Abstract
-
About the two predominant groups of microalgae, chlorophytes (green algae) and diatoms, and their respective primary carbon fixation methods
-
A diverse range of extreme environments where microalgae have been found, some of the characteristics of the microalgae found in these environments, and how to target different environments for algal product production
-
How microalgae are used in a conceptual biofuel production strategy including the various methods for growing, harvesting, and converting algal lipids and other products to biofuels and bioproducts
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Microalgae
- Hydrothermal Liquefaction
- East African Soda Lakes
- Carbon-concentrating Mechanism (CCMs)
- Soap Lake
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara What You Will Learn-
About the two predominant groups of microalgae, chlorophytes (green algae) and diatoms, and their respective primary carbon fixation methods
-
A diverse range of extreme environments where microalgae have been found, some of the characteristics of the microalgae found in these environments, and how to target different environments for algal product production
-
How microalgae are used in a conceptual biofuel production strategy including the various methods for growing, harvesting, and converting algal lipids and other products to biofuels and bioproducts
4.1 Introduction
Algal biomass represents a promising renewable energy system due to fast photoautotrophic growth rates, CO2 fixation, and accumulation of carbon storage metabolites which can be used as precursors for fuels and specialty chemicals; moreover, it offers a solution to offset the global dependence on conventional fuels. Currently, fuels make up approximately 66% of the global energy demand (Bhateria and Dhaka 2015). Correspondingly, extensive use of nonrenewable energy sources has increased the global carbon dioxide (CO2) concentration approximately 43% since the use of these fuels was significantly intensified since the Industrial Revolution, around 1750 (USEPA 2016). Fossil fuels are subject to volatile price swings based on geopolitical issues and availability of crude oil. As sources of crude oil become depleted, prices associated with fuels and other petroleum-derived products will experience rapid increases in response. Biofuels and other bio-products derived from microalgae have potential to contribute significantly to this market, yet how large their impact on the market will be remains to be seen (Hill et al. 2006).
Microalgae are oxygenic, phototrophic eukaryotes, which are abundantly found across diverse environments ranging from acidic hot springs to arctic ice and snow. Like other phototrophs, microalgae require light energy, water, and a few inorganic nutrients (carbon dioxide, nitrogen, phosphorus, iron, etc.) which they convert to biomass with diverse biochemical composition (Markou et al. 2014). There are several advantages to utilizing microalgae for biofuel production. First, microalgae have increased theoretical photosynthetic efficiency (10–12%) over terrestrial plants (4–6%) and high cell division rates (1–3 per day) which leads to overall improved biomass yield per unit area, which when paired with the ability to be cultivated continuously year-round further improves their productivity over terrestrial plants (Brennan and Owende 2010). Additionally, microalgae can be grown using brackish, salt, and wastewater sources reducing their demand for freshwater and can utilize nitrogen and phosphorus from agricultural, industrial, and municipal wastewaters as low-cost nutrient sources and as a method for remediation of the wastewater (Hu et al. 2008). Furthermore, algae have the potential to reduce carbon emissions if co-located with a power plant to sequester portions of the emitted CO2 before it enters the atmosphere (Schenk et al. 2008). Lastly, and perhaps most importantly, microalgae frequently have higher lipid content than terrestrial plants (Amin 2009), and with the combination of the other added benefits, often result in higher biofuel productivities on a per biomass basis. The inherent advantages to using microalgae for sustainable biofuel and bio-product formation are well known, though several bottlenecks still exist on the path from lab bench to full-scale production of microalgae.
One of the primary challenges associated with scale-up of biofuel production is algal species selection. For rapid growth in large-scale open systems, a robust species that tolerates moderate temperature, pH, and salinity changes must be selected to keep productivity high. Extremophilic algae are a compelling choice for biofuel production because of their innate ability to survive and even thrive on the boundary of extreme conditions (Seckbach 2007). Extremophilic microalgal strains have an added benefit of growing in conditions that inhibit growth of many competing microorganisms, which may allow higher biofuel productivity of targeted strains. Some strains isolated from alkaline or halophilic environments have been shown to contain very high concentrations of lipids, primarily in the form of triacylglycerol (TAG). Further, alkaline environments have greater flux of atmospheric CO2 into the algal growth medium, thus increasing inorganic carbon available for fixation. Therefore, extremophilic, microalgal strains have the potential to improve algal biofuel viability by providing a more cost-effective production with a greater potential for algal biodiesel productivity and decreased probability for significant contamination.
4.2 Microalgae
Algal biofuels are derived from two predominant groups, green algae and diatoms, both of which are unicellular, photosynthetic eukaryotes (Fig. 4.1). Some strains are known to store high concentrations of triacylglycerol (TAG) that can be converted into biodiesel. Diatom strain, RGd-1, was found to produce 30–40% (w/w) TAG and 70–80% (w/w) biofuel potential (BP) for ash-free dry weight (Moll et al. 2014). An isolate from the Heart Lake area of Yellowstone National Park, RGd-1, is able to grow in exceptionally high silica concentrations that are often inhibiting for marine diatoms (Hildebrand et al. 2012). Moll et al. (2014) found that RGd-1 maintained the best growth and TAG accumulation when grown in 2 mM Si, which is roughly an order of magnitude greater than the silica concentration in seawater. They went on to further stimulate TAG accumulation by adding 25 mM NaHCO3 just prior to nutrient depletion. The greatest lipid accumulation occurred during the stress of a combined Si and NO3− limitation with NaHCO3 addition which yielded a nearly a two-fold increase in TAG accumulation compared to Si limitation alone (Fig. 4.2). Further, NaHCO3 addition increased TAG accumulation compared to only nutrient limitation (Moll et al. 2014).
Chlorophytes (green algae) are thought to utilize the C3 photosynthesis pathway for carbon fixation, whereas diatoms, including Thalassiosira pseudonana, are thought to use the C3 and C4 pathways (Armbrust et al. 2004; Reinfelder et al. 2004; Roberts et al. 2007). However, both mechanisms utilize ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCo) to catalyze the first step in the Calvin cycle. RuBisCo has a relatively low affinity for CO2 and is less than half saturated under normal atmospheric conditions (Giordano et al. 2005). By consequence, microalgae have evolved carbon concentrating mechanisms (CCMs) to increase the carbon flux to RuBisCo (Kaplan and Reinhold 1999; Moroney and Somanchi 1999; Moroney and Ynalvez 2007). Algal CCMs are essentially a two-phase process: in the first phase, inorganic carbon is acquired from the environment and shuttled to the chloroplast, while during the second phase generates elevated HCO3 in the chloroplast stroma (Moroney and Ynalvez 2007). Microalgae have a number of carbonic anhydrases and bicarbonate transport channels to move inorganic carbon across the periplasmic membrane, through the cytosol, into the chloroplast, and convert the carbon to CO2 in the direct vicinity of the Rubisco in the pyrenoid. Interestingly, C4 pathways have the extra ability to convert HCO3 directly to a C4 organic acid molecule which is shuttled to the pyrenoid and reconverted to CO2 to be used by RuBisCo (Roberts et al. 2007; Radakovits et al. 2012).
Alkaline environments (e.g., Soap Lake, Washington) often have high bicarbonate ion concentrations. Given the current understanding of CCMs, it is not surprising that soda lakes are highly photosynthetically productive. Organisms that thrive in these extreme environments have physiological adaptations that allow them to be successful under conditions that would be lethal to other microorganisms. Diatoms are uniquely suited to living in alkaline environments due to their C4 metabolism. Evidence from the Phaeodactylum tricornutum and Thalassiosira pseudonana genomes indicates a propensity for C4 metabolism. Valenzuela et al. (2012) found evidence for P. tricornutum using C3 and C4 metabolism when dissolved inorganic carbon concentrations were low. Further, they found an increase in expression for P. tricornutum pyruvate carboxylase, malic enzyme, and malate dehydrogenase which indicates the presence of the C4 pathway (Valenzuela et al. 2012). This is advantageous by providing another pathway for CO2 fixation, especially given that C4 carboxylases are high affinity molecules allowing carbon to be concentrated in the chloroplast. As more extremophilic microalgae are isolated, identified, and characterized, further advances in biotechnology for biofuels and renewable biochemicals will become available.
Under replete growth conditions, microalgae capable of TAG accumulation will synthesis TAG during light hours and utilize the stored carbon for cellular maintenance during dark hours (Bigelow et al. 2011; Gardner et al. 2012, 2013b). However, when the cellular cycling is stressed or arrested due to nutrient limitation (Eustance et al. 2013; Stephenson et al. 2010; Valenzuela et al. 2013), environmental stress (e.g., pH, light, temperature stress) (Sharma et al. 2012; Gardner et al. 2010; Guckert and Cooksey 1990), or by chemical addition (Gardner et al. 2013a; Hunt et al. 2010), many algal strains will accumulate and maintain TAG vacuoles within the cell. Thus, industrial algal biofuel systems producing TAG as a biofuel substrate have to balance rapid growth with a means of impeding the cell cycle when the culture has reached a desired density (Borowitzka 1992; Griffiths and Harrison 2009). Typically, this is accomplished by timing cellular density with the depletion of nitrogen in the growth medium; however, this can often make the culture susceptible to contamination or predation from other microorganisms. However, use of extremophilic strains as an industrial algal biofuel platform is an under studied tactic and merits additional investigation focused on cellular cycling and TAG accumulation.
4.3 Extreme Environments
Microalgae have been isolated from extreme environments such as Arctic/Antarctic regions and acidic hot springs, as well as from alkaline and/or hypersaline environments. Examples of such environments include Yellowstone National Park; Soap Lake, Washington; Mono Lake, California; Great Salt Lake, Utah; and the East African Soda Lakes. In addition to providing a selective advantage for algal growth, with increased pH, there is increased CO2 solubility, leading to enhanced algal growth (Stumm and Morgan 2012). Soda lakes accumulate very high concentrations of sodium carbonate salts due to the limited Mg2+ and Ca2+ concentrations with pH 8–12 (Jones et al. 1998). The East African Soda Lakes are among the most productive lakes in the world with gross photosynthetic rates up to 36 g O2 m2 per day for Lake Nakuru, Kenya (Melack and Kilham 1974). Another example of a soda lake from which high lipid containing strains have been isolated is Soap Lake, Washington, which is pH 9.9 and contains very high concentrations of sodium carbonate and sodium bicarbonate at 6870 mg L−1 (0.7%) and 5209 mg L−1 (0.5%), respectively (Richards 2007). Halophiles are uniquely adapted to their environments by keeping high concentrations of intracellular K+ to compensate for the high extracellular Na+ concentrations. Pick et al. (1986) found that when Dunaliella salina was grown in 1–4 M NaCl, intracellular Na+ concentrations were 20–100 mM and K+ concentrations were 150–250 mM.
Witch Creek is an alkaline, freshwater creek located in the Heart Lake area of Yellowstone National Park (USA). Witch Creek is approximately two miles long and is fed by a combination of fresh groundwater and effluent channels (Fig. 4.3) from alkaline hot springs with high concentrations of metals such as arsenic (~300 ppb) and silicon (~72 ppm). Regular inputs from these thermal features into Witch Creek make the creek alkaline, leading to the growth of microorganisms including microalgae that are adapted to the alkaline conditions found in Witch Creek. Such microalgae have been isolated and characterized for biofuel applications.
4.3.1 Targeting Extremophiles
For microalgae, extremophilic organisms are those considered to have improved growth outside of “normal” environments. For microalgae, these defined “normal” environmental parameters are outlined as in Seckbach (2007) as those having an optimum temperature range between 4 and 40 °C, a pH range of 5–8.5, and a salinity range between that of fresh and salt water (0%–3.5%). The bulk of extremophilic microalgae species fall into the alkaliphile, acidophile, or halophile classifications although there are thermophilic microalgae as well (Seckbach 2007). Although the majority of microalgae species find their optimum growth somewhere in these defined ranges, extremophilic species have been targeted for use in biofuel production because of the generalized acceptance that product formation of lipids and other products is increased when the cell cycle is ceased and environmental stresses are implemented (Cooksey 2015; Markou and Nerantzis 2013). Table 4.1 highlights several extremophilic algae including acidophiles, alkaliphiles, psychrophiles, thermophiles, and halophiles and the conditions from which they were isolated.
4.3.2 Bioprospecting
Bioprospecting for potential strains that can be used for biodiesel production begins by matching desired growth conditions (e.g., high pH and salinity values) with natural environments that contain those conditions. In addition, locations for microbiological sampling are either on public or private property, and written permission to collect samples should be obtained for any samples collected. For finding extremophilic microalgae, find environments with a pH value below 5 or above pH 8.5, and a salinity range above that of salt water (3.5% w/w) up to sodium chloride saturation (35% w/w). For temperature limits, the upper limit for microalgal (eukaryotic) growth is approximately 57 °C (Seckbach 2007). However, around 45 °C phototrophic growth may be dominated by cyanobacterial species. Therefore, with regard to temperature, microalgal growth will be primarily in the mesophilic range (20–45 °C) (Madigan et al. 2008). As shown in Fig. 4.4, microbial and microalgal communities can change significantly over very short distances due to gradients in temperature, pH, salinity, or nutrient availability; therefore, care should be taken to characterize specific sampling locations for optimum selection of targeted microorganisms. Once areas have been targeted for sampling and written permission is obtained, samples can be taken from the area of interest and returned to the lab for isolation.
In the approach recommended here, samples should be disaggregated and inoculated into (5 mL) of various microalgal media types (e.g., Bold’s basal medium) to determine which would provide the best conditions for growth. Typically, standard media are not ideal for isolation of extremophilic microalgae, and so must be adjusted to higher or lower pH, higher salinity, or temperature that must be controlled at a higher or lower value. Samples should also be streaked for isolation on the appropriate solid growth media. Once the colonies have grown to sufficient size, individual, isolated colonies should be aseptically “picked” from the agar plate and inoculated into liquid medium (1 mL) and grown until they change the color of the medium (often green or brown, for green algae or diatoms, respectively) and subsequently transferred to liquid medium (5 mL). After approximately two weeks or once they have reached substantial growth, cultures should be streaked for isolation again and re-picked from agar plates for a total of three rounds of streaking for isolation and transferring to new medium to ensure isolation from other algal species. Following each round of isolation, strains should be observed under transmitted light microscopy to determine the cellular morphology.
Each isolated strain should be screened for TAG content by staining with Nile Red dye or Bodipy 505-515 and observed using epifluorescent microscopy (Cooksey et al. 1987). Fluorescence of lipid vacuoles appear bright yellow for Nile Red dye under epifluorescent light (Fig. 4.5) or green when stained with Bodipy. Strains that have the highest TAG content should be selected for further characterization, since these will likely have the highest biodiesel potential. Strains found to have high concentrations of TAG during the screening process should also have their growth rates measured, since a combination of fast growth rate and high lipid production is desirable. Strains that show faster growth rates and TAG production should be inoculated into approximately 150 mL of liquid medium in 250 mL baffled flasks and grown in triplicate at various temperatures to determine the optimal growth and TAG accumulation conditions for each strain.
4.4 Algae as Biofuels
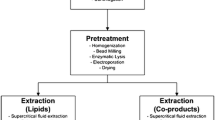
Manufacturing of biofuels and bioproducts from microalgae at industrial scale has many proposed methodologies for start-to-finish generation of targeted end products. Similar to a conventional petroleum refinery which makes multiple products and fuels from crude petroleum, a biorefinery would produce biofuels and other products from algal biomass. An operation such as this would have a variety of different steps, but the major sequences are as follows: cultivation of algae for biomass generation, harvesting of algal biomass, and extraction/conversion of algal biomass. Significant life cycle analyses and techno-economic analyses will be needed with regard to each step of this process to determine the most advantageous strategy for each phase of the operation (Davis et al. 2011).
Primary cultivation systems for the production of algal biomass are through the use of raceway ponds (Fig. 4.6) or photobioreactors (Fig. 4.7). Raceway ponds are typically large closed-loop ponds, in which microalgal culture is continuously circulated through a designed path, generally with the use of revolving paddle wheels (Chisti 2007). Algae are most often grown in large outdoor raceway ponds because it is one of the most cost-efficient ways to grow large quantities of algae (Schenk et al. 2008); however, raceway ponds can suffer from large evaporative losses and poor mass transfer properties for the application of CO2 (Terry and Raymond 1985). Still, the major caveat of this type of system is that it is non-sterile and has a potential for undesirable contamination from faster growing microorganisms that are not biofuel productive. One advantage of using extremophilic algae in outdoor raceway ponds is to create pond conditions that are favorable for extremophilic algal growth while being inhibitory for faster growing microorganisms that do not produce biodiesel precursors, but are relatively low cost to build and operate. Conversely, photobioreactors are collections of small-to-medium diameter (<10 cm) transparent tubes which are all oriented parallel to each other and usually stacked vertically to increase the reactor volume in a given footprint (Chisti 2007). Primary concerns regarding the use of photobioreactors are the design limitations on tube length, which is dependent on the degree of O2 production, CO2 depletion, and pH variation (Brennan and Owende 2010). While photobioreactors can cost more to build, they typically offer a more controlled environment and higher productivity than open raceway ponds (Sheehan et al. 1998).
Even dense cultures of microalgae require removal of excess water for downstream processing to some extent, and cost-effective and energy-efficient processes for the removal of water and subsequent harvesting of algal biomass are required for economical production of algal biofuels (Schenk et al. 2008). Harvesting of the algae is an energy-intensive step because many conversion pathways require the algal biomass to be at substantially higher concentrations than cultures grow in nature. Typically, even for extremely productive strains, biomass concentrations will not exceed 5% (w/w) suspensions while most conversion strategies require a minimum 20% biomass slurry and can require even more dewatering and drying. There are many different approaches to harvesting of algae, but the major developed strategies for harvesting algae are flocculation and sedimentation, filtration, and centrifugation. Each of these methods has advantages and disadvantages, and these methods are often used in combination to reach the desired final algae to water ratio. Flocculation and sedimentation is a routine method for harvesting algae which do not settle out in well-maintained reactors because of their small cell size (Bhateria and Dhaka 2015). Flocculation can be obtained through chemical additives such as alum, lime, polyacrylamide polymers, or surfactants. Following flocculation of the cells, the cells are allowed to settle, and excess water can be removed from the top of the cell sediment. Flocculation is also commonly used with dissolved air flotation (DAF) where the flocculated biomass is driven upward by the attachment of microbubbles where it can be collected at high concentration at the tank surface. Another common form of harvesting microalgae is centrifugation. It is likely that centrifugation will play a minor role in harvesting of culture where other harvesting methods fail to reach the desired algae content for slurry, as centrifugation can reach higher concentration biomass slurries than flocculation with sedimentation or other harvesting alternatives. However, centrifugation is an energy-intensive process which makes it seemingly unappealing for scale-up of algae cultivation for biofuels (Williams and Laurens 2010). Filtration is another possible alternative for harvesting algae, but has lost some appeal in scale-up from laboratory testing because of the potential for membrane or screen fouling as well as the labor-intensive process of operating such a system. Combinations of these practices for harvesting algae can reach biomass concentrations in the 20%–30% (w/w) range required for conversion methods utilizing wet biomass, but are not ideal for methods that require whole or dry biomass. If further dewatering is needed after the culture harvesting, a drying step will be necessary for removing excess water from algae paste or slurry. Thermal drying using methane drum dryers is most commonly practiced, but other oven-type dryers have been used, as well as solar drying and freeze-drying of algae slurry (Bhateria and Dhaka 2015).
Conversion of algal biomass can be accomplished through different methods which are generally categorized into two categories: thermochemical conversion or biochemical conversion (Amin 2009). From cultivated microalgal biomass, there are two major conversion strategies for making usable biofuels. The first, and more well known, is biochemical conversion. Most common for microalgal biodiesel production is the process of transesterification. Through the use of heat and an acid or base catalyst, algal lipids are converted to fatty acid methyl esters (FAMEs) and glycerol. These FAMEs are crude biodiesel and are similar in composition to those produced from transesterification of vegetable oils. The high lipid content in microalgae, primary TAG, makes transesterification an efficient process with production yields between 70% and 90%. While transesterification is primarily a straightforward chemical reaction, it falls into the biochemical conversion category because it does not require the significant energy requirements for high temperature and pressure systems typical with thermochemical conversion pathways. Another biochemical conversion pathway is fermentation of an algal slurry to produce ethanol. Fermentation of algae is a less common method for energy production, mostly because of the difficulties associated with the process of separating produced ethanol after the fermentation process as well as the relatively low starch content of microalgae compared with alternative lignocellulosic biomass. Still, fermentation of lipid extracted algae for conversion of the residual carbohydrates may offset costs associated with biofuel production from microalgae.
As an alternative to biochemical conversion, thermochemical conversion is currently being heavily studied for its application to microalgal biomass. Of the many different techniques for thermochemical conversion, hydrothermal liquefaction and pyrolysis are emerging as the two benchmark technologies, while gasification and hydrogenation will likely play smaller roles in utilizing all products from the conversion process (Amin 2009). Hydrothermal liquefaction is a process which uses subcritical water at moderate temperature and pressure (~300 °C and 10 MPa) to convert wet biomass into a liquid fuel called primary oil. This oil can be separated and purified using a solvent such as dichloromethane. Other products from hydrothermal liquefaction such as the aqueous and gas phases can be recycled to supply nutrients for more algae cultivation or used in a gasification or hydrogenation process for other products. Pyrolysis is another thermochemical conversion process to produce energy rich compounds such as biofuel, charcoal, and gaseous products from algal biomass. Short residence times and high temperatures (500 °C) are used to crack biomass into short chain molecules which can then be rapidly cooled into a liquid phase to produce biofuel. Pyrolysis has a high-energy input required because it requires algal biomass to be completely dried, adding the necessity for 20%–30% algal slurries to have all remaining residual water removed through one of the processes mentioned previously. There is still no general consensus on whether biochemical or thermochemical conversion processes will be the ultimate solution to producing biofuel from microalgae; however, life cycle analysis and techno-economic analysis considering the entire production of algae to biofuels is being completed with considerations for both types of conversion methods (Hise et al. 2016).
4.4.1 Other Secondary Products
Microalgae not only offer a source of sustainable biodiesel, but can be used to make an assortment of products such as food supplements, fertilizers, bioplastics, nutraceuticals, and cosmetics (Markou and Nerantzis 2013). For algal biofuels to be economically viable, additional coproducts will need to be formed in concert with biofuel. In particular, those products which have a combination of the highest yields and highest specific selling price (e.g., $/lb.) will be the optimum targets for coproducing with biofuels. Two examples of these higher value compounds are carotenoids and unsaturated fatty acids, which are both produced naturally by microalgae. Carotenoids are colorful pigments which can be used as food and feed additives, as well as nutraceutical supplements to promote health. The two most common carotenoids produced naturally by microalgae are β-carotene and astaxanthin (Markou and Nerantzis 2013). Two specific organisms produce these compounds in much greater quantity than any others studied, Dunaliella salina and Haematococcus pluvialis for β-carotene and astaxanthin production, respectively. Coincidentally, Dunaliella salina is a halophile with optimum sodium chloride concentrations between 10% and 27% depending on targeted growth regime, but also produces culture rich in β-carotene. Alternatively, Haematococcus pluvialis produces high concentrations of astaxanthin when environmentally stressed with sodium chloride concentrations in the range of 4%–6%. Furthermore, both these organisms can withstand high light environments and even can be induced to make more of their targeted carotenoids with light induced stress (Markou and Nerantzis 2013).
Mono- and poly-unsaturated fatty acids being another alternative secondary product, they must be valued separately from being simply a biofuel precursor. Depending on the intended application, omega-3 fatty acids such as α-linolenic acid or eicosapentaenoic acid can be sold as health supplements for much higher value as opposed to being converted to biofuel. Similarly, the monounsaturated fatty acid oleic acid is a valuable precursor to 9-decenoic acid, which can be used to create valuable products such as surfactants, lubricants, and polyester, amongst other things (Burns 2010). A feasible production strategy would make not only biofuels, but some other valuable secondary products as well.
Take Home Message
-
Microalgae are promising candidates for biodiesel production due to their fast growth rates, can be cultivated on nonarable land, can use brackish or wastewater, and avoid competing with food supplies.
-
Some microalgae strains accumulate high concentrations of TAG that can be converted into biodiesel.
-
Extremophilic algae are uniquely suited for growth because their growth conditions inhibit growth for most contaminating (biofuel nonproductive) microorganisms.
References Cited
Amin S (2009) Review on biofuel oil and gas production processes from microalgae. Energy Convers Manage 50(7):1834–1840. https://doi.org/10.1016/j.enconman.2009.03.001
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306(5693):79–86
Bhateria R, Dhaka R (2015) Algae as biofuel. Biofuels 5(6):607–631. https://doi.org/10.1080/17597269.2014.1003701
Bigelow NW, Hardin WR, Barker JP, Ryken SA, MacRae AC, Cattolico RA (2011) A comprehensive GC–MS sub-microscale assay for fatty acids and its applications. J Am Oil Chem Soc 88(9):1329–1338
Borowitzka MA (1990) The mass culture of Dunaliella salina. In: Regional workshop on the culture and utilization of seaweeds, Cebu City (Philippines), 27–31 Aug 1990
Borowitzka MA (1992) Algal biotechnology products and processes—matching science and economics. J Appl Phycol 4(3):267–279
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14(2):557–577. https://doi.org/10.1016/j.rser.2009.10.009
Burns NA (2010) Biomass--the next revolution in surfactants? Inform 21(727–729):779
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Cooksey KE (2015) Regulation of the initial events in microalgal triacylglycerol (TAG) synthesis: hypotheses. J Appl Phycol 27(4):1385–1387. https://doi.org/10.1007/s10811-014-0461-9
Cooksey KE, Guckert JB, Williams SA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6(6):333–345
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88(10):3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
Doemel WN, Brock T (1971) The physiological ecology of Cyanidium caldarium. Microbiology 67(1):17–32
Eustance E, Gardner RD, Moll KM, Menicucci J, Gerlach R, Peyton BM (2013) Growth, nitrogen utilization and biodiesel potential for two chlorophytes grown on ammonium, nitrate or urea. J Appl Phycol 25(6):1663–1677
Gardner R, Peters P, Peyton B, Cooksey KE (2010) Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta. J Appl Phycol 23(6):1005–1016. https://doi.org/10.1007/s10811-010-9633-4
Gardner RD, Cooksey KE, Mus F, Macur R, Moll K, Eustance E, Carlson RP, Gerlach R, Fields MW, Peyton BM (2012) Use of sodium bicarbonate to stimulate triacylglycerol accumulation in the chlorophyte Scenedesmus sp and the diatom Phaeodactylum tricornutum. J Appl Phycol 24(5):1311–1320. https://doi.org/10.1007/s10811-011-9782-0
Gardner RD, Lohman E, Gerlach R, Cooksey KE, Peyton BM (2013a) Comparison of CO(2) and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii. Biotechnol Bioeng 110(1):87–96. https://doi.org/10.1002/bit.24592
Gardner RD, Lohman EJ, Cooksey KE, Gerlach R, Peyton BM (2013b) Cellular cycling, carbon utilization, and photosynthetic oxygen production during bicarbonate-induced triacylglycerol accumulation in a Scenedesmus sp. Energies 6(11):6060–6076. https://doi.org/10.3390/en6116060
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56(1):99–131. https://doi.org/10.1146/annurev.arplant.56.032604.144052
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21(5):493–507. https://doi.org/10.1007/s10811-008-9392-7
Guckert JB, Cooksey KE (1990) Triglyceride accumulation and fatty acid profile changes in Chlorella (Chlorophyta) during high pH-induced cell cycle inhibition. J Phycol 26(1):72–79
Hildebrand M, Davis AK, Smith SR, Traller JC, Abbriano R (2012) The place of diatoms in the biofuels industry. Biofuels 3(2):221–240
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci 103(30):11206–11210. https://doi.org/10.1073/pnas.0604600103
Hise AM, Characklis GW, Kern J, Gerlach R, Viamajala S, Gardner RD, Vadlamani A (2016) Evaluating the relative impacts of operational and financial factors on the competitiveness of an algal biofuel production facility. Bioresour Technol 220:271–281
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
Hunt RW, Chinnasamy S, Bhatnagar A, Das K (2010) Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl Biochem Biotechnol 162(8):2400–2414
Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2(3):191–200
Kaplan A, Reinhold L (1999) CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Biol 50(1):539–570
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2008) Brock biology of microorganisms, 12th edn. Int Microbiol 11
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31(8):1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Melack JM, Kilham P (1974) Photosynthetic rates of phytoplankton in East African alkaline, saline lakes. Limnol Oceanogr 19(5):743–755
Moll KM, Gardner RD, Eustance EO, Gerlach R, Peyton BM (2014) Combining multiple nutrient stresses and bicarbonate addition to promote lipid accumulation in the diatom RGd-1. Algal Res 5:7–15. https://doi.org/10.1016/j.algal.2014.04.002
Moroney JV, Somanchi A (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119(1):9–16
Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6(8):1251–1259. https://doi.org/10.1128/EC.00064-07
Pick U, Karni L, Avron M (1986) Determination of ion content and ion fluxes in the halotolerant alga Dunaliella salina. Plant Physiol 81(1):92–96
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL, Posewitz MC (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun 3:686. https://doi.org/10.1038/ncomms1688
Reinfelder JR, Milligan AJ, Morel FM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135(4):2106–2111
Remias D, Lütz-Meindl U, Lütz C (2005) Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. Eur J Phycol 40(3):259–268
Richards AM (2007) Identification and structural characterization of siderophores produced by halophilic and alkaliphilic bacteria. Washington State University, Washington, DC
Roberts K, Granum E, Leegood RC, Raven JA (2007) Carbon acquisition by diatoms. Photosynth Res 93(1–3):79–88
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res 1(1):20–43. https://doi.org/10.1007/s12155-008-9008-8
Seckbach J (2007) Algae and cyanobacteria in extreme environments, vol 11. Springer, Heidelberg
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5(5):1532–1553
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the US Department of Energy’s aquatic species program: biodiesel from algae. National Renewable Energy Laboratory 328
Skorupa DJ, Castenholz RW, Mazurie A, Carey C, Rosenzweig F, McDermott TR (2014) In situ gene expression profiling of the thermoacidophilic alga Cyanidioschyzon in relation to visible and ultraviolet irradiance. Environ Microbiol 16(6):1627–1641
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1(1):47–58
Stumm W, Morgan JJ (2012) Aquatic chemistry: chemical equilibria and rates in natural waters, vol 126. Wiley, New York
Terry KL, Raymond LP (1985) System design for the autotrophic production of microalgae. Enzyme Microb Technol 7(10):474–487
USEPA (2016) Inventory of U.S. greenhouse gas emissions and sinks: 1990–2014. USEPA
Valenzuela J, Mazurie A, Carlson RP, Gerlach R, Cooksey KE, Peyton BM, Fields MW (2012) Potential role of multiple carbon fixation pathways during lipid accumulation in Phaeodactylum tricornutum. Biotechnol biofuels 5(1):1
Valenzuela J, Carlson R, Gerlach R, Cooksey K, Peyton BM, Bothner B, Fields MW (2013) Nutrient resupplementation arrests bio-oil accumulation in Phaeodactylum tricornutum. Appl Microbiol Biotechnol 97(15):7049–7059
Williams PJB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3(5):554. https://doi.org/10.1039/b924978h
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Moll, K.M., Pedersen, T.C., Gardner, R.D., Peyton, B.M. (2018). Biodiesel (Microalgae). In: Sani, R., Krishnaraj Rathinam, N. (eds) Extremophilic Microbial Processing of Lignocellulosic Feedstocks to Biofuels, Value-Added Products, and Usable Power. Springer, Cham. https://doi.org/10.1007/978-3-319-74459-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-74459-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74457-5
Online ISBN: 978-3-319-74459-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)