metabolized by a medium-chain acyl-CoA dehydrogenase, and short-chain acyl-CoA dehydrogenase degrades only four to six carbon acyl chains. Following this first oxidation step, the double bond in the saturated enoyl-CoA is hydrated and L-3-hydroxyacyl-CoA is formed. This intermediate gives rise to one molecule of NADH, following another round of oxidation. In the final step of β-oxidation, catalyzed by a β-ketothiolase, an acetyl-CoA and an acyl-CoA shortened by two carbon atoms reenter the cycle for another round of β-oxidation. In the final step, the acetyl groups of the newly formed acetyl-CoA are prone to be oxidized in the TCA cycle, producing reducing equivalents used in the ETC for energy purposes. In terms of energy production, each molecule of palmitate, the prototype sixteen-carbon fatty acid, yields eight molecules of acetyl-CoA and several reducing equivalents for ATP production. Additionally, when fatty acids have carbon chains above 20, as in the case of hexacosanoic acid (26:0), and/or in the case of branched-chain fatty acids, they are shuttled into peroxisomes for β-oxidation. As this process is not coupled to oxidative phosphorylation, it is less energetically efficient than its mitochondrial counterpart. On the other hand, it gives rise to the production of H2O2 that is further degraded by catalases. Mainly, the steps of peroxisomal β-oxidation are similar to the mitochondrial counterparts, although different enzymes are required.
5.2 Fatty Acid Synthesis
The metabolic networks associated to fatty acid synthesis and oxidation are regulated by different sets of enzymes and occur in different cellular compartments. Conversely to what happens with fatty acid degradation, the biosynthetic process occurs in the cytosol and it is dependent on the reducing equivalent NADPH (Fig. 1.8).
The first step of fatty acid synthesis consists of the carboxylation of the cytosolic acetyl-CoA into malonyl-CoA. The major source of cytosolic acetyl-CoA is citrate that is synthesized from oxaloacetate and acetyl-CoA within the mitochondria. In fact, this important intermediate is shuttled through the citrate transporter from mitochondria to cytosol. In the cytosol, citrate is cleaved by acetyl-CoA and oxaloacetate by ATP-citrate lyase and through the malate-aspartate shuttle (described in Sect. 1.3.4). Oxaloacetate can then be reduced to malate (using the cytosolic excess of NADH) and further oxidized to pyruvate by malic enzyme with the production of NADPH. Pyruvate is then transported to mitochondrial matrix. On the other hand, the formation of malonyl-CoA is mediated by acetyl-CoA carboxylase (ACC) at the expense of energy. The metabolite produced by the activity of this enzyme plays a crucial role in controlling the rate of fatty acid synthesis and fatty acid degradation, as it is able to inhibit CPTI and, therefore, translocation of fatty acids into the mitochondria. Additionally, intracellular levels of other molecules involved in fatty acid anabolism and catabolism, as citrate, AMP, and palmitoyl-CoA, also dictate the flux of degradation versus synthesis. These negative feedback-regulatory mechanisms are particularly important since they represent clearly the global status of cellular metabolic needs. Cytosolic citrate is a two-carbon biosynthetic precursor for fatty acid synthesis, and, consequently, in the presence of high intracellular quantities, the cells are primed to initiate anabolic programs that culminate with the increased lipid content. Furthermore, citrate is an allosteric activator of ACC and inhibitor of the glycolytic enzyme phophofructokinase-1, thus diverting cellular metabolism from the catabolism of organic fuels to the storage of fatty acids and glycogen by promoting increased levels of glucose-6-phosphate, substrate of glucose-6-phosphate dehydrogenase, and phosphoglucomutase, respectively. Likewise, the presence of higher levels of palmitoyl-CoA inhibits by feedback the activity of ACC. Furthermore, palmitoyl-CoA is also responsible for inhibiting the transport of citrate to the cytosol, as well as glucose-6-phosphate dehydrogenase that provides NADPH for fatty acid synthesis. ACC activity can also be regulated by covalent modifications, as phosphorylation, mainly via AMP-associated protein kinase (AMPK) activation (Hardie and Pan 2002; O’Neill et al. 2013). AMPK is an important intracellular sensor and one of the central regulators of cellular metabolism. In the presence of a high AMP/ATP ratio, AMPK is activated and, consequently, the downstream phosphorylation of ACC inhibits fatty acid synthesis, thus promoting β-oxidation and ATP generation. Therefore, any stimuli capable of enhancing ATP production and precursor availability indicate that there is no need for an additional source of energy, thus priming anabolic processes.
Following malonyl-CoA synthesis, the elongation of acyl chains and consequent formation of fatty acids occur in a four-step sequence (condensation, reduction, dehydration, and reduction). As stated previously, in opposition to β-oxidation, which uses NAD+ and FAD+ as electron acceptors in fatty acid synthesis, one molecule of NADPH is oxidized to NADP+ in each of the two reduction steps. Fatty acid synthase (FASN) is a multienzyme complex, which includes seven differentially functional catalytic sites. This complex is responsible for the catalysis of the final steps of the anabolic process originating palmitate. The synthesis of each molecule of palmitate requires eight molecules of acetyl-CoA as the primary biosynthetic precursor, as well as 14 molecules of NADPH and seven molecules of ATP. The NADPH precursors are the linkers between fatty acid synthesis and PPP pathway together with the action of malic enzyme. Further elongation and addition of saturated bonds are performed by different enzymes and are out of the scope of this chapter.
Most of newly synthesized fatty acids are either incorporated in triglycerides or in double-layer phospholipidic membranes. The chosen fate relies on current cellular requirements. In rapidly dividing cells, new membranes have to be promptly synthesized and, therefore, fatty acids are used as building blocks to produce phospholipids (Yao et al. 2016). However, in quiescent and in naïve cells, fatty acids may accumulate in the form of lipid bodies and constitute an energetic reservoir, available for degradation upon necessity (Knobloch et al. 2017; Lee et al. 2015; Yao et al. 2016).
6 Amino Acids
Amino acids are the building blocks of proteins and can be divided in essential, nonessential, and conditionally essential amino acids. Essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine) cannot be synthesized by the organism and, therefore, should be acquired through diet. On the contrary, dietary intake of nonessential amino acids such as alanine, aspartate, cysteine, and glutamate reduce the need of biosynthesis. Finally, some amino acids (as arginine, asparagine, glutamine, glycine, proline, serine, and tyrosine) are conditionally essential amino acids, which means that, in physiological conditions, these amino acids can be synthesized by the organisms and consequently dietary intake is not required. However, under certain situations, the net of synthesis may not fulfill the cellular/tissue/organ needs. When in excess, amino acids cannot be accumulated, as it happens with glucose (within glycogen) and fatty acids (within lipid bodies). Instead, amino acids are transformed into carbon skeletons and used as metabolic fuels or as biosynthetic precursor, depending on the cellular metabolic flux and regulation. A special highlight will be made for specific amino acids metabolism and their connection with the activation of intracellular signaling pathways.
6.1 Amino Acid Degradation: The Link Between the Urea Cycle and the TCA Cycle
Amino acid catabolism occurs mainly in liver, although in situations of fasting or prolonged exercise, the skeletal muscle may also use amino acids as a source of energy. In most cases, amino acid degradation starts with a transamination reaction that combines reversible amination and deamination. All transaminase reactions have the same mechanism and use pyridoxal phosphate (a derivative of vitamin B6) and transfer an amino group of an amino acid to a α-ketoacid to form a new amino acid. Aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) are the most studied transferases, responsible for the metabolism of aspartate and alanine. The transamination of aspartate and alanine originates oxaloacetate and pyruvate, respectively, which are then shifted to feed the TCA cycle together with glutamate. Furthermore, the newly formed glutamate suffers oxidative deamination by glutamate dehydrogenase and yields the ammonium ion (NH4+). Carbamoyl-phosphate synthetase further catalyzes the production of carbamoyl phosphate from ammonia, bicarbonate (HCO3−; derived from the hydration of carbon dioxide), and ATP. This reaction is the first committed step of the urea cycle, which is important in the removal of excess ammonia. This cycle was the first cyclic metabolic pathway to be described, initially proposed in 1932 by Hans Krebs and Kurt Henseleit. Due to the presence of an anhydride bond, carbamoyl phosphate has a high transfer potential, originating the transference of the carbamoyl group to ornithine, thus forming citrulline, in a reaction catalyzed by ornithine transcarbamylase. Citrulline is then transported to the cytoplasm and, upon reaction with aspartate, which is the donor of the second α-amino group, argininosuccinate is formed. This step is mediated by argininosuccinate synthetase and driven by the cleavage of ATP in AMP. Argininosuccinate is then cleaved by argininosuccinase to yield arginine and fumarate, which represents the aspartate-driven carbon skeleton. Finally, arginine is hydrolyzed by arginase, thus originating ornithine, which is transported back to the mitochondria (within an antiport system ornithine/citrulline), and urea.
As mentioned above, the catabolism or anabolism of the resulting α-ketoacid provides metabolic intermediates that link the urea cycle and amino acid catabolism with the TCA cycle or gluconeogenesis. The metabolic integration of these pathways occurs upon the synthesis of fumarate that may be hydrated into malate and consequently oxidized to oxaloacetate. This precursor has four possible fates: (1) transaminated back to aspartate, which is again used in the urea cycle; (2) used in the gluconeogenesis, (Sect. 1.4.1); (3) condensed with acetyl-CoA to yield citrate, which may then feed the TCA cycle or contribute to fatty acid synthesis (Sect. 1.5.2); and (4) converted into pyruvate, which also has several different fates accordingly to cellular metabolic active networks (Sect. 1.2.3).
6.2 Amino Acids in the Reprogramming of Cellular Metabolism and Function
Despite the diversity of amino acids, upon degradation the carbon skeletons are transformed into seven major metabolic intermediates: pyruvate, acetyl-CoA, oxaloacetate, fumarate, succinyl-CoA, acetoacetyl-CoA, and α-ketoglutarate. Ultimately, the carbon skeletons can be converted into glucose or enter the TCA cycle. In terms of characterization, amino acids that are degraded to acetyl-CoA and acetoacetyl-CoA are named ketogenic amino acids, since their catabolism may culminate with ketone bodies of fatty acid synthesis. Conversely, amino acids degraded to pyruvate, fumarate, oxaloacetate, and α-ketoglutarate are considered glucogenic amino acids as they can feed gluconeogenic processes. Leucine and lysine are purely ketogenic amino acids, while tryptophan, phenylalanine, tyrosine, and isoleucine can be both ketogenic and glucogenic amino acids. The remaining 14 amino acids are considered only glucogenic ones.
Associated with their metabolic profiles, some amino acids have been vastly studied regarding their role in cell biology and functioning. The best-known example is glutamine as described in 1935 by Hans Krebs. This amino acid has a vital role in organism homeostasis, as well as in energy-generating and biosynthetic purposes. Glutamine is transported to the intracellular milieu by several transporters, with SLC1A5 being the most studied one. Once inside the cell, glutamine is converted into glutamate by mitochondrial glutaminases in a process termed glutaminolysis. Glutamate may then follow one of two fates: it is either transformed into α-ketoglutarate by glutamate dehydrogenases providing a direct link with the TCA cycle, or it is used by aminotransferases as an amino group acceptor. The role of glutamine in cellular metabolic rewiring during activation or oncogenesis has been vastly explored, thus emphasizing the role of this amino acid in several biological processes (Altman et al. 2016). Tryptophan catabolism is an interesting example of how a single amino acid may in one hand increase the anaplerotic reactions of the TCA cycle and, on the other, culminate in the de novo production of NAD+ (Mesquita et al. 2016). Tryptophan degradation may yield acetoacetate, which is then converted into acetyl-CoA that directly enters the TCA cycle or is converted into citrate. However, tryptophan may be degraded by indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO) to feed the de novo biosynthesis of NAD+ (see Chap. 4 for more details). Arginine also has a vital role in the regulation of cellular functions and in the generation of immunomodulatory molecules that dictate the inflammatory state of the cell. As previously mentioned, this amino acid may be cleaved by arginase to yield ornithine and urea. Ornithine is important for the synthesis of polyamines, which have been vastly associated with tissue repair and anti-inflammatory phenotypes. On the other hand, arginine may be metabolized by nitric oxide synthase originating from nitric oxide (NO), an important microbicidal molecule associated with host defense mechanisms (Bronte and Zanovello 2005). In this reaction, citrulline is also produced, thus providing a direct link with the urea cycle.
Although amino acids are clearly involved in the metabolic choices and consequent reprogramming of cellular metabolism, it is known that through their action as signaling molecules, amino acids impact cell growth and proliferation. These effects are mainly mediated by amino acid sensing through the sensors mammalian target of rapamycin (mTOR) and general control non-derepressible 2 kinase (GCN2) (Gallinetti et al. 2013; Wolfson and Sabatini 2017; for more details, see Chaps. 3 and 12). Both sensors are regulated by nutrient availability and, more specifically, by amino acid starvation. Upon activation, these sensors signal to an increased uptake or synthesis of amino acids and proteins, which will support cell growth and proliferation. Branched chain amino acids (leucine, isoleucine, and valine) are capable of constitutively activating mTOR, which consequently activates the eukaryotic initiation factor 4E-binding protein 1 and the downstream target p70 S6 kinase. The subsequent cascades activate the transcription of specific mRNAs and signal for an initiation of protein synthesis and an arrest of its degradation. GCN2 activation during amino acid scarcity culminates in the phosphorylation of eIF2 and the adoption of specific transcriptional programs. Furthermore, GCN2 may also be activated while mTOR is inhibited and amino acid uptake is decreased (Averous et al. 2016), thus showing a crosstalk between these metabolic sensors.
Despite the diversity of the existing amino acids, recent works have highlighted the role of some of them in the regulation of cellular metabolism and its implications during the development of adequate responses (Ananieva 2015; Arts et al. 2016; Grohmann and Bronte 2010; O’Neill et al. 2016). However, additional studies are required to understand if the modulation of these central nodes can be used in distinct pathological contexts.
7 Conclusion
Metabolism comprises the biochemical basis of any biological function and as such spans all biological disciplines. The interconnection of metabolic networks from the cellular to the organismal level is essential for homeostatic function. Several metabolic pathways are highlighted as being the central of carbon or energy metabolic networks and as such have been extensive characterized in their metabolic as well as their regulatory constituents. In this chapter, we went through these central pathways emphasizing their co-regulatory aspects, in a simplified view for easy access and understanding. Therefore, only major metabolic events/pathways were covered.
References
Akram M (2014) Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 68:475–478
Altman BJ, Stine ZE, Dang CV (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16:619–634
Ananieva E (2015) Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J Biol Chem 6:281
Arts RJW, Novakovic B, ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng SC, Wang SY et al (2016) Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 24:807–819
Averous J, Lambert-Langlais S, Mesclon F, Carraro V, Parry L, Jousse C, Bruhat A, Maurin A-C, Pierre P, Proud CG et al (2016) GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci Rep 6:27698
Balaban RS (1990) Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol 258:377–389
Berg JM, Tymoczko JL, Stryer L (2002a) The glycolytic pathway is tightly controlled. In: Berg JM, Tymoczko JL, Stryer L (eds) Biochemistry. W.H. Freeman, New York
Berg JM, Tymoczko JL, Stryer L (2002b) Glycolysis is an energy-conversion pathway in many organisms. In: Berg JM, Tymoczko JL, Stryer L (eds) Biochemistry. W.H. Freeman, New York. section 16.1
Berg JM, Tymoczko JL, Stryer L (2002c) The metabolism of glucose-6-phosphate by the pentose phosphate pathway is coordinated with glycolysis. In: Berg JM, Tymoczko JL, Stryer L (eds) Biochemistry. W.H. Freeman, New York
Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M (2013) Porphyrin and heme metabolism and the porphyrias. Compr Physiol 3:365–401
Boulton RB (1996) Yeast and biochemistry of ethanol fermentation. In: Boulton RB, Singleton VL, Bisson LF, Kunkee RE (eds) Principles and practices of winemaking. Springer, Boston, MA, pp 102–192
Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5:641–654
Buchakjian MR, Kornbluth S (2010) The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol 11:715–727
Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A et al (2014) mTOR- and HIF-1-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684
Citric THE, Cycle A (2010) The citric acid cycle. In: Eastmond, PJ Graham (eds) pp 601–630
Dan Dunn J, Alvarez LAJ, Zhang X, Soldati T (2015) Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol 6:472–485
Déry MAC, Michaud MD, Richard DE (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37:535–540
Gallinetti J, Harputlugil E, Mitchell JR (2013) Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J 449:1–10
Grohmann U, Bronte V (2010) Control of immune response by amino acid metabolism. Immunol Rev 236:243–264
Hansford R (2002) Oxidative phosphorylation. Encycl Life Sci:1–8
Hardie DG, Pan DA (2002) Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30:1064–1070
Houten SM, Wanders RJA (2010) A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherted Metab Dis 33:469–477
Israelsen WJ, Vander Heiden MG (2015) Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol 43:43–51
Jiang B (2017) Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis 4:25–27
Jones W, Bianchi K (2015) Aerobic glycolysis: beyond proliferation. Front Immunol 6:227
Knobloch M, Pilz GA, Ghesquière B, Kovacs WJ, Wegleiter T, Moore DL, Hruzova M, Zamboni N, Carmeliet P, Jessberger S (2017) A fatty acid oxidation-dependent metabolic shift regulates adult neural stem cell activity. Cell Rep 20:2144–2155
Kornberg H (2000) Krebs and his trinity of cycles. Nat Rev Mol Cell Biol 1:225–228
Lane AN, Fan TWM (2015) Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 43:2466–2485
Lee J, Ellis JM, Wolfgang MJ (2015) Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep 10:266–279
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464
Mesquita I, Varela P, Belinha A, Gaifem J, Laforge M, Vergnes B, Estaquier J, Silvestre R (2016) Exploring NAD+ metabolism in host-pathogen interactions. Cell Mol Life Sci 73:1225–1236
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34:121–138
Nath S, Villadsen J (2015) Oxidative phosphorylation revisited. Biotechnol Bioeng 112:429–437
O’Neill HM, Holloway GP, Steinberg GR (2013) AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol 366:135–151
O’Neill LAJ, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16:553–565
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922
Patra KC, Hay N (2014) The pentose phosphate pathway and cancer. Trends Biochem Sci 39:347–354
Romero-Garcia S, Moreno-Altamirano MMB, Prado-Garcia H, Sánchez-García FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52
Senior AE, Nadanaciva S, Weber J (2002) The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim Biophys Acta – Bioenerg 1553:188–211
Thorens B, Mueckler M (2010) Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab 298:E141–E145
Wolfson RL, Sabatini DM (2017) The Dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab 26:301–309
Yang W, Lu Z (2013) Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett 339:153–158
Yao CH, Fowle-Grider R, Mahieu NG, Liu GY, Chen YJ, Wang R, Singh M, Potter GS, Gross RW, Schaefer J et al (2016) Exogenous fatty acids are the preferred source of membrane lipids in proliferating fibroblasts. Cell Chem Biol 23:483–493
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Mesquita, I., Rodrigues, F. (2018). Cellular Metabolism at a Glance. In: Silvestre, R., Torrado, E. (eds) Metabolic Interaction in Infection. Experientia Supplementum, vol 109. Springer, Cham. https://doi.org/10.1007/978-3-319-74932-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-74932-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74931-0
Online ISBN: 978-3-319-74932-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)