Abstract
This chapter is an introduction to nanotechnology and nanomaterials with emphasis on orthopedic applications. It covers different types of nanomaterials used in orthopedic applications including metals, polymers, ceramics, carbon materials and composites and their main structures and features. In addition, the significance of the nanomaterial surface and its toxicological effect on the success rate of implantation were discussed. This chapter also covers current applications of nanomaterials in bone tissue engineering. Finally, it presents characterizations of nanomaterials and their interactions with biological systems using advanced nanotechnology tools.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanotechnology
- Nanomaterial
- Orthopedic implant
- Nano-size
- Surface
- Toxicity
- Bone
- Characterization
- Atomic force microscopy
- FTIR
- fluorescent microscopy
1 Introduction: Nanotechnology and Nanomaterials
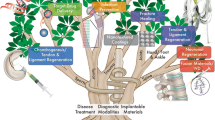
Nanotechnology can be defined as the science and engineering involved in the design, synthesis, characterization, and application of materials and devices at the nano-scale [1]. Today, nanotechnology is a rapidly expanding field, playing an important role in our society. It is changing our lifestyle in many fields, such as energy, environment, medicine, construction, transportation, and telecommunications. It brings a complex challenge to both theoretical and experimental sciences and provides a great opportunity for the development and welfare of human health [2]. The concept of nanotechnology started with the talk by Richard Feynman entitled “there is a plenty of room at the bottom” in 1959. Richard Feynman primarily highlighted immense capacity and potential of nanotechnology at an American Physical Society meeting at the California Institute of Technology [3]. Since then, nanotechnology has revolutionized science and played a significant role in every aspect of the society. Early applications of nanotechnology began with the crafting of glass for ceramic antiques, such as the Lycurgus Cup created in the fourth Century AD that generated color change due to the interaction of light with gold (Au), silver (Ag), copper (Cu), and iron (Fe) nanoparticles embedded in the glass [4]. Today, nanotechnology is integral in various fields of science, serving as the basis for instruments such as the field ion microscope and atomic force microscopes (AFM). The manipulation of individual atoms became possible with the introduction of the Scanning Tunneling Microscopy (STM) in the early 1980s. Nanotechnology has found its way into the marketplace since the early 2000s by allowing manufacturers to enhance materials employed in various applications such as a tennis racket, electronic devices, and sunscreen [5]. During the past decades, nanotechnology has achieved immense progress and recently it can be applied to the fields of medicine and biology to enhance drug delivery, medical diagnostics, and manufacturing processes [6]. There is a revolution in the application of nanotechnology in medicine because most biological molecules are in the nano-scale. Therefore, nanotechnology has been regarded as an emerging technology in medicine and is commonly referred to as nanomedicine [6]. In recent years, nanotechnology has been successfully applied in orthopedic implants , transforming drug delivery and medical diagnostic tools . The application of nanotechnology in orthopedic implants has resulted in incredible improvements in the treatment of bone defects and orthopedic trauma. Figure 1 illustrates the applications of nanotechnology in orthopedic surgery. Despite recent advances in nanotechnology, particularly within orthopedics, it seems that the potential of nanotechnology is vast and needs to be realized.
Generally, materials with a nano-sized topography or materials composed of the nano-sized structures are considered as nanomaterials. For instance, materials with a basic structural unit in the range of 1–100 nm, individual or multilayer surface coatings with a thickness in the range of 1–100 nm, crystal solids with the grain sizes between 1 and 100 nm, fibers with diameters in the range of 1–100 nm, and powders with average particle sizes between 1 and 100 nm are considered as “nanomaterials” [7, 8]. The application of nanotechnology and nanomaterials in hard/soft tissue engineering is a relatively new frontier research. So far, the application of nano-scale materials has been tested for a wide range of materials such as polymers, metals, composites, and ceramics. These nanomaterials show superior properties as compared to the micron-sized materials.
Over the last decades, nanomaterials have been highlighted as promising candidates for overcoming problems associated with the failure of conventional biomaterials and implants. Studies indicate that materials in the nano-scale exhibited excellent properties , such as superior mechanical, electrical, cytocompatible, catalytic, and magnetic properties as compared to their micron structured counterparts. Due to the unique physical properties and the greater surface area in a given volume, nanomaterials can be designed to mimic the natural environment of tissues to promote cell growth, tissue regeneration and even to reduce infection rates [9]. Nanomaterials have been also applied as coatings and scaffolding to provide a better interaction between the implant surface and surrounding tissue [10]. Therefore, nanomaterials can be selected as the next generation of implant coatings to enhance implant/tissue integration owing to their unique surface properties, which provide a more suitable environment for bone ingrowth [9]. Decreasing the likelihood of infection and improving scar appearance are a few examples of the advantages of using nanotechnology for implants [11]. It was reported that the application of a nanotexture material in total joint replacement improved osteoblast adhesion and osseointegration, hence increased the success rate of implantation [11].
Today there are many common and significant clinical problems of bone and joints such as bone fractures, bone cancer, and osteoarthritis. The National Center for Health Statistics (NCHS) reports a large number of patients with bone fractures, osteoarthritis and associated disorders annually [12]. According to the American Academy of Orthopedic Surgeons, there was about a 83% increase in hip replacements between 2000 and 2004 [12]. Although the need for orthopedic implants has been increased, the conventional implant materials last only 10–15 years on average. With this limitation, the rate of implant failures, such as implant loosening, osteolysis, inflammation, infection, and wear debris, is severely increased [11]. Therefore, there is an urgent need to design the new generation of cytocompatible bone and joint replacement candidates to regenerate bone tissue at the defect sites that will last the lifetime of the patient. The application of nanomaterials which are biomimetic with excellent mechanical properties has become an urgent requirement in orthopedics. Materials at the nano-scale exhibit unique surface properties such as surface wettability, surface chemistry, surface energy and surface topography due to the higher available surface area and roughness as compared to the micron-sized counterparts. It has been shown that the adhesion of specific proteins, such as fibronectin, vitronectin, and laminin, is directly related to the surface properties and bioactivity of implants [11]. In addition, osseointegration between implant materials and bone tissue is considered as a critical factor in designing successful orthopedic implants [1]. Studies have demonstrated that nanostructured materials with desirable cell adhesion properties can promote protein adhesion as well as new bone growth as an advantage of such nanomaterials as compared to conventional materials for tissue growth [1]. Therefore, various metals, polymers, nanophase ceramics and composite scaffolds have been designed for orthopedic applications via controlling surface properties. Many studies have indicated that nanostructured ceramics enhance friction and wear problems associated with joint replacement components [13, 14]. For instance, the application of nano-hydroxyapatite (HA) as a substitute, filler, coating, and composite material in orthopedics promotes biomineralization [15, 16].
Nanotechnology and nanomaterials have revolutionized many fields such as biology and medicine. Enhanced drug delivery, medical diagnostics, and bioengineering are few examples. Recently, researchers have demonstrated that nanotechnology and nanomaterials possess a huge potential for further applications in disease detection, intervention and particularly orthopedics [10]. Introducing a new dimension to technology and materials with the possibility of manipulating at the nano level has been regarded as an emerging science in many fields of competitive industries. This chapter provides a brief introduction to the nanotechnology and nanomaterials in orthopedic applications and tissue engineering. In addition, the toxicological effect of nanomaterials in the orthopedic applications is discussed. Finally, the last section of the chapter dedicated to the characterization of the orthopedic nanomaterials provides very critical information for the design and fabrication of these materials.
2 Nanomaterials Enhanced Orthopedic Implants
Applications of orthopedic implants are growing rapidly across the world. Many bone defects with low potential for regeneration are the result of trauma, tumor, infection or periprosthetic osteolysis and need to be surgically treated [17]. Surveys have shown that in the United States alone, there are over 600,000 joint replacements annually and about 80% of all transplantations are bone autografts and allografts [17]. However, bone autografts and allografts have shown many drawbacks such as immunological rejection, the risk of infection, and the high cost of operation [18]. The average lifetime of a conventional orthopedic implant is not permanent and unfortunately, there has been many cases of failed implants [19, 20]. Once an implant fails, revision surgeries are required, which increase pain, the cost of treatment, and added risk of post-surgery complications [18]. The poor surface interaction between the biomaterial and host tissue results in insufficient tissue regeneration around the biomaterials after implantation, which is one of the key factors of implant failure [21]. Therefore, an orthopedic implant must be inhabitable for osteoblast to proliferate the implant surface and regenerate new bone tissue [22]. By controlling surface properties , a broad range of ceramics, polymers, metals and composite scaffolds at the nano-scale have been designed for bone/cartilage tissue engineering applications [10]. Just like conventional or micro-structured materials, nanomaterials also can be classified as nanoparticles, nanocrystals, nanofibers, nanoclusters, nanotubes, nanowires, nanofilms, nanorods, etc. The success rate of bone tissue engineering products is strongly influenced by appropriate material selection and the fabrication method for producing a scaffold. So far, various natural and synthetic biomaterials such as ceramics, polymers, and a combination of them have been employed for bone tissue engineering applications [10]. Table 1 summarizes nano-sized materials used in orthopedic applications.
Although a wide range of materials at the micro-scale have been employed for orthopedic applications, nanomaterials have attracted considerable attention due to their specific properties to overcome the problems associated with conventional implant biomaterials [23, 24]. The biomimetic feature and physical and chemical properties of nanomaterials play a significant role in cell growth and tissue regeneration owing to the nano-scale dimension of natural tissue [25]. With recent advances in nanomaterials, novel orthopedic implants have been introduced with great potential for better osseointegration to efficiently stimulate new bone growth as compared to conventional implants [26].
Excellent physiochemical properties and nanostructured extracellular matrices (ECM) of nanomaterials can decrease infection and increase bone growth [27]. Investigations have shown that implants with a nano-scale surface roughness that mimic natural tissue promote more tissue growth than smooth implants [28]. In addition, it was demonstrated that nano-sized materials can be beneficial in promoting bone growth, or detrimental in promoting inflammation or infection [26]. Therefore, the design and fabrication of nanomaterials is a new confront in orthopedic applications which offers a new type of implant and scaffold that can mimic the complex and hierarchical structure of hard/soft tissues [26].
Controlling cell behavior is one of the important criteria in the design of materials for soft/hard tissue regeneration [29]. It is reported that nano-scale structures can control the orientation and alignment of cells and the mineralization of the collagen matrix [30]. In addition, nano-scale structures induce mineral deposition of calcium (Ca) and phosphorus (P) by osteoblasts from the culture media. Figure 2 shows scanning electron microscope (SEM) images of MG63 cells cultured on different surfaces of Ti for 1 day. The MG63 cells on the polished Ti surface were spindle-like and spreading. However, the cells on the nano-structured Ti surface have a flattened morphology with numerous filopodia actively attached on the surface. MG63 cells on the micro-structured Ti surface showed spindle-like sharpness and their lamellipodia attached mostly on the sides of the microholes, however, the filopodia adhered less to the surface directly. These images indicate that nano-structured Ti surface is more suitable for the cell growing and spreading as compared to the polished and micro-scale Ti surface [30].
SEM images of MG63 cells cultured for 1 day incubation on (a) the polished Ti surface, (b) the micro-roughened Ti surface, (c) the nano-network structured Ti surface, (X-2) are images with higher magnification taken of the area enclosed by the white square in (X-1). Scale bar = 50 μm (X-1); Scale bar = 5 μm (X-2). Images reprinted with permission of Liang et al. (2015). Copyright 2015 Elsevier Ltd. [30]
2.1 Significance of the Orthopedic Nanomaterial Surface
The physical and chemical properties of the implant surface have considerable effects on cell responses, cell attachment and cell migration [26]. Natural bone is a nano-structured composite which consists of a polymer matrix reinforced with nano-sized ceramic particles [10]. Investigations demonstrated that enhanced osteoconductivity , as well as osteoinduction, is achieved when the implant resembled bone in terms of its nano-scale features [55]. Therefore, sufficient osseointegration between bone tissue and implant material is considered as a significant criterion for designing of orthopedic implant materials. As illustrated in Fig. 2, a nanostructured implant with desirable surface properties can promote surface interaction with proteins, hence stimulate new bone growth as compared to the conventional materials [27]. Metals such as Ti, Ti alloys, cobalt-chromium (Co-Cr) alloys, and stainless steel are the most common materials for orthopedic implants due to their biocompatibility, excellent electrochemical as well as mechanical properties . However, metallic implants are prone to failure due to many factors including stress shielding and strain imbalances which may lead to implant loosening and eventual failure [56]. The favorable interaction between the implant surface and host tissue accelerates healing and increases implant longevity which results in a reduction in revision surgery [17]. It is believed that the lifespan of orthopedic implants is limited owing to the lack of investigation to understand cellular recognition of the proteins initially adsorbed by the implant surfaces. Therefore, to reduce failures of orthopedic implants, cellular processes that result in effective new bone growth must be investigated. The essential factors in cellular function including proliferation, high initial adhesion, and differentiation from non-Ca-depositing to Ca-depositing cells are considered as positive responses from osteoblasts. High activities of osteoblasts and osteoclasts are required for bone remodeling and providing healthy hard tissue-implant interactions. In contrast, poor activity leads to cell necrosis adjacent to the implant, which results in a higher risk of implant failure [57]. Due to the significance of cellular processes in orthopedic implants, many investigations have been done to modify the surface properties of implant biomaterials to increase the interaction with cells, hence, promote bone regeneration [58]. Different methods , such as hydrothermal treatment, electrochemical processing, sol-gel, sandblasting and chemical etching, have been applied for the surface modification of implants [58]. A recent study suggested that nanomaterials are suitable candidates to modify the surface features for orthopedic materials because they possess the appropriate environment for suitable tissue growth and reduced chronic inflammation as well as infection [59]. Surface nanostructuring and nanostructure coatings are considered as two major methods in surface modification at the nano-scale. Surface nanostructuring provides a nano-topography with large surface areas providing more binding sites to cell membrane receptors [60]. In addition, surface nanoarchitectures guide several molecular and biological processes at the implant/tissue interface, which results in greater biocompatibility of implants [61, 62]. One example of the effect of surface nanostructuring on the properties of commercially pure Ti (CP Ti) is shown in Fig. 3. The results reveal that the nanostructured topologies of titanium implants increase the bone-implant interfacial strength by improving the proliferation, differentiation, and development of the osteoblastic phenotype [26, 63]. In addition, it is demonstrated that the TiO2 thin film and TiO2 nanotubes improve cell viability, attachment, and proliferation, due to the high surface energy, a large number of particle-binding sites, and the topology mechanism [60]. Another alternative way for surface modification of implants at the nano-scale is to use nanostructured coatings and nanocomposites which affect the interaction of the bone/implant while tailoring their biological responses [26]. There are several studies on the preparation and characterization of nanocomposites, nanopolymers, nanoceramics, and carbon nanomaterials which indicate the significance of nanomaterial surfaces [64]. For instance, Safonov et al. have shown that Ti and stainless steel implants with nanostructured aluminum oxide coatings possess higher biocompatibility as compared to the non-coated surfaces owing to the hydrophilicity of the coated surface [65]. Rezazadeh et al. have reported that the incorporation of Ti nanotubes as a reinforcement in HA coatings significantly enhanced mechanical properties of the coating and improved the electrochemical properties of the metallic substrate [66, 67].
Effects of surface nanostructuring on the cell viability, differentiation, and bactericidal capacity of CP Ti. (a, b) SEM images of TiO2 nanotube layer and microporous Ti, respectively. (c, d) The nanostructuring effect on the MG-63 cell proliferation and ALP activity (*P < 0.05, n = 9). Images reprinted with permission of Xia et al. (2012). Copyright DOVE medical press [63]
Implantable biomaterials such as Co-Cr alloys and Ti alloys are widely applied for orthopedic applications to provide structural support as bone does [68]. With recent progress in nanotechnology, nanostructural materials have been introduced to orthopedic applications as novel implant materials with superior potential for osseointegration [11]. In comparison to the common materials, the desirable surface properties of nanomaterials efficiently promote new bone growth. In addition, it is reported that metallic implant materials with nanostructured surfaces enhance mechanical properties of the bone interface which is due to the enhanced available interlocking and anchor sites on the surface of the nanostructured implant in comparison to conventional implants. Figure 4 shows the mechanism by which metallic implant with a nanostructure possibly is superior to conventional materials for bone regeneration. As it is shown, the bioactive nano-surface of such implants can mimic those of natural bones to promote greater amounts of protein adsorption and efficiently stimulate new bone formation than conventional materials [11, 68].
Schematic illustration of the mechanism by which nanomaterials may be superior to conventional materials for bone regeneration. The bioactive surfaces of nanomaterials mimic those of natural bones to promote greater amounts of protein adsorption and efficiently stimulate more new bone formation than conventional materials. Images reprinted with permission of Zhang et al. (2009). Copyright 2008 Elsevier Ltd. [11]
3 Nanomaterials in Bone Tissue Engineering for Orthopedic Application
The main goal of bone tissue engineering is to develop biodegradable materials to be used as bone substitute for filling large bone defects. For this purpose, tissue substitutes are fabricated in the laboratory by combining artificial components such as polymers, metals, ceramics, composites and carbon materials with living cells. Subsequently, the fabricated tissue is implanted into a patient’s body to create, repair or replace natural tissues and/or organs [64]. In the past, tissue engineering with the incorporation of autografts and allografts had a variety of drawbacks for the patients [69]. Today, by controlling surface properties, a broad range of metals, polymers, nanophase ceramics and composite scaffolds is designed for tissue engineering applications [17]. In designing scaffolds as a temporary 3D matrix , biodegradability and the same functionality of the ECM network should be considered. Therefore, understanding the interaction between the cell and matrix is of paramount importance [70]. The functional roles of the native ECM scaffold are structural, biological, and biochemical. Structural : to play an important role in supporting cells and provide a substrate for cell migration and survival; Biological: to play a functional role which is required to separate growth factors and other chemical cues that regulate cell fate; and Biochemical : to offer bioactive peptide sequences which lead to receptor binding and activate intracellular signaling pathways [10].
Recently, it was reported that the size and topographical features of the structural elements in the ECM also need to be considered as key characteristics of the ECM [10]. Comprehensive consideration of structural properties of the scaffolds and their interactions with soft/hard tissue at the nano-scale range is needed for the successful design of scaffolds [71]. Lately, fabrication of biomimetic nanostructured tissue scaffolds which encapsulate cells for orthopedic applications such as bone and cartilage tissue has been tremendously increased [10]. The architecture of the scaffold defines the ultimate shape of new hard tissues by providing the necessary support for the cells to proliferate and differentiate [10]. Therefore, these scaffolds should be able to provide suitable diffusion of oxygen and nutrients to cells embedded within the scaffold as well as proper diffusion of waste from the cells [72].
Figure 5A shows the nanostructured hierarchal self-assembly of bone. Bone is a natural nanocomposite that consists of hard inorganic component (hydroxyapatite, HA, Ca10(PO4)6(OH)2) [66], a protein based soft hydrogel template (i.e., collagen, non-collagenous proteins (laminin, fibronectin, vitronectin) and water). 70% of the bone matrix is composed of nanocrystalline HA with the typical length and thickness of 20–80 nm and 2–5 nm, respectively [16]. Dense nanostructured ECM is rich in elastin fibers, collagen fibers and proteoglycans. However, cartilage is a low-rate regenerative tissue composed of a few chondrocytes. The absence of progenitor cells and vascular networks and lack of chondrocyte mobility in the dense ECM limit regenerative properties of cartilage [11]. Figure 5B illustrates nanophase Ti and nanocrystalline HA/helical rosette nanotubes (HRN) hydrogel scaffold . Recently, designing nanomaterials with sufficient mechanical properties which are also biomimetic in terms of their nanostructure has become very common to enhance bone cell and chondrocyte functions [11]. However, much of the current work of nanomaterials and nanotechnology is in the laboratory or in vivo testing. Therefore, more translational research is needed from basic science to practical applications [10].
The biomimetic advantages of nanomaterials . (a) The nanostructured hierarchal self-assembly of bone. (b) Nanophase titanium (top, the atomic force microscopy image) and nanocrystalline HA/HRN hydrogel scaffold (bottom, the SEM image). Images reprinted with permission of Zhang et al. (2009). Copyright 2008 Elsevier Ltd. [11]
4 Toxicological Effect of the Nanomaterials for Orthopedic Applications
Nanobiomaterials are new types of materials in terms of scale, properties, and functions that mimic the complex and hierarchical structure of the native hard/soft tissue. Owing to the nano dimensions of natural tissue of the body, the biomimetic features and unique physical and chemical properties of nanomaterials play a crucial role in stimulating cell growth as well as tissue regeneration. However, by the rapidly emerging science of nanotechnology, a main concern of the toxicity of nanomaterials on human health and environment has been raised [73]. Recently, nanotoxicology, the science of engineering nanostructures that deals with health threats or adverse effects on living organisms, has attracted considerable attention [26]. The diversity and unlimited potential applications of nanomaterials have posed a great challenge for their safety evaluation. It is believed that almost every material can be toxic at a high enough doses. However, the question is how toxic are nanomaterials at the potential concentrations used for their intended applications? Analytical methods such as measuring the concentrations of nanomaterials in the human body and environment are still at the beginning stage [74]. In the study of the toxicological effect of the nanoparticles, several research groups used different cell lines, culturing conditions, and incubation times. However, it is difficult to compare all the different findings and understand whether the cytotoxicity observed is physiologically relevant or not. Many biological models such as cells in culture, aquatic organisms including embryonic zebrafish, and whole-animal tests (such as rodents) have been applied to measure potential toxicological effects of chemicals [73]. Investigations show that some nanoparticles are able to translocate into different organs via the circulatory and lymphatic systems after entering the body [75]. There is a possibility of toxic responses of the nanoparticles generated from the degradation of nanomaterials in metallic artificial joint implants [76]. The formation of nano-scale debris may occur when orthopedic implants are subjected to physiological loading properties [77]. This may have many adverse effects such as exacerbation of asthma, chronic inflammatory, lung diseases, inflammation, fibrosis, metal fume fever, and carcinogenesis [78]. It has been reported that the tendency for toxicity increases with decreasing particles size [79]. Therefore, in the design and fabrication of nanomaterials, particularly in orthopedic applications, the biological effects of these nanomaterials and their interaction with living organisms should be studied. The toxicity of nanomaterials depends on the type of the base material, its size, and shape. The range of nanomaterials is very extensive in different compound classes such as metals, polymers, carbon, and composites. Hence, the effects of nanomaterial characteristics such as surface area, shape, and size on their toxic response need to be evaluated [80]. In a study conducted by Ribeiro et al., the adsorption of proteins to anatase nanoparticles in serum-containing medium was investigated [81]. Sodium dodecyl sulfate (SDS) gel electrophoresis demonstrated that by increasing the nanoparticle concentration, the protein adsorbed on anatase increased (Fig. 6a). The results indicate that the anatase bio-complexes had no influence on osteoblasts viability after exposure for 72 h. The percentage of live/dead cells was similar to the negative control (medium culture). The positive control (CuO) presented higher levels of dead cells (Fig. 6b). The observation shows that there are no significant differences in the population of necrotic and apoptotic cells exposed to the different concentrations of anatase nanoparticles [81]. It is believed that in order to determine the toxic effects of nanomaterials, more effort is required for in vivo evaluations, clinical trials, and toxicological surveys before it is commercialized for orthopedic applications.
Osteoblasts viability upon anatase nanoparticles exposure: (a) Illustration of SDS-PAGE gels and identified bands. (b) Live and dead assay of osteoblast viability after 72 h of nanoparticle exposure. Images reprinted with permission of Riberio et al. (2016). Copyright 2016 Nature Publishing Group [81]
5 Characterization of Orthopedic Nanomaterials
Properties of the nanomaterials used in orthopedics should be studied and well documented before and after their clinical applications. Depending on the interaction of these nanomaterials with the host tissue in the body, size, shape, crystal structure, elemental analysis, chemical bonding, and biocompatibility of these materials need to be investigated. Nanotechnology tools that can enable advanced characterization of nanomaterials and their interactions with biological systems have been recently developed. Such advanced tools can provide new insights for understanding the biocompatibility as well as non-toxicity of nanomaterials used in orthopedics and other medical applications. SEM and AFM are used mainly for the investigation of surface morphology at the nano-scale. In addition, Transmission Electron Microscopy (TEM) provides 2D and 3D images of 3D structures. It also reveals crystal structure information as well through electron diffraction, in a similar fashion with how a X-ray Diffractometer does. Via Electron Energy Loss Spectroscopy (EELS, attached to TEM) and Energy Dispersive X-Ray Spectrometry (EDS, as attached to either SEM and TEM), chemical analysis is carried out. Biocompatibility can be examined using either SEM or a Fluorescence Microscope. Chemical bonding information can be obtained via Fourier Transform Infrared Spectroscopy (FTIR) and Raman Spectroscopy. Investigation of the properties of the nanomaterials as synthesized or in environments mimicking real-life conditions provides critical information for optimization of the manufacturing parameters of these materials.
5.1 Size, Shape and Topological Information
5.1.1 Scanning Electron Microscopy
A conventional SEM is very powerful for the size and shape analysis of nanomaterials with resolutions down to 1 nm. SEM samples can be very large. Upon the interaction of primary electrons with the nanomaterial, secondary electrons are created at the surface providing topographical information of the sample. Backscattered electrons are also generated and they create atomic number related contrast. X-rays and Auger electrons are also emitted which are fingerprints for elements [82].
Ti is one of several metals, whose alloys are frequently employed as nanomaterials. In a recent work, Bhosle et al. carried out the anodization technique to create Ti-15Zr nanotubes in different anodization voltages [83]. According to the SEM images in Fig. 7, it was concluded that more electronic and ionic diffusion occurred at higher anodization voltages, causing an increase in the nanotube diameter. Voltages higher than 40 V resulted in sponge-like structures, even higher voltages caused dissolution of nanotubes. In addition to the size and shape of the nanomaterials, surface roughness is also a critical parameter to be controlled for the attachment or repulsion of the osteoblast cells, which has been reported via SEM imaging by both Nelson et al. [84] and Alves et al. [85]. Calcium phosphate can be utilized for drug delivery and to increase the bone mass for medicine and dentistry due to their osteoconductivity and biocompatibility [86]. In the work carried out by Nelson et al. [84], nano-amorphous calcium phosphate , nanocrystalline HA and conventional HA were fabricated via wet chemistry and hydrothermal treatment and pressed into compacts. It was concluded that the nano-amorphous calcium phosphate has larger specific surface area than non-crystalline HA. SEM images show the morphological changes of nanocrystalline HA to the conventional apatite. Peptide lysine-arginine-serine-arginine (KRSR) functionalization was carried out on nanoparticulate crystalline HA and investigated for osteoblast adhesion. They reported that compared to conventional HA and nano-amorphous calcium phosphate, nanocrystalline HA showed more widespread attachments.
Field Emission (FE)-SEM images of the Ti–15Zr substrates after anodization. The anodization was carried out at room temperature by using an electrolyte composed of 15 vol% water and 0.2 M ammonium fluoride in ethylene glycol solution. The nanotubular surfaces of substrates anodized at six different potentials—that is, 10, 20, 30, 40, 50 and 60 V—for a constant duration of 30 min were obtained. Panels (a), (b), (c), (d), (e) and (f) are the lower magnification images (×30,000) of anodization at 10, 20, 30, 40, 50 and 60 V, respectively. Panels (g), (h), (i), (j) and (k) are higher-magnification images (×50,000) of anodization at 10, 20, 30, 40 and 50 V conditions, respectively. Images reprinted with permission of Bhosle et al. (2017). Copyright 2017 ICE Publishing [83]
5.1.2 Fluorescence Microscopy
Fluorescence microscopy has been frequently used for biological research. Some fluorescence techniques can image with a 20–30 nm resolution and visualize molecular interactions in the samples [87]. What makes fluorescent microscopy favorable over optical imaging techniques is the use of fluorescent proteins. With the advancements of fluorescent proteins, which are not toxic to the cell, specific cells and tissues can be tagged. Also, advancements have increased the brightness of the fluorescence and the photostability [88]. Fluorescence is resistive to the effects of pH variation. In the recent work by Bhosle et al. biocompatibility tests were carried out by fixing and staining the MRC-5 cells with formaldehyde in phosphate buffered solution (PBS) and with Actin Red 555 Ready Probes , respectively [83]. According to the fluorescence images reported in Fig. 8, 20 and 30 V anodization showed well spreading of cells on nanotubes 1 day after seeding (Fig. 8b, c) compared to control samples (Fig. 8a), and spreading enhanced further as time passed (day 7) (Fig. 8f, g) compared to control sample (Fig. 8e). Cell spreading was observed for 40 V anodization after day 7 (Fig. 8h). It is concluded that the spreading of cells on the anodized samples is caused by the presence of well-organized actin stress fibers at the cortical areas of cells.
Fluorescence images of day 1 and day 7 of MRC-5 cells seeded on anodized samples. The effect of MRC-5 cells was studied on an anodized sample used as a control (a, e) and three different types of nanotubular surfaces—that is, (b, f) 20 V, (c, g) 30 V and (d, h) 40 V, where the date information is given as (day 1, day 7). Images reprinted with permission of Bhosle et al. (2017). Copyright 2017 ICE Publishing [83]
5.1.3 Atomic Force Microscopy
AFM gives very precise quantitative surface topography information. During the operation of the AFM, the tip of the cantilever touches the sample, bends and produces angle changes for the incoming laser and reflects the laser. Thus, surface topography maps are obtained with nanometer resolution [82]. Surface properties of the implants including the affinity to water, steric hindrance, and roughness affects the cell adhesion on the surfaces, as discussed by Park et al. [89]. Based on the AFM surface topography maps, the surface of the nanophase TiO2 is rougher than conventional TiO2.
5.2 Inner View, Crystal Structure, Chemical Information
5.2.1 Transmission Electron Microscopy
A modern TEM can resolve features with sizes lower than 0.1 nm, after the incorporation of aberration correctors. A conventional TEM provides a 2D projection image of a 3D object with a contrast depending on the thickness and density using a parallel coherent beam. With this, size and shape information of the nanoparticles can be obtained. Electrons reflect upon their interactions with atoms in specific planes of the crystals. This crystal structure information is a fingerprint for each material. With the electron holography technique , magnetic features of the materials can also be detected. When used in scanning mode, that is, scanning transmission electron microscope (STEM), similar to a SEM, electrons that reflect in high angles construct atomic number dependent images. Using this mode and EELS and EDS, atomic resolution chemical characterization can be done. EELS records the energy loss information for each electron, which is characteristic of elements, molecules, bonding or electronic structure. EDS records the energy of the X-ray produced, which again is a fingerprint for each element [90].
If the sample has organic materials or is hydrated with weak bonding, to eliminate the possible electron induced material damage, these nanomaterials should be imaged and characterized either as in liquid state or frozen state. Liquid state TEM involves either usage of a fluid flow holder or graphene liquid cells, in which liquids are encapsulated in between either two Si3N4 windows or two graphene layers, respectively [91, 92]. The former has the advantage of flowing in liquid to monitor the interaction of the nanomaterials in the shell with the flow, but is very thick, whereas the latter has no capability of flowing any liquid, but is very thin, making dynamic atomic resolution liquid imaging possible. These two techniques are capable of monitoring live nano-scale activities. Cryo-TEM is also very useful to preserve the structure at liquid nitrogen temperatures and to do static imaging with low sample damage due to the low temperature during imaging [93]. Colloidal samples can be simply cryo-plunged and thicker samples need to be high pressure frozen, cryo-microtomed and imaged in cryogenically or undergo freeze-substitution and prepared for conventional imaging. With the invention of the single particle analysis via cryo-TEM, multiple images of the homogeneously distributed nanomaterials are combined, grouped and processed to simulate a 3D object. Cryo-tomography is also a complementary tool for single particle analysis where the materials are tilted with discrete angles and images are collected at each tilting angle and processed to create a 3D object .
Metallic implants are known to undergo surface degradation when implanted in the human body and release debris and metallic ions which can cause inflammatory responses and foreign body reactions [81]. Considering the size of the nanoparticles, high-resolution imaging with spatially resolved characterization is necessary. Bio-nano interactions of anatase titania were recently investigated as a nanoparticle model which is widely used in consumer products as well orthopedic implants. STEM-EDS elemental maps showed the presence of Ca and P surrounding the nanoparticles for 10 μg/mL anatase titania (Fig. 9a–d) and 100 μg/mL anatase titania (Fig. 9e–h). HA and anatase crystal structure information was reported in the electron diffraction (Fig. 9i). Furthermore, when anatase was incubated with DMEM medium without fetal bovine serum and albumin stabilization, complexes form around the anatase in the form of protein and ion corona, which is monitored by the Ca-L, N-K and O-K edges by STEM-EELS (Fig. 9j).
Concentration dependency of anatase titania bio-complexes formation in the cell medium culture : 10 μg/mL anatase (a) Dark-field STEM image showing where the corresponding elemental maps were obtained; (b) STEM/EDS Ti-K map; (c) STEM/EDS Ca-K map; (d) STEM/EDS P-K map. 100 μg/mL anatase titania; (e) Dark-field STEM image showing where the corresponding elemental maps were obtained; (f) STEM/EDS Ti-K map; (g) STEM/EDS Ca-K map; and (h) STEM/EDS P-K map. (i) Diffraction pattern of {211} hydroxyapatite nanocrystal planes (marked as #) and anatase nanocrystal planes used for calibrating, d {101} = 0.351 nm (5, 7, 8 and 9). (j) EELS spectrum of 100 μg/ml of anatase titania in cell culture medium. Images reprinted with permission of Riberio et al. (2016). Copyright 2016 Nature Publishing Group [81]
TEM imaging was also utilized in the same work to verify the internalization of anatase titania particles by osteoblast cells. Typical secretory cells with organelles including the nucleus, chromatin, and cytoplasm containing mitochondria, Golgi complex, endoplasmic reticulum, vesicles, and vacuoles are reported in Fig. 10a. Exposure to 5 μg/ml and 100 μg/ml anatase titania are reported in Fig. 10b, c and Fig. 10d–f, respectively. With a lower concentration of anatase, ultrastructure resembles one of the non-treated cells with swollen mitochondria and structures resembling autophagolysosomes and anatase are seen in the cell periphery and as free in the cytoplasm (Fig. 10b) and mostly in mitochondria and inside the vesicles (Fig. 10c). With a higher concentration of anatase, more cell membrane disruption, bigger autophagolysosomes, and vacuoles in cell cytoplasm are reported (Fig. 10d). Anatase titania nanoparticles containing vesicles are seen in the cell cytoplasm and in the mitochondria (Fig. 10e) and in the nucleus (Fig. 10f). This technique is useful for the examination of localization of the internationalized bionanomaterials as well as nontoxicity evaluation of nanomaterials .
Electron micrographs of osteoblast cells : (a) Untreated cell. (b, c) Osteoblast cells treated with 5 μg/mL of anatase titania nanoparticles. Note the presence of nanoparticles distributed in cell cytoplasm (white arrows). In c we can observe anatase titania nanoparticles inside vesicles (arrowheads). Detail of these vesicles can be observed in the inset. (d–f) Osteoblast cells treated with 100 μg/mL of anatase titania nanoparticles. Note the large quantities of nanoparticles entering the cells (d) and internalized by cells (e). The inset shows nanoparticles next to the cell nucleus and inside mitochondria. In (f), anatase titania nanoparticles are inside the nucleus of the cell (black arrows). NPs nanoparticles, APL autophagolysosomes, M mitochondria, Nu nucleus, RER rough endoplasmic reticulum, SG secretory granules, and V vesicles. Images reprinted with permission of Riberio et al. (2016). Copyright 2016 Nature Publishing Group [81]
Several metal oxides including alumina and zirconia nanoparticles are used in the manufacturing of joint replacements due to their tribological properties and biocompatibility [94]. Thus, their synthesis and incorporation to the orthopedic implants are very critical. In previous work discussed in this section, the formation of a protein corona region around metal oxide nanoparticles was confirmed [81]. In separate work, both cryo (Fig. 11a) and in situ liquid electron microscopy (Fig. 11b) techniques, were used to confirm the presence of the hydration layer around alumina nanoparticles visually. Specifically, when the alumina nanoparticles are present in water, they form a hydration layer, which alters their behavior in colloidal solutions [95]. Via the EELS analysis of the O K-edge, these layers appeared to be a combination of Al2O3, Al(OH) and AlO(OH). With these images, these particles are seen to form agglomerates in water, which will affect their behavior during orthopedic applications.
(a) The hydration layer is manifested as a faint cloud covering the aggregated nanoparticles. Scale bar: 100 nm. The inset shows the schematics of the hydrated aggregate. (b) The hydration layer is manifested as a cloud enveloping aggregated nanospheres. These hydrated aggregates and surrounding liquid represent the new nanoparticles in the slurries. Scale bar: 100 nm. The inset shows schematics of the formed aggregate with the size and aspect ratio different from that of initial spherical particles. Images reprinted with permission of Firlar et al. (2015). Copyright 2015 Nature Publishing Group [95]
One of the most challenging issue for the manufacturing of biomaterials is that the synthesized biomaterials should be a perfect match in terms of the morphology . This can be carried out via 3D tomography with HR-STEM in which the images are collected with high resolution in 2D, tilt series are applied, and 3D reconstruction of the sample is conducted, as reported by Grandfield et al. [96]. In this particular example, the interfaces between the bone with HA and bone with TiO2 were investigated. Furthermore, a mesoporous TiO2 coating was employed for drug delivery. The advantage of the employment of STEM imaging is that it constructs the images depending on the Z-contrast, thus it becomes easier to differentiate the magnetite core from the surrounding HA. The surface of TiO2 was modified with a laser in that work. HA preferentially precipitated on the TiO2 surface through bonding. Collagen binding close to the implant was also reported. Furthermore, complete intermixing and encapsulation of TiO2 and HA were also observed. This is a good example of the visualization osseointegration between the bone and the implant. Through HAADF STEM images and 3D reconstruction of TiO2 on Ti implants, the interconnection of the pores in 3D was revealed, providing the basis for drug loading and release mechanisms.
5.2.2 X-Ray Diffraction Spectroscopy
X-ray diffraction (XRD) is another important crystal structure characterization method. It can be considered as an alternative to electron diffraction, which was discussed previously. XRD provides structural analysis of the sample, based on the diffraction of X-rays [97] as opposed to the diffracted electrons in electron diffraction in TEM. A beam of X-rays hit the sample, and the resulting spectrum gives peaks based on the direction of the diffracted X-rays, which correlates with the distribution of atoms in the crystal. The amount of the sample can be a lot larger in XRD compared to electron diffraction. XRD is commonly used in characterizing nanomaterials used in orthopedic applications because it analyzes the crystal structures and the phases [98, 99]. XRD is useful in coating experiments. With the previous XRD data knowledge of the coating material, the synthesis stages of the coatings on the substrates can be monitored [100].
Kuo et al. used XRD to analyze the crystallography of HA coatings at increasing temperatures [101]. The findings indicated that the main component of HA was dicalcium phosphate dihydrate at lower currents. However, at a higher current and higher temperatures, part of the HA underwent a phase transformation. By knowing when the HA changed its structure, they could determine the best temperature to deposit the coating. Webster et al. not only used XRD to characterize various HA formulations but also obtained the lattice patterns [102]. They were then able to compare the lattice parameters of all the variants of HA, which would help to determine other substituents that can be used to substitute Ca. Razavi et al. also used XRD to analyze the composition, but was specifically looking to see if the substrate would be corrosive if it was protected with magnesium oxide. Because the XRD pattern showed Mg2SiO4, this means that magnesium oxide did protect the substrate [103].
5.3 Chemical Bonding Information
5.3.1 Fourier Transform Infrared Spectroscopy
FTIR can be utilized to characterize the composition of the nanomaterials. FTIR identifies functional groups by measuring the absorption of the sample. Infrared radiation passes through the sample, where the sample then absorbs some of the radiation, and the rest will be transmitted [104]. The absorption peak is the result of the frequencies of the vibrations between the atoms of the sample. Then, the resulting spectrum will show the absorption peaks that represent the functional groups in the sample. The intensity of the peak can also quantitatively show the amount of that functional group that is present as compared to the other functional groups in the sample. All compounds have specific spectra because different compounds have a unique combination of atoms, resulting in different absorption peaks. Being able to identify functional groups is useful to confirm or identify unknown function groups in the resulting nanomaterial. A majority of research groups use FTIR to analyze the nanomaterial coatings on alloys for orthopedic implants. FTIR is commonly used in composition characterization of the synthesized coatings.
Cao et al. used FTIR to identify the phosphate and hydroxide group on their surface coating [105]. They treated Mg-Zn-Ca alloy by micro arc oxidation (MAO) , and then coated the treated alloy with TiO2. Therefore, the spectrum confirmed that the TiO2 layer reacted with the MAO coating. Razavi et al. similarly used MAO to coat magnesium (Mg) alloys with nanostructured diopside [103]. However, they used FTIR to evaluate in vitro bioactivity. After coating the alloys, they immersed the alloys in simulated body fluid for 672 h for analyzing the bioactivity of the coating. A precipitate layer was formed on the surface, which was analyzed using FTIR and found that it contained phosphate and carbonate groups. FTIR is a very helpful device to analyze the composition of a sample, and even to evaluate the molecular interaction in the sample [106]. Further nanomaterials characterization studies to identify the compositions of the coatings via FTIR were reported earlier [107,108,109], specifically to confirm if the synthesis of the coating is completed [110]. For instance, it is used to determine whether the sample is crystallized [111], or the original sample is altered over the time after process [112].
5.3.2 Raman Spectroscopy
Raman spectroscopy is similar to FTIR in that both spectra are based on the vibrations of molecules in the sample [113]. However, Raman spectroscopy has vibrational modes. Also, samples can be used without any preparation for analysis, making it a useful technique to monitor the sample before and after treatment [114]. Raman spectroscopy can be used complementary to FTIR for the detection of different functional groups.
Using Raman spectroscopy, Greer et al. evaluated the composition of Ti-based sol-gel [115]. However, they monitored the sample through the entire annealing process to analyze the structural phases. They could see the different phases as they increased the temperature. By knowing what phase the sample is in during the annealing process, they could determine when the annealing process was completed.
6 Summary
Future prospects for nanomaterials in orthopedic applications appear to be game changing. Although orthopedic implants have become more successful over the recent decades, they have not been perfected. Today there is a significant demand for the development of a bone substitute that is bioactive and exhibits mechanical and surface properties comparable with those of natural bone. Owing to the unique and exceptional physical and chemical properties of these nanomaterials, and furthermore their functions, specifically, enabling tissue regeneration, drug delivery, antimicrobial properties, enhancing bone growth, etc., they have been proposed as the next generation of orthopedic materials with high promise towards increasing the success rate of implantation by promoting osseointegration. The wide range of benefits of nanotechnology and nanomaterials in orthopedic applications can be realized even more, once further tests and studies concerning the nanotoxicity of such materials are completed. In addition, properties of the orthopedic nanomaterials and their interaction with host tissue need to be studied before and after their clinical applications. Currently, tools and techniques have been developed which enable advanced characterization of nanomaterials and their interactions with biological systems. The recent high success of such characterizations and tests will enable the use of broad range of nanostructured biomaterials for orthopedic applications specially targeted towards patients with certain medical conditions, and those with compromised bone tissues. Finally, it is safe to say that the promise of nanoscience and nanotechnology can positively change the future of orthopedic implants and those receiving or in need for them.
References
Tasker LH, Sparey-Taylor GJ, Nokes LDM. Applications of nanotechnology in orthopaedics. Clin Orthop Relat Res. 2007;456:243–9.
Di Sia P. Nanotechnology among innovation, health and risks. Proc Soc Behav Sci. 2017;237:1076–80.
Toumey C. Plenty of room, plenty of history. Nat Nanotechnol. 2009;4(12):783–4.
Mauro JC, Ellison AJ, Pye LD. Glass: the nanotechnology connection. Int J Appl Glas Sci. 2013;4(2):64–75.
Sakamoto JH, van de Ven AL, Godin B, Blanco E, Serda RE, Grattoni A, Ziemys A, Bouamrani A, Hu T, Ranganathan SI, De Rosa E, Martinez JO, Smid CA, Buchanan RM, Lee S-Y, Srinivasan S, Landry M, Meyn A, Tasciotti E, Liu X, Decuzzi P, Ferrari M. Enabling individualized therapy through nanotechnology. Pharmacol Res. 2010;62(2):57–89.
Mirza AZ, Siddiqui FA. Nanomedicine and drug delivery: a mini review. Int Nano Lett. 2014;4(1):94.
Chistiakov DA. Endogenous and exogenous stem cells: a role in lung repair and use in airway tissue engineering and transplantation. J Biomed Sci. 2010;17(1):92.
Wang M, Abbineni G, Clevenger A, Mao C, Xu S. Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomed Nanotechnol Biol Med. 2011;7(6):710–29.
Leonida MD, Kumar I. Nanomaterials, scaffolds, and skin tissue regeneration. Cham: Springer International Publishing; 2016. p. 103–16.
Parchi PD, Vittorio O, Andreani L, Piolanti N, Cirillo G, Iemma F, Hampel S, Lisanti M. How nanotechnology can really improve the future of orthopedic implants and scaffolds for bone and cartilage defects. J. Nanomed Biotherapeutic Discov. 2013;3(2):114. https://doi.org/10.4172/2155-983X.1000114.
Zhang L, Webster TJ. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today. 2009;4(1):66–80.
AAOS. Information about musculoskeletal conditions. Rosemont, IL: AAOS; 2013. p. 26.
Catledge SA, Thomas V, Vohra YK. Nanostructured diamond coatings for orthopaedic applications. Woodhead Publ Ser Biomater. 2013;2013:105–50.
Catledge SA, Fries MD, Vohra YK, Lacefield WR, Lemons JE, Woodard S, Venugopalan R. Nanostructured ceramics for biomedical implants. J Nanosci Nanotechnol. 2002;2(3–4):293–312.
Taheri MM, Abdul Kadir MR, Ahmad Shafie NK, Shokuhfar T, Assadian M, Rezazadeh Shirdar M. Green synthesis of silver nanoneedles using shallot and apricot tree gum. Trans Nonferrous Met Soc China. 2015;25(10):3286–90.
Taheri MM, Abdul Kadir MR, Shokuhfar T, Hamlekhan A, Shirdar MR, Naghizadeh F. Fluoridated hydroxyapatite nanorods as novel fillers for improving mechanical properties of dental composite: synthesis and application. Mater Des. 2015;82:119–25.
Christenson EM, Anseth KS, van den Beucken JJJP, Chan CK, Ercan B, Jansen JA, Laurencin CT, Li W-J, Murugan R, Nair LS, Ramakrishna S, Tuan RS, Webster TJ, Mikos AG. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25(1):11–22.
Sato M, Webster TJ. Nanobiotechnology: implications for the future of nanotechnology in orthopedic applications. Expert Rev Med Devices. 2004;1(1):105–14.
Sato M, Webster TJ. Orthopedic tissue engineering using nanomaterials. In Nanotechnologies for the life sciences. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2007.
Balasundaram G, Webster TJ, Akasaka T, Wroblewski BM, Ingham E, Kamali A, Stone MH, Ingham E, Montanaro L, Baquey CH. A perspective on nanophase materials for orthopedic implant applications. J Mater Chem. 2006;16(38):3737.
Marot D, Knezevic M, Novakovic G. Bone tissue engineering with human stem cells. Stem Cell Res Ther. 2010;1(2):10.
Boccaccini AR, Keim S, Ma R, Li Y, Zhitomirsky I. Electrophoretic deposition of biomaterials. J R Soc Interface. 2010;7(Suppl_5):S581–613.
Yang L, Zhang L, Webster TJ. Carbon nanostructures for orthopedic medical applications. Nanomedicine. 2011;6(7):1231–44.
Hodgkinson T, Yuan X-F, Bayat A. Adult stem cells in tissue engineering. Expert Rev Med Devices. 2009;6(6):621–40.
Chun YW, Webster TJ. The role of nanomedicine in growing tissues. Ann Biomed Eng. 2009;37(10):2034–47.
Simchi A, Mazaheri M, Eslahi N, Ordikhani F, Tamjid E. Nanomedicine applications in orthopedic medicine: state of the art. Int J Nanomedicine. 2015;10:6039.
Shirwaiker RA, Samberg ME, Cohen PH, Wysk RA, Monteiro-Riviere NA. Nanomaterials and synergistic low-intensity direct current (LIDC) stimulation technology for orthopedic implantable medical devices. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(3):191–204.
Khang D, Carpenter J, Chun YW, Pareta R, Webster TJ. Nanotechnology for regenerative medicine. Biomed Microdevices. 2010;12(4):575–87.
Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295(5557):1014–7.
Liang J, Song R, Huang Q, Yang Y, Lin L, Zhang Y, Jiang P, Duan H, Dong X, Lin C. Electrochemical construction of a bio-inspired micro/nano-textured structure with cell-sized microhole arrays on biomedical titanium to enhance bioactivity. Electrochim Acta. 2015;174:1149–59.
Mishnaevsky L, Levashov E, Valiev RZ, Segurado J, Sabirov I, Enikeev N, Prokoshkin S, Solov’yov AV, Korotitskiy A, Gutmanas E, Gotman I, Rabkin E, Psakh’e S, Dluhoš L, Seefeldt M, Smolin A. Nanostructured titanium-based materials for medical implants: modeling and development. Mater Sci Eng R Rep. 2014;81:1–19.
Yamanaka K, Mori M, Chiba A. Nanoarchitectured Co–Cr–Mo orthopedic implant alloys: nitrogen-enhanced nanostructural evolution and its effect on phase stability. Acta Biomater. 2013;9(4):6259–67.
Pauksch L, Hartmann S, Rohnke M, Szalay G, Alt V, Schnettler R, Lips KS. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014;10(1):439–49.
Krawczynska AT, Gloc M, Lublinska K. Intergranular corrosion resistance of nanostructured austenitic stainless steel. J Mater Sci. 2013;48(13):4517–23.
Mohandas G, Oskolkov N, McMahon MT, Walczak P, Janowski M. Porous tantalum and tantalum oxide nanoparticles for regenerative medicine. Acta Neurobiol Exp (Wars). 2014;74(2):188–96.
Schiavi J, Keller L, Morand D-N, De Isla N, Huck O, Lutz JC, Mainard D, Schwinté P, Benkirane-Jessel N. Active implant combining human stem cell microtissues and growth factors for bone-regenerative nanomedicine. Nanomedicine. 2015;10(5):753–63.
Levengood SL, Zhang M. Chitosan-based scaffolds for bone tissue engineering. J Mater Chem B Mater Biol Med. 2014;2(21):3161–84.
Lee P, Tran K, Chang W, Shelke NB, Kumbar SG, Yu X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: cartilage regeneration. J Biomed Nanotechnol. 2014;10(8):1469–79.
Zhang W, Zhu C, Ye D, Xu L, Zhang X, Wu Q, Zhang X, Kaplan DL, Jiang X. Porous silk scaffolds for delivery of growth factors and stem cells to enhance bone regeneration. PLoS One. 2014;9(7):e102371.
Gentile P, Chiono V, Carmagnola I, Hatton P. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–59.
Winkins S, Kamath M, Dhanasekaran M, Ahmed S. Polycaprolactone scaffold engineered for sustained release of resveratrol: therapeutic enhancement in bone tissue engineering. Int J Nanomedicine. 2013;9:183.
Xing Z-C, Han S-J, Shin Y-S, Koo T-H, Moon S, Jeong Y, Kang I-K. Enhanced osteoblast responses to poly(methyl methacrylate)/hydroxyapatite electrospun nanocomposites for bone tissue engineering. J Biomater Sci Polym Ed. 2012;24(1):61–76.
Lopes MS, Jardini AL, Filho RM. Poly (lactic acid) production for tissue engineering applications. Proc Eng. 2012;42:1402–13.
Evans NT, Torstrick FB, Lee CSD, Dupont KM, Safranski DL, Chang WA, Macedo AE, Lin ASP, Boothby JM, Whittingslow DC, Carson RA, Guldberg RE, Gall K. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015;13:159–67.
Wang P, Zhao L, Liu J, Weir MD, Zhou X, Xu HHK. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014;2:14017.
Zhou C, Deng C, Chen X, Zhao X, Chen Y, Fan Y, Zhang X. Mechanical and biological properties of the micro−/nano-grain functionally graded hydroxyapatite bioceramics for bone tissue engineering. J Mech Behav Biomed Mater. 2015;48:1–11.
Tamjid E, Bagheri R, Vossoughi M, Simchi A. Effect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone composites. Mater Sci Eng C. 2011;31(7):1526–33.
Price RL, Haberstroh KM, Webster TJ. Enhanced functions of osteoblasts on nanostructured surfaces of carbon and alumina. Med Biol Eng Comput. 2003;41(3):372–5.
Newman P, Minett A, Ellis-Behnke R, Zreiqat H. Carbon nanotubes: their potential and pitfalls for bone tissue regeneration and engineering. Nanomed Nanotechnol Biol Med. 2013;9(8):1139–58.
Zhao C, Lu X, Zanden C, Liu J. The promising application of graphene oxide as coating materials in orthopedic implants: preparation, characterization and cell behavior. Biomed Mater. 2015;10(1):15019.
Mansoorianfar M, Shokrgozar MA, Mehrjoo M, Tamjid E, Simchi A. Nanodiamonds for surface engineering of orthopedic implants: Enhanced biocompatibility in human osteosarcoma cell culture. Diam Relat Mater. 2013;40:107–14.
Hickey DJ, Ercan B, Sun L, Webster TJ. Adding MgO nanoparticles to hydroxyapatite–PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015;14:175–84.
Liao CZ, Li K, Wong HM, Tong WY, Yeung KWK, Tjong SC. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater Sci Eng C. 2013;33(3):1380–8.
Pishbin F, Mouriño V, Gilchrist JB, McComb DW, Kreppel S, Salih V, Ryan MP, Boccaccini AR. Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 2013;9(7):7469–79.
Longmire MR, Ogawa M, Choyke PL, Kobayashi H. Biologically optimized nanosized molecules and particles: more than just size. Bioconjug Chem. 2011;22(6):993–1000.
Katz D, Kany J, Valenti P, Sauzières P, Gleyze P, El Kholti K. New design of a cementless glenoid component in unconstrained shoulder arthroplasty: a prospective medium-term analysis of 143 cases. Eur J Orthop Surg Traumatol. 2013;23(1):27–34.
Cobelli N, Scharf B, Crisi GM, Hardin J, Santambrogio L. Mediators of the inflammatory response to joint replacement devices. Nat Rev Rheumatol. 2011;7(10):600–8.
Izman S, Rafiq M, Anwar M, Nazim EM, Rosliza R, Shah A, Hass MA. Surface modification techniques for biomedical grade of titanium alloys: oxidation, carburization and ion implantation processes. In: Nurul Amin AKM, editor. Titanium alloys—towards achieving enhanced properties for diversified applications. London: InTech; 2012.
Wang Q, Yang X, Liu D, Zhao J. Fabrication, characterization and photocatalytic properties of Ag nanoparticles modified TiO2 NTs. J Alloys Compd. 2012;527:106–11.
Lin L, Wang H, Ni M, Rui Y, Cheng T-Y, Cheng C-K, Pan X, Li G, Lin C. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J Orthop Transl. 2014;2(1):35–42.
Kasemo B, Gold J. Implant surfaces and interface processes. Adv Dent Res. 1999;13(1):8–20.
Bauer S, Schmuki P, von der Mark K, Park J. Engineering biocompatible implant surfaces. Prog Mater Sci. 2013;58(3):261–326.
Xia L, Feng B, Wang P, Ding S, Liu Z, Zhou J, Yu R. In vitro and in vivo studies of surface-structured implants for bone formation. Int J Nanomedicine. 2012;7:4873–81.
Mazaheri M, Eslahi N, Ordikhani F, Tamjid E, Simchi A. Nanomedicine applications in orthopedic medicine: state of the art. Int J Nanomedicine. 2015;10:6039–53.
Safonov V, Zykova A, Smolik J, Rogowska R, Lukyanchenko V, Kolesnikov D. Modification of implant material surface properties by means of oxide nano-structured coatings deposition. Appl Surf Sci. 2014;310:174–9.
Rezazadeh Shirdar M, Sudin I, Taheri MM, Keyvanfar A, Yusop MZM, kadir MRA. A novel hydroxyapatite composite reinforced with titanium nanotubes coated on Co–Cr-based alloy. Vacuum. 2015;122:82–9.
Rezazadeh Shirdar M, Taheri MM, Moradifard H, Keyvanfar A, Shafaghat A, Shokuhfar T, Izman S. Hydroxyapatite–titania nanotube composite as a coating layer on Co–Cr-based implants: Mechanical and electrochemical optimization. Ceram Int. 2016;42(6):6942–54.
Breme H, Biehl V, Reger N, Gawalt E. Chapter 1a Metallic biomaterials: introduction. In: Black J, Hastings G, editors. Handbook of biomaterial properties. New York, NY: Springer New York; 2016. p. 151–8.
Smith GD, Knutsen G, Richardson JB. A clinical review of cartilage repair techniques. J Bone Joint Surg Br. 2005;87B(4):445–9.
Mafi P, Hindocha S, Mafi R, Khan WS. Evaluation of biological protein-based collagen scaffolds in cartilage and musculoskeletal tissue engineering—a systematic review of the literature. Curr Stem Cell Res Ther. 2012;7(4):302–9.
Cartmell S. Controlled release scaffolds for bone tissue engineering. J Pharm Sci. 2009;98(2):430–41.
Li Z, Kawashita M. Current progress in inorganic artificial biomaterials. J Artif Organs. 2011;14(3):163–70.
Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(1):1–35.
Majestic BJ, Erdakos GB, Lewandowski M, Oliver KD, Willis RD, Kleindienst TE, Bhave PV. A review of selected engineered nanoparticles in the atmosphere: sources, transformations, and techniques for sampling and analysis. Int J Occup Environ Health. 2010;16(4):488–507.
Bakand S, Hayes A, Dechsakulthorn F. Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal Toxicol. 2012;24(2):125–35.
Madl AK, Kovochich M, Liong M, Finley BL, Paustenbach DJ, Oberdörster G. Toxicology of wear particles of cobalt-chromium alloy metal-on-metal hip implants. Part II: importance of physicochemical properties and dose in animal and in vitro studies as a basis for risk assessment. Nanomed Nanotechnol Biol Med. 2015;11(5):1285–98.
Webster TJ, Ahn ES. Nanostructured biomaterials for tissue engineering bone. Adv Biochem Eng Biotechnol. 2007;103:275–308.
Cheng L-C, Jiang X, Wang J, Chen C, Liu R-S. Nano-bio effects: interaction of nanomaterials with cells. Nanoscale. 2013;5(9):3547.
Fan AM, Alexeeff G. Nanotechnology and nanomaterials: toxicology, risk assessment, and regulations. J Nanosci Nanotechnol. 2010;10(12):8646–57.
Fischer HC, Chan WC. Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol. 2007;18(6):565–71.
Ribeiro AR, Gemini-Piperni S, Travassos R, Lemgruber L, C Silva R, Rossi AL, Farina M, Anselme K, Shokuhfar T, Shahbazian-Yassar R, Borojevic R, Rocha LA, Werckmann J, Granjeiro JM. Trojan-like internalization of anatase titanium dioxide nanoparticles by human osteoblast cells. Sci Rep. 2016;6:23615.
Goldstein J, Newbury D, Joy DC, Michael J, Ritchie NWM, Scott JH. Scanning electron microscopy and X-ray microanalysis. New York: Springer; 2017.
Bhosle S, Patel S, Taheril MM, Sukotjo C, Shokuhfar T. Electrochemical anodisation of Ti–15Zr implant: effect of different voltages and times. Surf Innov. 2017;5(1):82–9.
Nelson M, Balasundaram G, Webster TJ. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. Int J Nanomedicine. 2006;1(3):339–49.
Alves SA, Patel SB, Sukotjo C, Mathew MT, Filho PN, Celis J-P, Rocha LA, Shokuhfar T. Synthesis of calcium-phosphorous doped TiO2 nanotubes by anodization and reverse polarization: a promising strategy for an efficient biofunctional implant surface. Appl Surf Sci. 2017;399:682–701.
Wang M. Bioactive materials and processing. In: Biomaterials and tissue engineering. Heidelberg: Springer; 2004. p. 1–87.
Bates M, Huang B, Dempsey GT, Zhuang X. S-Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science. 2007;317(5845):1749–53.
Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2(6):444–56.
Park MR, Banks MK, Applegate B, Webster TJ. Influence of nanophase titania topography on bacterial attachment and metabolism. Int J Nanomedicine. 2008;3(4):497–504.
Williams DB, Carter CB. Transmission electron microscopy: a textbook for materials science. Boston, MA: Springer US; 2009.
Evans JE, Jungjohann KL, Browning ND, Arslan I. Controlled growth of nanoparticles from solution with in situ liquid transmission electron microscopy. Nano Lett. 2011;11(7):2809–13.
Wang C, Qiao Q, Shokuhfar T, Klie RF. High-resolution electron microscopy and spectroscopy of ferritin in biocompatible graphene liquid cells and graphene sandwiches. Adv Mater. 2014;26(21):3410–4.
Mandelkow E, Mandelkow E, Milligan RA. Microtubule dynamics and microtubule caps: a time-resolved cryo- electron microscopy study. J Cell Biol. 1991;114(5):977–91.
Dalla Pria P. Evolution and new application of the alumina ceramics in joint replacement. Eur J Orthop Surg Traumatol. 2007;17(3):253–6.
Firlar E, Çınar S, Kashyap S, Akinc M, Prozorov T. Direct visualization of the hydration layer on alumina nanoparticles with the fluid cell STEM in situ. Sci Rep. 2015;5:9830.
Grandfield K, Palmquist A, Engqvist H. High-resolution three-dimensional probes of biomaterials and their interfaces. Philos Trans R Soc A Math Phys Eng Sci. 2012;370:1337–51.
Nelson SA. X-ray crystallography. Sci Am. 2010;219(1):1–6.
Feng B, Weng J, Yang BC, Qu SX, Zhang XD. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials. 2003;24(25):4663–70.
Eslami N, Mahmoodian R, Hamdi M, Khatir NM, Herliansyah MK, Rafieerad AR. Study the synthesis, characterization and immersion of dense and porous bovine hydroxyapatite structures in Hank’s balanced salt solution. JOM. 2017;69(4):691–8.
Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, Hardouin P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49(2):155–66.
Kuo MC, Yen SK. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater Sci Eng C. 2002;20(1–2):153–60.
Webster TJ, Massa-Schlueter EA, Smith JL, Slamovich EB. Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials. 2004;25(11):2111–21.
Razavi M, Fathi M, Savabi O, Vashaee D, Tayebi L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater Sci Eng C. 2014;41:168–77.
Ganzoury MA, Allam NK, Nicolet T, All C. Introduction to fourier transform infrared spectrometry. Renew Sust Energ Rev. 2015;50:1–8.
Cao G, Wang L, Fu Z, Hu J, Guan S, Zhang C, Wang L, Zhu S. Chemically anchoring of TiO2 coating on OH-terminated Mg 3(PO3)2 surface and its influence on the in vitro degradation resistance of Mg-Zn-Ca alloy. Appl Surf Sci. 2014;308:38–42.
Ambre A, Katti KS, Katti DR. In situ mineralized hydroxyapatite on amino acid modified nanoclays as novel bone biomaterials. Mater Sci Eng C. 2011;31(5):1017–29.
Yazdimamaghani M, Razavi M, Vashaee D, Tayebi L. Development and degradation behavior of magnesium scaffolds coated with polycaprolactone for bone tissue engineering. Mater Lett. 2014;132:106–10.
Cordero-Arias L, Boccaccini AR, Virtanen S. Electrochemical behavior of nanostructured TiO2/alginate composite coating on magnesium alloy AZ91D via electrophoretic deposition. Surf Coat Technol. 2015;265:212–7.
Shi J, Dong LL, He F, Zhao S, Yang GL. Osteoblast responses to thin nanohydroxyapatite coated on roughened titanium surfaces deposited by an electrochemical process. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(5):e311–6.
Killion J a, Geever LM, Devine DM, Higginbotham CL. Fabrication and in vitro biological evaluation of photopolymerisable hydroxyapatite hydrogel composites for bone regeneration. J Biomater Appl. 2014;28(8):1274–83.
Rojaee R, Fathi M, Raeissi K, Taherian M. Electrophoretic deposition of bioactive glass nanopowders on magnesium based alloy for biomedical applications. Ceram Int. 2014;40(6):7879–88.
Deeken CR, Bachman SL, Ramshaw BJ, Grant SA. Characterization of bionanocomposite scaffolds comprised of mercaptoethylamine-functionalized gold nanoparticles crosslinked to acellular porcine tissue. J Mater Sci Mater Med. 2012;23(2):537–46.
Cerruti M, Sahai N. Silicate biomaterials for orthopaedic and dental implants. Rev Miner Geochem. 2006;64(1):283–313.
Bertoluzza A, Fagnano C, Monti P, Simoni R, Tinti A, Tosi MR, Caramazza R. Raman spectroscopy in the study of biocompatibility. Clin Mater. 1992;9(1):49–68.
Greer AIM, Lim TS, Brydone AS, Gadegaard N. Mechanical compatibility of sol-gel annealing with titanium for orthopaedic prostheses. J Mater Sci Mater Med. 2016;27(1):1–6.
Acknowledgement
The authors are grateful to the National Science Foundation, CAREER award DMR-1564950 for providing partial financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Shokuhfar, T., Firlar, E., Shirdar, M.R., Taheri, M.M. (2017). Orthopedic Nanomaterials. In: Li, B., Webster, T. (eds) Orthopedic Biomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-73664-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-73664-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73663-1
Online ISBN: 978-3-319-73664-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)