Abstract
Cellular organization is possible through metabolic processes that ensure the construction of biomass and energy supply. Central metabolic pathways use sensors to monitor nutrient availability and environmental changes. Metabolic sensors orchestrate environment-driven metabolic adaptations according to the cellular condition. Although the role of metabolism in the immune response has been established for many decades, only in recent years have researchers turned their interest to investigate metabolic changes in immune cells, setting the field of immunometabolism. Early in vitro studies were instrumental in uncovering mechanisms that induce a metabolic reprogramming required for an effective immune response, mainly in macrophages and dendritic cells. Also, in vitro studies have shown a metabolic shift during T cell activation and proliferation. However, such approaches may not reflect the metabolic complexity within an integrated organism with changes in partial oxygen pressure, pH, inflammation and nutrient gradient. Besides that, metabolic changes can affect both microenvironments and promote systemic inter-organ communication. Therefore, the immunometabolic study at specific sites is crucial to understand the outcome of infections, tumors, non-infectious inflammation and tissue repair. In this chapter, we will address the metabolic reprogramming in tissue-specific immune cells during host infection. At first, we will introduce the functioning of the immune system in barrier tissues that are the gateway to pathogens.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Barrier Tissues as Major Sites for Pathogen Entrance
Barrier tissues are in close contact with the external environment and exposed to a variety of antigens. These sites are endowed with characteristics that differentiate them from the other tissues due to their protective mechanisms against external agents. Such functions place the barrier tissues as the first line of host response to changes in homeostasis, including skin, oral cavity, respiratory, genitourinary and gastrointestinal tracts. Since these sites are highly exposed to environmental antigens, commensal microbes and pathogens, a selective barrier is crucial to limit the entry of external contents to the organism. The firewall mechanisms include cells and their products that act physically or actively to protect the organism. Besides, the barrier-associated immune system promotes effective immune surveillance, preventing exacerbated inflammatory responses, as well as tolerating commensal and innocuous antigens [8]. Next, we describe the barrier components that contribute to tissue-specific immunity and how episodes of infection could influence the metabolic reprogramming of immune cells.
The different tissues comprising the host barriers share structural components such as epithelial cells connected by tight junctions controlling tissue permeability, antimicrobial peptide-secreting cells, antibody-secreting B cells and other specialized epithelial cells supporting the chemical barrier. Also, a full network of immune cells covering mononuclear phagocytes, innate and adaptive lymphocytes, is in charge of monitoring the barriers. According to the organization of such components, the mucosal tissues are classified into two types: I and II (Table 1). Type I mucosal surfaces are covered by a single columnar epithelium, as seen in the small and large intestine, pseudostratified epithelia of the respiratory tract and the female upper reproductive tract. Common features comprise mucus-secreting goblet cells and polymeric immunoglobulin receptor (pIgR) expression at the basolateral surface. The pIgR binds to polymeric Immunoglobulin A (pIgA) secreted by plasma cells and releases the secretory IgA into the lumen. Hence, IgA is the main protective antibody in type I mucosal surfaces and contributes to the immune firewall by neutralizing toxins and microbial-associated antigens [45]. Innate and adaptive immune cells in the type I mucosal tissues are classically found in the mucosa-associated lymphoid tissue (MALT), while type II mucosal surfaces are characterized by sparse clusters of immune cells in the submucosa. Type II mucosal surfaces, including the lower female reproductive tract, eyes and mouth, are characterized by a protective stratified squamous epithelial layer (keratinocytes), which shares many features with the skin. However, type II mucosal surfaces are protected by mucous and usually do not undergo terminal cornification. Also, the absence of pIgR in keratinocytes from stratified epithelial impairs IgA secretion into the lumen. Therefore, IgG is the main antibody found on the type II mucosa [44]. Despite the similarities among the firewall mechanisms, each barrier has unique characteristics providing the immune tone of tissue-specific responses, as depicted below.
1.1 Skin Barrier
In order to prevent physical insults and pathogen entrance, the body is covered by a keratinized stratified epithelium comprising the epidermis and dermis. Immunocompetent cells are located in both compartments, and the dead cells compose the stratum corneum that forms the outermost layer protecting the skin from injuries and infection. The skin surface holds a variety of microbial communities that shapes the immunological tone of the tissue [7, 69]. By controlling the expression of innate immune components by keratinocytes, e. g. cathelicidins, β-defensins and other antimicrobial peptides (AMPs), the skin-resident microbiota protects the host from the colonization by pathogenic species [32, 66]. Also, the skin commensals modulate the production of IL-17A and IFN-γ by local T cells that are involved in both host defense and inflammatory diseases [68, 69]. Through the expression of Toll-like receptors (TLRs), keratinocytes favor the differentiation of CD4 T lymphocytes to the T helper (Th) 1 profile and the production of interferons (IFNs), IL-1β, IL-18 and IL-6 that coordinate leukocyte traffic to the skin [60]. A specialized subset of mononuclear phagocyte, the Langerhans cells, projects the dendrites towards the outermost layers to uptake antigens and stimulate the adaptive immune response. In the dermis, mast cells store large amounts of substances in granules, such as cathelicidins, histamine and proteinases, that contribute to resident dendritic cell maturation and the immune dialogue with T cells [94].
1.2 Airway Barrier
Human lungs consume up to 11, 500L of air each day, therefore exposed to an enormous amount of particulates, as dust, smoke, pollution, pollen and aerosols [86]. The immune system associated with the airways ensures that inappropriate responses are not initiated against such particulate innocuous antigens, preventing tissue damage and inflammatory disorders [5, 86]. However, lung immunity also needs to be effective due to the massive surface of the epithelial area in contract with the environment and the intimate association of an extensive blood capillaries network, which turns the respiratory tract into a major portal for pathogen entrance.
The continuous rhythmic movements of the cilia and constant production of a thin mucous layer by goblet cells afford the first barrier protection by trapping airborne particles and removing them out of the lung. The immune system associated with the respiratory tract consists of a specialized network of lung-resident innate cells, comprising epithelial cells, macrophages, dendritic cells and innate lymphoid cells (mostly from type 1 and 2 subsets), interacting with local stromal cells. Alveolar macrophages (AMs) are thought to account for 90–95% of lung immune cells during homeostatic conditions and are critical for the phagocytosis of invading microorganisms and also for the production of transforming growth factor-β (TGF-β) to maintain the homeostasis [5, 110]. AMs also phagocytes particulate matter, dying cells and cellular debris, limiting lung inflammation and promoting tissue repair after the clearance of infection. In addition to TGF-β, retinoic acid metabolized by AMs can convert naïve or activated T cells into regulatory T cells [96], crucial to control immune responses against lung disease. Pathogens that evade the aforementioned innate mechanisms of immunity require the activation of T cell subsets for the ultimate control of infection, and DCs are crucial for stimulation and differentiation of specialized T cell subsets. In this context, surveillance of the lung environment and uptake of foreign airborne particles are controlled by lung-resident bronchial, interstitial, and alveolar macrophages, as well as by conventional, plasmacytoid, and monocyte-derived dendritic cells.
1.3 Urinary Tract Barrier
The urinary tract consists of the kidneys, bladder, ureters and urethra, and, except for the urethra, most of it is considered to be a sterile microenvironment. In addition to the anatomical barrier and mucus layer [36], as protective mechanisms, the urinary tract has soluble components secreted in the urine, such as the glycoprotein plaque uroplatins, which protect against microbial colonization. [109]. Also, epithelial and immune cells residing in the urinary tract reinforce protection against infections that, in the urinary tract, are mainly caused by intestinal microorganisms. The epithelial cells secrete several soluble components, such as the cytokines IL-1, IL-6 [95] and IL-8 [67], promoting the enrollment of phagocytes to the bladder or kidney [2], uromodulin prevents interactions between pathogens and epithelial cells and antimicrobial factors inhibit microbial growth, e. g. neutrophil gelatinase-associated lipocalin, AMPs, and pentraxins. In the early stage of urinary tract infections (UTIs), neutrophils are recruited into the bladder to promote the bacterial clearance [38]. Resident macrophages in the bladder act as sentinels secreting the chemokines such as CXC-chemokine ligand 1 and 2 and macrophage migration inhibitory factor (MIF) to recruit and stimulates transepithelial movement of neutrophils and other immune cells to combat the pathogens [88]. Mast cells also play a pivotal sentinel role during UTIs, due to the release of pre-stored proinflammatory mediators such as TNF, histamine and several chemokines [1]. During the resolution phase of infection, mast cells secrete IL-10 that have been associated with the regeneration of the bladder epithelium [17]. Of note, while the innate cells of the urinary tract are highly reactive in the presence of microbes, the adaptive immunity, particularly in the bladder, tends to be limited. In the context of adaptive immunity, the arrival of the infectious agent in the kidneys leads to the production of specific antibodies but a robust activation of adaptive immunity is not seen in the bladder [55, 79]. The limited ability of the bladder-associated immune system to become activated and differentiate into memory cells could be a main cause for the return of UTIs, which may be linked to the increase of local IL-10 production by mast cells.
1.4 Oral and Intestinal Barriers
The oral mucosa is lined by non-keratinized stratified squamous epithelia, while regions with mechanical stimulation or mastication injury have a protective layer of keratin. The junctional epithelium (JE) that lines the teeth is an extremely vulnerable site in the oral barrier. The JE connection to the tooth is highly permeable to the passage of fluid containing host protective factors, including plasma proteins, cytokines, immunoglobulins, and cells. Notably, the neutrophils are the main cells recruited to the gingival crevice and form a “defense wall” in the subgingival biofilm. [22]. In parallel, the gingival mononuclear phagocyte set comprises a complex network of DCs, macrophages, and recruited monocytes. Particularly, macrophages mediate the main antimicrobial functions at the gingiva but can also participate in healing and continuous tissue repair [57]. In the gingiva, also reside innate lymphoid cells (ILC), natural killer (NK) cells, B cells and T helper 17 (Th17) cells. Recently, it has been demonstrated that Th17 cells in the gingiva increase with age in response to local damage from chewing. [26]. IL-17 produced by Th17 cells is crucial for innate antimicrobial defense mechanisms, such as β-defensin 3, crucial for oral antifungal immunity. In addition to immune cells, saliva also comprises a plethora of antimicrobial agents, such as immunoglobulins, lysozyme, lactoferrin and peroxidases [42].
Moving forward towards the gastrointestinal tract, the intestine is specifically adapted to support (1) the colonization of commensal microorganisms, essential to promote the digestion and absorption of dietary nutrients, (2) tolerance towards innocuous environmental antigens and (3) immunity against infectious challenges. Notably, the gut epithelial and immune cells detect the microbiota components and its metabolites to maintain the tone of the tissue-specific immune response (Fig. 1). The primary firewall preventing pathogen and pathobiont invasion in the gut is the mucus layer. Ranging from a thin to a thick layer along the gastrointestinal mucosa, this multi-component structure integrates an intricated network of mucin glycoproteins along with the presence of AMPs and neutralizing IgA antibodies. Underneath the mucus layer, the intestinal lamina propria is covered by an epithelial cell monolayer. The intestinal epithelium is organized into crypts and villi, being the crypt compartment organized as invagination in the underlying lamina propria and responsible for the epithelium regeneration and microbicidal activity by AMPs-producing Paneth cells [76]. Among the enterocytes, which recognizes and respond to microbial components via pattern recognition receptors, several other cell subsets with immunological properties are found in the epithelial layer, including goblet cells (mucus producers), intraepithelial lymphocytes, DCs, macrophages, Paneth cells, Microfold (M) cells (transport of luminal particles), enteroendocrine cells (hormone producers) and tuft cells (initiation of type 2 immunity) [33, 106]. The crosstalk between this immunological compartment and the MALT is crucial for the full activation of gut immunity.
The intestinal barrier comprises physical, chemical and active structures. The physical barrier includes the epithelium and a mucus layer. Along with epithelial cells, other cell subtypes contribute to the defense of the mucosa. The antimicrobial peptides and the immunoglobulin A (IgA) provide chemical strength to prevent the pathogen invasion and the host immune cells act as an active barrier, controlling the homeostasis and responding to invading microorganisms
In the intestine, the MALT is named as gut-associated lymphoid tissue (GALT) and comprises subepithelial lymphoid aggregates, such as Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs). DCs, T and B cells in the GALT, immediately beneath the follicle-associated epithelium, are exposed to luminal antigens transported by M cells [76]. Specialized subsets of CD103-expressing DCs in the lamina propria and PPs produce high levels of IL-10, TGF-β and retinoic acid, therefore promoting the differentiation of regulatory T cells and the expression of gut-homing molecules CCR9 and α4β7 on activated lymphocytes [48, 64]. The regulatory T cells induced in the gut mucosa are essential for the tolerance towards environmental antigens and commensal microbiota. In contrast, lower concentrations of TGF-β, and IL-6 in the mesenteric milieu induce the differentiation of Th17 cells [114]. Both, Th17 and regulatory T cells are the canonical responses in the gut mucosa shaped by the resident microbiota and its metabolites, such as short chain fatty acids [49, 50]. In contrast to the immunomodulatory effects of the regulatory T cells, the IL-17A produced by Th17 cells acts on intestinal epithelium to promote barrier function. In addition, together with IL-17F, IL-21, IL-22, TNF-α, IL-17 is critical in controlling extracellular infections. Th17 and regulatory T cells can also be converted into follicular T helper cells that support intestinal IgA production [40, 103]. Recently, adipose tissue depots adjacent to the intestine, particularly the mesentery, have been described as an important reservoir for memory T cells activated towards intestinal antigens [37]. As discussed later in this chapter, the adipose tissue provides a unique environment for the long-term survival of memory T cells that undergo metabolic reprogramming. In this context, the interplay between the metabolites, microbiota, epithelial and immune cells is critical to shaping the tissue-specific responses during homeostasis and in the face of infectious challenges. The disruption of this fine-tuned balance directly impacts the canonical pathways of the gut immune response and defines the outcome of infection and inflammatory diseases.
2 Consequences of Infection for the Tissue Immune Homeostasis
As described previously in this chapter, the barrier-associated immune system is endowed with tissue-specific mechanisms to ensure immunity, tolerance towards innocuous challenges and protection against tissue injury. However, defined pathogens can escape the firewall mechanisms and disrupt the tissue homeostasis, initiating infection and inflammatory process. Success in combating infectious disease consists of activating an innate immune response to prevent the growth of pathogens, induce an inflammatory response and promote the generation of adaptive immunity. However, pathogens are able to modulate the immune response to ensure their survival, which usually promotes a decrease in the tolerogenic response and increases the pro-inflammatory profile in the barrier tissues. For instance, years ago, several studies demonstrated that the protozoa Leishmania spp. has developed mechanisms to subvert macrophage killing through the modification of host cytokine expression such as IL-1 and TNF-α, downmodulation of major histocompatibility complex (MHC) class I and II by IFN-γ and reduction of ROS production [15, 23, 81].
Not only protozoan but also several other pathogens are capable of subverting macrophage or neutrophil microbicidal function, including bacteria and fungi. In the urogenital tract, for instance, the uropathogenic Escherichia coli has been shown to suppress NF-κB activation in urothelial cells, decreasing cytokine secretion and increasing apoptosis [10, 43]. Salmonella typhimurium infection by the oral route impairs the adaptive immune response by promoting MHC class II ubiquitination in infected DCs, decreasing antigen presentation to CD4 T cells, and favoring bacterial survival [56]. Fungal species such as Candida spp. are part of the microbiome, but are considered pathobionts. Of these species, C. albicans is the most relevant, promoting oral thrush and vulvovaginal candidiasis. Neutrophils and macrophages have a relevant role in controlling mucosal fungal infections. However, studies have shown that C. albicans could escape neutrophils (NETose) and extracellular neutrophil traps (NETs) that release DNAse into the extracellular medium [112] and form biofilms [52], which renders fungal cells less accessible to immune cells. C. albicans also influence polarization of macrophages from a classic phenotype toward an alternative phenotype, thereby reducing antimicrobial response and promoting fungal survival. These are just a few examples about how pathogens evade specific cellular pathways of immunity. However, infections can compromise not only the functioning of defined immune cell subsets but also may interfere in the entire firewall mechanism.
Indeed, it has been described that a single gastrointestinal episode of infection with the bacteria Yersinia pseudotuberculosis can lead to chronic inflammation in several tissues, particularly the mesentery, long-term after the clearance of the pathogen. The infection impairs the immune dialog in the gut by changing the function of mesenteric lymphatic vessels. As a result, migratory DCs in the gut are deviated from the gut-lymph node migratory route, which permanently compromises the canonical regulatory responses in the mucosa, impacting mucosal immunity and promoting chronic inflammation [30]. Another example of immunological scar driven by infection, is the oral infection with Toxoplasma gondi. Although the systemic infection with T. gondii triggers a transient activation of IFN-γ- and TNF-producing Th1 cells, essential to infection control, there is also a persistent thymic atrophy and decrease in the naïve CD4 T lymphocyte pool. The result of the loss of thymic architecture supports chronic T. gondii infection and compromises resistance to other pathogens [54]. In the context of immune modulation, in 2019, two complementary studies demonstrated that measles virus (MV) infection, efficiently transmitted by aerosol or respiratory droplets, diminishes preexisting antibodies to previously encountered pathogens, a phenomenon called “immunological amnesia” [62], due to depletion of expanded B cell memory clones, and inability to restore the naïve B cell pool [77], and consequently, increasing vulnerability to future infections.
Therefore, despite the sophisticated mechanisms of barrier immunity, pathogens evolved to subvert the immune system and establish infection. For a very long time, the studies on host–pathogen interaction at barrier tissues had almost exclusively focused on the immunological aspects of the responses. However, only recently, the involvement of host and pathogen metabolic reprogramming began to be appreciated for understanding the outcome of infection. Indeed, the immune system adapts to the infectious environment to obtain nutrients used in cellular functions. The presence of pathogens at barrier sites, such as the lung and intestine, interfere with immune cell metabolism and pathogens modulate host cell metabolism to their advantage. In the next sections, will be discussed how cells of the innate and adaptive immune systems induce a metabolic reprogramming necessary for antimicrobial molecules production and cell activation, proliferation and differentiation. Such changes also allow tissue repair and the generation of memory cells against future pathogen encounters.
3 Effects of Infection in the Immune Cell Metabolism
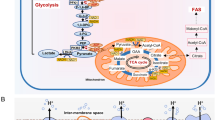
The cellular metabolic state is directly regulated by the surrounding microenvironment. Metabolic changes occur as an alternative to maintain homeostasis and control infections. However, metabolism also reflects the cell activation profile and, consequently, supports the immune response against pathogens. Metabolic processes are divided into anabolic processes, gathering macromolecules, and catabolic processes with the disruption of macromolecules. The catabolic process provides carbon and nitrogen sources, mainly through the use of glucose and glutamine, for the biosynthesis of nucleotides, amino acids, and lipids. In this way, the immune cell supports its activation and proliferation process. Each immune cell type has its own metabolic choice depending on its energy requirement and nutrient demand. For this purpose, cells have specific nutrient sensors and transcription of metabolic enzymes that assist in targeting the metabolism [58]. In the absence of glucose and glutamine, immune cells use fatty acids and amino acids as new sources of nutrients. Cellular energy is obtained through a catabolic process of high (oxidative phosphorylation) and low (glycolysis) efficiency. Quiescent cells use glucose degradation to generate pyruvate that supplies Tricarboxylic acid cycle (TCA) and oxidative phosphorylation. Unlike resting cells, classically activated M1 macrophages, DCs and effector T cells induce a metabolic reprogramming to support cellular biosynthetic processes termed “Warburg effect”, often driven by aerobic glycolysis [25] (Fig. 2). Therefore, the uptake of nutrients is crucial to supply the cell demand for energy and favor metabolic shift. Likewise, pathogens use the same nutrients for their maintenance, which drives the competition for the available glucose [102].
Metabolic reprogramming in immune cells during infection. Quiescent cells maintain the glucose influx to supply the TCA cycle. Upon infection, innate immune cells increase glycolytic flux that culminate in lactate production even with oxygen availability (Warburg effect) and decrease in TCA cycle. TCA - Tricarboxylic acid cycle
Microbial compounds such as lipopolysaccharide (LPS) are recognized by Toll-like receptors (TLRs) in immune cells. This engagement induces a metabolic reprogramming in the innate immune cells to supply their current needs. In addition to microbial products, the inflammatory milieu drives cell differentiation and metabolism. Macrophage differentiation occurs according to the production of cytokines at the inflamed site into cell subsets that diverge mainly in their metabolic state. The cytokine IL-4 increases mitochondrial respiration and oxidation of fatty acids that culminate in the production of Arginase-1 (Arg-1) in macrophages named M2. However, IFN-γ increases the glycolytic pathway and the production of antimicrobial peptides such as the superoxide burst (ROS) and reactive nitrogen intermediates (NO)[101], characterizing a population of pro-inflammatory macrophages also known as M1. Although the polarization of macrophages is important for the outcome of the infection, the front line in combating pathogens at mucosal sites are tissue-resident macrophages (TRMs) that are specialized immune sentinels. In this way, TRMs change their metabolic reprogramming according to the environment. Upon IL4 stimulation, alveolar and peritoneal macrophages can undergo STAT6 phosphorylation, but only peritoneal macrophages upregulate Arg-1 [99]. Therefore, the escape mechanisms used by pathogens in TRMs may differ depending on the entry barrier. Indeed, the TRMs metabolism is mostly unknown due to its heterogeneity in barrier tissues, making this a crucial issue that must be addressed in future studies on cell metabolic reprogramming.
Like macrophages, the activation of DCs, mast cells and neutrophils increases the glycolytic pathway and fatty acid synthesis. In addition to switching to the glycolytic pathway, metabolic reprogramming of innate immunity cells triggers several metabolic changes after the pathogen recognition. The infected cell activates the phosphoinositide-3 kinase/Akt (PI3K / Akt) pathway to induce a signaling cascade that supports metabolic reprogramming. PI3K promotes phosphatidylinositol-3,4,5-trisphosphate (PIP3) which conscripts Akt to be activated by Phosphoinositide-dependent kinase 1 (PDK1) [31]. Phosphorylated Akt prompts the glucose transporter 1 (GLUT1), hexokinase 2 (HK2), and phosphofructokinase 1 (PFK1) expression. Next, Akt induces ATP-dependent citrate lyase (ACL), which converts citrate in the cytosol into oxaloacetate (OAA) and Acetyl-CoA. The metabolic switch to the glycolytic pathway reduces pyruvate entry into mitochondrial TCA and increases lactate production. In this context, the TCA pathway is supplied by the increase in glutaminolysis, providing α-ketoglutarate (α-KG) and, consequently, precursors of amino acids. Thus, the citrate released into the cytosol is converted to OAA and acetyl-CoA for the synthesis of lipids and fatty acids. The citrate is also a precursor for the itaconate acid [61], which has antimicrobial functions against intracellular bacteria from lung barriers such as Mycobacterium tuberculosis and Legionella pneumophila; or intestinal barriers such as Salmonella enterica [59]. Still, citrate is also used to develop reactive oxygen species (ROS), nitric oxide (NO) and prostaglandins. The accumulation of succinate induced by high levels of pyruvate kinase M2 (PKM2) after LPS recognition, stabilizes hypoxia-inducible factor 1 alpha (HIF1a) allowing translocation to the nucleus and inducing IL1 transcription [100]. Oxidized succinate induces mROS production and also assists in HIF1a-mediated IL1b mRNA expression. In contrast, glucose 6-phosphate triggers the Pentose Phosphate Pathway (PPP), which culminates in reducing equivalent NADPH and NADH, that will be used for the generation of ROS and NO (Fig. 3).
Metabolic reprogramming in innate immune cells during infection. Microbial compounds increase glycolysis through Akt activation and induce PKM2 dimers and tetramers. PKM2 dimers translocate to the nucleus and contribute to IL1β expression. Accumulated succinate stabilizes HIF1α to drive IL1β expression and citrate promotes NO, ROS and itaconate production
The recognition of molecular patterns is crucial for inducing metabolic reprogramming during infection. Notably, it has been observed that defined stimuli, such as β-glucan and Bacillus Calmette-Guérin (BCG), recognized by innate immune cells induce long-term functional reprogramming, which is referred to as “trained immunity”, which is an innate version for immunological memory [77, 85]. The changes occur through epigenetic reprogramming due to histones acetylation and methylation in monocyte, macrophage or NK cells that culminate in an increase in cytokine production and metabolic shift [85]. Therefore, trained immunity increases responsiveness to second infections for weeks and the ability to eliminate the pathogen.
4 Metabolic Reprogramming of T Cells to Protect Against Infections
Similar to innate immune cells, activated T cells also induce metabolic reprogramming to support cell biosynthesis. After activation by antigen and costimulatory molecules, T cells increase glycolysis and oxidative phosphorylation (OXPHOS) to supply the energy demand. High expression of glucose transporters (Glut1) supports glucose uptake to maintain the functional glycolytic pathway. In addition, Glut1 expression increases T cell proliferation, cytokine production and cell survival. In naïve T cells, Glut1 expression is low and is increased after activation of TCR and CD28-mediated Akt-dependent and independent pathways [46]. T-cell glycolytic metabolism is restrained by the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) activity and transcription factors targeting metabolic reprogramming, such as HIF1 and c-Myc [91, 107]. In addition, under low nutrient availability, T cells adapt to low glucose levels, increasing glutaminolysis through glutamine uptake [11]. Glutamine uptake is controlled by the ERK / MAPK pathway during T lymphocyte activation [16].
Effector T cells maintain the glycolytic pathway after differentiation, while Foxp3 + regulatory T cells decrease glucose consumption and prefer to use derived fatty acids to support oxidative metabolism [34]. Besides its own metabolism, the maintenance and generation of regulatory cells in barrier sites depend on homeostatic environmental control. The production of TGFβ, prostaglandin E2 (PGE2) and IL-10 by the mucosal epithelium, down regulates the immune response and induces regulatory T cells [4, 53, 74]. As discussed previously, the presence of regulatory T cells is essential for mucosal tolerance, and the mucosal surface portrays a complex system that must work to contain harmful pathogens, but at the same time, it must inhibit the immune response against commensal microorganisms, proper antigens and nutrients. Thereby, mucosal components provide significant metabolic changes, mainly targeting tissue hypoxia for environmental control or supporting metabolic shift in T cells. Intraepithelial cells detect the pathogen and promote metabolic changes in TCRγδ intestinal intraepithelial lymphocytes, increasing the glycolytic pathway to sustain the maintenance of the intestinal epithelial barrier against enteric pathogens [41]. However, T-cell subpopulations have their own energy demand and metabolic reprogramming to perform their immune function.
Contrary to Foxp3 + regulatory T cells, type 1 regulatory T cells (Tr1) maintain the glycolytic pathway as effector T cells. Meanwhile, memory T cells predominantly use fatty acid oxidation to support oxidative phosphorylation (OxPhos), and glucose is used to generate mitochondrial citrate (Fig. 4). However, in an infectious context, the metabolic reprogramming of T cells depends on the environmental conditions and available nutrients that result in efficient activation, regulation and memory generation. Chronic infections such as Mycobacterium tuberculosis, mitochondrial dysfunction in CD8 T cells result in greater dependence on glycolysis and show bioenergetic deficiencies [84]. However, for memory T cells generation, the glycolytic pathway is reduced, and the AMP-activated protein kinase (AMPK) activity and OxPhos are increased for homeostatic maintenance.
Metabolic pathway in T cell subsets. A. Naive T cells use glucose and glutamine to maintain the oxphos pathway. B. Effector and Tr1 cells increase glycolysis and produce lactate. Intermediate components support the synthesis of nucleotides (Nuc), amino acids (AA) and fatty acids (FA). C. Foxp3+ T regs use extracellular FA to support oxphos by fatty acid oxidation (FAO). D. Memory T cells use glucose to increase TCA intermediate components to support fatty acid synthesis (FAS). Glycerol and FA are used to fuel the oxphos pathway. Blue cells show oxidative metabolism and red cells show glycolytic metabolism
5 Modulation of Immune Cell Metabolism by Pathogens
As discussed previously, immune cells that compose an active barrier at sites of high exposure to pathogens control infections and maintain the body's homeostasis. For this, immune cells need to change the metabolism and provide energy even with the drop of nutrients in the environment. In contrast, pathogens have mechanisms that act as an opposite force for their survival within the host. Intracellular pathogens, such as viruses and some bacteria, are able to reprogram host cell metabolism to increase the demand for nutrients and allow their replication. Elseways, extracellular pathogens modulate the immune system to improve the production of essential metabolites for their survival. Herein, we will discuss how pathogens modulate the metabolism of immune cells and determine their permanence during infection.
The limited resources of cellular energy during the pathogen-host interaction lead to a race against time in obtaining nutrients for the cellular machinery of both. In addition, metabolic changes in the host cell may favor the pathogen's permanence due to the evasion of the immune system. In this way, metabolic changes act as key mechanisms for the survival of some species, mainly by increasing glucose and lactate in the host cell. Some protozoa use the host's glucose as a primary carbon source, such as Trypanosoma brucei and Plasmodium falciparum [75, 89]. The extracellular pathogenic bacterium group A Streptococcus (GAS) causes an endoplasmic reticulum stress to host cells. This stress increases asparagine (ASN) synthetase expression that promotes GAS proliferation, while proliferation-linked genes are downregulated in the absence of ASN [6]. Another extracellular pathogen, the Citrobacter rodentium, modulates innate immunity and microbiota composition, impairing cellular bioenergetics [65].
In general, the modulation of host metabolism guarantees nutrients and evasion of the immune system to increase the pathogen survival. Thereby, the metabolic shift in the host cell promotes the intracellular survival of Mycobacterium tuberculosis [9], Legionella pneumophila [29], Brucella abortus [20] or Chlamydia trachomatis [83]. Cancer cells, known to induce the Warburg effect, are more susceptible to Listeria monocytogenes replication, suggesting that metabolic reprogramming is a favorable mechanism for the survival of this bacterium [35]. Among the many infections that interfere with the host metabolism, a group of pathogenic bacteria, known as Intracellular Bacterial Pathogens (IBPs), survive and proliferate inside vacuoles or in the cytosol for long periods. Because IBPs are heterotrophic, i.e., metabolism depends on carbon sources for energy,they use both the host carbon and nitrogen. An interesting fact is a possible association between intracellular carbon metabolism and virulence gene expression. It is known that specific regulators can control these genes that sense metabolites generated by the metabolism of carbon and nitrogen [27]. As a primary carbon source, IBPs use glucose from immune cells for their metabolic activities [13]. However, defined bacteria need a “biopartite metabolism” because they have more difficulty in using only glucose. These IBPs use several sources of carbon from the host to generate energy and stay within the host cells. Among them, it is possible to highlight TCA intermediaries such as succinate and malate, amino acids, fatty acids and also products derived from glucose such as glycerol (-3P), pyruvate and lactate [28]. Due to the evolutionary adaptation of the pathogen, some components in the metabolic reprogramming of the host cell, are used as an escape and survival mechanism. Alternatively, pathogen virulence factors interact with central regulators of host metabolism (Fig. 5). The activation of PI3K/Akt pathway is downmodulated by the Src homology 2 (SH2) domain-containing inositol-5′-phosphatase (SHIP) protein that promotes Francisella tularensis to escape into the cytosol [3, 19]. Still, the motility and phagocytosis of the host cell are controlled by molecular mechanisms developed by IBPs subverting the metabolism of phosphoinositides [78]. In experimental model, the infection with Yersinia enterocolitica led to HIF-1 in Peyer's patches [39]. Furthermore, Salmonella requires the fatty acid regulator PPARδ to establish a metabolic environment that favors a long-term permanence. Under cellular stress, defined metabolic strategies to obtain nutrients also promote the elimination of intracellular pathogens. These cells reach high levels of AMP/ATP by reducing the production of ATP, which in turn induces the AMP-activated protein kinase (AMPK) activation. Therefore, AMPK regulates the autophagic process, efficient autophagosome maturation [47]. Thus, autophagy is used as a self-degrading process to obtain energy, and hence intracellular pathogens can be eliminated as well. However, pathogens have machinery capable of modulating autophagy in the host, either by changing the maturation of the phagosome such as Mycobacteria [93], Legionella [90], Brucella [97] and Salmonella [108], or escaping from those vacuoles into the cytoplasm as in Shigella [72] and Listeria monocytogenes infection [63]. Therefore, pathogens evade host defense mechanisms not only by interfering in the microbicide function of immune cells, but also, in their metabolic pathways.
Intracellular bacterial pathogens (IBPs) modulate central metabolic regulators in the host cells. Bacterial components activate or alter metabolic pathways to evade the immune system and maintain its survival. The main metabolic mediators affected by IBPs are the Pi3K/Akt/mTOR pathway, HIF1α, fatty acid regulators and AMPK
In general, the viruses perform both lytic cycles, with high replication and viral load, as well as latent and persistent within the cell. Therefore, these conditions have different metabolic needs of the host cell [21]. During viral replication, the virus depends on high demand for nucleotides, amino acids and ATP (Fig. 6). Therefore, changes in glycolytic pathways, fatty acid synthesis (FAS), glutaminolysis and Pentose Phosphate Pathway (PPP) are observed. The targeting of glucose to PPP supports the production of nucleotides. In addition, pyruvate can be converted to lactate or enter the TCA pathway. Citrate can be transported to the cytoplasm via the FAS pathway. On the other side, glutamine enters the infected cell and supports the TCA cycle [87]. Therefore, the catabolism of suitable carbon sources in infected cells is crucial for viral replication, enabling the synthesis of nucleic acids and the viral envelope. For instance, the infection of monocytes with acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggers mitochondrial ROS production, capable of HIF-1α stabilization, which promotes the expression of glycolytic genes and IL-1β, responsible to monocytes metabolic reprogramming to aerobic glycolysis (Warburg effect) and T cell dysfunction, respectively. These data may explain how diabetic individuals with uncontrolled glucose levels are more susceptible to develop the severe form of Coronavirus disease 2019 (COVID-19), the pandemic that affected the world's population in 2020 [18].
Virus infection modulates host cell metabolism to support its replication. Viruses promote metabolic changes in host cells to increase the production of Nucleotides (Nuc), amino acids (AA) and ATP that support viral replication. Entry into the glycolytic pathway is a critical mechanism to supply the demand for viral components
Another example of metabolic reprogramming in the context of viral infection is the HIV. The susceptibility of different subtypes of CD4 T cells to HIV-1 infection is associated with high cellular metabolic activity. In fact, independently of their activation phenotype, CD4 T cells with high oxidative phosphorylation and glycolysis are more infected by HIV as compared to CD4 T cells harboring other metabolic profiles [105]. As exemplified above, different viruses are capable of infecting specific barrier tissues, such as COVID-19 respiratory tract infection and HIV-1 genital tract infection. However, regardless of the virus or the infected barrier site, one of the characteristics observed is the ability that these viruses to interfere within the metabolism of immune cells in favor of their survival and replication. Most of the studies regarding immune cell metabolism were performed in vitro, and now this knowledge needs to be integrated into the context of tissue-specific immune responses. Therefore, we need to better understand how the cellular metabolic reprogramming imposed by different types of pathogens can interfere, especially in the long-term, in the barrier immune response and its function, given the relevance of the balance between expansion and contraction of effector immune response in these tissues and their role as pathogen entrance sites.
6 Therapy Targeting Cell Metabolism to Control Infections
Considering that intracellular as well as extracellular pathogens can promote the metabolic reprogramming of the host immune cells, either through the modification of the cellular immune response profile or even the bioavailability of nutrients, it is plausible to suggest that therapies targeting metabolic pathways may be important to better target the immune response to fight the infectious agent and return homeostasis. In contrast to the pathogen-targeted traditional antimicrobial therapy, these therapeutic approaches would target the host metabolism.
For instance, the first line of oral drugs for controlling glucose metabolism is metformin, that activates AMP kinase, therefore lowering blood glucose concentrations by decreasing hepatic gluconeogenesis and improving glucose cell uptake. Because of this, metformin is commonly used to treat diabetes. One of the anti-inflammatory effects associated with metformin seen in LPS-activated macrophages is the inhibition ROS generation through reverse electron transport at Complex I and inhibition of IL-1β production [51]. Another one is to induce the inhibition of Complex I, which consequently reduces intracellular ATP production and activation of AMP-activated protein kinase (AMPK) [98, 113]. In contrast, in an AMPK- independent manner, metformin has been shown to inhibit the mammalian target of rapamycin complex I (mTORCI) in T cells and prevents the expression of the transcription factors c-Myc and Hif-1α [111]. Although metformin treatment is associated with a decreased risk of developing sepsis in humans [92],in experimental model it has been suggested that metformin may increase C. albicans infections by reducing macrophages responses to this opportunistic fungal infection [104]. Another anti-inflammatory drug is dimethyl fumarate, which promotes the expression of antioxidant genes by stabilizing the expression of the transcription factor Nuclear Factor (erythroid-derived 2) like 2 (Nrf2) [24] and is usually prescribed for treating multiple sclerosis (MS) [12] and psoriasis [14]. The Nrf2 regulates the pentose phosphate route in macrophages, reducing inflammatory macrophages response and induces lipid metabolism alterations in MS patients. In addition, fungal β-glucan administration is demonstrated to induce trained immunity characterized by increased glycolysis and partial reversion of LPS-induced immunoparalysis in monocytes isolated from human blood [71]. Moreover, oral glucan administration also demonstrated increased survival in experimental models challenged with S. aureus, as well as C. albicans, demonstrating that trained immunity is not a pathogen-specific response [82]. The world has been witnessing a constant emergence of new pathogens against which there may be no effective antimicrobial drugs. Therefore, the manipulation of metabolic cell reprogramming may represent a new promising approach for treating infectious diseases.
Abbreviations
- ACL:
-
ATP-dependent Citrate Lyase
- AMPK:
-
AMP-activated protein kinase
- AMPs:
-
Antimicrobial peptides
- AMs:
-
Alveolar macrophages
- Arg -1:
-
Arginase-1
- ASN:
-
Asparagine
- BCG:
-
Baccillus Calmette-Guérin
- COVID-19:
-
Coronavirus disease 2019
- DCs:
-
Dendritic cells
- FAS:
-
Fatty Acid Synthesis
- GALT:
-
Gut-Associated Lymphoid Tissue
- GAS:
-
Group A Streptococcus
- GLUT1:
-
Glucose Transporter 1
- HIF:
-
αHypoxia-Inducible Factor 1-Alpha
- HK2:
-
Hexokinase 2
- IBPs:
-
Intracellular Bacterial Pathogens
- IFNs:
-
Interferons
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- IL:
-
Interleukin
- ILCs:
-
Innate lymphoid cells
- ILFs:
-
Isolated Lymphoid Follicles
- JE:
-
Junctional epithelium
- LPS:
-
Lipopolysaccharide
- MALT:
-
Mucosa-associated lymphoid tissue
- MHC:
-
Major Histocompatibility Complex
- MIF:
-
Migration Inhibitory Factor
- MS:
-
Multiple Sclerosis
- mTOR:
-
Mammalian Target of Rapamycin
- mTORC1:
-
Mammalian Target of Rapamycin complex 1
- MV:
-
Measles Virus
- NETs:
-
Neutrophil Extracellular Traps
- NK:
-
Natural killer
- NLRP3:
-
NLR family pyrin domain containing 3
- NO:
-
Nitric Oxide
- Nrf2:
-
Nuclear Factor erythroid-derived 2
- OAA:
-
Oxaloacetate
- OXPHOS:
-
Oxidative Phosphorylation
- PAMPs:
-
PathogenAssociated Molecular Patterns
- PDK1 :
-
Phosphoinositide-Dependent Kinase 1
- PFK1 :
-
Phosphofructokinase 1
- PGE2:
-
Prostaglandin E2
- PI3K :
-
Phosphoinositide-3 Kinase
- pIgA:
-
Polymeric Immunoglobulin A
- pIgR:
-
Polymeric immunoglobulin receptor
- PIP3 :
-
Phosphatidylinositol-3,4,5-Trisphosphate
- PKM2:
-
Pyruvate Kinase M2
- PPARδ:
-
Peroxisome Proliferator-Activated Receptor Gamma
- PPP:
-
Pentose Phosphate Pathway
- PPs:
-
Peyer’s Patches
- PRRs:
-
Pattern Recognition Receptors
- ROS :
-
Reactive Oxygen Species
- SARS-CoV-2:
-
Respiratory Syndrome Coronavirus 2
- SH2:
-
Src Homology 2
- TAC:
-
Tricarboxylic Acid Cycle
- TGF-β:
-
Transforming growth factor-β
- Th:
-
T helper
- TLRs:
-
Toll-like receptors
- Tr1:
-
Type 1 Regulatory T cells
- Tregs:
-
Regulatory T cells
- TRMs :
-
Tissue-Resident Macrophages
- UTIs:
-
Urinary tract infections
- α-KG:
-
α-Ketoglutarate
References
Abraham SN, ST John A L (2010) Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10:440–452
Agace WW, Hedges SR, Ceska M, Svanborg C (1993) Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest 92:780–785
Asare R, Kwaik YA (2010) Exploitation of host cell biology and evasion of immunity by francisella tularensis. Front Microbiol 1:145
Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM (2005) Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175:1483–1490
Barlow JL, McKenzie ANJ (2019) Innate Lymphoid Cells of the Lung. Annu Rev Physiol 81:429–452
Baruch M, Belotserkovsky I, Hertzog BB, Ravins M, Dov E, McIver KS, le Breton YS, Zhou Y, Cheng CY, Hanski E (2014) An extracellular bacterial pathogen modulates host metabolism to regulate its own sensing and proliferation. Cell 156:97–108
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141
Belkaid Y, Harrison OJ (2017) Homeostatic Immunity and the Microbiota. Immunity 46:562–576
Billig S, Schneefeld M, Huber C, Grassl GA, Eisenreich W, Bange FC (2017) Lactate oxidation facilitates growth of Mycobacterium tuberculosis in human macrophages. Sci Rep 7:6484
Billips BK, Schaeffer AJ, Klumpp DJ (2008) Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun 76:3891–3900
Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, Raissi TC, van der Windt GJ, Viollet B, Pearce EL, Pelletier J, Piccirillo CA, Krawczyk CM, Divangahi M, Jones RG (2015) The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42:41–54
Bomprezzi R (2015) Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 8:20–30
Bowden SD, Rowley G, Hinton JC, Thompson A (2009) Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar typhimurium. Infect Immun 77:3117–3126
Bruck J, Dringen R, Amasuno A, Pau-Charles I, Ghoreschi K (2018) A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol 27:611–624
Buchmuller-Rouiller Y, Mauel J (1987) Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun 55:587–593
Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA (2010) Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185:1037–1044
Chan, C. Y., ST John AL, Abraham SN (2013) Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 38:349–359
Codo AC, Davanzo GG, de Brito Monteiro L, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CA, Crunfli F, Restrepo JL (2020) Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1alpha/Glycolysis-Dependent Axis. Cell Metab 32:498–499
Cremer TJ, Butchar JP, Tridandapani S (2011) Francisella Subverts Innate Immune Signaling: Focus On PI3K/Akt. Front Microbiol 5:13
Czyż DM, Willett JW, Crosson S (2017) Brucella abortus Induces a Warburg Shift in Host Metabolism That Is Linked to Enhanced Intracellular Survival of the Pathogen. J Bacteriol, 199
Delgado T, Sanchez EL, Camarda R, Lagunoff M. (2012)Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog, 8 e1002866.
Delima AJ, van Dyke TE (2003) Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000(31):55–76
Descoteaux A, Matlashewski G (1989) c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol 9:5223–5227
Deshmukh P, Unni S, Krishnappa G, Padmanabhan B (2017) The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev 9:41–56
Donnelly RP, Finlay DK (2015) Glucose, glycolysis and lymphocyte responses. Mol Immunol 68:513–519
Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, Calderon G, Hong BY, Break TJ, Bowdish DM, Lionakis MS, Jones SA, Trinchieri G, Diaz PI, Belkaid Y, Konkel JE, Moutsopoulos NM (2017) On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 46:133–147
Eisenreich W, Heesemann J, Rudel T, Goebel W (2013) Metabolic host responses to infection by intracellular bacterial pathogens. Front Cell Infect Microbiol 3:24
Eisenreich W, Rudel T, Heesemann J, Goebel W (2017) To Eat and to Be Eaten: Mutual Metabolic Adaptations of Immune Cells and Intracellular Bacterial Pathogens upon Infection. Front Cell Infect Microbiol 7:316
ESCOLL, P., SONG, O. R., VIANA, F., STEINER, B., LAGACHE, T., OLIVO-MARIN, J. C., IMPENS, F., BRODIN, P., HILBI, H. & BUCHRIESER, C. 2017. Legionella pneumophila Modulates Mitochondrial Dynamics to Trigger Metabolic Repurposing of Infected Macrophages. Cell Host Microbe, 22, 302–316 e7.
Fonseca DM, Hand TW, Han SJ, Gerner MY, GLATMAN ZARETSKY, A., BYRD, A. L., HARRISON, O. J., ORTIZ, A. M., QUINONES, M., TRINCHIERI, G., BRENCHLEY, J. M., BRODSKY, I. E., GERMAIN, R. N., RANDOLPH, G. J. & BELKAID, Y. (2015) Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell 163:354–366
FRANKE, T. F. 2008. Intracellular signaling by Akt: bound to be specific. Sci Signal, 1, pe29.
Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12:503–516
Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P (2016) Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529:226–230
Gerriets VA, Rathmell JC (2012) Metabolic pathways in T cell fate and function. Trends Immunol 33:168–173
GILLMAIER, N., GOTZ, A., SCHULZ, A., EISENREICH, W. & GOEBEL, W. 2012. Metabolic responses of primary and transformed cells to intracellular Listeria monocytogenes. PLoS One, 7, e52378.
Grist M, Chakraborty J (1994) Identification of a mucin layer in the urinary bladder. Urology 44:26–33
HAN, S. J., GLATMAN ZARETSKY, A., ANDRADE-OLIVEIRA, V., COLLINS, N., DZUTSEV, A., SHAIK, J., MORAIS DA FONSECA, D., HARRISON, O. J., TAMOUTOUNOUR, S., BYRD, A. L., SMELKINSON, M., BOULADOUX, N., BLISKA, J. B., BRENCHLEY, J. M., BRODSKY, I. E. & BELKAID, Y. 2017. White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity, 47, 1154–1168 e6.
Haraoka M, Hang L, Frendeus B, Godaly G, Burdick M, Strieter R, Svanborg C (1999) Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis 180:1220–1229
Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, Neumann D, Colgan SP, Kempf VA (2008) Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134:756–767
Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B (2013) Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol 14:372–379
HOYTEMA VAN KONIJNENBURG, D. P., REIS, B. S., PEDICORD, V. A., FARACHE, J., VICTORA, G. D. & MUCIDA, D. 2017. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell, 171, 783–794 e13.
Humphrey SP, Williamson RT (2001) A review of saliva: normal composition, flow, and function. J Prosthet Dent 85:162–169
Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ (2005) Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun 73:3999–4006
IWASAKI, A. (2007) Mucosal dendritic cells. Annu Rev Immunol 25:381–418
IWASAKI, A. (2010) Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 10:699–711
Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC (2008) Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol 180:4476–4486
Jang M, Park R, Kim H, Namkoong S, Jo D, Huh YH, Jang IS, Lee JI, Park J (2018) AMPK contributes to autophagosome maturation and lysosomal fusion. Sci Rep 8:12637
Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W (2003) Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med 198:963–969
Kayama H, Okumura R, Takeda K (2020) Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu Rev Immunol 38:23–48
Kayama H, Takeda K (2020) Manipulation of epithelial integrity and mucosal immunity by host and microbiota-derived metabolites. Eur J Immunol 50:921–931
Kelly B, Tannahill GM, Murphy MP, O’Neill LA (2015) Metformin Inhibits the Production of Reactive Oxygen Species from NADH: Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1beta (IL-1beta) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. J Biol Chem 290:20348–20359
KERNIEN, J. F., JOHNSON, C. J. & NETT, J. E. 2017. Conserved Inhibition of Neutrophil Extracellular Trap Release by Clinical Candida albicans Biofilms. J Fungi (Basel), 3.
Konkel JE, Chen W (2011) Balancing acts: the role of TGF-beta in the mucosal immune system. Trends Mol Med 17:668–676
Kugler DG, Flomerfelt FA, Costa DL, Laky K, Kamenyeva O, Mittelstadt PR, Gress RE, Rosshart SP, Rehermann B, Ashwell JD, Sher A, Jankovic D (2016) Systemic toxoplasma infection triggers a long-term defect in the generation and function of naive T lymphocytes. J Exp Med 213:3041–3056
LACERDA MARIANO, L. & INGERSOLL, M. A. (2020) The immune response to infection in the bladder. Nat Rev Urol 17:439–458
Lapaque N, Hutchinson JL, Jones DC, Meresse S, Holden DW, Trowsdale J, Kelly AP (2009) Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc Natl Acad Sci U S A 106:14052–14057
Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159:1312–1326
Loftus RM, Finlay DK (2016) Immunometabolism: Cellular Metabolism Turns Immune Regulator. J Biol Chem 291:1–10
Luan HH, Medzhitov R (2016) Food Fight: Role of Itaconate and Other Metabolites in Antimicrobial Defense. Cell Metab 24:379–387
Martinon F, Mayor A, Tschopp J (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265
Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K (2013) Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A 110:7820–7825
Mina MJ, Kula T, Leng Y, Li M, de Vries RD, Knip M, Siljander H, Rewers M, Choy DF, Wilson MS, Larman HB, Nelson AN, Griffin DE, de Swart RL, Elledge SJ (2019) Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 366:599–606
Mitchell G, Cheng MI, Chen C, Nguyen BN, Whiteley AT, Kianian S, Cox JS, Green DR, McDonald KL, Portnoy DA (2018) Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc Natl Acad Sci U S A 115:E210–E217
Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160
Mullineaux-Sanders C, Sanchez-Garrido J, Hopkins EGD, Shenoy AR, Barry R, Frankel G (2019) Citrobacter rodentium-host-microbiota interactions: immunity, bioenergetics and metabolism. Nat Rev Microbiol 17:701–715
Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL (2002) Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol 119:1090–1095
Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ (2015) Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci U S A 112:E871–E880
Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y (2015) Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520:104–108
Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y (2012) Compartmentalized control of skin immunity by resident commensals. Science 337:1115–1119
NETEA, M. G., JOOSTEN, L. A., LATZ, E., MILLS, K. H., NATOLI, G., STUNNENBERG, H. G., O'NEILL, L. A. & XAVIER, R. J. 2016. Trained immunity: A program of innate immune memory in health and disease. Science, 352, aaf1098.
NOVAKOVIC, B., HABIBI, E., WANG, S. Y., ARTS, R. J. W., DAVAR, R., MEGCHELENBRINK, W., KIM, B., KUZNETSOVA, T., KOX, M., ZWAAG, J., MATARESE, F., VAN HEERINGEN, S. J., JANSSEN-MEGENS, E. M., SHARIFI, N., WANG, C., KERAMATI, F., SCHOONENBERG, V., FLICEK, P., CLARKE, L., PICKKERS, P., HEATH, S., GUT, I., NETEA, M. G., MARTENS, J. H. A., LOGIE, C. & STUNNENBERG, H. G. 2016. beta-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell, 167, 1354–1368 e14.
Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C (2005) Escape of intracellular Shigella from autophagy. Science 307:727–731
OHNO, H. (2016) Intestinal M cells. J Biochem 159:151–160
Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, Lin CS, Jobin C, Brand S, Sotlar K, Wada K, Katayama K, Nakajima A, Mizuguchi H, Kawasaki K, Nagata K, Muller W, Snapper SB, Schreiber S, Kaser A, Zeissig S, Blumberg RS (2014) Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509:497–502
Olszewski KL, Llinas M (2011) Central carbon metabolism of Plasmodium parasites. Mol Biochem Parasitol 175:95–103
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153
Petrova VN, Sawatsky B, Han AX, Laksono BM, Walz L, Parker E, Pieper K, Anderson CA, de Vries RD (2019) Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol, 4
Pizarro-Cerda J, Cossart P (2004) Subversion of phosphoinositide metabolism by intracellular bacterial pathogens. Nat Cell Biol 6:1026–1033
Ratner JJ, Thomas VL, Sanford BA, Forland M (1981) Bacteria-specific antibody in the urine of patients with acute pyelonephritis and cystitis. J Infect Dis 143:404–412
REINER, N. E. (1987) Parasite accessory cell interactions in murine leishmaniasis. I. Evasion and stimulus-dependent suppression of the macrophage interleukin 1 response by Leishmania donovani. J Immunol 138:1919–1925
Reiner NE, Ng W, McMaster WR (1987) Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J Immunol 138:1926–1932
Rice PJ, Adams EL, Ozment-Skelton T, Gonzalez AJ, Goldman MP, Lockhart BE, Barker LA, Breuel KF, Deponti WK, Kalbfleisch JH, Ensley HE, Brown GD, Gordon S, Williams DL (2005) Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther 314:1079–1086
Rother M, Teixeira da Costa AR, Zietlow R, Meyer TF, Rudel T (2014) Modulation of Host Cell Metabolism by Chlamydia trachomatis. Microbiol Spectr, 7
Russell SL, Lamprecht DA, Mandizvo T, Jones TT, Naidoo V, Addicott KW, Moodley C, Ngcobo B, Crossman DK, Wells G, Steyn AJ (2019) Compromised Metabolic Reprogramming Is an Early Indicator of CD8(+) T Cell Dysfunction during Chronic Mycobacterium tuberculosis Infection. Cell Rep, 29 3564–3579 e5
Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JW, Joosten LA, Wijmenga C, Martens JH, Xavier RJ, Logie C, Netea MG, Stunnenberg HG (2014) Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345:1251086
Saluzzo S, Gorki AD, Rana BMJ, Martins R, Scanlon S, Starkl P, Lakovits K, Hladik A, Korosec A, Sharif O, Warszawska JM, Jolin H, Mesteri I, McKenzie ANJ, Knapp S (2017) First-Breath-Induced Type 2 Pathways Shape the Lung Immune Environment. Cell Rep 18:1893–1905
Sanchez EL, Lagunoff M (2015) Viral activation of cellular metabolism. Virology 479–480:609–618
Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Grone HJ, Garbi N, Kastenmuller W, Knolle PA, Kurts C, Engel DR (2014) Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156:456–468
Shah-Simpson S, Lentini G, Dumoulin PC, Burleigh BA (2017) Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog, 13 e1006747
Sherwood RK, Roy CR (2016) Autophagy Evasion and Endoplasmic Reticulum Subversion: The Yin and Yang of Legionella Intracellular Infection. Annu Rev Microbiol 70:413–433
Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208:1367–1376
Shih CJ, Wu YL, Chao PW, Kuo SC, Yang CY, Li SY, Ou SM, Chen YT (2015) Association between Use of Oral Anti-Diabetic Drugs and the Risk of Sepsis: A Nested Case-Control Study. Sci Rep 5:15260
Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, Lee SH, Lee ZW, Cho SN, Kim JM, Friedman RL (2020) Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog, 6 e1001230
Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170:3037–3045
Song J, Duncan MJ, Li G, Chan C, Grady R, Stapleton A, Abraham SN (2007) A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog, 3 e60
Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M (2013) Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med 210:775–788
Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J (2012) Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45
Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B (2011) Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54:3101–3110
Svedberg FR, Brown SL, Krauss MZ, Campbell L, Sharpe C, Clausen M, Howell GJ, Clark H, Madsen J, Evans CM, Sutherland TE, Ivens AC, Thornton DJ, Grencis RK, Hussell T, Cunoosamy DM, Cook PC, Macdonald AS (2019) The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat Immunol 20:571–580
Tannahill GM, Curtis AM, Adamik J, Palsson-Mcdermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA (2013) Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496:238–242
Thapa B, Lee K (2019) Metabolic influence on macrophage polarization and pathogenesis. BMB Rep 52:360–372
Traven A. Naderer T (2019) Central metabolic interactions of immune cells and microbes: prospects for defeating infections. EMBO Rep, 20, e47995.
Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S (2009) Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science 323:1488–1492
Tucey TM, Verma J, Harrison PF, Snelgrove SL, Lo TL, Scherer AK, Barugahare AA, Powell DR, Wheeler RT, Hickey MJ, Beilharz TH (2018) Glucose Homeostasis Is Important for Immune Cell Viability during Candida Challenge and Host Survival of Systemic Fungal Infection. Cell Metab, 27, 988–1006 e7
Valle-Casuso JC, Angin M, Volant S, Passaes C, Monceaux V, Mikhailova A, Bourdic K, Avettand-Fenoel V, Boufassa F, Sitbon M, Lambotte O (2019) Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4(+) T Cells and Offers an Opportunity to Tackle Infection. Cell Metab, 29, 611–626 e5
von Moltke J, Ji M, Liang HE, Locksley RM (2016) Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529:221–225
Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35:871–882
Wu S, Shen Y, Zhang S, Xiao Y, Shi S (2020) Salmonella Interacts With Autophagy to Offense or Defense. Front Microbiol 11:721
Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT (2009) Uroplakins in urothelial biology, function, and disease. Kidney Int 75:1153–1165
Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B, Greter M (2017) The Cytokine TGF-beta Promotes the Development and Homeostasis of Alveolar Macrophages. Immunity, 47, 903–912 e4
Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA (2014) Adenosine-mono-phosphate-activated protein kinase-independent effects of metformin in T cells. PLoS One, 9, e106710
Zhang X, Zhao S, Sun L, Li W, Wei Q, Ashman RB, Hu Y (2017) Different virulence of candida albicans is attributed to the ability of escape from neutrophil extracellular traps by secretion of DNase. Am J Transl Res 9:50–62
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174
Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF (2008) TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453:236–240
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Salles, É.M., Pizzolante, B.C., da Fonseca, D.M. (2022). Metabolic Reprogramming and Infectious Diseases. In: Camara, N.O.S., Alves-Filho, J.C., Moraes-Vieira, P.M.M.d., Andrade-Oliveira, V. (eds) Essential Aspects of Immunometabolism in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-86684-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-86684-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86683-9
Online ISBN: 978-3-030-86684-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)