Abstract
American tegumentary leishmaniasis is an endemic anthropozoonosis undergoing expansion on the American continent. The disease is caused by several Leishmania species and thus there are intraspecific parasitological dissimilarities that may generate different pathologies. Furthermore, in America Leishmania spp. has diverse reservoirs (that may change continuously) and can use various vectors to infect humans and mammals. Antimonials are the drugs of choice for the treatment of American tegumentary leishmaniasis; however, their efficacy is not predictable, and this may be linked to parasite drug resistance. This is further complicated by the fact that the etiological parasitic species in America belong to both the Leishmania and the Viannia subgeni. For all these reasons, the identification of the etiological infectious agent—up to the species level—is fundamental for precise clinical diagnosis, treatment, and prognosis and for control of the disease. The present chapter offers a description of American tegumentary leishmaniasis, a fundamental piece of knowledge for the comprehension of the challenges we face for leishmaniasis in times of drug resistance. As a way to better understand the unique scenario that America offers for leishmaniasis, some data related to the figures present in the Old World will be presented.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- American tegumentary leishmaniasis
- Drug resistance
- Endemic anthropozoonosis
- Species identification
- Clinical diagnosis
- Disease control
- Dermatological syndrome
1 Introduction
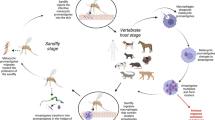
Leishmaniasis is an infectious disease caused by flagellate protozoa of the genus Leishmania (L.). The disease is transmitted to humans through the bite of an insect vector, the sand fly. Depending on the vertebrate reservoir found in a specific geographic zone, the disease is classified as zoonotic or anthroponotic. Leishmaniasis is characterized by a spectrum of clinical, histopathological, and immunological features linked to the pathogenicity of the infecting parasite as well as to the immunological response of the host. As stated in various chapters of the present volume, clinical manifestations of leishmaniasis include lesions in the skin and/or the mucous membranes or invasion of visceral organs [1].
Leishmania infections range in severity from asymptomatic lesions to disfiguring tegumentary leishmaniasis and fatal visceral leishmaniasis. Dermotrophic strains cause American tegumentary leishmaniasis (ATL) characterized by a spectrum of clinical manifestations including localized cutaneous (LCL), diffuse cutaneous (DCL), disseminated (DL), and mucocutaneous (MCL) leishmaniasis [1, 2].
The main species (in 86–98% of cases) causing the limited clinical manifestations (characteristic ulcerative lesion) of leishmaniasis in the Old World are L. (L.) major, L. (L.) tropica, L. (L.) aethiopica, and some zymodemes of L. (L.) infantum. The lesions usually heal spontaneously in periods from 3 months to 2 years or may evolve to a relapsing lesion known as leishmaniasis recidiva cutis (L. (L.) tropica) or to DCL (L. (L.) aethiopica) [3]. L. (L.) donovani visceral infections may develop into post-Kala-azar dermic leishmaniasis (PKDL) [4]. A detailed description of the clinical manifestations of Old World cutaneous leishmaniasis is found in a recently published comprehensive review [3].
Most of the species that cause tegumentary leishmaniasis occur in the New World. They are numerous and belong to both the Leishmania (L.) and Viannia (V.) subgeni, being L. (V.) braziliensis the most prevalent species, followed by L. (L.) amazonensis and L. (L.) mexicana, L. (V.) guyanensis, and L. (V.) panamensis. Other types of Leishmania that may also produce the disease in America are L. (L.) pifanoi, L. (L.) shawi, L. (L.) venezuelensis, L. lainsoni, and L. (V.) peruviana [2]. This variety in the species responsible for New World ATL determines the diverse clinical manifestations of the disease, including the aggressive and destructive MCL [1]. The comprehensive review written by Goto and Lindoso [1] offers a detailed description of the clinical manifestations of New World cutaneous leishmaniasis.
2 Epidemiology, Classification of Leishmania, Vectors
Tegumentary leishmaniasis is endemic in 82 countries all over the world, with approximately 1.5 million cases per year. Africa hosts most of the reported cases, then comes cases found in the Middle East, and finally those found in Latin America, being Chile the only country, which has not reported cases [1, 5]. Around 70–75% of global incidence occur only in ten countries: Afghanistan, Algeria, Brazil, Colombia, Costa Rica, Ethiopia, the Islamic Republic of Iran, Peru, Sudan, and the Syrian Arab Republic. [6, 7]. Approximately 35,000 cases of mucosal leishmaniasis occur annually, mainly in Brazil, Peru, and Bolivia [8], and for CL high-burden countries, the population at risk of CL varies between 14% and 100%, and all together 399 million people are at risk of CL [7].
The disease is a dermatological syndrome. It is diagnosed in 3.3% of the skin-related infections present in tourists that visit Latin America [9]. Cases predominate among agricultural workers, followed by students and finally housewives and children; its incidence in males is higher, possibly due to their greater risk of vector exposure caused by their type of (outdoors) work.
Risk factors to emergence and spread of tegumentary leishmaniasis include environmental factors (temperature and water storage, irrigation habits, deforestation, climate changes), immunosuppression (HIV or organ transplant), the use of immunosuppression therapy, and appearance of drug resistance. There is also an increased incidence in leishmaniasis in traveling people. Finally, war, people displacement by geopolitical problems, poor socioeconomic status, and low-level household also contribute to spread the disease [10].
The Leishmania species as well as the immune status of the host determine the clinical features of ATL. The initial lesion appears at the site where the insect bites. The incubation period lasts 2 weeks to 3 months. The initial lesion is a small, itchy, erythematous papule or nodule that eventually results in the enlargement of the draining lymph node. This initial wound may heal spontaneously; alternatively, it may evolve after several weeks, to patent disease with different clinical features [11].
Due to the diversity of the species that may co-exist in some geographical areas, correlation between clinical features of the disease and the infecting species of Leishmania is not straightforward; this is further complicated by the fact that the laboratory procedures needed for species identification are complex and sophisticated. These facts, as well as the range of drug sensitivities expressed by New World Leishmania, constitute a challenge for the prognosis of ATL [1].
As clearly described in the introduction of this volume, the Leishmania parasite alternates between two extreme environments to which the parasite must adapt, i.e., the mammalian host (amastigotes, without flagellum) and the insect vector (promastigote, flagellar form). Successful transmission occurs when the parasitized vector sucks blood from a vertebrate and inoculates promastigotes present in the proboscis. As the parasite enters the vertebrate circulation, the parasite is phagocytosed by macrophages. Although phlebotomine sand flies (Phlebotomus and Lutzomyia) transmit the disease, only anthropophilic Lutzomyia (~30 species), distributed all over America, can potentially function as vectors for Leishmania [5].
Regarding reservoirs, it is fundamental to differentiate zoonotic leishmaniasis, in which the reservoirs are wild or domestic animals, and anthroponotic leishmaniasis, when humans constitute the main host. This latter form of transmission is typical but not exclusive for the VL produced by L. (L.) donovani, and the LCL caused by L. (L.) tropica, in the Old World but is not common in America [5].
In America, many vertebrates have been identified as reservoirs: the sloth (Choloepus (C.) didactylus) for L (V.). guyanensis and C. Hoffmani for L. (V.) panamensis, the opossum (Didelphis marsupialis) for L. (V.) guyanensis, the rice rats (Oryzomis capito), and the agouti (Dasyprocta Nectomys) for L. (L.) amazonensis. Also, several rodents’ species function as reservoirs for L. (L.) mexicana and the rat (Rattus rattus) for L. (V.) guyanensis [12,13,14,15,16].
3 Clinical Spectrum, Immune and Pathologic Consequences of ATL
ATL may occur in three general forms with a range of clinical, histological, and immunological features that differ among them. LCL is located at one end of the spectrum and occurs in immune-competent patients. It is characterized by one or a few usually ulcerated lesions. The anergic DCL is located at the other end of the spectrum and is characterized by the clinical expression of numerous nodules, non-ulcerated papules, and plaques. Mucocutaneous lesions are located in the intermediate area of the spectrum, with extensive lesions prone to relapse [1, 17].
3.1 Localized Cutaneous Leishmaniasis (LCL)
LCL (Fig. 8.1a) is the most prevalent form of the disease and is caused by dermotropic Leishmania species [18]. Both Vianna and Leishmania subgeni produce it. The lesions, varying in number from one to ten, appear in an exposed area of the body surface. The established lesion is a well-delimited round, painless ulcer, with raised edges and a central crust, sometimes hemorrhagic. It starts as an erythematous papule after the bite of the vector. It grows and, in a few weeks, develops into an ulcer with little secretion but purulent if a secondary infection builds up. It may occur also as papules that surround the primary ulcer and may be accompanied by inflamed lymphatic tracts and nodes. The ulcers may heal spontaneously, leaving a hypopigmented, smooth, thin scar. The host–parasite balance, as well as other undefined factors, determines the evolution to other forms of the disease [3, 19, 20].
The ulcer differentiates to a typical epithelioid granuloma with a mixed pattern of Th1 and Th0 cytokines and a predominance of a Th1 response. Nodules and plaques on the skin may be flat; in the ulcers, the skin is abruptly lost producing epidermal hyperplasia. A macrophage infiltrate with epithelioid differentiation occupies the dermis, and a variable number of lymphoid cells and plasma cells (including a moderate number of Langerhans type giant cells) surround and/or invade the macrophage infiltration. The patients are normally immune-competent and develop a positive Montenegro test [1, 21]. For differential diagnosis, the following diseases should be considered: piodermitis, sporotrichosis, chromomycosis, skin cancer, cutaneous tuberculosis, and varicose ulcers and traumatic ulcers.
3.2 Leishmaniasis Recidiva Cutis (LRC)
LRC (Fig. 8.1b) is rare in the New World and in the Old World is associated with infections produced by L. (L.) tropica. Characteristic papular and vesicular lesions appear, in or around the healed scar. Most of the identified parasites that produce this form of the disease in the New World belong to the subgeni Viannia [22], but L. (L.) amazonensis in Brazil [23] and L. (V.) panamensis in Ecuador [24] can produce it [1].
3.3 Diffuse Cutaneous Leishmaniasis (DCL)
DCL is a true anergic form of tegumentary leishmaniasis characterized by the presence of nodular lesions that do not ulcerate (Fig. 8.1c) [25, 26]. This uncommon (described in Brazil, Mexico, Venezuela, the Dominican Republic, and Colombia) presentation of leishmaniasis is characterized by a lack of a cell-mediated immune response, although it may produce protective antibodies. It is caused by parasites of the subgeni Leishmania, i.e., L. (L.) mexicana, and L. (L.) amazonensis in the New World and by L. (L.) aethiopica in the Old World.
DCL seems to eclose mainly in childhood, beginning the early manifestations before the age of 15. It is believed that this predisposition is related to genetic and metabolic individual factors [27]. Some authors refer that an initial LCL lesion may be the origin of the spread of parasites by lymphatic and hematic means and that the subsequent inhibition of specific cellular immunity may lead to DCL appearance [28].
In early stages, the disease is characterized by the presence of papules, plaques, or erythematous nodules generally in localized skin areas. These lesions (full of parasites probably due to the Th2 immune response) may be asymmetrical, affecting a single extremity, or may be symmetrical but limited only to the upper or lower limbs [19, 25]. The lesions ulcerate if they suffer trauma, and invasion of the nasal mucosa occurs once the clinical disease becomes severe. This form of the disease is not accompanied by a strong inflammatory reaction. In DCL the initial sores relapse with the formation of nodules on the edge of the scar that remains with little changes over months or years and abruptly spread through the body surface.
Histological sections demonstrate atrophy of the epidermis, with dermo-epidermal boundary rectification. A dense macrophage infiltration invades the dermis, accompanied by a moderate amount of vacuolated lymphoid and plasma cells. The inflammation reaches the subcutaneous tissue, and vacuolated macrophages contain a large number of parasites [1].
Cytokines and accessory signals on the skin decline; this situation compromises the function of antigen-presenting cells and induces a parasite-specific anergy. The granuloma is characterized by a predominantly Th2 response, with a high percentage of naive T cells that react against the parasite. The Montenegro test is negative [21]. In rare occasions, the initial diagnosis is positive but then becomes negative [19, 29, 30]. The titers of anti-Leishmania antibodies are high but decrease after treatment, a response that does not reveal a protective activity [31]. For differential diagnosis, the following diseases should be considered: lepromatous leprosy, cutaneous neurofibromatosis, lymphomas, and xanthomatosis.
3.4 Disseminated Leishmaniasis (DL)
DL (Fig. 8.1d) is characterized by the presence of multiple (10–300) pleomorphic small lesions, mainly acneiform and papular, in two noncontiguous areas of the body [20]. In 29% of cases, at least a mucocutaneous lesion is found. The clinical outcome includes a verrucous plaque, sarcoid, chronic ulcers with poor response to treatment and relapse with extensive lesions with a variable immunological response. It is produced by parasites of the subgeni Leishmania and Viannia. However, there are areas in northeast Brazil where L. (V.) braziliensis has been the only species found in infected patients [32].
As for DCL, some authors refer that an initial LCL lesion may be the origin of the spread of parasites by lymphatic and hematic means and that the subsequent inhibition of specific cellular immunity may lead to DL appearance [26, 28]. The lesions develop transformations similar to those found in LCL, and the epithelioid differentiation of the epidermis concurs with epithelial proliferation, hyperkeratosis, parakeratosis, and scale-crusts. The parasites appear in varying numbers and must be sought within macrophages. Also, similar to LCL and in contrast to DCL, the infection is not age related, and it is mainly a result of the exposure of the host to the infected vector and to the immune response of the patients [28, 31].
DL pathogenesis is not still fully dilucidated; however, the absence of a cell-mediated immune response, with decreased CD4+ T cell titers in peripheral blood, and a poor response by these cells to the Leishmania antigen seem to be a common feature. In DL patients, epidermal Langerhans cells are not frequent, and the granuloma has a mixed pattern of Th1 and Th2 cytokines. The Montenegro test has been reported to be negative depending on the geographical area where the patient lives; thus it has been claimed to be negative in Brazil and positive in Venezuela [21]. For the differential diagnosis, the following diseases should be considered: skin tuberculosis, chromomycosis, sporotrichosis, sarcoidosis, and leprosy.
3.5 Mucocutaneous Leishmaniasis (MCL)
One of the most severe forms of damage that occur in leishmaniasis involves the upper respiratory tract mucosa. It includes metastases by way of blood vessels or lymphatic system or by expansion of a face LCL [1, 31]. MCL appears years after the onset of cutaneous leishmaniasis and is characterized by the destruction of the walls of oral–nasal and pharyngeal cavities, potentially evolving to disfiguring lesions. The initial symptoms are mild and include nasal inflammation and stuffiness; ulceration and perforation of the nose septum could slowly ensue. The lesion may extend to the face, the soft palate, the pharynx, or the larynx. A cutaneous lesion can accompany the mucocutaneous lesion. L. (V.) braziliensis is the etiological agent in most cases, but species like L. (V.) panamensis, L. (V.) guyanensis, L. (L.) amazonensis, and L. (L.) major may also cause MCL [20].
The epidemiological data demonstrate that 5–7% of patients with LCL develop MCL [31, 33]. However, the frequency of MCL varies according to geographical location: In Brazil, it varies from 0.4% in the south [34, 35] to 1.4% in the central region [20] and to 2.7% in the northeast [36]. In the Andean countries, MCL may represent 7.1% of the registered cases of leishmaniasis [37]; Bolivia exhibits a high frequency of 20%, Ecuador a medium frequency of 7.7% [38], Colombia a low frequency of 2.3%, and Venezuela a very low frequency of 0.4% [37]. Most patients are over 40 years of age, although this form of the disease may also affect children [1].
The clinical manifestations begin with nasal obstruction, rhinorrhea, mucocutaneous bleeding, and shedding of serous crusts, impaired olfaction, and cacosmia. Physical examination at the beginning of the disease demonstrates erythema and infiltration in the nasal mucosa, mainly in the septum and inferior turbinate. If the disease develops without diagnosis and treatment, it progresses to an ulcer with serous crusts, surrounded by diffuse infiltrations of the mucosa (because of a poor definition of the granuloma); it may compromise the cartilaginous septum and produce drilling and deformation and even the total destruction of the septum giving the appearance of “tapir nose.” The discharge of the nose can occasionally be purulent, due to bacterial infections and polypoid degeneration of the nasal mucosa.
These features are accompanied with significant shrinkage of the nasal wing and collapse of the corresponding nostril. Sometimes the acute inflammatory processes that occur around the nasal vestibule produce severe pain that could compromise the maxillary region of the affected side [17, 33, 39, 40]. At advanced stages of the disease, a destruction of the midface may occur.
In some cases, invasion of the nose and palate occurs; the patients report a feeling of “fullness” in the mouth, toothache, teeth loss, and spontaneous bleeding of the gums. These lesions grow profusely and may compromise the upper lip; they may also produce indurations, infiltration, and ulceration of the hard palate, amputation of the uvula, and lesions of the soft palate. Additionally, dysphagia, open rhinolalia, and regurgitation of food, as well as damage of the laryngeal structures such as epiglottis, ventricular bands, and vocal cords, may occur. Finally, the upper airway may also be compromised due to the tension produced by the formation of a granuloma in the mucosa and subsequent fibrosis; some cases may even require tracheotomy. In severe cases, there is deterioration of the patient’s general condition and even death if the compromise of the respiratory tract is serious [1].
Histological sections support a diffuse mixed infiltrate [1]. The macrophage infiltrate differentiates into an epithelioid tissue with low densities of parasites [17, 30]). Langerhans cells (CD1a+) and CD83+ cells cannot be found in the epithelium [30, 41]. This situation might reflect the migration of Langerhans cells to the lymph node, or the action of the parasites on Langerhans cells during the chronic phase of the disease, circumstances that may cause an inadequate and deficient transduction of the signals necessary for an adequate immune response. In the epidermis, there is a strong expression of major histocompatibility complex (MHC)-II and intercellular adhesion molecule 1 (ICAM)-1, which confirms the state of hypersensitivity of this clinical form of leishmaniasis. The MCL granuloma expresses a mixed pattern of cytokine production (Th1/Th2, and a high CD4/CD8 ratio) [42, 43].
The Montenegro test reaction is strongly positive (Restrepo 1980). Leishmania antibody levels are variable and correlate with the extent of the patient’s clinical profile [19, 31]. For differential diagnosis, the following diseases should be considered: in the nasal area, trauma, bacterial infections, syphilis, cocaine use, chromium poisoning, half-facial malignant granuloma, paracoccidioidomycosis, nasal polyps, rhinosporidiosis, leprosy, and squamous and basal cell carcinoma and in the palate and larynx carcinoma, paracoccidioidomycosis, and tuberculosis.
The number of diseases with which MCL should be differentially diagnosed is high; therefore, it is fundamental to carry out further examinations. These tests must include fungal serology, intradermal tests, mycological studies, mycobacteria, chest X-ray, nose and paranasal sinuses tomography, and histopathological analysis. Additionally, there may be complications such as conjunctival lesions with distortion of the palpebral fissure and, in rare cases, loss of the eyesight. Moreover, healing processes can lead to a decreased size of the mouth and airways that hinder feeding and breathing. Finally, extension of the lesion at the base of the skull with bacterial infection can cause meningitis or osteomyelitis.
3.6 Tegumentary Leishmaniasis in HIV-Infected Patients
HIV/Leishmania co-infection has been reported in 35 countries. In the Old World, there are reports of PKDL in HIV-infected patients [5, 44]. In the New World, the manifestations can be similar to those found in non-immunosuppressed patients with no signs of aggravation, but they can be quite unusual. A full description of this problem is covered in Chaps. 5 and 6, this same volume; therefore the theme will not be discussed in detail herein.
4 Diagnosis and Treatment
Diagnosis of ATL is relatively simple, and in most cases the demonstration of the parasite by direct methods after clinical suspicion is sufficient to establish the treatment. The diagnosis cannot be intuitive but has to be confirmatory of the parasite (etiological agent) or its antigen(s) in the lesion. These forms of diagnosis are called direct, while those immunological tests used if the direct approaches fail are called indirect parameters of diagnosis [45].
The sensitivity of the direct examination tests is low (50–70% in the Old World, 15–30%, in the New World, where chronic cases and MCL are frequent). The detection level is higher, reaching 44–58% by culturing the biopsies and 38–52% by injection into hamsters [1, 46,47,48].
On the other hand, serodiagnosis includes a set of indirect methods seldom used for the diagnosis of LCL in the Old World because the results may be variable, the sensitivity of the tests is low, and there may be cross-reactivity with other infections. Unfortunately, the sensitivities of these methods are not better for New World leishmaniasis. However, still they are in use. The most commonly used assays for ATL serodiagnosis are thus the indirect immunofluorescence assay (IIFA) and the enzyme-linked immunosorbent assay (ELISA) [1, 46,47,48,49]. In ATL, the anti-Leishmania antibody levels do not remain high after treatment; this means that positive results of serologic diagnostic method generally indicate current infection.
Excluding direct microscopic examination of biopsies, the additional diagnostic methods require a complex laboratory structure and technical skills, as well as longer times to obtain the results [1]. Furthermore, the approaches to detect the etiological agent have low sensitivity and do not always identify the Leishmania species. Recent efforts aim to develop assays to detect the parasite DNA in the patients [5].
Among the variety of molecular approaches developed for the diagnosis of leishmaniasis and the identification of the etiological agent, the polymerase chain reaction (PCR) assay is considered one of the best methods. It is based on the complementarity that exists between the two strands of DNA. The method relies on cycles of repeated heating and cooling of DNA melting and its enzymatic replication in the presence of primers, which are short DNA fragments containing sequences complementary to the target region. This cycling enables selective and repeated amplification and eventually the identification of the infecting Leishmania species [1, 50, 51].
Finally, the anti-Leishmania delayed-type hypersensitivity or Montenegro skin test diagnoses Leishmania infection, and therefore is used in epidemiological studies to determine infection prevalence. The test does not distinguish between present and past infections, and thus its importance as a diagnostic tool is questionable for people living in endemic areas. The test is positive in patients with more than 19 months of treatment [48, 52, 53] and in 75% of non-infected individuals, with no disease manifestation in the past, but living in an endemic area [52]. This test may be useful, however, for the diagnosis in travelers that do not normally live in endemic areas.
The treatment of leishmaniasis must include the thorough cleaning of the lesions with topical antiseptics and the treatment of secondary bacterial infections with topical and/or mouth antibiotics. Afterward, the patient should be treated with the adequate chemotherapy to kill the parasite. Alternatively, attempts to develop an immunotherapy against leishmaniasis have been performed in many laboratories and places including Venezuela [54, 55]. The data suggest that immunotherapy might be an excellent therapy for LCL, with few side effects and low-cost administration. However, further studies are needed to confirm the results. Finally the surgical reconstruction of the sequelae in nasal pyramid and portion of the upper lip skin is advisable to do it after confirming that there is no active disease for a period of 1 year or longer.
5 Challenges of ATL in the Era of Drug Resistance
ATL is a serious public health problem in America both in rural and urban areas; its incidence has dramatically increased in the last two decades. ATL affects zones considered endemic for leishmaniasis, but it is also increasing in travelers living in non-endemic parts who have visited endemic areas [1]. Furthermore, co-infection is an additional concern because of its increasing rates, either by HIV, by additional parasites like T. cruzi or helminths, or the special case of co-infection represented by Leishmania RNA viruses, or LRV, which are endosymbionts reported so far essentially in Latin America and frequently associated with treatment failure. These issues are thoroughly described in Chaps. 4 and 6 from the present volume. This means that fighting against leishmaniasis must be among priority programs related to endemic and epidemic diseases that must integrate other pathogens and monitoring conditions and must also incorporate public and private institutions, scientific societies, and affected communities.
Diagnosis seems to be a dilemma due to the variety of Leishmania species that produce ATL. This is especially true for L. (V.) braziliensis in LCL and LMC patients as the parasite is scarce in the tissues. For this reason, main goals to be reached must include the use of homogeneous protocols for Leishmania antigen purification according to validated protocols with quality control analysis; additionally, the cutoff determination of the diagnosis method for leishmanina must be performed in order to homogenize the criteria of positive and negative readings. When talking about direct microscopy and PCR, a lot of discussion still exist. Microscopy on a smear is more frequently used since it means a speedy (<1 h) result. Molecular diagnosis is much more sensitive than microscopy. However, specificity depends on the performance of each laboratory, the selected target, and the selected protocol, many of them in house protocols with an intrinsical variability evidenced when the protocol is transferred from one lab to another, highlighting the lack of consensus that exist.
Tegumentary leishmaniasis therapy in America is mostly restricted to the use of antimonials (SbV) and more recently miltefosine (MIL) for some types of LCL. However, in Latin America, the efficacy of this medicament is rather unpredictable with 7% treatment failure in Bolivia, 16% in Brazil, 23.9% in Peru, and up to 39% in Colombia [1]. Furthermore, the guidelines for regional implementation are unfortunately not homogeneous [1, 56]. This all means that therapeutic failure, defined as the clinical phenotype in which the patient does not improve at the end of a treatment (absence of response), or in which the clinical symptoms reappear after the initial cure (relapse), is a real challenge that should be clearly differentiated from clinical resistance in order to avoid the ambiguity of both meanings.
Drug resistance represents an intrinsic characteristic of parasites with a significantly lower susceptibility to a drug than that of their susceptible counterparts. Drug resistance is an adaptive trait. Exposure to drugs (e.g., due to external factors like suboptimal doses or poor quality of the medicaments that induces the expression and function of ATP-binding cassette (ABC) transporters and proteins) promotes an increase in the frequency of occurrence of this phenotype, and although it is expressed in the patient, the associated phenotype must be confirmed experimentally evaluated in parasites isolated from the lesion [56,57,58].
On the other hand, treatment failure is a multifactorial complex phenomenon. Drug, host, and parasite factors may contribute to it. In the case of American field strains of Leishmania (but not only, as beautifully described in Chaps. 4 and 15 of this volume), special attention should be paid to the variable intrinsic drug sensitivity usually related to species-specific issues as is the case of the Viannia subgenus already described, as well as to epigenetic features that may change different functions in the parasites. This means that the specific contribution of the parasite physiology to treatment failure is difficult to address [59,60,61]. This is especially true since as has been described in various chapters of this volume (Introduction, Chaps. 4 and 15), the in vitro data is normally obtained using the extracellular form of the parasite (the promastigotes) and seldom using its intracellular form (the amastigotes), and results are infrequently compared to the treatment outcome of patients from whom parasites are isolated.
However, it is fundamental to find easy tools to be used in the common clinical laboratory to evaluate if relapses that occur in patients associate with metabolic changes that might be associated to the fitness of infecting isolates. In such isolates (isolated from patient suffering DCL and refractory to SbV), a correlation between glucose uptake and plasma membrane potential has been evaluated. The results were compared with those obtained from reference strains and demonstrated that Leishmania parasites (L. (L.) amazonensis and L. (L.) mexicana) causing DCL incorporate glucose at an efficient rate, albeit without significant changes in the plasma membrane potential as their corresponding reference strains. One isolate did not change its accumulation rate of glucose compared to its reference strain and expressed a less polarized membrane potential insensitive to mitochondrial inhibitors, thus suggesting a metabolic dysfunction in this isolate. Further validation of the concepts herein established and whether or not the third isolate corresponds with a drug-resistant phenotype needs to be demonstrated at the genetic level [62, 63].
In the case of ATL, especially in Latin America, this is further complicated due to the many infecting species of Leishmania, including parasites of subgeni Leishmania and Viannia. In fact, isolates of L. (V.) braziliensis with lower susceptibility to SbV have been reported even before the start of treatment, although they have probably never been in contact with the drug (s) [64]. It is not clear if this difference is due to an intrinsic unresponsiveness to the drugs, expressed by members of the Viannia subgenus, but certainly constitutes an issue that should recall our attention and emphasize that the contribution of the parasite to therapeutic failure could not only correspond to the expression of drug resistance. That is, the existence of additional phenotypes could be determinant for the phenomenon of therapeutic failure. Unfortunately, and again returning to the experimental determination of this phenomena, these phenotypes are not necessarily easy to identify in the available systems and therefore and is fundamental to describe specific cellular markers easy to evaluate in the clinical laboratory, a situation that challenges the classical view of how the factors responsible for that therapeutic failure are evaluated [59,60,61, 63, 64].
In summary, Old World leishmaniasis has a better therapeutic outcome, except when caused by L. (L.) aethiopica, than New World leishmaniasis where therapeutic responses are mixed. This all means that treatment guidelines and protocols have to be reevaluated on a global basis considering the huge differences between Old and New World leishmaniasis [1], that the concept of monotherapy with regard to resistance has to be reevaluated, and that diagnosis and satisfactory treatment are imperative challenges for the adequate outcome in ATL, especially in an era of drug resistance.
References
Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous Leishmaniasis. Exp Rev Anti Infect Ther. 2010;8:419–33.
Souza AS, Giudice A, Pereira JM, Guimaraes LH, et al. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-alpha production. BMC Infect Dis. 2010;10:209.
Akilov OE, Khachemoune A, Hasan T. Clinical manifestations and classification of Old World cutaneous leishmaniasis. Int J Dermatol. 2007;46:132–42.
Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, et al. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3:87–98.
World Health Organization. Control of the leishmaniasis. Technical report series 949. 2010.
Alvar J, Velez ID, Bern C, Herrero M, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671.
World Health Organization. Wkly Epidemiol Rec. 2016;91:285–96.
Savoia D. Recent updates and perspectives on Leishmaniasis. J Infect Dev Ctries. 2015;9(6):588–96.
Gautret P, Schlagenhauf P, Gaudart J, Castelli F, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe and for the GeoSentinel Surveillance Network. Emerg Infect Dis. 2009;15:1783–90.
Oryan A, Akbari M. Worldwide risk factors in leishmaniasis. Asian Pac J Trop Med. 2016;9(10):925–32.
Machado P, Araújo C, Da Silva AT, Almeida RP, et al. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clin Infect Dis. 2002;34:E69–73.
Feliciangeli MD. Vectors of leishmaniasis in Venezuela. Parassitologia. 1991;33:229–36.
Feliciangeli MD, Rodriguez N, Bravo A, Arias F, et al. Vectors of cutaneous leishmaniasis in north-central Venezuela. Med Vet Entomol. 1994;8:317–24.
Feliciangeli MD, Rabinovich J. Abundance of Lutzomyia ovallensi but not Lu. Gomezi (Diptera:Psychodidae) correlated with cutaneous leishmaniasis incidence in north-central Venezuela. Med Vet Entomol. 1998;12:121–31.
De Lima H, Rodriguez N, De Guglielmo Z, Rodriguez A, et al. Cotton rats and black rats as possible reservoirs of cutaneous leishmaniasis in an endemic area in Lara State, Venezuela. Mem Inst Oswaldo Cruz. 2002;97:169–74.
Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35(11–12):1169–80.
Ridley DS, Marsden PD, Cuba CC, Barreto AC. A histological classification of mucocutaneous leishmaniasis in Brazil and its clinical evaluation. Trans R Soc Trop Med Hyg. 1980;74:508.
Scarisbrick JJ, Chiodini PL, Watson J, Moody A, et al. Clinical features and diagnosis of 42 travellers with cutaneous leishmaniasis. Travel Med Infect Dis. 2006;4:14–21.
Convit J, Ulrich M, Fernández CT, Tapia FJ, et al. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1993;87:444–8.
Reithinger R, Dujardin JC, Louzir H, Pirmez C, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96.
Restrepo Isaza M. La reacción de Montenegro en la epidemiología de la leishmaniasis sudamericana. Bol Of Sanit Panam. 1980;89:130.
Oliveira-Neto MP, Mattos M, Souza CS, Fernandes O, et al. Leishmaniasis recidiva cutis in New World cutaneous leishmaniasis. Internat J Dermatol. 1998;37:846–9.
Bittencourt AL, Costa JM, Carvalho EM, Barral A. Leishmaniasis recidiva cutis in American cutaneous leishmaniasis. Internat J Dermatol. 1993;32:802–5.
Calvopina M, Uezato H, Gomez EA, Korenaga M, et al. Leishmaniasis recidiva cutis due to Leishmania (Viannia) panamensis in subtropical Ecuador: isoenzymatic characterization. Internat J Dermatol. 2006;45:116–20.
Barral A, Costa JM, Bittencourt AL, Barral-Netto M, et al. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Internat J Dermatol. 1995;34:474–9.
Ortega Moreno ME, Lugo DA, Belizario Ochoa DC, Galindo Martinez WA, et al. Comparación clínica de la Leishmaniasis Cutánea Difusa y Leishmaniasis Diseminada en Venezuela. Dermatol Venez. 2013;51:29–35.
Zerpa O, Convit J. Leishmaniasis Cutánea Difusa en Venezuela. Gaz Méd Bahía. 2009;79(Supl. 3):30–4.
Jiménez A, Vásquez DA, Albarracín N, Vélez ID. Leishmaniasis Diseminada en Colombia: Reporte de un caso. Dermatol Venez. 2012;50(2):46–9.
Tapia FJ, Cáceres-Dittmar G, Sánchez MA, Fernández CT, et al. Adhesion molecules in lesions of American cutaneous leishmaniasis. Exp Dermatol. 1994;3:17–22.
Castés M1, Tapia FJ. Immunopathology of American tegumentary leishmaniasis. Acta Cient Venez. 1998;49(1):42–56.
Ulrich M, Rodriguez V, Centeno M, Convit J. Differing antibody IgG isotypes in the polar forms of leprosy and cutaneous leishmaniasis characterized by antigen specific T cell anergy. Clin Exp Immunol. 1995;100:54–8.
Turetz ML, Machado PR, Ko AI, Alves F, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186:1829–34.
el-Hassan AM, Meredith SEO, Yagi HI, Khalil EAG, et al. Sudanese mucocutaneous leishmaniasis: epidemiology, clinical features, diagnosis, immune responses and treatment. Trans R Soc Trop Med Hyg. 1995;89:647–52.
Grimaldi G Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. American J Trop Med Hyg. 1989;41:687–725.
de Castro EA, Luz E, Telles FQ, Pandey A, et al. Eco-epidemiological survey of Leishmania (Viannia) braziliensis American cutaneous and mucocutaneous leishmaniasis in Ribeira Valley River, Parana State, Brazil. Acta Trop. 2005;93:141–9.
Jones TC, Johnson WD Jr, Barretto AC, Lago E, et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987;156:73–83.
Davies CR, Reithinger R, Campbell-Lendrum D, Feliciangeli D, et al. The epidemiology and control of leishmaniasis in Andean countries. Cad Saude Publica. 2000;16:925–50.
Garcia AL, Parrado R, Rojas E, Delgado R, et al. Leishmaniases in Bolivia: comprehensive review and current status. Am J Trop Med Hyg. 2009;80:704–11.
Marsden PD. Mucocutaneous leishmaniasis (“Espundia” Escomel, 1911). Trans R Soc Trop Med Hyg. 1986;80:859–76.
Marsden PD, Nonata RH. Mucocutaneous leishmaniasis-a review of clinical aspects. Rev Soc Bras Med Trop. 1975;IX:325–6.
Sanchez MA, Caceres-Dittmar G, Oriol O, Mosca W, et al. Epidermal Langerhans cells and dendritic epidermal T cells in murine cutaneous leishmaniasis. Immunocytochemical study. Acta Microsc. 1993;2:180–7.
Moll H. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Inst Mitt. 1997;99:73–8.
Moll H. The role of dendritic cells at the early stages of Leishmania infection. Adv Exp Med Biol. 2000;479:163–73.
Puig L, Pradinaud R. Leishmania and HIV co-infection: dermatological manifestations. Ann Trop Med Parasitol. 2003;97:107–14.
Stuart K, Brun R, Croft S, Fairlamb A, et al. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–10.
Cuba CA, Marsden P, Barreto AC, Rocha R, et al. Diagnóstico parasitológico e inmunológico de leishmaniasis tegumentaria Americana. Rev Med Exp. 1980;17:1–4.
Weigle KA, De Davalos M, Heredia P, Molineros R, et al. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–96.
Reed SG. Diagnosis of leishmaniasis. Clin Dermatol. 1996;14:471–8.
Bray RS, Lainson R. The immunology and serology of leishmaniasis: the fluorescent antibody staining technique. Trans R Soc Trop Med Hyg. 1965;59:535–44.
Osman OF, Oskam L, Zijlstra EE, Kroon NC, et al. Evaluation of PCR for diagnosis of visceral leishmaniasis. J Clin Microbiol. 1997;35(10):2454–7.
Rodríguez N, Guzman B, Rodas A, Takiff H, et al. Diagnosis of cutaneous leishmaniasis and species discrimination of parasites by PCR and hybridization. J Clin Microbiol. 1994;32(9):2246–52.
Sassi A, Louzir H, Ben Salah A, Mokni M, et al. Leishmanin skin test lymphoproliferative responses and cytokine production after symptomatic or asymptomatic Leishmania major infection in Tunisia. Clin Exp Immunol. 1999;116:127–32.
Shaw JJ, Lainson R. Leishmaniasis in Brazil: X. Some observations of intradermal reactions to different trypanosomatid antigens of patients suffering from cutaneous and mucocutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1975;69:323–35.
Convit J, Castellanos PL, Ulrich M, Castes M, et al. Immunotherapy of localized, intermediate, and diffuse forms of American cutaneous leishmaniasis. J Infect Dis. 1989;160:104–15.
Convit J. Leishmaniasis immunological and clinical aspects and vaccines in Venezuela. Clin Dermatol. 1996;14:479–87.
Yardley V, Ortuno N, Llanos-Cuentas A, Chappuis F, et al. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J Infect Dis. 2006;194:1168–75.
Yardley V, Croft SL, De Doncker S, Dujardin JC, et al. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am J Trop Med Hyg. 2005;73:272–5.
Rijal S, Yardley V, Chappuis F, Decuypere S, et al. Antimonial treatment of visceral leishmaniasis: are current in vitro susceptibility assays adequate for prognosis of in vivo therapy outcome? Microbes Infect. 2007;9:529–35.
Ponte-Sucre A. Physiological consequences of drug resistance in Leishmania and their relevance for chemotherapy. Kinetoplastid Biol Dis. 2003;2:14.
Natera S, Machuca C, Padrón-Nieves M, Romero A, et al. Leishmania sp.: proficiency of drug resistant parasites. Int J Antimicrob Agents. 2007;29:637–42.
Padrón-Nieves M, Díaz E, Romero A, Machuca C, et al. Valor pronóstico de los cambios fisiológicos asociados a la quimio-resistencia en Leishmania. VITAE Academia Biomédica Digital (33). 2007.
Padrón-Nieves M, Machuca C, Díaz E, Cotrim P, et al. Correlation between glucose uptake and membrane potential in Leishmania parasites isolated from DCL patients with therapeutic failure: a proof of concept. Parasitol Res. 2014;113(6):2121–8.
Padrón-Nieves M, Ponte-Sucre A. Marcadores de resistencia en Leishmania: susceptibilidad in vitro a drogas leishmanicidas vs. retención de calceina en aislados de pacientes venezolanos con leishmaniasis cutanea difusa. Arch Venez Farmacol y Ter. 2015;32:29–33.
Vanaerschot M, Dumetz F, Roy S, Ponte-Sucre A, et al. Treatment failure in leishmaniasis: drug-resistance or another (epi) phenotype? Expert Rev Anti Infect Ther. 2014;12:937–46.
Acknowledgments
The authors are grateful for the financing support received from the Coordination for Research, Faculty of Medicine, UCV and the Council for Scientific and Humanistic Research (CDCH), Universidad Central de Venezuela. Likewise, they are grateful for the support conferred by the Alexander von Humboldt Foundation and the University of Würzburg through the Siebold-Collegium Institute for Advanced Studies, Germany, to Alicia Ponte-Sucre.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Zerpa, O., Padrón-Nieves, M., Ponte-Sucre, A. (2018). American Tegumentary Leishmaniasis. In: Ponte-Sucre, A., Padrón-Nieves, M. (eds) Drug Resistance in Leishmania Parasites. Springer, Cham. https://doi.org/10.1007/978-3-319-74186-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-74186-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74185-7
Online ISBN: 978-3-319-74186-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)