Abstract
Ascorbic acid (AsA) is oxidized to monodehydroascorbate (MDHA), which dissociates to form dehydroascorbate (DHA) instead of detoxifying reactive oxygen species (ROS). MDHA and DHA are directly reduced to AsA by two reductases, monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR), respectively. They contribute to maintaining AsA contents and its redox status, which are dependent on the rate of its biosynthesis and recycling. The primary functions of MDHAR and DHAR appear to recycle AsA in the AsA-glutathione (GSH) cycles for diminishing ROS produced during photosynthesis in leaves. In fruits, MDHAR and DHAR might function in a complementary manner to maintain the AsA redox status during fruit development and ripening. Also, MDHAR and DHAR function as part of the AsA-GSH cycles in the different plant cellular compartments, like chloroplasts, mitochondria, and peroxisomes. Taking into account the physiological functions of AsA in plants, MDHAR and DHAR as AsA regenerators are paid much attention in engineering of stress tolerance and nutrient values. Transgenic plants overexpressing MDHAR and DHAR exhibit an increase in AsA contents and enhanced stress tolerance. This chapter focuses on the primary structures and gene expressions of plant MDHAR and DHAR isozymes as well as their contributions to the AsA contents in the leaves and fruits of plants. Also, this chapter provides the information about the roles of MDHAR and DHAR in the chloroplast, the cytosol, the guard cells, and stress tolerance in plants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Ascorbic acid-glutathione cycle
- Ascorbic acid recycling
- Dehydroascorbate reductase
- Environmental stress
- Monodehydroascorbate reductase
- Oxidative stress tolerance
1 Introduction
Ascorbic acid (AsA) is the major antioxidant of plants, and it detoxifies reactive oxygen species (ROS) such as singlet oxygen, superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2). When removing H2O2, AsA is oxidized to monodehydroascorbate (MDHA) by ascorbate peroxidase and followed by nonenzymatic dissociation to form dehydroascorbate (DHA) . In AsA recycling (Noctor and Foyer 1998), after AsA is oxidized to MDHA and DHA , two types of reductases reduce MDHA and DHA respectively back to AsA. The ratio of total AsA (reduced and oxidized AsA) and DHA relates to cellular redox status. Considering the physiological functions of AsA in plants, it is essential to maintain the AsA redox status under condition increasing ROS production, such as light, extreme temperature, metal or salt stress (Davey et al. 2000; Conklin 2001; Anjum et al. 2014). Thus, the AsA redox status, as well as its contents in cells, is a significant indicator of physiological properties to stress tolerance, and it depends on the balance of AsA biosynthesis and recycling. In AsA recycling as described above, two reductases participate in the reduction of oxidized AsA; one of the reductases is monodehydroascorbate reductase (MDHAR; EC 1.6.5.4), which can reduce MDHA to AsA using NAD(P)H as a reductant (Fig. 1; Noctor and Foyer 1998). The other is dehydroascorbate reductase (DHAR; EC 1.8.5.1) for reducing DHA to AsA in a reaction requiring glutathione (GSH) as a reductant. MDHAR and DHAR, as well as AsA biosynthesis enzymes, are essential enzymes to maintain the AsA redox status (Leterrier et al. 2005; Lunde et al. 2006; Tang and Yang 2013; Zhang et al. 2015). As shown in Fig. 1, the AsA recycling is a component of the AsA-GSH cycle . In the AsA-GSH cycle, oxidized glutathione (GSSG), which comes from GSH used by DHAR to recycle AsA from DHA, is reduced by glutathione reductase using NADPH as a reductant. If DHA does not get back to AsA, it spontaneously hydrolyzes to 2,3-diketogulonic acid, which results in irreversible AsA loss. Since irreversible DHA oxidation causes the decrease of AsA contents, the efficiencies of AsA recycling are important in maintaining not only AsA redox status but also AsA contents in cells. In plants, the primary structures of MDHAR and DHAR are determined, and they can be classified into several groups based on their primary structures. In leaves, MDHAR and DHAR mainly participate in scavenging ROS in photosynthesis. The gene expressions of MDHAR and DHAR during fruit development and ripening suggest that MDHAR and DHAR have a complementary relationship in maintaining the AsA redox status. Acerola (Malpighia glabra) fruits, containing high AsA contents (Badejo et al. 2009; Eltelib et al. 2011), possess high DHAR activities compared to other plants with relatively low AsA contents (Hossain and Asada 1984; Dipierro and Borraccino 1991; Kato et al. 1997; Shimaoka et al. 2000; Eltayeb et al. 2006). Thus, the AsA recycling appears to contribute high AsA contents in acerola fruits . Under abiotic stress conditions accompanied by ROS accumulation, the gene expressions of MDHAR and DHAR are enhanced in plants, suggesting that they improve the AsA redox status by the AsA recycling from MDHA and DHA. And transgenic plants overexpressing MDHAR and DHAR exhibit enhanced oxidative stress tolerance, even slight increase of AsA contents and AsA redox status (Kwon et al. 2003; Amako et al. 2006; Eltayeb et al. 2006, 2007, 2011; Ushimaru et al. 2006; Li et al. 2010a; Yin et al. 2010; Qin et al. 2011; Eltelib et al. 2012; Chang et al. 2017). On the other hand, biotic stress by pathogen reduced the gene expression of MDHAR in wheat leaves. Moreover, the suppression of MDHAR in wheat leaves improved resistance to pathogen infection (Feng et al. 2014a, b). Under biotic stress, ROS serve as not only the toxic substance but also pathogen resistive element (Wang et al. 2007). Thus, the regulation of MDHAR and DHAR expressions may be of importance for resistance to abiotic and biotic stresses through ROS regulation by the AsA-GSH cycles.
Ascorbic acid recycling by the ascorbate-glutathione cycle through DHAR and MDHAR. After oxidization of AsA to monodehydroascorbate (MDHA), monodehydroascorbate reductase (MDHAR) converts MDHA to AsA, or MDHA disproportionates nonenzymatically to dehydroascorbate (DHA). Dehydroascorbate reductase (DHAR) reduces DHA to AsA using glutathione (GSH) as the reductant. Oxidized glutathione (GSSG) is reduced by glutathione reductase (GR) to GSH using NADHP as the reductant. DHA will spontaneously hydrolyze to 2,3-diketoguloninc acid unless salvaged by DHAR. Total AsA represents the inclusion of reduced (AsA) and oxidized AsA (MDHA and DHA)
2 The Primary Structures of Ascorbic Acid Recycling Enzymes in Plants
In plants, there are many isozymes of MDHAR and DHAR, and their primary structures have been deposited at public database sites , such as National Center for Biotechnology Information (U.S. National Library of Medicine) and DNA Data Bank of Japan (Japan, National Institute of Genetics). As described in the previous section, MDHAR is the first enzyme to recycle oxidized AsA (MDHA). MDHAR contains the FAD-NAD-binding sites, which play a significant role in its enzyme activity. The alignment of the primary structures of plant MDHAR shows they are classified into four groups (Fig. 2a: Leterrier et al. 2005; Lunde et al. 2006). Each group of the plant MDHAR appears to localize at different cell compartments: cytosol, peroxisome, chloroplast, and mitochondrion. The MDHAR isozymes which localize in chloroplasts, mitochondria, and peroxisomes have specific extensions at their N-terminus or C-terminus . These extensions act as a transit peptide for mitochondrial or chloroplast targeting (N-terminus) and peroxisomal targeting (C-terminus). In Arabidopsis, six MDHAR isozymes (AtMDHAR) were found in the genome (Leterrier et al. 2005; Lisenbee et al. 2005). Two of them, AtMDHAR1 (gene accession number; At3g52880) and AtMDHAR4 (gene accession number; At3g27820) localize in the peroxisome and possess specific C-terminal sequences, which are similar to the type-1 peroxisomal targeting signal (PTS1) and membrane peroxisomal targeting signals, respectively (Lisenbee et al. 2005). Another two which arise from a single gene (gene accession number; At1g639400) at different transcription initiation sites, AtMDHAR5 (gene accession number; D84417) and AtMDHAR6 (gene accession number; NP_564818) with an N-terminal extended region, are targeted to mitochondria and chloroplasts, respectively (Obara et al. 2002). The other two, AtMDHAR2 (At5g03630) and AtMDHAR3 (At3g09940), localize in the cytosol with no sequence characterized by subcellular localizations (Lisenbee et al. 2005). These six isozymes of Arabidopsis MDHAR exist in different cell compartments. On the other hand, DHAR is the other enzyme to recycle oxidized AsA (DHA) and prevents DHA from being spontaneously hydrolyzed to 2,3-diketogulonic acid. Plant DHAR contains a highly conserved DHAR peptide motif (CxxS), corresponding to thiol-dependent redox sites in thiol-disulfide oxidoreductase enzymes, and it is involved in redox function (Fomenko and Gladyshev 2002). Unlikely to plant MDHAR, the phylogenetic tree shows the plant DHAR isozymes are classified into two groups (Fig. 2b). In Arabidopsis, three DHAR isozymes, AtDHAR1 (gene accession number; At1g19570), AtDHAR2 (gene accession number; At1g75270), and AtDHAR3 (gene accession number; At5g16710), were found in the genome (Yoshida et al. 2006; Noshi et al. 2017). Based on the primary structures of the plant MDHAR isozymes, two (AtDHAR1 and AtDHAR2) of three Arabidopsis DHAR are classified into the same group. AtDHAR1 and AtDHAR2 having no targeting signal like sequence, they seemed to localize in the cytosol (Yoshida et al. 2006; Grefen et al. 2010). However, AtDHAR1 was reported to localize in peroxisomes practically (Reumann et al. 2009). AtDHAR3 only possesses a specific N-terminal extension, similar to chloroplast targeting signal, and was found to localize in the chloroplast (Noshi et al. 2016). Zea mays also has four DHAR isozymes (ZmDHAR) in the genome. One DHAR (ZmDHAR2) possesses a similar sequence to chloroplast targeting signal, and another two isozymes (ZmDHAR1 and ZmDHAR3) don’t contain any targeting signal . However, the other one (ZmDHAR4) contains signal peptide like sequence and localizes in vacuole (Zhang et al. 2015). In Pisum sativum, DHAR were reported to localize in mitochondria as well as peroxisome (Jimenez et al. 1997). Taking into account the multiplicity of plant MDHAR and DHAR isozymes, the MDHAR and DHAR isozymes function differentially at their localization sites in plants.
Phylogenetic tree of plant MDHAR and DHAR. Phylogenetic trees based upon protein sequences of plant MDHAR (a) and DHAR (b) are presented. The dotted-line box indicated each clade in the phylogenetic trees of plant MDHAR and DHAR. For abbreviations and accession numbers see the following, and accession numbers are given in parentheses. MDHAR; Arabidopsis thaliana, AtMDHAR1 (AAM83213), AtMDHAR2 (NP_568125), AtMDHAR3 (NP_566361), AtMDHAR4 (AAM91734), AtMDHAR5 (BAA12349), AtMDHAR6 (NP_564818); Brassica napus, BrMDHAR1 (XP_013663253), BrMDHAR2 (XP_013749992), BrMDHAR3 (XP_013681036), BrMDHAR4 (XP_013724800), BrMDHAR5 (XP_013740977), BrMDHAR6 (XP_013646118), BrMDHAR7 (XP_013686116); Glycine max, GmMDHAR1 (XP_003557022), GmMDHAR2 (XP_006599107), GmMDHAR3 (XP_003553831), GmMDHAR4 (XP_006584627); Oryza sativa, OsMDHAR1 (BAS80528), OsMDHAR2 (BAT06666), OsMDHAR3 (BAT09471); Solanum lycopersicum, SlMDHAR1 (NP_001234013), SlMDHAR2 (NP_001318117), SlMDHAR3 (AAZ66138), SlMDHAR4 (ADJ21816). DHAR; Arabidopsis thaliana, AtDHAR1 (NP_173387), AtDHAR2 (NP_177662), AtDHAR3 (NP_568336); Brassica napus, BnDHAR1 (No. CDY29420), BnDHAR2 (CDY62535), BnDHAR3 (CDY62535), BnDHAR4 (CDX85490); Brassica rapa, BrDHAR1 (XP_009149443), BrDHAR2 (XP_009128088), BrDHAR3 (XP_009128089), BrDHAR4 (XP_009126134); Glycine max, GmDHAR1 (NP_001236937), GmDHAR2 (KRH36212), GmDHAR3 (KRG97934), GmDHAR4 (KRH30940); Oryza sativa, OsDHAR1 (BAS91971), OsDHAR2 (BAS96927); Solanum lycopersicum, SlDHAR1 (AAY47048), SlDHAR2 (AAY47049); Solanum tuberosum, StDHAR1 (ABX26128), StDHAR2 (ACJ70069). Zea mays, ZmDHAR1 (AIQ78396), ZmDHAR2 (AIQ78394), ZmDHAR3 (NP_001151414)
3 The Role of Ascorbic Acid Recycling Enzymes in Regulating Ascorbic Acid Contents and its Redox State
Leaf tissues abundantly contain AsA, especially in chloroplasts (Foyer et al. 1983; Foyer and Lelandais 1996). Light induces the photosynthesis in chloroplasts. In the photosynthesis process, ROS are unexpectedly generated when electrons from reduced ferredoxin of the photosynthetic electron transport chain at photosystem I transfer to O2 instead of to NADP. In chloroplasts , AsA is essential as a substrate for APX in the AsA-GSH cycle, which detoxifies ROS through successive oxidation and reduction with GSH and NADPH. Since MDHAR and DHAR participate in the AsA-GSH cycle, they play important roles in the regulation of cellular redox status to scavenge ROS in photosynthesis. In tobacco (Nicotiana tabacum), the levels of enzyme activities and proteins of DHAR were highest in the youngest leaves and declined along with leaf aging (Chen and Gallie 2006). The fluctuation in DHAR activities primarily correlated with the change in the chlorophyll contents and the rate of CO2 assimilation. The overexpression of DHAR also decreased the levels of lipid peroxidation, whereas the suppression of DHAR expression increased the levels of lipid peroxidation . Moreover, DHAR can affect plant growth development as well as the diminishment of ROS. In rice (Oryza sativa), the overexpression of OsDHAR1 exhibited better growth development, phenotypes, and rice yield, including grain yield and biomass (Kim et al. 2013). In potato (Solanum tuberosum) possessing two DHAR isozymes (StDHAR1 and StDHAR2), the StDHAR1 was highly expressed in tubers, whereas the expression of StDHAR2 was high in leaves (Qin et al. 2011). Their primary structures suggest StDHAR1 and StDHAR2 localize in cytosol and chloroplast, respectively. The overexpression of StDHAR1 significantly increased AsA contents in leaves and tubers. In contrast, StDHAR2 overexpression resulted in the increase of DHAR activities and AsA contents only in leaves and did not fluctuate them in tubers, suggesting that StDHAR2 might need to be activated by posttranslational processing in chloroplasts. StDHAR1 and StDHAR2 might play important roles in improving the AsA contents at different organs and cell compartments. MDHAR and DHAR expressed not only leaves but also roots. During development of the taproots of carrot and radish, the AsA contents were gradually decreased (Xu et al. 2013; Wang et al. 2015). MDHAR and DHAR may be involved in the fluctuation of AsA contents in root, although their physiological function remains unknown.
Fruit is a primary source of AsA for humans, which cannot synthesize their own AsA due to a lack of l-gluconolactone oxidase . Thus, AsA accumulation during fruit development and ripening has been paid attention to in recent years. The patterns of AsA accumulation during fruit development and ripening depend on plant species. Several studies have reported that the AsA contents and AsA redox status in fruits can be modulated during fruit development and ripening. Acerola (Malpighia glabra) is a tropical fruit containing high AsA contents. The AsA content in green fruits is the highest during fruit ripening, and it decreases significantly as ripening progresses (Badejo et al. 2009). In acerola fruits, the high gene expression levels of the AsA biosynthesis enzymes involved in the Smirnoff–Wheeler were observed during fruit ripening, suggesting that the Smirnoff–Wheeler pathway mainly contributes to high AsA contents in acerola fruit (Badejo et al. 2009). In acerola fruits, AsA contents are decreased during fruit ripening, whereas the ratio of reduced AsA to DHA is increased. Concerning AsA recycling, MDHAR activities were greatly higher than DHAR activities in acerola fruits. MDHAR activities are increased gradually and significantly as ripening progressed, although DHAR activities increased at the early and intermediate stages of ripening, and then decreased dramatically at the later stages (Eltelib et al. 2011). The one of each DHAR and MDHAR isozymes were cloned from acerola, and the gene expression of the DHAR was correlated with its enzyme activities during fruit ripening. The MDHAR mainly expressed at overripe fruits, against high MDHAR activities through fruit ripening. On the other hand, the gene expression of the MDHAR was consistent with enzyme activities in other tissues: root, stem, and young and mature leaves. Furthermore, overexpression of the isolated MDHAR leads to the increase of AsA contents in tobacco plants (Eltelib et al. 2012), suggesting that the MDHAR function mainly in other tissue than fruit. However, acerola fruits possess high MDHAR activities and its activities were increased with fruit ripening. Considering the existence of several MDHAR isozymes in Arabidopsis, other MDHAR isozymes may mainly function to maintain the AsA redox status during fruit ripening in acerola. Similar AsA accumulation patterns have been reported in the fruits of blueberry (Vaccinium corymbosum) (Liu et al. 2015) and kiwifruit (Actinidia deliciosa) (Li et al. 2010b) and the pulps of citrus such as Navel orange (Citrus sinensis) and Satsuma mandarin (Citrus unshiu) (Lado et al. 2015). In blueberry and kiwifruit (Li et al. 2010b; Liu et al. 2015), the AsA contents were higher in green fruits and decreased as fruit ripening, and the gene expression of MDHAR increased in fruit ripening, while that of DHAR decreased. Also, comparative analysis with AsA accumulation patterns was performed with two blueberry cultivars, “Bluecrop ” and “Berkeley.” In the two cultivars, although the AsA accumulation patterns were similar to each other, the decrement of AsA content in “Berkeley” fruit was more significant during fruit ripening than in “Bluecrop” fruit, which was consistent with the gene expressions of MDHAR and DHAR. The results suggest the higher efficiency of AsA recycling was partially responsible for the higher AsA accumulation in “Bluecrop.” In pulps of two citrus fruits, Satsuma Owari Mandarin and Washington Navel orange fruits, the AsA contents were decreased as fruit development and ripening progress, and the AsA contents in oranges were about twofold higher than in mandarins (Lado et al. 2015). The gene expression levels of three citrus MDHAR isozymes (MDHAR1, MDHAR2, MDHAR3) were increased during fruit maturation, suggesting the turnover of AsA in the pulp of oranges and mandarins was enhanced during fruit ripening. In oranges, the high expression levels of citrus MDHAR3 at the early stages of fruit development and citrus MDHAR1 and MDHAR2 during fruit ripening were observed, compared with those in mandarins. Thus, it may be assumed that the coordinated expression of the MDHAR isozymes may contribute to maintaining the AsA recycling in the pulp of fruits accumulating the high AsA contents. In contrast to AsA accumulation patterns of acerola, blueberry, and kiwifruit, the AsA contents of strawberry (Fragaria × ananassa) (Agius et al. 2003; Cruz-Rus et al. 2011), tomato (Ioannidi et al. 2009), and chestnut rose (Rosa roxburghii Tratt) were increased during fruit ripening. In strawberry, the gene expression level of MDHAR was the highest in red fruits and was correlated with the increase in AsA contents during fruit ripening. On the other hand, the gene expression of DHAR was high in green fruits of strawberry. Chestnut rose, one of the high AsA accumulated plants, contained high AsA contents in matured fruits and the AsA redox status was also enhanced during fruit ripening. The gene expression levels and enzyme activities of DHAR significantly correlated with AsA accumulation during fruit ripening, indicating DHAR contributes to the buildup of high AsA contents in chestnut rose fruits. In fruits, each plant MDHAR and DHAR isozymes contribute to AsA recycling at different phases of the ripening process. The influences of MDHAR and DHAR on AsA accumulation during fruit development and ripening vary among plant species. However, MDHAR and DHAR appear to have a complementary relationship in maintaining the AsA redox status in fruit development and ripening.
4 The Role of Ascorbic Acid Recycling Enzymes in the Chloroplast, the Cytosol, and the Guard Cell
Aerobic metabolism continuously produces ROS at the different plant cellular compartments, like chloroplasts, mitochondria, and peroxisomes. Thus, the AsA redox status is important to scavenge ROS produced under both normal and stress conditions in those cellular compartments (Gallie 2013). As part of the AsA-GSH cycle, MDHAR and DHAR isozymes can localize in these organelles. The chloroplast is a major source of ROS production in plants and produces ROS during the photosynthesis process as described in the previous section. Chloroplasts possess the light harvesting system in thylakoid membranes. The photosystems, photosystem I (PSI) and photosystem II (PSII) , form the core of the light harvesting system and are the primary sources of ROS production. In the photosynthesis process, after the PSI reduces ferredoxin (Fd), photoreduced Fd transfers electrons to NADP+, which is used for reduction of CO2 in the Calvin–Benson cycle. However, especially when NADP+ is limited, excess photoreduced Fd can transfer electrons to O2 instead of NADP+, which leads to ROS production in the stroma of chloroplasts. Thus, the regulation of the PSII reduction is necessary to maintain electron flow through the PSI and to prevent over-reduction of Fd. H2O2 is produced not only under normal conditions but also by oxidative stress. Under water deficit conditions like drought, salinity, and high temperature , intake of CO2 is restricted because of stomatal closure, and excess light leads to the formation of ROS mainly at the PSI as well as PSII. At the PSI, superoxide radical is formed from O2 by photoreduced Fd, and then it is converted to H2O2 by iron-containing superoxide dismutase (FeSOD) at thylakoid membranes via the Mehler reaction (Miller et al. 2010). The superoxide radical generated at the PSII is converted into more toxic ROS like hydroxyl radical via H2O2 by the Fenton reaction at the Fe-S centers of the PSII. Hydroxyl radical can harm different cellular components by lipid peroxidation, protein damage, and membrane destruction. At the PSII, singlet oxygen is also produced by the imbalance between light harvesting and energy utilization under environmental stress conditions, as well as by the over-reduction of the PSII. Singlet oxygen can severely damage the PSI and PSII. As described above, chloroplast is a major source of ROS production in plants. To detoxify ROS, AsA presents in millimolar concentrations in chloroplasts (Smirnoff 2000). In the stroma of chloroplasts, AsA is used as a reductant in a reaction of stromal APX for the reduction of H2O2, which is converted by copper-zinc superoxide dismutase (CuSOD) from the superoxide radical generated at the PSI, along with oxidation of AsA to MDHA. The AsA recycling is more essential than AsA biosynthesis to detoxify ROS and to protect from photodamage in chloroplasts because AsA is synthesized in mitochondria (Ostergaard et al. 1997), not in chloroplasts. The photoreduced Fd, which donates electrons to NADP+ in a reaction of Fd-NADP+ reductase, can also donate electrons to MDHA to back to AsA in the stroma as part of the thylakoid scavenging system (Miyake and Asada 1994). In addition to the AsA recycling by Fd, the MDHAR and DHAR with N-terminal targeting sequences are localized in chloroplasts, and they participate in the AsA recycling as part of the AsA-GSH cycle in the stroma of chloroplasts. In the lumen of chloroplasts, to prevent the over-reduction of the PSII, AsA donates electrons to the oxygen-evolving complex in the PSII. And also, AsA is used as a cofactor for violaxanthin de-epoxidase (VDE) (Eskling et al. 1997), which catalyzes the conversion of violaxanthin to zeaxanthin in the xanthophyll cycle. The xanthophyll cycle works for the dissipation of excess absorbed excitation energy during non-photochemical quenching. If not reduced immediately to AsA, MDHA disproportionates to AsA and DHA. Because neither Fd nor MDHAR are localized, MDHA cannot be recycled to AsA in the lumen of chloroplasts. Besides, in the lumen of chloroplasts, the disproportionation of MDHA is fast due to the low pH during light exposure (Asada 1999; Mano et al. 2004). Thus, MDHA produced in the lumen disproportionates to AsA and DHA, then DHA is transported to the stroma and recycled to AsA. In chloroplasts, the MDHAR and DHAR isozymes play important roles in maintaining AsA contents as stromal scavenging system.
Peroxisomes are single membrane spherical organelles and are also a major source of H2O2 production by their oxidative metabolism (del Rio et al. 2006; Palma et al. 2009). In peroxisomes, the β-oxidation of fatty acid produces H2O2 as a by-product of lipid catabolism. Superoxide radical is also produced at the two different locations of peroxisomes : peroxisomal matrix and membrane. As described above, when the water availability is low and stomata remains closed under stress condition, the ratio of CO2 to O2 is reduced considerably, which causes increased photorespiration leading to glycolate formation. The glycolate is oxidized by the glycolate oxidase in peroxisomes to produce H2O2 during photorespiration (Noctor et al. 2002). In order to detoxify H2O2, peroxisomes possess catalase to convert H2O2 to H2O and O2 in the peroxisomal matrix. In addition to catalase, peroxisomes possess APX and MDHAR in the peroxisomal membranes, and the APX and MDHAR cooperate with each other on the AsA-dependent electron transfer system to detoxify H2O2. H2O2 passes freely through membranes, and the H2O2 escaping from the peroxisomes is reduced by a peroxisomal membrane-bound APX and MDHAR using AsA as a reductant. In the seedlings of the Arabidopsis sugar-dependent2 (sdp2) mutant, which is deficient in the MDHAR localized to the peroxisomal membrane (Eastmond 2007), the H2O2 level was elevated, and the oil body proteins and lipids were oxidized, because of the low AsA contents. Moreover, the oxidation damage in the seedlings of the sdp2 mutant caused the inactivation of the triacylglycerol (TAG) lipase which is associated with the oil body membranes. In peroxisomes, all of catalase, APX, and MDHAR are essential to the peroxisomal antioxidant system in the seedlings. The main role of MDHAR appears to prevent H2O2 from escaping into the cytosol. The H2O2 escaping from the peroxisomes appears to lead to the inactivation of TAG hydrolysis in oil bodies and prevents the seedlings from producing energy for initial postgerminative growth. Thus, the MDHAR located in peroxisomes plays a crucial role in growth of seedlings.
Mitochondrion is involved in respiration and photorespiration and is also a source of ROS production as well as chloroplast and peroxisome . Although the ROS production in the mitochondria is less than those in light-exposed chloroplasts or in peroxisomes, mitochondria are the major sources of ROS under the dark, or in nongreen tissues (Szarka et al. 2012). The inner membrane of mitochondrion performs oxidative phosphorylation and energy-linked ion translocation. And energy capture, transduction, and utilization are achieved via a number of reactions in the inner membrane. The electron transport chain in mitochondria is the major factor to reduce O2 to form ROS. The role of manganese-containing superoxide dismutase is well known to protect from ROS induced by oxidative stress in mitochondria. In mitochondria, l-galactono-γ-lactone dehydrogenase presents at the inner mitochondrial membrane and catalyzes the conversion of l-galactono-γ-lactone to AsA, which is the last step of AsA biosynthesis in plants (Ostergaard et al. 1997). AsA, which is synthesized in the mitochondria, is not only transported to other cell compartments to detoxify ROS but also used as an electron donor to the electron transport chain in the mitochondria. In mitochondria, the presence of the AsA-GSH cycle was confirmed in some plants such as Pisum sativum (Jimenez et al. 1997) and Arabidopsis (Chew et al. 2003). DHA is generated in the intermembrane space by APX, and DHA can be transported to the mitochondrial matrix, where the AsA-GSH cycle is able to recycle DHA to AsA. In order to fuel these reactions, the reductants are supplied in the form of NADH and NADPH from the tricarboxylic acid cycle (Szarka et al. 2013).
In guard cells, ROS serve as secondary messenger for controlling gas exchange in leaves, and H2O2 can regulate the opening and closing of the stomatal pores. The signaling of abscisic acid (ABA) induces H2O2 production to facilitate the closing of the stomatal pores under water stress conditions. As described above, the photosynthesis process can induce the fluctuation of H2O2 contents during day and night along with the movement of the stomatal pores. The AsA redox status is of importance to the movement of the stomatal pores because AsA is used by APX to convert H2O2 to H2O in cells (Gallie 2013). In tobacco, the increase in the AsA redox status and the decrease in the H2O2 contents in the guard cells were achieved in the DHAR-overexpression plants (Chen and Gallie 2004). The DHAR-overexpression plants showed a higher percentage of open stomata, an increase in total open stomatal area, and increased transpiration. Also, the guard cells with an increase in the AsA redox status were less responsive to H2O2 or ABA signaling, and the plants exhibited greater water loss under drought conditions. On the other hand, the DHAR-suppression plant showed an increase in the H2O2 contents in the guard cells and a reduction in total open stomatal area, and consequently, the plant exhibited an increase of drought tolerance. Thus, DHAR can regulate the opening and closing of stomatal pores mediated by the AsA redox status through the AsA recycling in the guard cells.
5 Contribution of Ascorbic Acid Recycling Enzymes to Environmental Stress Tolerance
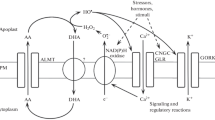
In general, salt, drought, cold/freeze, and high light intensity cause oxidative stresses at different compartments in cells (Mittler et al. 2004; Choudhury et al. 2013). And ROS generations such as H2O2 are promoted under oxidative stress conditions. The AsA-GSH cycle plays a significant role in oxidative stress tolerance by removing ROS generated in cells (Noctor and Foyer 1998; Mittler et al. 2004; Foyer and Noctor 2005). As shown in Figs. 1 and 3, MDHAR and DHAR are the components of the AsA-GSH cycle and they contribute to scavenging and detoxifying of ROS with AsA and GSH. APX catalyzes the reduction of H2O2 with simultaneous oxidation of AsA, which resulted in generation of MDHA as a primary product and followed by oxidation of MDHA to DHA. MDHAR and DHAR reduce two oxidized AsA, MDHA and DHA, respectively, before hydrolyzing to 2,3-diketogulonic acid. Therefore, MDHAR and DHAR are essential enzymes to maintain AsA contents as well as redox status, especially under oxidative stress conditions. As described in the previous section, there are many MDHAR and DHAR isozymes in plants (Leterrier et al. 2005; Lunde et al. 2006; Zhang et al. 2015). And they localize in various cell compartments such as chloroplast, mitochondrion, peroxisome, and cytosol. Thus, MDHAR and DHAR work together to protect from oxidative stress by ROS at different cell compartments. In response to oxidative stresses, the expression levels of MDHAR and DHAR are influenced, although their expression regulations remain unknown. Salt stress is a major limiting factor to plant growth and productivity along with ROS production in plant cells. As described above, MDHAR is the first enzyme getting back oxidized AsA to AsA. In Avicennia marina which is a highly salt tolerant species, MDHAR gene was inducibly expressed in salt stressed leaves (Kavitha et al. 2010). Temperature is also one of the factors that not only affects plant growth and development but also causes oxidative stress. Low temperature-induced ROS accumulation has been widely reported in plants (Suzuki and Mittler 2006). And MDHAR activities in tomato fruits were increased by cold stress (Stevens et al. 2008). In addition to oxidative stresses caused by salt and cold, the gene expression of MDHAR in Chinese cabbage was increased by oxidative stress induced by H2O2, salicylic acid, paraquat, and ozone (Yoon et al. 2004). The gene expression of DHAR is also induced by oxidative stress. In rice (Urano et al. 2000) and acerola (Eltelib et al. 2011), DHAR activities and expressions were increased under high- and low-temperature conditions. Moreover, the gene expression of DHAR in Jatropha curcas was also induced by oxidative stress such as treatments of PEG, NaCl, and H2O2 (Chang et al. 2017). The expressions of MDHAR and DHAR in response to oxidative stress in plants suggest that both of MDHAR and DHAR play roles in protecting cell components from oxidative stress through maintaining the AsA redox status. Thus, the overexpressions of MDHAR and DHAR can enhance oxidative stress tolerance. The overexpression of MDHAR in the cytosol of tobacco (Eltayeb et al. 2007, Eltelib et al. 2012) significantly increased AsA contents and AsA redox status as well as MDHAR activities, compared to wild-type tobacco. And the transgenic plants overexpressing MDHAR showed enhanced tolerance to oxidative stress by ozone, salt, and drought. The overexpression of human DHAR in the cytosol of tobacco slightly but significantly increased only AsA redox status but not AsA contents (Kwon et al. 2003). In contrast to human DHAR, the overexpression of rice DHAR in Arabidopsis slightly increased only AsA contents but not AsA redox status (Ushimaru et al. 2006). In spite of slight increase of AsA contents and AsA redox status by overexpressing DHAR, the transgenic plants with high DHAR activities enhanced tolerance to oxidative stress such as H2O2, methyl viologen, NaCl, and ozone (Kwon et al. 2003; Ushimaru et al. 2006; Chang et al. 2017). Compared to MDHAR, the overexpression of DHAR seems to provide no significant influence on AsA contents and AsA redox status in plants. However, under aluminum (Al) stress leading to accumulation of ROS in roots, tobacco plants overexpressing DHAR in the cytosol showed lower H2O2 contents, less lipid peroxidation, and lower level of oxidative DNA damage, compared with transgenic tobacco overexpressing MDHAR and wild-type tobacco (Yin et al. 2010). Al stress causes ROS accumulation mainly in the apoplast of roots. Because of the acidic pH (pH 5–6) where MDHA is unstable in contrast to DHA (Asada 1999), MDHA in the apoplast of roots tend to be spontaneously oxidized to DHA (Fig. 3). Because DHAR is absent in the apoplast, DHA can be reduced to AsA by DHAR after transported into the symplast , where DHAR exists. Thus, the tobacco plants overexpressing DHAR have higher tolerance to Al stress, compared to tobacco overexpressing MDHAR. MDHAR and DHAR play important roles in enhancing stress tolerance against environmental stress according to their localization sites. However, interestingly, there is a report about 2,4,6-trinitrotoluene (TNT) toxicity mediated by MDHAR (Johnston et al. 2015). TNT has a high toxicity and induces ROS accumulation in mitochondria. The suppression of MDHAR targeting to mitochondria leads to enhance tolerance to TNT, which is converted to a nitro radical by MDHAR in mitochondria. Also, the recovery of the MDHAR expression in the mutants reduces the tolerance to TNT.
Overview of ascorbate recycling in plant cells under oxidative stress. Under oxidative stress conditions by light, salt, drought, and cold/freeze, ROS generations such as H2O2 are promoted. Ascorbate peroxidase (APX) catalyzes the reduction of H2O2 with the oxidation of AsA, which resulted in generation of MDHA as a primary product and followed by oxidation of MDHA to DHA. The AsA recycling plays a significant role in oxidative stress tolerance by removing ROS generated in cells. In apoplast, MDHA is rather unstable because of the acidic condition, and MDHA tend to be spontaneously oxidized to DHA. After transporting into the symplast, where DHAR exists, DHAR will reduce DHA to AsA (Adapted from Yin et al. 2010)
ROS are accumulated in responses to biotic stress as well as abiotic stress in plants, and cause damage to the cell. However, under biotic stress conditions, ROS act as not only harmful compounds but also valuable substances, because ROS seem to directly kill the invading pathogen and serve as secondary messengers regulating pathogen defense responses (Tripathy and Oelmuller 2012; Choudhury et al. 2013). Thus, the cellular ROS levels may have substantial properties to pathogen defense. It was reported that Arabidopsis mutant vitamin c (vtc), which is deficient in AsA contents, enhanced resistance to pathogens (Barth et al. 2004). When virulent Pseudomonas syringae and Peronospora parasitica were infected with the Arabidopsis vtc mutants, the growth of the bacterial or fungal pathogen was substantially suppressed, compared to wild type. Besides, the expressions of the pathogen-related proteins were strongly induced by the infection of P. syringae and P. parasitica in the Arabidopsis vtc mutants along with high salicylic acid contents. The AsA contents seem to affect defense responses against pathogens in plant cells. Considering functional roles of the AsA-GSH cycles, The AsA-GSH cycles can be a regulator of cellular ROS contents in order to stimulate the redox-regulated plant defense (Noctor and Foyer 1998). Thus, MDHAR and DHAR must be involved in plant–pathogen interactions through maintaining the AsA redox status in plants. Puccinia striiformis f. sp. tritici (Pst) causes wheat stripe rust, which is one of the serious diseases in wheat. In wheat (Triticum aestivum L.), there are two MDHAR isoforms: TaMDHAR2 and TaMDHAR4. TaMDHAR2 gene is isolated as a target gene of PN-2013, one of microRNAs (miRNAs) , in a Pst resistant wheat cultivar. miRNAs are known as regulator of the gene expression of the target gene at the posttranslational level by degrading target mRNA or repressing gene translation (Feng et al. 2014b). When the wheat cultivar was challenged by Pst, the expression of PN-2013 was induced, and then that of TaMDHAR2 was decreased. The negative correlation of PN-2013 expression with the TaMDHAR2 expression suggests that PN-2013 suppress the gene expression of TaMDHAR2 in response to Pst. Similar expression response was reported in the incompatible interaction between wheat and pathogen (Feng et al. 2014a). The expression of TaMDHAR4 was decreased at the early stage of inoculation with a Pst race CYR23 (incompatible interaction), while no significant change was observed in the compatible interaction with another Pst race CYP31. Based on primary structures, TaMDHAR2 and TaMDHAR4 are expected to exist in cytosol and peroxisomes, respectively. Plant peroxisomes play essential roles in plant–pathogen interaction (McCartney et al. 2005) as well as photorespiration detoxification reaction and plant hormone synthesis (Hayashi and Nishimura 2003; Hu et al. 2012). TaMDHAR2 and TaMDHAR4 function at different cell compartments in plant–pathogen interactions. Besides, the suppression of TaMDHAR2 and TaMDHAR4 showed improved resistance to Pst in wheat (Feng et al. 2014a, b). Thus, MDHAR could contribute to pathogen defense through regulation of ROS metabolism by the AsA-GSH cycles.
6 Conclusion
AsA functions as an antioxidant which is essential for photosynthesis and stress response in order to remove generated ROS. Therefore, AsA contents and its redox status are of importance to health and stress tolerance of plants. And because of its nutrient values for human, which cannot produce AsA by themselves, the AsA contents in vegetables and fruits have been paid attention to for a long time. Although the AsA biosynthesis appears to be a primary factor in determining and regulating the AsA contents in plants, the AsA contents as well as its redox status are the result of the balance between its biosynthesis and recycling. In the AsA-GSH cycles including AsA recycling, two reductases, MDHAR and DHAR, reduce MDHA and DHA to AsA, respectively. In this chapter, at first, the primary structures of MDHAR and DHAR as well as their gene expressions in leaves and fruits are focused. Plants possess the multiple isozymes of MDHAR and DHAR, and the isozymes of MDHAR and DHAR are classified into some groups, based on their primary structures. They localize at different cell compartments: cytosol, chloroplast, mitochondrion, peroxisome, and vacuole. Considering that the overexpression of DHAR localizing in chloroplasts did not increase the DHAR activities in potato tubers, some MDHAR and DHAR isozymes might need to be posttranslationally modified in its localization site. The differences in their cellular localization and posttranslational modification suggest the isozymes function at different cell compartments in plants. In leaves, the gene expression patterns of MDHAR and DHAR suggest they are mainly involved in the diminishment of ROS produced during photosynthesis. MDHAR and DHAR also function during fruit development and ripening. There are mostly two types of the AsA accumulation model during fruit development and ripening. One is that the AsA is accumulated at the highest levels in green fruits and its contents decrease as fruit maturation, while the other shows opposite properties of AsA accumulation. Thus, the contribution and influence of MDHAR and DHAR on the AsA recycling during fruit development and ripening vary among plant species. And their gene expression patterns suggest that they have a complementary relationship in maintaining the AsA redox status.
Aerobic metabolism constantly generates ROS under both normal and stress condition at the different plant cellular compartments, like chloroplasts, mitochondria, and peroxisomes. Chloroplast is a major source of ROS production in plants. To ensure the continuous survival of plants under stress conditions, controlling and scavenging the ROS in the chloroplasts are very essential. AsA present in millimolar concentrations in chloroplasts (Smirnoff 2000). However, AsA is synthesized not in chloroplasts but in the mitochondria (Ostergaard et al. 1997) and transported to other cell compartments to eliminate ROS. Thus, MDHAR and DHAR isozymes are essential to scavenge ROS as part of the AsA-GSH cycles in chloroplasts, mitochondria, and peroxisomes. In addition, the AsA recycling has sometimes greater effect than the AsA biosynthesis especially under environmental stress, because the increase of AsA contents by AsA biosynthesis cannot occur in hours when plants suffer environmental stresses (Bartoli et al. 2006). Therefore, the efficiency of the AsA recycling is of importance to regulate cellular ROS contents under stress conditions. In fact, transgenic plants overexpressing MDHAR and DHAR show enhanced tolerance to oxidative stress, in spite of slight increase of AsA contents. However, ROS acts as not only toxic substances to damage cell components but also signal elements involved in pathogen defense. In pathogen infection, the gene expression of MDHAR was suppressed in the wheat leaves, and the suppression of MDHAR enhanced the resistance to pathogen stress. According to the environmental condition, MDHAR and DHAR contribute to stress tolerance through regulation of ROS metabolism by the AsA-GSH cycles.
In plants, the AsA contents are the results of balance of AsA biosynthesis and its recycling. Thus, the AsA contents can depend on the ability of AsA recycling as well as that of its biosynthesis in plants. The AsA recycling can serve as two integrated means by the effect on the AsA contents in plants, one is to control plant health and development, and the other is engineering of the improving with nutrient values and stress tolerance. The mechanisms of gene expressions of MDHAR and DHAR would be of great interest to manipulating the AsA contents in plants.
References
Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat Biotechnol 21(2):177–181
Amako K, Ushimaru T, Ishikawa A, Ogishi Y, Kishimoto R, Goda K (2006) Heterologous expression of dehydroascorbate reductase from rice and its application to determination of dehydroascorbate concentrations. J Nutr Sci Vitaminol (Tokyo) 52(2):89–95
Anjum NA, Gill SS, Gill R, Hasanuzzaman M, Duarte AC, Pereira E, Ahmad I, Tuteja R, Tuteja N (2014) Metal/metalloid stress tolerance in plants: role of ascorbate, its redox couple, and associated enzymes. Protoplasma 251(6):1265–1283
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Badejo AA, Fujikawa Y, Esaka M (2009) Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J Plant Physiol 166(6):652–660
Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134(4):1784–1792
Bartoli CG, Yu J, Gomez F, Fernandez L, Mcintosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57(8):1621–1631
Chang LM, Sun H, Yang H, Wang XH, Su ZZ, Chen F, Wei W (2017) Over-expression of dehydroascorbate reductase enhances oxidative stress tolerance in tobacco. Electron J Biotechnol 25:1–8
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16(5):1143–1162
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142(2):775–787
Chew O, Whelan J, Millar AH (2003) Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J Biol Chem 278(47):46869–46877
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8(4):e23681
Conklin PL (2001) Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ 24(4):383–394
Cruz-Rus E, Amaya I, Sánchez-Sevilla JF, Botella MA, Valpuesta V (2011) Regulation of ʟ-ascorbic acid content in strawberry fruits. J Exp Bot 62(12):4191–4201
Davey MW, Van Montagu M, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J (2000) Plant ʟ-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric 80(7):825–860
Del Rio LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol 141(2):330–335
Dipierro S, Borraccino G (1991) Dehydroascorbate reductase from potato-tubers. Phytochemistry 30(2):427–429
Eastmond PJ (2007) MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell 19(4):1376–1387
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K (2006) Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol Plant 127(1):57–65
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225(5):1255–1264
Eltayeb AE, Yamamoto S, Habora MEE, Yin L, Tsujimoto H, Tanaka K (2011) Transgenic potato overexpressing Arabidopsis cytosolic AtDHAR1 showed higher tolerance to herbicide, drought and salt stresses. Breed Sci 61(1):3–10
Eltelib HA, Badejo AA, Fujikawa Y, Esaka M (2011) Gene expression of monodehydroascorbate reductase and dehydroascorbate reductase during fruit ripening and in response to environmental stresses in acerola (Malpighia glabra). J Plant Physiol 168(6):619–627
Eltelib HA, Fujikawa Y, Esaka M (2012) Overexpression of the acerola (Malpighia glabra) monodehydroascorbate reductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S Afr J Bot 78:295–301
Eskling M, Arvidsson P, Akerlunde HE (1997) The xanthophyll cycle, its regulation and components. Physiol Plant 100(4):806–816
Feng H, Liu W, Zhang Q, Wang X, Wang X, Duan X, Li F, Huang L, Kang Z (2014a) TaMDHAR4, a monodehydroascorbate reductase gene participates in the interactions between wheat and Puccinia striiformis f. sp. tritici. Plant Physiol Biochem 76:7–16
Feng H, Wang X, Zhang Q, Fu Y, Feng C, Wang B, Huang L, Kang Z (2014b) Monodehydroascorbate reductase gene, regulated by the wheat PN-2013 miRNA, contributes to adult wheat plant resistance to stripe rust through ROS metabolism. Biochim Biophys Acta 1839(1):1–12
Fomenko DE, Gladyshev VN (2002) CxxS: fold-independent redox motif revealed by genome-wide searches for thiol/disulfide oxidoreductase function. Protein Sci 11(10):2285–2296
Foyer CH, Lelandais M (1996) A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol 148(3–4):391–398
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17(7):1866–1875
Foyer C, Rowell J, Walker D (1983) Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157(3):239–244
Gallie DR (2013) The role of ʟ-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot 64(2):443–443
Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64(2):355–365
Hayashi M, Nishimura M (2003) Entering a new era of research on plant peroxisomes. Curr Opin Plant Biol 6(6):577–582
Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25(1):85–92
Hu JP, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK (2012) Plant peroxisomes: biogenesis and function. Plant Cell 24(6):2279–2303
Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Jimenez A, Hernandez JA, Del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114(1):275–284
Johnston EJ, Rylott EL, Beynon E, Lorenz A, Chechik V, Bruce NC (2015) Monodehydroascorbate reductase mediates TNT toxicity in plants. Science 349(6252):1072–1075
Kato Y, Urano J, Maki Y, Ushimaru T (1997) Purification and characterization of dehydroascorbate reductase from rice. Plant Cell Physiol 38(2):173–178
Kavitha K, George S, Venkataraman G, Parida A (2010) A salt-inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochimie 92(10):1321–1329
Kim YS, Kim IS, Bae MJ, Choe YH, Kim YH, Park HM, Kang HG, Yoon HS (2013) Homologous expression of cytosolic dehydroascorbate reductase increases grain yield and biomass under paddy field conditions in transgenic rice (Oryza sativa L. japonica). Planta 237(6):1613–1625
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160(4):347–353
Lado J, Alos E, Rodrigo MJ, Zacarias L (2015) Light avoidance reduces ascorbic acid accumulation in the peel of Citrus fruit. Plant Sci 231:138–147
Leterrier M, Corpas FJ, Barroso JB, Sandalio LM, Del Río LA (2005) Peroxisomal monodehydroascorbate reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiol 138(4):2111–2123
Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW (2010a) Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant 139(4):421–434
Li M, Ma F, Liang D, Li J, Wang Y (2010b) Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi. PLoS One 5(12):e14281
Lisenbee CS, Lingard MJ, Trelease RN (2005) Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J 43(6):900–914
Liu F, Wang L, Gu L, Zhao W, Su H, Cheng X (2015) Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem 188:399–405
Lunde C, Baumann U, Shirley NJ, Drew DP, Fincher GB (2006) Gene structure and expression pattern analysis of three monodehydroascorbate reductase (Mdhar) genes in Physcomitrella patens: implications for the evolution of the MDHAR family in plants. Plant Mol Biol 60(2):259–275
Mano J, Hideg E, Asada K (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys 429(1):71–80
Mccartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT (2005) Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 17(12):3513–3531
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction of the monodehydroascorbate radical in spinach thylakoids. Plant Cell Physiol 35(4):539–549
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89(7):841–850
Noshi M, Hatanaka R, Tanabe N, Terai Y, Maruta T, Shigeoka S (2016) Redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase is required for high-light stress tolerance in Arabidopsis. Biosci Biotechnol Biochem 80(5):870–877
Noshi M, Yamada H, Hatanaka R, Tanabe N, Tamoi M, Shigeoka S (2017) Arabidopsis dehydroascorbate reductase 1 and 2 modulate redox states of ascorbate-glutathione cycle in the cytosol in response to photooxidative stress. Biosci Biotechnol Biochem 81(3):523–533
Obara K, Sumi K, Fukuda H (2002) The use of multiple transcription starts causes the dual targeting of Arabidopsis putative monodehydroascorbate reductase to both mitochondria and chloroplasts. Plant Cell Physiol 43(7):697–705
Ostergaard J, Persiau G, Davey MW, Bauw G, Van Montagu M (1997) Isolation of a cDNA coding for ʟ-galactono-gamma-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. Purification, characterization, cDNA cloning, and expression in yeast. J Biol Chem 272(48):30009–30016
Palma JM, Corpas FJ, Del Rio LA (2009) Proteome of plant peroxisomes: new perspectives on the role of these organelles in cell biology. Proteomics 9(9):2301–2312
Qin A, Shi Q, Yu X (2011) Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol Biol Rep 38(3):1557–1566
Reumann S, Quan S, Aung K, Yang P, Manandhar-Shrestha K, Holbrook D, Linka N, Switzenberg R, Wilkerson CG, Weber AP, Olsen LJ, Hu J (2009) In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol 150(1):125–143
Shimaoka T, Yokota A, Miyake C (2000) Purification and characterization of chloroplast dehydroascorbate reductase from spinach leaves. Plant Cell Physiol 41(10):1110–1118
Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol 3(3):229–235
Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M (2008) Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ 31(8):1086–1096
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126(1):45–51
Szarka A, Tomasskovics B, Banhegyi G (2012) The ascorbate-glutathione-alpha-tocopherol triad in abiotic stress response. Int J Mol Sci 13(4):4458–4483
Szarka A, Banhegyi G, Asard H (2013) The inter-relationship of ascorbate transport, metabolism and mitochondrial, plastidic respiration. Antioxid Redox Signal 19(9):1036–1044
Tang ZX, Yang HL (2013) Functional divergence and catalytic properties of dehydroascorbate reductase family proteins from Populus tomentosa. Mol Biol Rep 40(8):5105–5114
Tripathy BC, Oelmuller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7(12):1621–1633
Urano J, Nakagawa T, Maki Y, Masumura T, Tanaka K, Murata N, Ushimaru T (2000) Molecular cloning and characterization of a rice dehydroascorbate reductase. FEBS Lett 466(1):107–111
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163(11):1179–1184
Wang C-F, Huang L-L, Buchenauer H, Han Q-M, Zhang H-C, Kang Z-S (2007) Histochemical studies on the accumulation of reactive oxygen species (O2 − and H2O2) in the incompatible and compatible interaction of wheat Puccinia striiformis f. sp. tritici. Physiol Mol Plant Pathol 71(4–6):230–239
Wang GL, Xu ZS, Wang F, Li MY, Tan GF, Xiong AS (2015) Regulation of ascorbic acid biosynthesis and recycling during root development in carrot (Daucus carota L.) Plant Physiol Biochem 94:10–18
Xu Y, Zhu X, Chen Y, Gong Y, Liu L (2013) Expression profiling of genes involved in ascorbate biosynthesis and recycling during fleshy root development in radish. Plant Physiol Biochem 70:269–277
Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231(3):609–621
Yoon HS, Lee H, Lee IA, Kim KY, Jo J (2004) Molecular cloning of the monodehydroascorbate reductase gene from Brassica campestris and analysis of its mRNA level in response to oxidative stress. Biochim Biophys Acta 1658(3):181–186
Yoshida S, Tamaoki M, Shikano T, Nakajima N, Ogawa D, Ioki M, Aono M, Kubo A, Kamada H, Inoue Y, Saji H (2006) Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol 47(2):304–308
Zhang YJ, Wang W, Yang HL, Li Y, Kang XY, Wang XR, Yang ZL (2015) Molecular properties and functional divergence of the dehydroascorbate reductase gene family in lower and higher plants. PLoS One 10(12):e0145038
Acknowledgements
This work was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Grant-in-Aid for Scientific Research; JSPS KAKENHI Grant Numbers JP15K07394, JP24580143, JP21580113, JP19580108, and for Grant-in-Aid for JSPS Research Fellow; JSPS KAKENHI Grant Number JP17J00827.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Suekawa, M., Fujikawa, Y., Esaka, M. (2017). Physiological Role of Ascorbic Acid Recycling Enzymes in Plants. In: Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A. (eds) Ascorbic Acid in Plant Growth, Development and Stress Tolerance. Springer, Cham. https://doi.org/10.1007/978-3-319-74057-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-74057-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74056-0
Online ISBN: 978-3-319-74057-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)