Abstract
Plants confront fluctuating and in some cases intense environmental conditions, such as changes in irradiation, water availability, extreme temperatures, mineral nutrient accessibility, and air pollutants exposition among others. In order to face abiotic stress situations, the redox buffer capacity, mainly represented by ascorbic acid (AsA) and glutathione (GSH) pools, is involved in growth–stress responses crossroad. These compounds are associated in a set of reactions known as AsA-GSH cycle. The main function of the AsA-GSH cycle originally observed was the detoxification of reactive oxygen species (ROS) in different subcellular compartments such as chloroplast, mitochondria, or cytosol. More recently, the crucial participation of the AsA-GSH cycle in the optimization of photosynthesis was established. In addition, these antioxidants are considered essential components of cell signaling pathways triggering adaptive plant responses. The role of AsA-GSH cycle is analyzed regarding the ability of plants to overcome some selected abiotic stress situations.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Life is supported through a continuous movement of electrons in mitochondria and chloroplasts allowing energy production. These electron flows affect the equilibrium of interconvertible redox couples such as NAD+/NADH and NADP+/NADPH and in turn impacts cellular redox status (Foyer and Noctor 2009). The balance between oxidized and reduced forms of glutathione (GSSG/2GSH couple) in the cell is considered to be the major thiol-disulfide cellular redox buffer and could be used as an indicator for the redox environment of the cell. Taking into consideration its redox potential and concentration, ascorbic acid (AsA) acts as the final antioxidant and its recovery depends on GSH, thus both species are interconnected (Foyer and Noctor 2011). The subcellular localization, transport, and participation of both redox couples in physiological processes, and their contribution to adaptive responses of plants under abiotic stress situations will be discussed in this chapter.
2 AsA and GSH Functions
2.1 As Antioxidants
The formation of reactive oxygen species (ROS) is continuous and constitutes an integrated part of the metabolism (Table 1). Although ROS are often associated with stressful situations that can lead to cell death, they also have an important role transmitting information allowing appropriate cellular responses to developmental and environmental changes (Foyer and Noctor 2005). Plant cells are endowed with antioxidant systems to prevent the extension of the oxidative reactions by ROS and other oxidative species formed mainly across mitochondrial and chloroplastic electron transport. These systems include enzymatic and non-enzymatic detoxifying mechanisms. GSH (l-γ-glutamyl-l-cysteinyl-glycine ) and AsA are the most abundant non-enzymatic antioxidants; they can reach intracellular concentrations from 5 to 20 mM, respectively (Asada and Takahashi 1987). Even though both species have been historically known as antioxidants, their functions in living organisms greatly exceed this role. GSH is the most abundant low molecular thiol in plants, discovered in 1926 (Hunter and Eagles 1926), and later associated with powerful antioxidant functions against lipid peroxides and ROS in general. AsA, in turn, was discovered by Szent-Gyorgyi by the same time (Buettner and Schafer 2006) and later identified as vitamin C. It is considered the terminal small molecule antioxidant in biological systems (Sharma and Buettner 1993), acting as a natural reductant of free radical species.
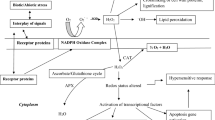
In addition, AsA and GSH are closely related by the so-called AsA-GSH cycle (Fig. 1). When H2O2 is generated by the metabolic pathways mainly in chloroplasts, peroxisomes, and mitochondria, AsA is oxidized to the radical monodehydroascorbate (MDHA ) by the reaction catalyzed by ascorbate peroxidase (APX, EC 1.11.1.7) in order to transform this reactive species into water. The radical MDHA is less reactive than other free radicals and disproportionates spontaneously to dehydroascorbate (DHA ) and AsA. Then, AsA is regenerated from DHA by the action of the enzyme DHA reductase (DHAR, EC 1.8.5.1) that requires GSH. The GSSG thus formed is then reconverted to GSH by the action of the enzyme glutathione reductase (GR, EC 1.6.4.2) that uses NADPH as cofactor (Fig. 1). GR is in charge to maintain high GSH/GSSG ratio in close relationship with NADPH/NADP+ ratio (Diaz-Vivancos et al. 2015).
Scheme showing the consumption and regeneration of AsA and GSH, and the enzymes involved in ascorbate-glutathione (AsA-GSH) cycle. Reactive oxygen species are generated in chloroplasts, mitochondria, peroxisomes, cytosol, and apoplast (Table 1). APX Ascorbate peroxidase, DHAR dehydroascorbate reductase, MDHAR monodehydroascorbate reductase, GR glutathione reductase
In addition, the enzyme MDHA reductase (MDHAR, EC 1.6.5.4) catalyzes the reduction of MDHA to AsA, requiring NAD(P)H. This enzyme has different isoforms present in cytosol, chloroplasts, mitochondria, and peroxisomes and is associated with plasma membrane too.
It has been demonstrated that the AsA-GSH cycle takes place in mitochondria, chloroplasts, peroxisomes, and cytosol (Jiménez et al. 1997) since the enzymes mentioned above are present in these subcellular compartments. APX has multiple isoforms that are found in the chloroplast stroma (Nakano and Asada 1981), associated to chloroplast thylakoids (close to PSI) (Groden and Beck 1979; Miyake and Asada 1992), cytosol, mitochondria, and peroxisomes (Jiménez et al. 1997). In leaves about 70–80% of the AsA-dependent H2O2 scavenging enzymes are located in the chloroplasts. NADPH-dependent GR is present in the chloroplast stroma (Foyer and Halliwell 1976) as in other compartments. GR activity inclines the couple GSH/GSSG towards GSSG reduction in thermodynamic equilibrium with NADPH/NADP+ ratio (Jocelyn 1972). As a consequence, GSH is mostly in the reduced form and reaches concentration of 3.5 mM in chloroplasts (Foyer and Halliwell 1976). This allows keeping a redox environment suitable for the activation and stabilization of Calvin cycle and the reduction of DHA to AsA. The GSH:GSSG ratio is usually in 20:1 (Mhamdi et al. 2010), with variations in specific compartments, being higher in cytosol and lower in vacuole (Meyer et al. 2007; Queval et al. 2011).
Besides the enzymatic reduction of DHA to AsA by DHAR, it is worth to mention that GSH is able to reduce DHA non-enzymatically at pH higher than 7 (Foyer and Halliwell 1976), conditions that can be reached in the stroma of illuminated chloroplasts. On the other hand, some of the AsA-GSH cycle enzymes were found to be active in the apoplast (Podgórska et al. 2017).
2.2 Other Functions of AsA and GSH
Being GSH the major reduced thiol, there is a close relationship between sulfur plant status and GSH content. GSH acts as the main long-distance transport form of reduced sulfur from leaves to organs with high demand for protein synthesis, and it is also proposed that GSH acts as a signal transported via phloem from shoot, inhibiting sulfate uptake in roots, thus regulating plant sulfur homeostasis (Rennenberg and Herschbach 2014). Both, external sulfate uptake and assimilation, are inhibited by GSH through effects on specific transporters and enzymes (Buchner et al. 2004). GSH acts as the electron donor in the reduction of adenosine 5′-phosphosulfate to sulfite in plastids; however, in a complex relationship the same step is also inhibited by GSH decreasing adenosine 5′-phosphosulfate reductase (APR) activity, at protein and mRNA level (Vauclare et al. 2002).
GSH participates in heavy metal and xenobiotic detoxification. Conjugation of GSH to a number of xenobiotics is catalyzed by glutathione S-transferase enzymes. A variety of hydrophobic compounds, such as herbicides, become more water soluble and less toxic than the original compounds after GSH conjugation, being the mechanism of detoxification and the basis of resistance of some plant species to certain herbicides (e.g., chloroacetanilides and triazines ) (Labrou et al. 2005). GSH is also the precursor in the synthesis of phytochelatins [(γ-Glu-Cys)n-Gly, n = 2–11], which are important in detoxifying certain heavy metals such as copper (Cu), cadmium (Cd), and zinc (Zn), since after chelation they are accumulated in the vacuole. Plant cells respond to high concentrations of heavy metals by increasing the synthesis of phytochelatins and decreasing their GSH levels (Tukendorf and Rauser 1990; Dixon et al. 1998; Edwards et al. 2000; Sheehan et al. 2001).
GSH forms part of a complex regulatory network underlying growth, stress tolerance, and it is also necessary for certain developmental steps, such as gametogenesis, seed development, and postembryonic growth, evidenced through the use of Arabidopsis thaliana mutants in which the absence of GSH causes embryo lethality (Cairns et al. 2006). These results point out that GSH is essential for normal plant development.
GSH is required in the regulation of the plant cell cycle through the control of the G1–S transition. The recruitment of GSH into the nucleus in the G1 phase affects the redox state of the cytoplasm, where GSH concentration decreases, modifying the expression of transcripts associated with proteins involved in the oxidative signaling and stress tolerance (Diaz-Vivancos et al. 2010). GSH movement between cellular compartments has a profound effect on localized redox state, affecting the redox-sensitive motifs (i.e., cysteine residues, metal cofactors) of cell cycle-regulatory proteins. Thus, it has been suggested that the balance between ROS production and subsequent removal through the action of antioxidants impacts on cell proliferation (Menon and Goswami 2007). Similarly, vtc2 mutants with low AsA show increased level of oxidation in the nuclei and a delay in the progress of the cell cycle in the proliferation zone of embryonic roots (de Simone et al. 2017).
GSH is also required for the synthesis of pathogen defense-related molecules and disease resistance, protecting against biotic stress (Noctor et al. 2012). In addition, GSH is a source of sulfur for the synthesis of secondary metabolites, and it is found in high concentrations in nitrogen-fixing nodules, where GSH or homoglutathione , have proved to be essential for proper development of the root nodules during the interaction of legumes and rhizobia (Frendo et al. 2005). GSH protects against a broad spectrum of abiotic stress in addition to heavy metal, including drought, salinity, UV radiation, cold, and heat (Srivalli and Khanna-Chopra 2008).
One of the most important protein modification, known as protein thiolation, is produced through the redox signaling pathway mediated by dithiol/disulfide transitions carried out by the activity of thioredoxins (TRX) , and participates in plant cell death-mediated defense responses (Kuźniak et al. 2013). One specific form of protein thiolation that depends on the presence of GSH is called S-glutathionylation , considered an important redox post-translational mechanism regulating protein activity. In addition, an important interaction of GSH consists in the reaction with nitric oxide (NO) leading to the formation of S-nitrosoglutathione (GSNO) which constitutes a form to transport the bioactive molecule NO and also involves the modulation of protein activity through the post-translational pathway known as S-nitrosylation (Lyndermayr et al. 2005).
Besides its antioxidant function, AsA participates in many physiological processes such as photosynthesis, hormone biosynthesis, growth and development regulation; and in plant acclimation responses to adverse environmental conditions. The wide collection of AsA mutants has facilitated the identification and description of the processes in which AsA is involved.
In the photosynthetic apparatus, AsA plays an important role in photoprotection either as a direct antioxidant, scavenging ROS, or as a cofactor of enzymes of the xanthophyll cycle and electron donor. The xanthophyll cycle constitutes the non-photochemical quenching (NPQ) , an essential process by which excess light energy is dissipated in order to avoid photoinhibition. When plants are exposed to high light intensity, light harvesting complexes receive more energy that could be driven to photosynthesis through electron transport chain. In other words, to prevent photooxidative damage mainly of photosystem II, zeaxanthin, located at antenna complexes, is able, in conjunction with low pH, to dissipate energy safely as heat and drive excited chlorophyll to its basal state (Demmig-Adams and Adams 1996; Jahns and Holzwarth 2012). Zeaxanthin is synthesized by the reaction catalyzed by violaxanthin de-epoxidase. This thylakoid lumen located enzyme requires AsA as cofactor and low pH to be active (Hager and Holocher 1994; Bratt et al. 1995). In addition, AsA can act as an alternative photosystem II electron donor and thus, support electron transport when oxygen-evolving complexes are inactivated (Tóth et al. 2009).
AsA is also involved in biosynthetic pathways including dioxygenases in their steps. These enzymes require Fe2+ and AsA as cofactors (Prescott and John 1996). One example is the 1-aminocyclopropane-1-carboxylate oxidase which catalyzes the final step of ethylene synthesis (Smith et al. 1992). Other dioxygenases participate in gibberellin (GA) biosynthesis. Gibberellin 20-oxidase and GA20-3β-hydroxylase use AsA as co-substrate (Lange 1994; Smith et al. 1990). Dioxygenases are also present in secondary plant metabolism pathways as flavonoids and alkaloids biosynthesis. Flavanone 3-hydroxylase catalyzes the formation of dihydroflavonols which are intermediates in the biosynthesis of many secondary metabolites such as flavonols , anthocyanidins , and proanthocyanidins , and it requires AsA (Britsch and Grisebach 1986). AsA is also needed for the activity of anthocyanidin synthase, flavone synthase I, and flavonol synthase (Saito et al. 1999; Britsch 1990; Britsch et al. 1981).
Moreover, AsA plays an important role in plant growth and stress responses through hormone signaling. It has been reported that A. thaliana plants deficient in AsA synthesis (vtc1) showed increased abscisic acid (ABA) levels (Pastori et al. 2003; Kerchev et al. 2011). This leads to plant growth arrest. In leaves, water deficit leads to an increase in ABA levels, triggering H2O2 accumulation and the subsequent stomatal closure (Zhang et al. 2001). In this process, the AsA levels and its redox status are crucial in order to reach the H2O2 level needed to trigger stomatal movement (Chen and Gallie 2004).
Apoplastic AsA participates in cell growth and cell signaling (Horemans et al. 1998). Shoot growth in response to auxin involves the AsA redox state; when reduced apoplastic AsA is diminished, there is a loss of response to auxin (Pignocchi et al. 2006). It is worth to mention here that the AsA oxidase is involved in regulating the apoplastic AsA redox state by catalyzing its oxidation to MDHA (Pignocchi et al. 2003). AsA influences cell-wall composition and mechanical characteristics as cofactor of prolyl hydroxylases enzymes that catalyze the formation of hydroxyproline-rich glycoproteins (HRGPs) present in the cell wall (De Tullio et al. 1999).
3 Subcellular Localization and Transport of AsA and GSH
The first step of GSH synthesis occurs in plastids; cysteine and glutamate are converted in γ-glutamylcysteine (γ-EC) by the enzyme γ-EC synthetase (Wachter et al. 2005). The second and final step adds glycine and converts γ-EC in GSH through the activity of glutathione synthetase (GSHS). Both γ-EC and GSH can be transported outside plastids; in fact, it is assumed that GSH synthesis occurred mostly in the cytosol where GSHS is also present (Noctor et al. 2012; Lim et al. 2014). Cytosol and plastids have similar GSH concentration, around 3.2–3.5 mM (Krueger et al. 2010), and GSH concentration is clearly higher in leaves than in roots (Noctor et al. 2002). In agreement, sulfate reduction is several times higher in green leaves than in other plant organs, and the reaction is strongly stimulated by light (Fankhauser and Brunold 1978). This light enhancement is to be expected because of the requirement for GSH and ferredoxin as reductants for adenosine-5′-phosphosulfate and sulfite, respectively.
In some plant species, glycine is replaced by alanine or serine in the γ-glutamylcysteinylglycine tripeptide , as in homo-GSH (γ-Glu-Cys-β-Ala), found in many legumes or hydroxymethyl GSH (γ-Glu-Cys-Ser) present in cereals, with similar functions to GSH (Marschner 2012).
Compartment-specific alterations in GSH levels impact on metabolism and defenses, and it is worth to note that a measurement of total content of GSH and GSSG in tissues does not necessarily reflect the redox environment in subcellular compartments such as the nucleus, mitochondria, or chloroplasts (Diaz-Vivancos et al. 2015). As an example, GSH content found in trichomes of Arabidopsis resulted two or three times higher than in basal and epidermal cells (Gutierrez-Alcala et al. 2000). GSH concentration in different compartments or cell types is controlled through its biosynthesis, redox state, use, degradation, and transport (Foyer et al. 2001). Long-distance transport of GSH occurs since it has been found in xylem and phloem (Schneider et al. 1994), being the most abundant thiol in phloem, and exchange between phloem and xylem sap also takes place (Mendoza-Cózatl et al. 2011).
Plasma-membrane transport systems for GSH and GSSG are important in maintaining adequate levels in vacuole and apoplast. In particular, in apoplast GSH reaches low concentration with a probable role in defense against pathogens (Vanacker et al. 1998). If GSSG is generated in apoplast, it should be reduced after re-entering to the cytosol since there is no enzyme for GSH recovery in apoplast. It was also demonstrated a recycling of GSH and its efflux from the root cells with a net increase in the external medium; however, the presence of GSH transporter in the plasma membrane has not been demonstrated (Ferretti et al. 2009). Efflux of GSH from cells probably occurs through MRPs (multidrug-resistance-associated protein) belonging to the ATP binding cassette (ABC) protein family, known as ATP-driven pumps for GS-X and GSH across membranes in animals (Foyer et al. 2001). GSH uptake through high and low affinity components was characterized in several plant species, and also a specific symport system exists for GSSG uptake (Foyer et al. 2001). Chloroplasts are able to uptake GSH, in addition to synthesize it (Noctor et al. 2002), while GS-X and GSSG are transported into the vacuole detoxifying conjugated xenobiotics from the cytosol. Enzymatic activities acts recycling GSH/GSSG to amino acids in apoplast and vacuole, both compartments with low GSH:GSSG ratios (Foyer et al. 2001).
In mammalians, it has been suggested a role for GSH in the export of iron from cells, mainly through the formation of GSH-Fe-NO complexes that could be transported by MRP system (Richardson and Lok 2008). Similar compounds, with the structure (NO)2-Fe-GSH have shown adequate membrane permeability (Ueno et al. 1999), and could have a role delivering a safe form of Fe in plants (Buet and Simontacchi 2015; Ramirez et al. 2011).
AsA is a ubiquitous molecule, it is found in all cellular compartments, cytosol, mitochondria, chloroplasts, vacuole, peroxisomes, and apoplast. Its concentration ranges from 2 mM in the vacuole to 20 mM in cytosol. In spite of its abundance and its presence in most cell compartments, it took almost 50 years to elucidate its synthesis pathway in plants. Nowadays, this biosynthetic pathway is known as the Smirnoff-Wheeler pathway (Please see Chap. 6) (Fig. 1).
Since the final step of AsA synthesis takes place in mitochondria, it needs to be transported to the rest of plant cell compartments. AsA is capable of crossing the plasma membrane through AsA/DHA-specific transporters/exchangers that showed a greater affinity for DHA (Horemans et al. 1997). The AsA/DHA exchanger in the plasma membrane is particularly important given that the DHAR and the enzymes of the AsA-GSH cycle are not present in the apoplast. This exchanger uptakes DHA into the cytosol and translocate AsA to the apoplast, and it is essential to maintain AsA levels and redox homeostasis owing to the mentioned processes where it is involved. In this regard, it has been identified high redox potential b-type cytochrome (known as cytb 561) that is present in the plasma membrane of higher plants (Asard et al. 2001; Nanasato et al. 2005). It is able to operate such as transmembrane MDHAR, using cytoplasmic AsA as the electron donor and apoplastic MDHA as the electron acceptor (Nanasato et al. 2005; Horemans et al. 1994). Another isoforms of this cytochrome are located in the tonoplast (Griesen et al. 2004). In chloroplasts, AsA is taken up as the monoanionic form through a saturable carrier in the chloroplast envelope that presents a relatively low affinity (5 mM) (Foyer and Lelandais 1996). Recently, Miyaji and collaborators (2015) showed that the phosphate transporter AtPHT4;4 functions as an AsA transporter in the chloroplast envelop enabling the AsA entrance from the cytosol. A small fraction (10–20% of chloroplastic AsA) reaches the thylakoid lumen through a fast process but none transporter has been described.
It has been reported that AsA can be transported over long distances from source tissues to newly and non-photosynthetic tissues. It can be taken up by roots and transported by xylem and translocated from leaves via phloem (Mozafar and Oertli 1993; Franceschi and Tarlyn 2002; Tedone et al. 2004). In phloem they are also present the enzymes that participate in AsA biosynthesis with the exception of l-galactono-γ-lactone dehydrogenase (Hancock et al. 2003). This could be relevant in the supply of AsA precursors to sink tissues, but this contribution remains to be elucidated.
4 Role of AsA-GSH Cycle Under Stress Conditions
4.1 General Considerations
Plants are subjected to continuous changes of the environment. Normal fluctuations of irradiance, temperature, and other external factors affect processes such as photosynthesis or respiration. Small or large changes in the physiological status may occur during the day, as for example, the reduction in the water status of the leaf cells as a result of increasing evaporation conditions at midday due to temperature and light variations. Additionally, plants may be affected in the long term by the restriction or excess of resources such as water or mineral elements. Beyond the extension and kind of environmental changes, physiological disorders provoke a common feature: the increase in ROS generation (Miller et al. 2010). Under conditions where the amount of photons reaching the leaves is in excess compared to that required for CO2 fixation, the ROS production increases (Asada 2006). This involves that part of the reducing equivalents getting in excess into the photosynthetic electron transport chain may be derived to the univalent reduction of O2 forming ROS (Asada 2006). Consequently, ROS steady-state concentration may be continuously changing in chloroplasts (but also in other subcellular compartments). AsA-GSH cycle links plant metabolism with ROS production through the detoxification of H2O2 in chloroplast, mitochondria, or peroxisomes (Foyer and Halliwell 1976; Asada 1999). This formation of ROS can be modulated under harmful surrounding conditions by AsA-GSH cycle channeling the excess of reducing power to the safety formation of water (Asada 1999). Oxidative modifications may take place as a consequence of increased level of ROS especially under extreme environmental conditions. Increases in the oxidized state of antioxidants, oxidative damage to macromolecules or ROS steady-state levels were originally considered as detrimental for different physiological processes but now arise a new role as signaling function for them in plants (Foyer et al. 2017). These changes in the antioxidant-ROS network can be conceived as signals defining the fate of plant cells.
Foyer and Noctor (2016) proposed that the antioxidant defense is designed to modulate the accumulation of ROS instead of completely abolishing them. This concept is particularly important for physiological processes requiring a redox stimulus. Sensing mechanisms of ROS production may control plant growth and development. Physiological processes such as abscission zone formation, root hair growth or stomata closure depend on the ROS generation mediated by NADPH oxidases (Sakamoto et al. 2008; Jones et al. 2007; Kwak et al. 2003).
This association of plant metabolism with the steady-state levels of ROS (or eventually changes in the redox state of antioxidants or in the increment of oxidative damage to macromolecules) may represent integrators of signals from the environment with plant functions (e.g., hormone-related processes). An interaction of redox signaling with hormones such as auxins for the control of plant development and growth has been proposed (Schippers et al. 2016). Depending on the developmental stage, this network defines plant physiological status such as growth or dormancy establishment. This is illustrated in tree buds presenting high AsA content in a reduced state during active growth in spring/summer and a low concentration of AsA in a highly oxidized state during the rest period in autumn/winter (Gergoff Grozeff and Bartoli 2014).
Researchers asked themselves what are the protective processes exerted by antioxidants in this scenario. It is suggested that the antioxidant network may regulate ROS production keeping a steady state of ROS high enough to trigger cell protecting mechanisms (e.g., hormone action, gene expression, or others) (Noctor et al. 2017). Alternatively, it may control the extension of oxidative damage to macromolecules that would participate as specific signals depending on the subcellular compartment where they are generated (Møller et al. 2007). In addition, these reactions produce changes in the oxidized/reduced ratio of AsA or GSH that may be good indicators/sensors of redox status of plant cells (Noctor et al. 2016). All this components may act in conjunction for triggering adaptive responses of plant metabolism to a challenging environment.
4.2 The Role of AsA-GSH Cycle Under Different Abiotic Stress
4.2.1 Drought
Since ROS and antioxidants (enzymatic and non-enzymatic components) form a network that may be conceived as a sensor of environmental conditions, the contribution of AsA-GSH cycle on plant tolerance was largely studied under challenging environments in several species. The closure of stomata aiming at water loss avoidance under drought conditions entails lowering CO2 uptake limiting Calvin cycle, leading to a typical increase in ROS production in plant cells (Noctor et al. 2014). The participation of AsA and GSH in both antioxidant and signaling functions has been demonstrated by several experimental evidences. Leaves of a wheat cultivar with high concentration of AsA show lower oxidative damage compared with leaves of a cultivar with low AsA under drought conditions (Bartoli et al. 2004). Plants with increased AsA content display higher photochemical quenching and non-photochemical quenching than plants with lower AsA (Tambussi et al. 2000) demonstrating the role of AsA-GSH cycle ameliorating photosynthetic activity under water stress. The GSH/GSSG ratio can be determined by the use of the redox-sensitive GFP. It was reported that this antioxidant becomes more oxidized during drought (Jubany-Mari et al. 2010) suggesting a signaling function of this redox couple during abiotic stress conditions (Noctor et al. 2014).
4.2.2 Salinity
Salinity in plants induces nutritional alterations as a consequence of ion toxicity as well as osmotic stress. The increments in Na+ concentrations are toxic, producing a decrease in the uptake and concentration of K+ due to the chemical similarity of Na+ and K+, leading to a metabolic disorder (Acosta-Motos et al. 2017). Besides ROS production as by-products of plant metabolism, abiotic stresses induce the activity of the apoplastic NADPH oxidase catalyzing O2 − formation that then originates H2O2. Evidences demonstrate that this NADPH oxidase-dependent synthesis of ROS is crucial for the adaptive responses of plants to salinity such as regulation of Na+/K+ balance (Ma et al. 2012). NADPH oxidase inhibitors avoid the induction of antioxidant enzymes, particularly APX and GR under saline conditions (Ben Rejeb et al. 2015). Furthermore, the expression of genes involved in the early response of plants to salt stress is also induced by H2O2 treatment (Schmidt et al. 2013). These experiments, among many others, demonstrate the active role of AsA and GSH in the response of plants to saline stress.
4.2.3 Light
Chloroplasts constitute the main source of ROS in leaves derived from photosynthetic activity (Foyer and Noctor 2016). AsA-GSH cycle has a crucial role keeping compatible steady-state levels of ROS with plant metabolism under increasing irradiance conditions. Tomato plants with very low AsA present a severe oxidative damage when they are moved from a low to a high light environment (Baldet et al. 2013). Mutant plants lacking stromal and thylakoidal APX are prone to photooxidative damage under high light conditions (Kangasjärvi et al. 2008). Furthermore, distinct gene expression and enzyme activity are observed after high irradiance treatments in these mutants compared with wild type plants.
Besides the amount of light, the red/far red light ratio (R/FR) indicates the quality of light reaching the surface of the leaves. High or low R/FR represents a signal of sunny or shadow conditions, respectively, and determines the accumulation of both high or low AsA and GSH concentrations (Bartoli et al. 2009). Increments of both antioxidants under high R/FR illustrate a plant response to avoid the potential risk of oxidative damage under a sunny light environment.
These results demonstrate the involvement of APX and antioxidants in both photoprotection and redox signaling functions as integrants of the AsA-GSH cycle under changing light conditions.
4.2.4 Chilling
Low temperatures induce photooxidative stress in plants, especially in those species adapted to a warm climate. Light-dependent increments in ROS production and reductions in antioxidant (i.e., AsA and GSH) concentrations have been observed under chilling conditions (Wise and Naylor 1987). Plants with lower capacity to synthesize AsA (Wang et al. 2013; Yang et al. 2017) or with decreased activity of thylakoid APX (Duan et al. 2012) or GR (Shu et al. 2011) are more susceptible to chilling stress. On the other hand, H2O2 treatments improve the tolerance of plants exposed to subsequent low temperatures giving an evidence of signaling participation of ROS in the adaptive response (Yu et al. 2003; Wang et al. 2010a). Furthermore, plants with reduced thylakoidal APX activity show increased expression of stress related genes showing compensatory increments of other antioxidants (Duan et al. 2012). As previously mentioned for other abiotic stresses, the antioxidant-ROS network has protective and signaling functions in plants under low temperatures.
4.2.5 Heat Shock
Heat shock induces oxidative stress in plants (Larkindale and Knight 2002). Mustard plant seedlings exposed to an elevated temperature treatment show increments in H2O2 production followed by increments in the AsA and GSH concentration and activities of antioxidant enzymes such as APX and GR (Dat et al. 1998a, b). This exposition to high temperature induces thermotolerance (Dat et al. 1998a, b). Furthermore, non-lethal heat-shock treatments are applied to increase abiotic stress tolerance and have been proved to be useful to extend the postharvest life of different plant organs. For example, mild heat treatment (40 °C for 3.5 min) produces an increase in mitochondrial H2O2 in spinach leaves keeping high reduced/oxidized ratio of AsA and GSH, and extends their storage in the dark after detachment (Gómez et al. 2008). However, heat-shock treatment at higher temperatures (55 °C for 10 min) provokes reduction in the concentrations of AsA and GSH and a high Fe-dependent ROS production (non-mitochondrial source) triggering root cell death (Distéfano et al. 2017). Notably, this cell death is prevented with external GSH supplementation (Distéfano et al. 2017). These results suggest that the extension of the abiotic stress (and hence, oxidative stress) determines the recovery or death of plant cells.
5 Manipulating AsA and GSH Levels in Plants: A Promise to Deal with Abiotic Stress Improving Yield and Health Properties of Fruits and Vegetables
In recent years, there has been an increasing concern in consuming fruits and vegetables due to the importance for human nutrition and health. Fruits and vegetables, the main source of antioxidants in the diet, are associated with a lower risk of degenerative diseases, being a great opportunity improving health by increasing consumption (Ames et al. 1993). Due to the significance of antioxidants for plant productivity and stress tolerance, and also for human health, the manipulation of AsA and GSH metabolism in plants has become an important issue of research.
Under this scenario, genetic engineering strategies may provide a promising tool to optimize tolerance traits (Cushman and Bohnert 2000). Genetic engineering may increase the quantity of certain compound (or group of compounds) changing the expression of a gene (or genes), overcoming rate-limiting enzymatic steps in the biosynthetic pathways (Verpoorte and Memelink 2002). The recent advances regarding AsA synthesis pathways and biotechnology make feasible the possibility to improve AsA levels in plants (Zhang et al. 2007). Bulley et al. (2009) employing expression studies in kiwifruit, and gene over expression in Arabidopsis, reported that GDP-l-galactose guanyltransferase is a major regulating point in AsA biosynthesis through the l-galactose pathway (Fig. 2). These studies highlighted the potential importance of this rate-limiting step for breeding experiments, and showed the necessity to combine expression studies with other physiological experiments, since gene expression may not reflect enzyme activity. Baldet et al. (2013) also described that several tomato mutant lines defective in genes encoding enzymes from the AsA biosynthetic pathway (GDP-D-mannose pyrophosphorylase and the GDP-l-galactose phosphorylase genes) showed reduced AsA content and suffered from severe bleaching upon exposure to high light intensity.
Biosynthetic pathways for GSH and AsA. The GSH synthesis pathway is outlined in orange. ECS corresponds to the γ-EC synthetase and GSHS, to the GSH synthetase. This latter enzyme is present in chloroplasts and cytosol. The main AsA synthesis pathway in leaves is outlined in blue. VTC1 corresponds to the GDP-mannose pyrophosphorylase VTC2 and VTC5 to GDP-l-galactose phosphorylase, VTC4 to l-galactose-1-phosphate phosphatase, L-GalDH to l-galactose dehydrogenase, and L-GalLDH, to the l-galactono-γ-lactone dehydrogenase. This latter enzyme is located in the inner mitochondrial membrane and delivers electrons to the mitochondrial electron transport chain
The main efforts to enhance AsA content in plants have been focused in increasing not only the biosynthetic pathway enzymes, but also in improving the regeneration of the oxidized forms of this antioxidant (Lorence and Nessler 2007; Zhang et al. 2007). In addition, other strategies are destined to inhibiting degradation, introducing alternative pathways [by exogenous novel gene(s)], or switching the localization of key enzymes [summarized by Zhang et al. (2007)].
Regarding GSH, as its synthesis involves three amino acids and two enzymes in two steps ATP-dependent reactions (Fig. 2), the manipulation of GSH metabolism may be suitable also for crop species. However, manipulation of GSH partitioning seems difficult due to the limitation on the GSH high affinity transporters identification (Maughan and Foyer 2006). Also, being cysteine supply the key limiting factor for GSH synthesis, it should be essential to take into account solutions that also modulate sulfur assimilation for further enhance the capacity of plants to synthesize GSH (Maughan and Foyer 2006).
In order to achieve successful strategies, information at multiple levels including genes, enzymes, compartmentalization, transport, and accumulation should be encouraged (Verpoorte and Memelink 2002). Also, the regulation of multiple genes or the combination of more than one strategy would be better than the manipulation of a single gene or enzyme (Zhang et al. 2007). This could be particularly interesting in the case of AsA and GSH due to their connected metabolism. In this sense, Le Martret et al. (2011) have observed that the simultaneous expression of DHAR and GR led to a more pronounced reduction of H2O2 levels in leaf discs, than in lines expressing DHAR alone, improving chilling tolerance. Expression of both enzymes also conferred methyl viologen-induced oxidative stress tolerance (Le Martret et al. 2011).
The potential to counteract environmental stress became relevant due to the concern about climatic change. Plant productivity is limited by different kind of stresses. Most of the limitations can be attributed to the abiotic stresses that can appear during the lifetime of the plants, such as drought, salinity, extreme temperatures, light, UV radiation, heavy metals, nutrient deficiency, air pollution, herbicides, and many others (Boyer 1982; Bhattachrjee 2005). The understanding of the processes involved in the stressed plant physiology can lead to the development of new tools to manage stress situations and the design of strategies to overcome or prevent the damage (Nelson et al. 1998). In this context, some genetic strategies developed to manipulate AsA and GSH concentrations in plants leading to increased tolerance to abiotic stress are summarized in Table 2.
Abiotic stress studies also comprise an interesting source of information to develop new tools to manipulate vegetables and fruits during postharvest, specially related to oxidative stress (Cisneros-Zevallos 2003; Toivonen 2003a, b). The analysis of the reports about pre- and postharvest biology field in fruits and vegetables shows several works dealing with the effect of abiotic stress in perishables (Pedreschi and Lurie 2015). Fruit ripening involves the coordinated expression of hundreds of genes, as it can be seen in peach (The International Peach Genome Initiative 2013) or in tomato (The Tomato Genome Consortium 2012). To understand several processes involved in growth and development, the abiotic stress physiology can give us some new insights of complex processes such as fruit ripening (Gapper et al. 2013). Actually, some strategies designed to improve the content of antioxidant compounds in plants may involve the establishment of stress conditions increasing plant defense responses, raising the content of specific compounds. These strategies may involve, in some cases, the employment of a low (or sublethal) dose of an agent capable of inducing a physical or chemical stress (Costa et al. 2006). Postharvest treatments with UV radiation increased AsA content in some fruits like tomato (Jagadeesh et al. 2011) and apple (Hagen et al. 2007). UV-C treatments in strawberry fruits enhanced the activities of GR, MDHAR, and DHAR enzymes, increasing GSH and GSSG, and delaying the fruit decay comparing to control (Erkan et al. 2008).
Overall, the knowledge of plant responses to abiotic stress at molecular and metabolic level should converge to improve plant breeding strategies leading to crops best adapted to fluctuating environmental conditions. However, not only genetic engineering approaches, but also combined postharvest strategies should converge to obtain optimal antioxidant levels improving nutritional and health properties of fruits and vegetables.
6 Conclusions
Plant growth, productivity, and nutritional quality are affected by constant challenges from the surrounding. ROS-antioxidant networks are integrated to cell metabolism, and constitute crucial signals for important plant decisions, including developmental and adaptive processes. In this sense, plants show a tight interaction of ROS and antioxidants with hormonal signaling.
Evidence from the last years shows that enhancement of the own antioxidant defenses through the modulation of genes affecting the AsA-GSH cycle improves the performance of plants when they face a broad spectrum of environmental stressful conditions. This points out a positive association between the redox state of antioxidants with the capacity to withstand oxidative stress.
References
Acosta-Motos J, Ortuño M, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco M, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A 90:7915–7922
Aono M, Kubo A, Saji H, Tanaka K, Kondo N (1993) Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplast glutathione reductase activity. Plant Cell Physiol 34:129–135
Aono M, Saji H, Sakamoto A, Tanaka K, Kondo N, Tanaka K (1995) Paraquat tolerance of transgenic Nicotiana tabacum with enhanced activities of glutathione reductase and superoxide dismutase. Plant Cell Physiol 36:1687–1691
Asada K, Takahashi M (1987) Production and scavenging of active oxygen species in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier Science Publishers, Amsterdam, pp 227–287
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asard H, Kapila J, Verelst W, Bérczi A (2001) Higher-plant plasma membrane cytochrome b561: a protein in search of a function. Protoplasma 217:77–93
Baldet P, Bres C, Okabe Y, Mauxion JP, Just D, Bournonville C, Ferrand C, Mori K, Ezure H, Rothan C (2013) Investigating the role of vitamin C in tomato through TILLING identification of ascorbate-deficient tomato mutants. Plant Biotechnol 30:308–314
Bartoli CG, Gómez F, Martínez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.) J Exp Bot 55:1663–1669
Bartoli CG, Tambussi EA, Fanello DD, Foyer CH (2009) Control of ascorbic acid synthesis and accumulation by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett 583:118–122
Ben Rejeb K, Benzarti M, Debez A, Bailly C, Savouré A, Abdelly C (2015) NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J Plant Physiol 174:5–15
Bhattachrjee S (2005) Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transducation in plants. Curr Sci 89:1113–1121
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Bratt CE, Arvidsson P-O, Carlsson M, Akerlund H-E (1995) Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res 45:169–175
Britsch L, Grisebach H (1986) Purification and characterization of (2S)-flavanone-3-hydroxylase from Petunia hybrida. Eur J Biochem 156:569–577
Britsch L, Heller W, Grisebach H (1981) Conversion of flavanone to flavone, dihydroflavonol and flavonol with an enzyme system from cell cultures of parsley. Z Naturforsch 36:742–750
Britsch L (1990) Purification and characterization of flavone synthase I, a 2-oxoglutarate-dependent desaturase. Arch Biochem Biophys 282:152–160
Buchner P, Takahashi H, Hawkesford MJ (2004) Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J Exp Bot 55:1765–1773
Buet A, Simontacchi M (2015) Nitric oxide and plant iron homeostasis. Ann N Y Acad Sci 1340:39–46
Buettner G, Schafer F (2006) Albert Szent-Györgyi: vitamin C identification. Biochemist 28:31–33
Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactoseguanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60:765–778
Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ (2006) Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol 141:446–455
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci U S A 100:3525–3530
Chen Z, Gallie DR (2005) Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol 138:1673–1689
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16:1143–1162
Cisneros-Zevallos L (2003) The use of controlled postharvest abiotic stress as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J Food Sci 68:1560–1565
Costa L, Vicente AR, Civello PM, Chaves AR, Martínez GA (2006) UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol Technol 39:204–210
Cushman JC, Bohnert HJ (2000) Genomic approaches to plant stress tolerance. Curr Opin Plant Biol 3:117–124
Dat JF, Foyer CH, Scott IM (1998a) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118:1455–1461
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998b) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357
De Simone A, Hubbard R, Viñegra de la Torre N, Velappan Y, Wilson M, Considine MJ, WJJ S, Foyer CH (2017) Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis Thaliana. Antioxid Redox Signal 27(18):1505–1519. https://doi.org/10.1089/ars.2016.6959
De Tullio MC, Paciolla C, DallaVecchia F, Rascio N, D’Emerico S, De Gara L, Liso R, Arrigoni O (1999) Changes in onion root development induced by the inhibition of peptidyl-prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209:424–434
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Diaz-Vivancos P, De Simone A, Kiddle G, Foyer CH (2015) Glutathione - linking cell proliferation to oxidative stress. Free Radic Biol Med 89:1154–1164
Diaz-Vivancos P, Dong Y, Ziegler K, Markovic J, Pallardó FV, Pellny TK, Verrier PJ, Foyer CH (2010) Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J 64:825–838
Distéfano AM, Martin MV, Córdoba JP, Bellido AM, D’Ippólito S, Colman SL, Soto D, Roldán JA, Bartoli CG, Zabaleta EJ, Fiol DF, Stockwell BR, Dixon SJ, Pagnussat GC (2017) Heat stress induces ferroptosis-like cell death in plants. J Cell Biol 216:463–476
Dixon DP, Cummins I, Cole DJ, Edwards R (1998) Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol 1:258–266
Duan M, Ma N-N, Li D, Deng Y-S, Kong F-Y, Lv W, Meng Q-W (2012) Antisense-mediated suppression of tomato thylakoidal ascorbate peroxidase influences anti-oxidant network during chilling stress. Plant Physiol Biochem 58:37–45
Edwards R, Dixon DP, Walnot V (2000) Plant glutathione S- transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5:193–198
Eltelib HA, Fujikawa Y, Esaka M (2012) Overexpression of the acerola (Malpighia glabra) monodehydroascorbatereductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S Afr J Bot 78:295–301
Erkan M, Wang SY, Wang CY (2008) Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol Technol 48:163–171
Fankhauser H, Brunold C (1978) Localization of adenosine 5′-phosphosulfate sulfotransferase in spinach leaves. Planta 143:285–289
Ferretti M, Destro T, Tosatto SCE, La Rocca N, Rascio N, Masi A (2009) Gamma-glutamyltransferase in the cell wall participates in extracellular glutathione salvage from the root apoplast. New Phytol 181:115–126
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Lelandais M, Galap C, Kunert KJ (1991) Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol 97:863–872
Foyer CH, Lelandais M (1996) A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol 148:391–398
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:865–905
Foyer CH, Noctor G (2016) Stress-triggered redox signaling: what’s in pROSpect? Plant Cell Environ 39:951–964
Foyer CH, Ruban AV, Noctor G (2017) Viewing oxidative stress through the lens of oxidative signaling rather than damage. Biochem J 474:877–883
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109:1047–1057
Foyer CH, Theodoulou FL, Delrot S (2001) The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci 6:486–492
Franceschi VR, Tarlyn NM (2002) L-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiol 130:649–656
Frendo P, Harrison J, Norman C, Hernandez Jimenez MJ, Van de Sype G, Gilabert A, Puppo A (2005) Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol Plant-Microbe Interact 18:254–259
Gapper NE, McQuinn RP, Giovaninni JJ (2013) Molecular and genetic regulation of fruit ripening. Plant Mol Biol 82:575–591
Gergoff Grozeff G, Bartoli CG (2014) Participation of ascorbic acid in the dormancy establishment of poplar lateral branch buds. J Forest Res 19:301–304
Gómez F, Fernández L, Gergoff G, Guiamet JJ, Chaves A, Bartoli CG (2008) Heat shock increases mitochondrial H2O2 production and extends postharvest life of spinach leaves. Postharvest Biol Technol 49:229–234
Griesen D, Su D, Bérczi A, Asard H (2004) Localization of an ascorbate-reducible cytochrome b561 in the plant tonoplast. Plant Physiol 134:726–734
Groden D, Beck E (1979) H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta 546:426–435
Gutierrez-Alcala G, Gotor C, Meyer AJ, Fricker M, Vega JM, Romero LC (2000) Glutathione biosynthesis in Arabidopsis trichome cells. Proc Natl Acad Sci U S A 97:11108–11113
Hagen SF, Borge GIA, Bengtsson GB, Bilger W, Berge A, Haffner K, Solhaug KA (2007) Phenolic contents and other health and sensory related properties of apple fruit (Borkh., cv. Aroma): effect of postharvest UV-B irradiation. Postharvest Biol Technol 45:1–10
Hager A, Holocher K (1994) Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta 192:581–589
Hancock RD, McRae D, Haupt S, Viola R (2003) Synthesis of L-ascorbic acid in the phloem. BMC Plant Biol 3:7
Horemans N, Asard H, Caubergs RJ (1998) Carrier mediated uptake of dehydroascorbate into higher plant plasma membrane vesicles shows trans-stimulation. FEBS Lett 421:41–44
Horemans N, Asard H, Caubergs RJ (1997) The ascorbate carrier of higher plant plasma membranes preferentially translocates the fully oxidized (dehydroascorbate) molecule. Plant Physiol 114:1247–1253
Horemans N, Asard H, Caubergs RJ (1994) The role of ascorbate free radical as an electron acceptor to cytochrome b-mediated trans-plasma membrane electron transport in higher plants. Plant Physiol 104:1455–1458
Hunter G, Eagles BA (1926) Glutathione. A critical study. J Biol Chem 72:147
Jagadeesh SL, Charles MT, Gariépy Y, Goyette B, Raghavan VGS, Vigneault C (2011) Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioprocess Technol 4:1463–1472
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 2012:182–193
Jiménez A, Hernández JA, del Río LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Jocelyn PC (1972) Biochemistry of the thiol group. Academic Press, New York
Jones MA, Raymong MJ, Yang Z, Smirnoff N (2007) NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends onROP GTPase. J Exp Bot 58:1261–1270
Jubany-Mari T, Alegre-Batlle L, Jiang K, Feldman LJ (2010) Use of a redox-sensing GFP (c-roGFP1) for real-time monitoring of cytosol redox status in Arabidopsis thaliana water-stressed plants. FEBS Lett 584:889–897
Kangasjärvi S, Lepistö A, Hännikäinen K, Piippo M, Luomala E-M, Aro E-M, Rintamäki E (2008) Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem J 412:275–285
Kavitha K, George S, Venkataraman G, Parida A (2010) A salt-inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochemie 10:1321–1329
Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier PJ, Hancock RD, Foyer CH (2011) The transcription factor ABI-4 is required for the ascorbic acid- dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23:3319–3334
Krueger S, Donath A, Lopez-Martin MC, Hoefgen R, Gotor C, Hesse H (2010) Impact of sulphur starvation on cysteine biosynthesis in T-DNA mutants deficient for compartment-specific serine-acetyltransferase. Amino Acids 39:1029–1042
Kuźniak E, Kaźmierczak A, Wielanek M, Głowacki R, Kornas A (2013) Involvement of salicylic acid, glutathione and protein S-thiolation in plant cell death-mediated defence response of Mesembryanthemum crystallinum against Botrytis cinerea. Plant Physiol Biochem 63:30–38
Kwak JM, Mori I, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genesfunction in ROS-dependent ABA signaling. EMBO J 22:2623–2633
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbatereductase gene. J Plant Physiol 160:347–353
Labrou NE, Karavangeli M, Tsaftaris A, Clonis YD (2005) Kinetic analysis of maize glutathione S-transferase I catalysing the detoxification from chloroacetanilide herbicides. Planta 222:91–97
Lange T (1994) Purification and partial amino-acid sequence of gibberellin 20-oxidase from Cucurbita maxima L. endosperm. Planta 195:108–115
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Le Martret B, Poage M, Shiel K, Nugent GD, Dix PJ (2011) Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J 9:661–673
Lim B, Paternak M, Meyer AJ, Cobbett CS (2014) Restricting glutamycysteinesynthetase activity to the cytosol or glutathione biosynthesis to the plastids is sufficient for normal plant development and stress tolerance. Plant Biol 16:58–67
Liu W, An H-M, Yang M (2013) Overexpression of Rosa roxburghii l-galactono-1,4-lactone dehydrogenase in tobacco plant enhances ascorbate accumulation and abiotic stress tolerance. Acta Physiol Plant 35:1617–1624
Lorence A, Nessler CL (2007) Chapter 8: Pathway engineering of the plant vitamin C metabolic network. In: Verpoorte R, Alfermann AW, Johnson TS (eds) Applications of plant metabolic engineering. Springer, The Netherlands, pp 197–217
Lyndermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot 63:305–317
Marschner P (2012) Chapter 3: Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier, San Diego, pp 49–70
Maughan S, Foyer CH (2006) Engineering and genetic approaches to modulating the glutathione network in plants. Physiol Plant 126:382–397
Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI (2011) Long-distance transport, vacuolar sequestration and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14:554–562
Menon SG, Goswami PC (2007) A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26:1101–1109
Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52:973–986
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Miyaji T, Kuromori T, Takeuchi Y, Yamaji N, Yokosho K, Shimazawa A, Sugimoto E, Omote H, Ma JF, Shinozaki K, Moriyama Y (2015) AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nature 6:5928
Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33:541–553
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Mozafar A, Oertli JJ (1993) Vitamin C (ascorbic acid): uptake and metabolism by soybean. J Plant Physiol 141:316–321
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nanasato Y, Akashi K, Yokota A (2005) Co-expression of cytochrome b561 and ascorbate oxidase in leaves of wild watermelon under drought and high light conditions. Plant Cell Physiol 46:1515–1524
Nelson DE, Rammesmayer G, Bohnert HJ (1998) Regulation of cell-specific inositol metabolism and transport in plant salinity tolerance. Plant Cell 10:753–764
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160
Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164:1636–1648
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez- Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Noctor G, Reichheldb J-P, Foyer CH (2017) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol. https://doi.org/10.1016/j.semcdb.2017.07.013
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53:1283–1304
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Pedreschi R, Lurie S (2015) Advances and current challenges in understanding postharvest abiotic stresses in perishables. Postharvest Biol Technol 107:77–89
Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH (2003) The function of ascorbate oxidase in tobacco. Plant Physiol 132:1631–1641
Pignocchi C, Kiddle G, Hernández I, Foster SJ, Asensi A, Taybi T, Barnes J, Foyer CH (2006) Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol 141:423–435
Podgórska A, Burian M, Szal B (2017) Extra-cellular but extra-ordinarily important for cells: apoplastic reactive oxygen species metabolism. Front Plant Sci 8:1353
Prescott AG, John P (1996) DIOXYGENASES: molecular structure and role in plant metabolism. Annu Rev Plant Physiol Plant Mol Biol 47:245–271
Queval G, Jaillard D, Zechmann B, Noctor G (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34:21–32
Ramirez L, Simontacchi M, Murgia I, Zabaleta E, Lamattina L (2011) Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: a well equipped team to preserve plant iron homeostasis. Plant Sci 181:582–592
Rennenberg H, Herschbach C (2014) A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J Exp Bot 65:5711–5724
Richardson DR, Lok HC (2008) The nitric oxide-iron interplay in mammalian cells: transport and storage storage of dinitrosyl iron complexes. Biochim Biophys Acta 1780:638–651
Saito K, Kobayashi M, Gong Z, Tanaka Y, Yamazaki M (1999) Direct evidence for anthocyanidin synthase as a 2-oxoglutarate-dependent oxygenase: molecular cloning and functional expression of cDNA from a red forma of Perillafrutescens. Plant J 17:181–189
Sakamoto M, Munemura I, Tomita R, Kobayashi K (2008) Involvement of hydrogen peroxide in leaf abscissionsignaling, revealed by analysis with an in vitro abscissionsystem in Capsicum plants. Plant J 56:13–27
Santolini J, André F, Jeandroz S, Wendehenne D (2017) Nitric oxide nitric oxide synthase in plants: where do we stand? Nitric Oxide 63:30–38
Schippers JHM, Foyer CH, van Dongen JT (2016) Redox regulation in shoot growth, SAM maintenance and flowering. Curr Opin Plant Biol 29:121–128
Schmidt R, Mieulet D, Hubberten H-M, Obata T, Hoefgen R, Fernie AR, Fisahn J, San Segundo B, Guiderdoni E, Schippers JHM, Mueller-Roeber B (2013) Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 25:2115–2131
Schneider A, Schatten T, Rennenberg H (1994) Exchange between phloem and xylem during long distance transport of glutathione in spruce trees (Picea abies [Karst.] L.) J Exp Bot 45:457–462
Sharma M, Buettner G (1993) Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic Biol Med 14:649–653
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360(1):16
Shu D-F, Wang L-Y, Duan M, Deng Y-S, Meng Q-W (2011) Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol Biochem 49:1228–1237
Smith JJ, Ververidis P, John P (1992) Characterization of the ethylene-forming enzyme partially purified from melon. Phytochemistry 31:1485–1494
Smith VA, Gaskin P, MacMillan J (1990) Partial purification and characterization of the Gibberellin A20 3β-hydroxylase from seeds of Phaseolus vulgaris. Plant Physiol 94:1390–1401
Srivalli S, Khanna-Chopra R (2008) Role of glutathione in abiotic stress tolerance. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stress in plants. Springer, Heidelberg, pp 207–225
Tambussi E, Bartoli CG, Guiamet JJ, Beltrano J, Araus JL (2000) Oxidative damage to thylakoid proteins in water-stressed leaves of wheat (Triticum aestivum L.) Physiol Plant 108:398–404
Tedone L, Hancock RD, Alberino S, Haupt S, Viola R (2004) Long-distance transport of L-ascorbic acid in potato. BMC Plant Biol 4:16
The International Peach Genome Initiative (2013) The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet 45:487–494
The Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485:635–641
Toivonen PMA (2003a) Effects of storage conditions and postharvest procedures on oxidative stress in fruits and vegetables. In: Hodges DM (ed) Postharvest oxidative stress in horticultural crops. Food Product Press, New York, pp 69–90
Toivonen PMA (2003b) Postharvest treatments to control oxidative stress in fruits and vegetables. In: Hodges DM (ed) Postharvest oxidative stress in horticultural crops. Food Product Press, New York, pp 225–246
Tóth SZ, Puthur JT, Nagy V, Garab G (2009) Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol 149:1568–1578
Tukendorf A, Rauser WE (1990) Changes in glutathione and phytochelatins in roots of maize seedlings exposed to cadmium. Plant Sci 70:155–166
Ueno T, Suzuki Y, Fujii S, Vanin AF, Yoshimura T (1999) In vivo distribution and behavior of paramagnetic dinitrosyldithiolato iron complex in the abdomen of mouse. Free Radic Res 31:525–534
Upadhyaya CP, Venkatesh J, Gururani MA, Asnin L, Sharma K, Ajappala H, Park SW (2011) Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett 33:2297–2307
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Vanacker H, Carver TLW, Foyer CH (1998) Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol 117:1103–1114
Vauclare P, Kopriva S, Fell D, Sutter M, Sticher L, von Ballmoos P, Krähenbühl U, Op den Camp R, Brunold C (2002) Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible that ATP sulphurylase to negative control by thiols. Plant J 31:729–740
Verpoorte R, Memelink J (2002) Engineering secondary metabolite production in plants. Curr Opin Biotechnol 13:181–187
Wachter A, Wolf S, Steininger H, Bogs J, Rausch T (2005) Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 41:15–30
Wang L-Y, Li D, Deng Y-S, Lv W, Meng Q-W (2013) Antisense-mediated depletion of tomato GDP-l-galactosephosphorylase increases susceptibility to chilling stress. J Plant Physiol 170:303–314
Wang Y, Li J, Wang J, Li Z (2010a) Exogenous H2O2 improves the chilling tolerance of manilagrass and mascarenegrass by activating the antioxidative system. Plant Growth Regul 61:195–204
Wang Z, Xiao Y, Chen W, Tang K, Zhang L (2010b) Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol 52:400–409
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation. Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83:278–282
Yang D-Y, Li M, Ma N-N, Yang X-H, Meng Q-W (2017) Tomato SlGGP-LIKE gene participates in plant responses to chilling stress and pathogenic infection. Plant Physiol Biochem 112:218–226
Yu C-W, Murphy TM, Lin C-H (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Zhang L, Wang Z, Xia Y, Kai G, Chen W, Tang K (2007) Metabolic engineering of plant L-ascorbic acid biosynthesis: recent trends and applications. Crit Rev Biotechnol 27:173–182
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhu YL, Pilon-Smits EA, Jouanin L, Terry N (1999) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119:73–80
Acknowledgements
Authors are researchers at CONICET and are grateful to CONICET, FONCyT, and Universidad Nacional de La Plata (Argentina) for supporting the work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Bartoli, C.G., Buet, A., Gergoff Grozeff, G., Galatro, A., Simontacchi, M. (2017). Ascorbate-Glutathione Cycle and Abiotic Stress Tolerance in Plants. In: Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A. (eds) Ascorbic Acid in Plant Growth, Development and Stress Tolerance. Springer, Cham. https://doi.org/10.1007/978-3-319-74057-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-74057-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74056-0
Online ISBN: 978-3-319-74057-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)