Abstract
Damage control vascular surgery incorporates strategies to achieve rapid control of hemorrhage while mitigating ischemia by establishing adequate end-organ perfusion in an abbreviated initial intervention. Vascular damage control surgery functions in concert with damage control resuscitation focused on the correction of physiologic derangements and metabolic acidosis, the correction of coagulopathy, appropriate blood product transfusion, and active patient warming measures to ameliorate hypothermia. Ultimately, the patient’s physiology dictates the technical feasibility of vascular intervention and determines operative planning with respect to injury management. Surgeon experience and technical familiarity with vascular injury and location of the vascular injury are significant factors that alter patient outcomes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
11.1 Introduction
Damage control vascular surgery incorporates strategies to achieve rapid control of hemorrhage while mitigating ischemia by establishing adequate end-organ perfusion in an abbreviated initial intervention. Vascular damage control surgery functions in concert with damage control resuscitation focused on the correction of physiologic derangements and metabolic acidosis, the correction of coagulopathy, appropriate blood product transfusion, and active patient warming measures to ameliorate hypothermia. Ultimately, the patient’s physiology dictates the technical feasibility of vascular intervention and determines operative planning with respect to injury management. Surgeon experience and technical familiarity with vascular injury and location of the vascular injury are significant factors that alter patient outcomes.
The military has extensive experience with damage control vascular surgery strategies. Hemorrhage has been identified as a leading cause of preventable death on the modern battlefield [1,2,3,4]. Analysis from the recent wars in Iraq and Afghanistan has demonstrated that hemorrhage was the underlying physiologic insult in 90% of potentially survivable battlefield injuries [1]. The current incidence of wartime vascular injury on the modern battlefield has significantly increased compared to past conflicts [5,6,7,8,9,10,11,12,13,14,15,16]. Because of this increased incidence, the military offers a unique perspective with regard to damage control techniques and strategies. Reports from DeBakey, Hughes, and Rich [5,6,7,8,9,10,11] laid the foundation for the characterization of wartime vascular injury and demonstrated the feasibility regarding the management of complex, often devastating, injuries. Subsequent reports have continued to define and describe additional surgical adjuncts and implementation of strategies across the continuum of the modern battlefield. The Golden Hour Offset Surgical Treatment Team (GHOST-T) initiative positions forward surgical treatment and resuscitative teams within a 60-min medical evacuation radius from combat elements. These small units provide combat support and perform damage control surgery and resuscitation. Following the completion of damage control maneuvers, patients are rapidly transported to the next echelon of military medical care.
Rapid hemorrhage control and alleviation of end-organ ischemia are the central tenets to damage control vascular surgery. Hemorrhage control techniques include intracavitary packing for solid organ injury and pelvic packing following severe pelvic fractures. These techniques have an important role in initial trauma laparotomy. Peripheral vascular hemorrhage control includes the use of tourniquets, ligation of bleeding vessels, and primary amputation of the mangled extremity. Mitigation of end-organ ischemia focuses on the use of temporary vascular shunts (TVS) and revascularization strategies. Endovascular capabilities have extended the therapeutic options for vascular trauma with adjuncts that include resuscitative balloon occlusion of the aorta (REBOA), primary stenting for central vascular injuries, and coil embolization techniques. REBOA has demonstrated clinical feasibility and is an effective means of proactive aortic control for patients in end-stage hemorrhagic shock [17,18,19,20,21,22,23]. An extended discussion regarding endovascular principles and procedures is beyond the scope of this chapter, and a more detailed description can be found in the endovascular damage control surgery section. This chapter on open damage control vascular surgery will focus on hemorrhage control techniques, temporary revascularization strategies, revascularization operations, and specific technical considerations regarding vascular injury management.
11.2 Hemorrhage Control
11.2.1 Tourniquets
Death from compressible hemorrhage remains a significant cause of mortality during modern combat operations [2, 3, 24]. The tourniquet has become a ubiquitous lifesaving tool in the military. Minimal training and familiarity are required for effective utilization of an extremity tourniquet making it an ideal prehospital intervention. Combat medics operating in a forward, austere environment deploy tourniquets in the prehospital setting to reduce hemorrhage from compressible extremity injury. The tourniquet is associated with improved combat casualty survival and low complication rates [25,26,27,28,29,30,31,32,33]. Kragh et al. [25] reviewed 232 combat casualties with major limb trauma and reported on 428 tourniquets applied on 309 injured limbs. This report demonstrated a significant mortality reduction following early prehospital tourniquet application compared to delayed use once the patient had reached a military treatment facility (MTF) (mortality of 11–24%, respectively). Early use of tourniquets prior to the onset of hemorrhagic shock was associated with improved survival and no limb loss demonstrating the safety and efficacy of tourniquet application. Additionally, tourniquet duration was not associated with increased morbidity. A subsequent report form Kragh et al. [30] reviewed the military’s experience from 2001 to 2010 with a retrospective review of tourniquet use during combat operations. In total, 4297 combat casualties were identified, and tourniquets were applied in 1272 casualties. Interestingly, the additional experience with tourniquets, as well as an understanding of the efficacy, resulted in an increase in the use of tourniquets from 4% in 2001 to 40% in 2010. Survival rates with tourniquet use also increased from 2004 to 2010 despite the simultaneous increase in injury severity. Beekley et al. [31] reviewed data from 3444 injured casualties during Operation Iraqi Freedom in 2004. One hundred sixty-five patients were identified with a major vascular injury to an extremity, traumatic amputations, or annotation of a prehospital tourniquet placement. Of this cohort, 67 (40%) patients arrived to the Role 3 combat support hospital with a tourniquet in place. Tourniquet use resulted in effective hemorrhage control on arrival to the MTF. In summary, tourniquets have demonstrated clear clinical utility in the prehospital phase of casualty care preventing life-threatening extremity hemorrhage when applied early, and the use of tourniquets has revealed an overall low complication profile. Tourniquets remain a critical prehospital adjunct for the mitigation of exsanguinating hemorrhage from compressible extremity hemorrhage.
The civilian literature reflects similar trends regarding the efficacy and safety of tourniquet use in vascular injury. Inaba et al. [34] retrospectively reported on 87 civilian trauma patients at the Level 1 trauma center that had a tourniquet applied in the prehospital setting, emergency room, or operating room. Eighty-one percent of patients demonstrated a major vascular injury. One identified difference in military versus civilian trauma management indicated that civilian patients exist in a system with more constant and rapid transport times [34]. Therefore, the overall incidence and implementation of tourniquets are reduced compared to military trauma which occurs in more austere environments. Interestingly, the civilian literature prehospital tourniquet rate varies from 5.6% to 50.6% depending on the study [34, 35]. In the face of explosive blast injuries as the primary mechanism of injury and increased wartime experience, the military rate of prehospital tourniquet application is 40% [30].
11.2.2 Ligation
Selective vessel ligation remains a viable and appropriate damage control option. In patients who present in extremis with severe physiology derangement, ligation offers rapid hemorrhage control and does not necessarily exclude future revascularization options. Patient physiology and surgeon experience contribute to the surgical plan significantly. At times, initial ligation, followed by rapid casualty evacuation to a higher echelon of care, allows for reexploration and TVS placement for central vascular injuries. Conversely, selective peripheral vascular injury ligation remains an acceptable method of hemorrhage control.
In a review of vascular surgery procedures in recent combat, ligation and reconstruction were observed in nearly equal proportions for the treatment of battlefield vascular trauma [16]. This fact represents the utility of vessel ligation as a damage control maneuver. Burkhardt et al. [36] reported outcomes after a selective approach to revascularization for the distal lower extremity. This report reviewed 1332 patients with combat-related vascular injuries and characterized the management of 135 tibial-level disruptions or occlusions. Selective revascularization of isolated tibial-level arterial injury was the predominant technical approach reported, and 83% of limb salvage patients were managed without arterial reconstruction. Arterial ligation remains an effective damage control option in the context of single tibial-vessel injury. However, patients with complete or persistent ischemia should be considered for revascularization.
11.2.3 Primary Amputation
Primary amputation should be considered for non-salvageable extremity injury with complex, multisystem trauma in a damage control setting. Additionally, for patients in extremis who are unable to tolerate an attempt at temporary revascularization, primary amputation is an appropriate option. Stannard et al. [15] reviewed 1203 service personnel injured in combat and identified 110 vascular injuries. The overall amputation rate among all patients with extremity vascular injury was 47%.The patient cohort included in this analysis underwent damage control maneuver after sustaining significant limb trauma with a high mangled extremity severity score (MESS). Blast ordinance is the most common mechanism of injury in modern combat. Due to the destructive nature of these weapons, extremity injury frequently presents as a non-salvageable limb. The decision for a primary amputation is often straightforward in these cases. However, civilian reports have demonstrated that the incorporation of a multidisciplinary decision-making process offers the patient significant insight following the injury [35, 37].
11.3 Temporary Revascularization
11.3.1 Temporary Vascular Shunt
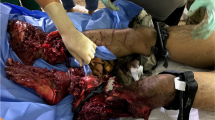
Utilization of a temporary vascular shunt (TVS) during damage control maneuvers is a well-described method to accomplish restoration of flow and end-organ perfusion in vascular trauma [38,39,40,41,42,43,44,45,46,47,48]. Shunts for arterial injury allow for temporary preservation of distal end-organ perfusion (Fig. 11.1). If the TVS is placed in the peripheral arterial distribution, the end organ at risk is the extremity itself. Shunting of venous injury provides necessary drainage of blood and subsequent reduction of venous hypertension that compounds tissue ischemia and bleeding.
Utilization of a temporary vascular shunt (TVS) for damage control management of severe ulnar and radial artery injury. Following resuscitation and physiologic improvement, the patient underwent brachial artery to radial artery bypass with the reversed greater saphenous vein (rGSV) and an interposition bypass from the proximal ulnar artery to distal ulnar artery with rGSV
Rasmussen et al. [39] described a contemporary wartime experience with TVS as a damage control adjunct during Operation Iraqi Freedom at Balad Air Base, Iraq. This report included 30 TVS inserted for arterial (87%) and venous injury (13%). TVS patency was reported for proximal or central vascular injuries at 86% and distal or peripheral shunting at 12%. No systemic heparin was used following shunt insertion, and no shunt complications were reported. Limb salvage, determined by early preservation of a viable limb, occurred in 92% of the casualties. Utilization of a TVS represents a damage control adjunct that is safe and effective with respect to establishing distal perfusion and extending the window of opportunity for limb salvage. Chambers et al. [40] reviewed 582 traumatic injuries in 293 combat casualties treated by a Marine Corps forward resuscitative surgical system team from 2004 to 2005. This reviewed identified 66 casualties that sustained a major vascular injury. Of these, 29 arterial and venous injuries were managed with a TVS representing 44% shunt utilization. Shunt patency was reported at 78%, and limb salvage was achieved in 85% of injured patients.
Taller et al. [41] reported on 610 combat trauma patients treated over a 7-month period in Iraq. In total, 37 patients sustained 73 major traumatic vascular injuries with 26 TVS inserted for limb salvage. Regarding shunt placement, 36% of the injuries were initially managed with TVS insertion in the prehospital setting. Reported TVS patency was 96%, and early limb salvage was achieved in all patients who underwent temporary revascularization with TVS. Gifford et al. [42] published a retrospective database review incorporating the Balad Vascular Registry, Walter Reed Vascular Registry, and Joint Theater Trauma System (now consolidated into the Department of Defense Trauma Registry). Failure of limb salvage was the primary endpoint. Two groups were established for analysis, the TVS group and a matched control group, in which no TVS was utilized. In the TVS group, 61 injured US troops sustained 64 arterial injuries (64 arterial stents inserted) and 25 concomitant venous injuries (14 venous stents inserted). In the control group, 60 injured patients sustained 61 arterial injuries and 23 concomitant venous injuries. After propensity score adjustment, there was a trend suggesting a reduced risk of amputation with TVS; however the primary endpoint of limb salvage was 78% in the TVS group and 77% in the control group. Associated orthopedic injury, an elevated mangled extremity severity score, and venous ligation were identified as independent risk factors for amputation. The military’s experience with TVS suggests that this damage control adjunct is an effect technique to temporarily provide distal perfusion. In a porcine model of limb ischemia, early TVS insertion protected the injured extremity from further ischemic insult and reduced circulating markers of tissue injury [43]. Preservation of perfusion allows for an attempt at limb salvage.
The civilian experience with TVS demonstrates similar technical success and efficacy. Subramanian et al. [44] reported on the 10-year experience of a Level I trauma center, in a large retrospective review of TVS. This report included 786 patients treated for vascular injury. Indications for shunt placement included significant physiology derangement requiring the need for a damage control treatment strategy and utilization of the TVS at the initial operation in preparation for a staged, definite vascular repair. In total, 73 patients had 108 TVS inserted. This represents 9% TVS usage in the management of vascular trauma compared to 44% in a wartime application. Shunt patency was reported at 91%, and the limb salvage rate was 74%. The multicenter shunt study group reported on the use of temporary vascular shunts performed at several high-volume Level I trauma centers [45]. This report detailed the largest multicenter aggregate of patients in the civilian literature who underwent damage control vascular surgery with TVS. This retrospective study identified 213 vascular injuries (201 patients) requiring TVS in a cohort of 7385 patients (2.7% aggregate shunt insertion rate). Of the 213 TVS, 95% of the shunts were used for arterial injuries. Shunting of the extremity occurred in 75% of patients, and the superficial femoral artery was the most common location for shunt placement (24%), followed by the popliteal artery (19%) and brachial artery (13%). This civilian report demonstrated excellent TVS patency with shunt thrombosis recorded at only 5.6% and minimal TVS complications with TVS dislodgement at 1.4%. TVS were implemented in a damage control treatment strategy in 63% of patients and used in concomitant orthopedic and vascular injury in the remaining 36% of patients. A 96% limb salvage rate was achieved. Systemic heparin was only used in 22% on shunted patients. The use of heparin was not associated with a reduced incidence of shunt thrombosis. This report demonstrated no independent predictors for shunt thrombosis. Granchi et al. [46] described the long-term effectiveness of TVS without systemic heparinization. In this report, 19 patients demonstrated TVS patency with no shunt thrombosis reported, and the average shunt dwell time was greater than 10 h. Overall limb salvage was reported at 89%. In the severely injured patient, multiple simultaneous injury is common. Frequently, these additional injuries represent a contraindication to therapeutic anticoagulation or, at least, limit the ability to anticoagulated. Review of available data suggests that anticoagulation is not required during shunting.
Insertion of a TVS requires technical familiarity as well as experience with the relative arterial and venous system anatomy at risk. Generally, proximal or central vascular control and distal or peripheral vascular control must be achieved at the location of injury. Once vascular control has been established, the surgeon must assess distal perfusion. Typically, the injured vessel can be forward-bled and back-bled to confirm uninterrupted flow. If flow is not visualized, balloon thromboembolectomy catheters are passed to remove thrombus. The shunt is subsequently inserted into the distal or peripheral vascular bed and allowed to back-bleed. Next, the TVS is inserted into the proximal or central vessel. Flow is generally confirmed with continuous-wave Doppler. The shunt is secured into position with heavy silk suture to prevent dislodgement. Table 11.1 describes several commercially available vascular shunts.
11.3.2 Temporary Synthetic Conduit
Autologous vein remains the standard bypass conduit for traumatic vascular injuries. Devastating combat blast injuries can render an ischemic limb with no suitable autologous conduit. The use of prosthetic graft for reconstruction of military and civilian vascular injuries has demonstrated feasibility as a damage control adjunct allowing for reestablishing distal perfusion in some scenarios (Fig. 11.2). Feliciano et al. [47] reported on 206 patients with 236 polytetrafluoroethylene (PTFE) grafts inserted in traumatic vascular wounds. PTFE was found to be an acceptable conduit for interposition grafting of segmental arterial defects; however long-term follow-up and determination of long-term patency were lacking. This early study demonstrated the feasibility of using synthetic conduit in repair of vascular trauma. Vertrees et al. [48] described 95 emergent bypasses performed for military vascular injuries. Fourteen bypasses were constructed with polytetrafluoroethylene (PTFE). Indications for the use of PTFE included major vessel segmental loss (79%), pseudoaneurysm (7%), and vein graft disruption (14%). This study reported 79% of prosthetic grafts maintained short-term patency allowing for patient stabilization, continued medical evacuation, and eventual definitive revascularization with autologous conduit. Four PTFE grafts (29%) required explantation for presumed infection. No prosthetic graft disruptions were reported, and no patients required amputation due to prosthetic graft failure. Secondary hemorrhage related to prosthetic vascular graft anastomotic disruption was reviewed by Greer et al. [49]. Combat-related vascular injuries are frequently associated with heavy contamination and soft tissue devastation resulting in a high risk of infection [50, 51]. This report included 181 US casualties sustaining arterial injury treated with bypass grafting for limb salvage identified. Autologous venous conduit was used in 97% of arterial repairs. Only six patients (3%) underwent reconstruction with prosthetic conduit. Anastomotic disruption was reported in 6% of repairs; all disruptions occurred at the arterial vein graft anastomosis. Infection was the cause of the disruption process. Watson et al. [52] identified 3569 vascular injuries in US service personnel. Four hundred thirty-five (12%) were managed with interposition bypass graft reconstruction with 410 autologous vein grafts and 25 expanded polytetrafluorethylene (PTFE) grafts. This retrospective cohort comparison demonstrated that PTFE had similar effectiveness and durability when compared to autologous conduit. However, the use of prosthetic conduit resulted in higher rates of complications. The utilization of synthetic conduit has demonstrated technical and clinical success regarding limb preservation following severe low extremity injury. When feasible, temporary synthetic conduit should be explanted and autologous bypass completed.
This combat casualty sustained a devastating penetrating injury to the right lower extremity with injuries to the femoral vessels and femur. The patient was initially managed with temporary bypass using polytetrafluorethylene (PTFE) and external fixation of the femur fracture. The black arrow identifies the PTFE conduits for the femoral artery and vein injuries. The angiogram depicts the definitive common femoral artery reconstruction and bypass (tunneled anterolateral) with the autologous greater saphenous vein
11.4 Revascularization
Definitive revascularization has a limited role in damage control vascular surgery, often because of the time, technical experience, and operative support required for such measures. Primary repair of minor vascular trauma can be performed rapidly without physiologic consequences. In 1946, DeBakey [5] commented that “therapeutic measures designed to save the limb are applicable, at best, in not more than 20% of cases” on the battlefield. Hughes and Rich [6,7,8,9,10,11] demonstrated the feasibility of complex vascular repair as an option for combat-related vascular injuries. During modern combat, nearly 50% of vascular injuries sustained in battle are now managed with repair or bypass which confirms that the window of opportunity for limb salvage has been extended [16, 53, 54]. Advanced methods of flow preservation and elaborate revascularization have been successfully performed in a forward, austere environment in conjunction with damage control resuscitation [12, 13, 55]. Fox et al. [55] reported on 16 combat casualties that underwent 20 vascular reconstructions for upper and lower extremity major vascular injuries for limb salvage. Routine fasciotomy and stabilization of concomitant orthopedic injury were performed. Damage control resuscitation resulted in physiologic recovery and avoided the lethal triad of hypothermia, coagulopathy, and progressive acidosis. Reported median operative time was 4.5 h for revascularization. This report documented the technical success of 19 saphenous vein bypass grafts and 1 synthetic bypass graft performed for definitive revascularization. Fasciotomies remain a critical adjunct when considering revascularization [56].
11.5 Specific Damage Control Vascular Surgery Considerations
11.5.1 General Principles
The fundamental principles regarding the management of vascular injury include adequate exposure, proximal and distal control, debridement to viable tissue, shunting, revascularization, or ligation. Damage control vascular decisions must account for patient physiology, concomitant injuries, anatomic location of the injured vessels, and available resources. Frequently, the most challenging aspect in the management of vascular injury relates to the anatomic exposure. Primary repair, construction of an anastomosis, and shunt placement are generally considered a straightforward technical exercise. However, in the context of devastating tissue destruction, concomitant injuries, hematoma formation, and significantly distorted anatomic landmarks, the identification and subsequent exposure of these vascular injuries can be challenging for even an experienced surgeon. The following sections will discuss general diagnostic considerations and vessel-specific exposures. It is important to note that the patient’s physiology dictates the surgical plan and should be considered prior to implementing temporary or definitive revascularization.
11.5.2 Carotid Artery
Penetrating cervical trauma involving the carotid artery remains a challenging vascular injury. The modern incidence of wartime cervical vascular injury is 8% [16]. Injury to the carotid injury can result in life-threatening exsanguinating hemorrhage, significant cervical hematoma formation with airway compromise, and devastating neurologic complications. Hemorrhage and occlusion are indications for intervention. When feasible, contrast CTA should be performed. CTA facilitates the triage process, improves operative planning, and records baseline neurologic imaging. The patient’s physiologic status determines the surgical plan with respect to revascularization. TVS has a clear utility during damage control maneuvers and also during the definitive revascularization allowing for continued cerebral perfusion to potentially ischemic neurons.
Exposure of the carotid artery is through an incision at the anterior margin of the sternocleidomastoid muscle, ipsilateral to the injury. The platysma muscle is divided and the sternocleidomastoid muscle reflected posterolaterally. The internal jugular vein is mobilized laterally following ligation of the common facial vein thereby exposing the carotid artery bifurcation. If feasible, the common carotid artery is exposed proximal to the hematoma or injured segment of the vessel and controlled with a vessel loop secured by a Rummel tourniquet. In the absence of uncontrolled hemorrhage, there is no need to tighten down the Rummel tourniquet. The dissection proceeds distal into the zone of injury. If bleeding is encountered, the Rummel tourniquet can be cinched down, or a vascular clamp can be placed. Back bleeding from the internal carotid artery is a favorable sign and can be controlled with a small clamp or a vessel loop. It is important to recognize that distal thrombosis of the internal carotid artery results in poor or no back bleeding. If this is encountered, carefully passing a 2–3 French embolectomy catheter can remove the thrombus and restore appropriate back bleeding. Aggressive catheter manipulation can result in a carotid-cavernous fistula; therefore, great care should be practiced.
Following vascular control of the proximal common carotid artery and distal internal carotid artery, the injury is explored. A TVS should be placed to maintain perfusion while the injury is explored and options considered. With respect to TVS placement, the shunt should be placed into the internal carotid artery and secured with a vessel loop allowing back bleeding through the shunt. In order to secure the proximal shunt, in sequence, the shunt is placed in the common carotid artery through the Rummel tourniquet. As the shunt advances into the common carotid artery, the Rummel tourniquet is tightened down fully securing the shunt in place. Repair of carotid artery injuries typically requires placement of an interposition greater saphenous vein graft, although primary repair or vein patch angioplasty can be performed for less severe injuries. To perform the interposition graft over the TVS, the proximal end is removed using the DeBakey clamp to occlude the common carotid artery. The vein graft is placed over the shunt (i.e., shunt in the vein graft lumen). The proximal shunt is reinserted into the common carotid artery and secured with the Rummel device using the previously described sequence. After flow is restored in the shunt, the distal vein graft anastomosis is performed using 6-0 Prolene suture to the edge of the normal internal carotid. Next, the proximal anastomosis to the common is started also with 6-0 Prolene suture. When the anastomosis is nearly completed, the shunt is removed through the remaining anastomotic opening, first removing the distal TVS from the internal carotid artery observing back bleeding followed by the proximal extent of the TVS observing appropriate forward bleeding. The anastomosis is completed. Alternatively, the reconstruction can be performed without a shunt; however, this exposes the ipsilateral hemisphere to prolonged ischemia. Regardless of whether or not a shunt is used, the mean arterial pressure should be kept above 90 mmHg during the repair to optimize cerebral perfusion. If no other life-threatening injuries are present, a small amount of systemic heparin (50u/kg) is recommended along with generous flushing of the repair with heparinized saline to prevent platelet aggregation and clot formation. Ligation of the internal carotid artery is an acceptable damage control maneuver to stop hemorrhage but has an acute stroke rate of 30–50%.
11.5.3 Subclavian Artery
Management of injury to the subclavian artery requires technical familiarity with exposure of the involved portion of the vessel. The central right subclavian artery is approached through a median sternotomy, while the central left subclavian artery is approached through a high left anterolateral thoracotomy. The mid-subclavian artery can be exposed through a supraclavicular approach following division of the clavicular head of sternocleidomastoid muscle and scalene fat pad, identification of the phrenic nerve, and subsequent division of the anterior scalene muscle. The supraclavicular approach can be a meticulous, time-consuming dissection given the critical associated structures in the surgical field. Alternatively, the mid- and distal subclavian arteries can be exposed and controlled through a combined supraclavicular and infraclavicular incisions. There is no requirement to obtain proximal vascular control within the surgical field of injury; using separate incisions through non-traumatized tissues can expedite rapid vascular control. In a hemodynamically unstable patient, initial proximal control obtained via sternotomy or thoracotomy will allow for more rapid vascular control than use of the more time-consuming supraclavicular approach. Because of the technical challenges with exposure, the utility of temporary vascular shunts in this injury pattern is limited. Additionally, interposition graft using 6–8 mm PTFE or Dacron is sometimes required for subclavian artery repair. Endovascular intervention for this injury pattern allows for rapid definitive repair without the morbidity of the surgical approach.
11.5.4 Axillary Artery
Control of the proximal axillary artery is best accomplished through an ipsilateral supraclavicular incision (proximal control via the subclavian artery), although the axillary artery itself is exposed through an infraclavicular approach. The infraclavicular exposure includes division of the clavipectoral fascia and the blunt separation of the fibers of the pectoralis major muscle. The axillary vein is the first structure encountered in the axillary sheath. The axillary artery lies deep to the vein; mobilization and caudal retraction of the axillary vein will expose the first segment of the axillary artery. The pectoralis minor muscle can be retracted laterally or divided. Repair of the axillary artery most commonly involves an interposition graft using reversed saphenous vein. TVS are of significant utility for delayed reconstruction in the damage control setting.
11.5.5 Brachial Artery
The brachial artery and median nerve travel within the brachial sheath and are exposed through a medial incision in the upper arm in the bicipital groove. The median nerve is the most superficial structure encountered upon entering the brachial sheath. The ulnar nerve runs posterior to the artery which is surrounded by paired deep brachial veins. Repair of the brachial artery is most commonly accomplished using primary repair, reversed saphenous vein interposition graft, or TVS allowing for delayed reconstruction. Although it may be possible to ligate the brachial artery distal to the origin of the profunda brachii artery and maintain a viable arm and hand, this proposition is based on intact collateral circulation. Unfortunately, collaterals from the shoulder and profunda brachii artery are often damaged in the setting of penetrating blast wounds, and therefore maintenance of flow through the brachial artery with a TVS or definitive vascular repair is advised. Ligation or primary amputation is an acceptable damage control maneuver if there is not time for shunting or the patient is in extremis.
11.5.6 Thoracic and Abdominal Aorta
Management of penetrating injury to the thoracic and abdominal aorta is rare given the prehospital lethality of this injury. Wartime estimates reported a combined incidence of aortic injury at 2.9% [16]. Initial management of thoracic hemorrhage in the setting of penetrating trauma is directed by chest tube location and output in conjunction with the patient’s physiology. The descending thoracic aorta is approached through the left anterior-lateral thoracotomy. An initial left thoracotomy can be extended into the right chest extending across the sternum (i.e., “clamshell” thoracotomy). Aortic control proximal and distal to the injury or hematoma must be obtained including isolation or control of any intercostal arteries in this segment. Aortic clamps are used to arrest flow in this segment, and the hematoma is entered with debridement of the injured aorta. An adequate length of the aorta must be debrided to allow placement of a large-caliber synthetic conduit (20 mm–26 mm Dacron graft) positioned end to end to the proximal and distal segments of the uninjured aorta. Endovascular management of blunt aortic injury to the thoracic aorta (i.e., partial aortic transection or pseudoaneurysm formation) in a patient who has demonstrated temporary physiologic stability allows for definitive repair without the morbidity of thoracotomy and improved outcomes [57].
Blunt and penetrating injuries to the abdominal aorta present as a central, zone I retroperitoneal hematoma. The surgical management of zone I retroperitoneal hematomas should be based upon the distribution of the hematoma. Supra-mesocolic, zone I retroperitoneal hematomas are best approached via a left medial-visceral rotation (Mattox maneuver) which exposes the supraceliac, paravisceral, and infrarenal segments of the abdominal aorta. Infra-mesocolic, zone I retroperitoneal hematomas can be approached via a standard transabdominal, transperitoneal approach with transverse colon cranial retraction and small bowel evisceration or with a right medial-visceral rotation (Cattell-Braasch maneuver) exposing the infrarenal aorta and inferior vena cava. Proximal and distal aortic control is paramount during surgical management. Proximal control is rapidly obtained in the supraceliac position and obtained through the gastrohepatic ligament by retracting the esophagus to the left and dividing the diaphragmatic crus. Alternatively, the Mattox maneuver exposes the supraceliac aorta from the lateral position, enabling proximal control as well. The iliac vessels or distal aorta are subsequently controlled, providing isolation before entering the hematoma. Repair techniques for the aorta and its branch vessels range from primary pledgetted closure to replacement with a Dacron interposition graft and depend upon the degree of injury.
11.5.7 Inferior Vena Cava
Intracavitary injury to the inferior vena cava (IVC) can result in massive hemorrhage and hemodynamic instability. The inferior vena cava is approached in the abdomen by performing the Cattell-Braasch and extended Kocher maneuvers. Mobilization of the liver is required to visualize the retro-hepatic vena cava. The lumbar venous tributaries into the injured segment of the IVC should be controlled to allow for comprehensive isolation. Because repair of the IVC is likely to require intermittent occlusion (i.e., sponge sticks or vascular clamps) or ligation in extreme cases, central venous access should be established above the diaphragm to allow effective volume resuscitation. If temporary occlusion of the IVC results in significant hypotension, the adjacent abdominal aorta may be temporarily occluded to support central pressures while continued resuscitation takes place. Repair of longitudinal injuries to the IVC can be accomplished with a running venorrhaphy provided that the residual lumen is not narrowed more than 50%. In instances where longitudinal repair will result in greater than 50% stenosis of the IVC, patch angioplasty or resection and interposition graft using ePTFE or Dacron is preferable. Ligation of the infrarenal IVC is acceptable as a damage control maneuver, although this carries a significant risk of mortality and major morbidity in the form of decreased cardiac preload and significant lower extremity edema. If infrarenal IVC ligation is needed, bilateral lower extremity fasciotomies must be completed in order to reduce the risk for compartment syndrome. Suprarenal occlusion of the IVC is generally not compatible with survival and should be considered a measure of last resort [58].
11.5.8 Common, External, and Hypogastric Iliac Arteries
Iliac artery injuries generally present as a zone III or pelvic hematoma with or without extremity ischemia. Exploration of the zone III hematoma should be performed following proximal control of the infrarenal abdominal aorta and the contralateral common iliac artery, if feasible. The distal external iliac artery should be identified as it exits the pelvis at the inguinal ligament at a position free from the hematoma formation. The hypogastric (internal iliac) artery may not be initially controlled or visualized before exploring the hematoma. The inability to initially control all bleeding from the hematoma necessitates preparation including multiple suction devices, Fogarty occlusion balloons, direct tamponade strategies or devices, and alerting anesthesia regarding the need for continued resuscitation during exploration. After proximal and distal control of the common and external iliac arteries is obtained, the hematoma is entered which facilitates exposure and clamping of the hypogastric artery and the injured vessel(s). Common and external artery injuries can be controlled and managed with a TVS as needed or repaired with interposition grafting using saphenous vein or prosthetic conduit (6–8 mm ePTFE or Dacron). In an unstable patient or a patient where there is significant contamination of the surgical field, shunt placement with delayed definitive repair or reconstruction is appropriate. If the primary injury is to the hypogastric artery, it can be ligated. Bleeding from associated iliac veins may be severe and difficult to expose. The common or external iliac artery may be divided if necessary to facilitate exposure of the iliac vein, followed by subsequent repair of the artery. Endovascular adjuncts, such as selective embolization of a bleeding hypogastric artery, are an option, particularly in blunt trauma with associated pelvic fracture.
11.5.9 Common and Superficial Femoral Artery
Injury to the common femoral artery is often fatal as hemorrhage control at this anatomic location is often difficult. Additionally, injury to the common femoral artery and superficial femoral artery represents the second most common anatomic location for wartime vascular trauma [16]. Surgeon experience and familiarity in damage control maneuvers for rapid vascular control of the femoral vessels are critical. Exposure of the common femoral artery is obtained through a longitudinal incision above the artery approximately 2 cm lateral to the pubic tubercle at the inguinal ligament. A technical point in exposing the common femoral artery is extending the incision cranial enough so that the inguinal ligament can be identified first in a consistent and familiar manner. Shunting with a TVS can be performed in conjunction with damage control maneuvers. However, distal common femoral artery injuries at the bifurcation of the superficial femoral artery and profunda artery represent a unique challenge with respect to maintaining forward perfusion to both structures. Every attempt should be made to maintain flow into the profunda femoris artery, although the feasibility of this will depend upon the pattern of injury and surgeon experience with more complicated vascular reconstruction. Alternatively, proximal control can be obtained in the retroperitoneum (i.e., external iliac artery) through the cranial extension of the groin incision or by using a limited transverse-oblique incision in the lower abdomen cranial to the inguinal ligament. After a transverse-oblique skin incision, the external and internal oblique aponeuroses are divided. The transversus abdominus muscle and transversalis fascia are opened allowing entrance into the retroperitoneum. The plane between peritoneum and retroperitoneum is developed, and the peritoneal contents are reflected cephalad, exposing the external iliac vessels along the medial border of the psoas muscle. Proximal vascular control is obtained at the external iliac artery.
Exposure of the distal superficial femoral artery is performed through a medial thigh incision and the adductors of the leg (i.e., adductor magnus). Exposure is facilitated by placing a lift or “bump” below the knee which allows the superficial femoral artery, sartorius muscle, and adductors to be suspended improving separation. Entry into the fascia of the lower thigh is performed at the anterior margin of the sartorius muscle which is subsequently reflected posteriorly. Exposure is facilitated with the surgeon seated looking across the dissection field with lights positioned directly over the shoulder if they do not have a headlight available. When exposing the superficial femoral artery, it is important to recognize the femoral vein which is in close proximity to the artery. Repair of superficial femoral artery injury is best performed by reversed saphenous vein interposition graft from the uninjured leg. Shunting of the superficial femoral artery is appropriate during damage control procedures.
11.5.10 Profunda Femoris Artery
The profunda femoris artery provides perfusion to the musculature of the thigh. Exposure of the proximal profunda femoris artery is obtained through a longitudinal incision used to expose the common femoral artery. Mid- and distal segments of the profunda femoris artery are exposed through a vertical incision made parallel to the lateral border of the sartorius muscle. The sartorius muscle is retracted medially and the rectus femoris is retracted laterally to expose the mid- and distal segments. Proximal profunda injuries should be repaired with reversed saphenous vein interposition graft. This is especially important if there is question about the integrity of the superficial femoral or popliteal vessels. In the setting of a compromised superficial femoral artery, flow through the profunda femoris is critical to allow healing of subsequent lower extremity wounds and amputations. In a patient who sustains a devastating blast injury with a non-salvageable lower extremity (i.e., above-knee traumatic amputation), the superficial femoral artery can be used as a conduit in order to maintain the integrity of the profunda femoris artery. If patency of the superficial femoral artery can be confirmed, ligation of mid- and distal profunda femoris arterial injuries is acceptable.
11.5.11 Popliteal Artery
Popliteal artery and vein injuries were identified in 9% of traumatic injuries during the wars in Iraq and Afghanistan [16]. Injuries in the popliteal space are exposed through a medial incision. The dissection is extended from cephalad to caudad at the medial aspect of the knee and is facilitated by a lift or “bump” under the calf of the leg with the knee flexed. When exposing caudal portion of the popliteal space, the bump is placed under the thigh. Natural dissection planes exist in exposing the above-knee popliteal artery with the exception of the need to divide the fibers of the adductor magnus which envelop the distal superficial femoral artery (Hunter’s canal). Similarly, a natural dissection plane exists into the popliteal space for the below-knee popliteal artery; however, added exposure can be accomplished by division of the gastrocnemius and soleus muscle fibers from the medial tibial condyle thereby allowing a lengthy exposure of the below-knee popliteal artery and the origins of the anterior tibial artery and the tibial-peroneal trunk. To completely expose the popliteal space, the medial attachment of the pes anserinus (conjoined tendons of the sartorius, semitendinosus, semimembranosus, and gracilis) to the medial condyle of the tibia can be divided. When feasible, the pes anserinus should be reconstructed given its significant role in medial knee stabilization. Weitlaner retractors, cerebellar retractors, and flexible Adson-Beckman or Henly popliteal retractors with detachable side blades are necessary to expose the popliteal space. Typically, the medial head of the gastrocnemius can be retracted down using one of these devices and does not need to be divided. TVS are of significant utility in damage control management of popliteal artery injuries. Reconstruction generally incorporates the use of autologous greater saphenous vein when feasible.
11.5.12 Tibial Arteries
Peripheral vascular injury to the lower extremity continues to represent the most common injury pattern encountered throughout military history [5,6,7,8,9,10,11,12,13,14,15,16]. During modern warfare, tibial-level vascular injuries are present in 21% of wounded casualties. The recommended approach to tibial artery injury is one of selective repair. Because of their distal location and redundant nature, isolated and multiple tibial artery injuries can be ligated without adverse outcomes. As long as one tibial artery remains uninjured and patent to the ankle, no additional tests or repairs are required. This selective approach to tibial repair has been shown to be effective, confirming that although tibial injuries can be ligated, there is a distinct injury pattern which requires repair [36]. TVS can be inserted into tibial vessels although shunt patency is lower than that in more proximal vessels. The anterior tibial artery is exposed through an anterolateral longitudinal incision midway between the tibia and fibula. The fascia along the lateral border of the anterior tibialis muscle is divided, and the plane between the anterior tibialis and extensor digitorum longus muscles is developed. The anterior tibial artery lies deep along the interosseous membrane. Exposure of the posterior tibial artery in the deep compartment of the leg is through a medial incision with a lift or “bump” under the knee or thigh. A longitudinal incision is made 2 cm posterior to the posterior margin of the tibia. Division of the tibial attachments of the soleus muscle in the proximal and mid-leg and posterior retraction of the soleus exposes the artery. Reconstruction of a peroneal artery injury is rarely required, and ligation is adequate. Importantly, tibial reconstruction is technically more challenging and time-consuming because of the smaller size of the vessels. Like other vascular repairs, tibial reconstruction should not be undertaken if the patient has other life-threatening injuries or is in extremis.
11.6 Summary
Vascular damage control surgery emphasizes immediate hemorrhage control and mitigation of ischemia (and subsequent complications related to end-organ ischemia) with restoration of perfusion.
References
Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7.
Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, Butler FK. Causes of death in US special operations forces in the global war on terrorism 2001–2004. Ann Surg. 2007;245:986–91.
Bellamy RF. The cause of death in conventional land warfare: implications for combat casualty care research. Mil Med. 1984;149:55–62.
Kelly JF, Ritenour AE, McLaughlin DF, et al. Injury severity and causes of death from operation Iraqi freedom and operation enduring freedom: 2003–2004. J Trauma. 2008;64(suppl):s21–7.
DeBakey ME, Simeone FA. Battle injuries of the arteries in world war II. Ann Surg. 1946;123:534–79.
Hughes CW. The primary repair of wounds of major arteries; an analysis of experience in Korea in 1953. Ann Surg. 1955;141:297–303.
Hughes CW. Acute vascular trauma in Korean war casualties; an analysis of 180 cases. Surg Gynecol Obstet. 1954;99:91–100.
Jahnke EJ Jr, Hughes CW, Howard JM. The rationale of arterial repair on the battlefield. Am J Surg. 1954;87:396–401.
Rich NM, Hughes CW. Vietnam vascular registry: a preliminary report. Surgery. 1969;65:218–26.
Rich NM, Baugh JH, Hughes CW. Popliteal artery injuries in Vietnam. Am J Surg. 1969;118:531–14.
Rich NM, Baugh JH, Hughes CW. Acute arterial injuries in Vietnam: 1,000 cases. J Trauma. 1970;10:359–69.
Woodward EB, Clouse WD, Eliason JE, et al. Penetrating femoropopliteal injury during modern warfare: experience of the balad vascular registry. J Vasc Surg. 2007;47:1259–65.
Clouse WD, Rasmussen TE, Peck MA, et al. In-theater management of vascular injury: 2 years of balad vascular registry. J Am Coll Surg. 2007;204:625–32.
Rasmussen TE, Clouse WD, Jenkins DH, et al. Echelons of care and the management of wartime vascular injury: a report from the 332nd EMDG/air force theater hospital, Balad Air Base, Iraq. Persp Vasc Surg Endovasc Ther. 2006;18(2):91–9.
Stannard A, Brown K, Benson C, Clasper J, Midwinter M, Tai NR. Outcome after vascular trauma in a deployed military trauma system. Br J Surg. 2011;98(2):228–34.
White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg. 2011;253(6):1184–9.
White JM, Cannon JW, Stannard A, Markov NP, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400–9.
Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71(6):1869–72.
Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, Rasmussen TE. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848–56.
Scott DJ, Eliason JL, Villamaria C, Morrison JJ, Houston R 4th, Spencer JR, Rasmussen TE. A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg. 2013;75(1):122–8.
Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, Holcomb JB, Scalea TM, Rasmussen TE. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75(3):506–11.
Morrison JJ, Ross JD, Houston R, Watson JD, Sokol KK, Rasmussen TE. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of noncompressible torso hemorrhage. Shock. 2014;41(2):130–7.
Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80(2):324–34.
Mabry RL, Holcomb JB, Baker AM, Cloonan CC, Uhorchak JM, Perkins DE, Canfield AJ, Hagmann JH. United States Army rangers in Somalia: an analysis of combat casualties on an urban battlefield. J Trauma. 2000;49(3):515–28; discussion 528–9.
Kragh JF Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Practical use of emergency tourniquets to stop bleeding in major limb trauma. J Trauma. 2008;64(Suppl 2):S38–9.
Kragh JF Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249(1):1–7.
Kragh JF Jr, Littrel ML, Jones JA, Walters TJ, Baer DG, Wade CE, Holcomb JB. Battle casualty survival with emergency tourniquet use to stop limb bleeding. J Emerg Med. 2011;41(6):590–7.
Kragh JF Jr, O’Neill ML, Walters TJ, Jones JA, Baer DG, Gershman LK, Wade CE, Holcomb JB. Minor morbidity with emergency tourniquet use to stop bleeding in severe limb trauma: research, history, and reconciling advocates and abolitionists. Mil Med. 2011;176(7):817–23.
Kragh JF Jr, Cooper A, Aden JK, Dubick MA, Baer DG, Wade CE, Blackbourne LH. Survey of trauma registry data on tourniquet use in pediatric war casualties. Pediatr Emerg Care. 2012;28(12):1361–5.
Kragh JF Jr, Dubick MA, Aden JK, McKeague AL, Rasmussen TE, Baer DG, Blackbourne LH. U.S. military use of tourniquets from 2001 to 2010. Prehosp Emerg Care. 2015;19(2):184–90.
Beekley AC, Sebesta JA, Blackbourne LH, Herbert GS, Kauvar DS, Baer DG, Walters TJ, Mullenix PS, Holcomb JB, 31st Combat Support Hospital Research Group. Prehospital tourniquet use in operation Iraqi freedom: effect on hemorrhage control and outcomes. J Trauma. 2008;64(2 Suppl):S28–37; discussion S37.
King DR, van der Wilden G, Kragh JF Jr, Blackbourne LH. Forward assessment of 79 prehospital battlefield tourniquets used in the current war. J Spec Oper Med. 2012;12(4):33–8.
Lairet JR, Bebarta VS, Burns CJ, Lairet KF, Rasmussen TE, Renz EM, King BT, Fernandez W, Gerhardt R, Butler F. Prehospital interventions performed in a combat zone: a prospective multicenter study of 1,003 combat wounded. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S38–2.
Inaba K, Siboni S, Resnick S, Zhu J, Wong MD, Haltmeier T, Benjamin E, Demetriades D. Tourniquet use for civilian extremity trauma. J Trauma Acute Care Surg. 2015;79(2):232–7.
Fortuna G, DuBose JJ, Mendelsberg R, Inaba K, Haider A, Joseph B, Skarupa D, Selleck MJ, OʼCallaghan TA, Charlton-Ouw K, Lower Extremity Vascular Repairs Outcome Group. Contemporary outcomes of lower extremity vascular repairs extending below the knee: a multicenter retrospective study. J Trauma Acute Care Surg. 2016;81(1):63–70.
Burkhardt GE, Cox M, Clouse WD, Porras C, Gifford SM, Williams K, Propper BW, Rasmussen TE. Outcomes of selective tibial artery repair following combat-related extremity injury. J Vasc Surg. 2010;52(1):91–6.
Liang NL, Alarcon LH, Jeyabalan G, Avgerinos ED, Makaroun MS, Chaer RA. Contemporary outcomes of civilian lower extremity arterial trauma. J Vasc Surg. 2016;64(3):731–6.
Eger M, Golcman L, Goldstein A, Hirsch M. The use of a temporary shunt in the management of arterial vascular injuries. Surg Gynecol Obstet. 1971;132:67–70.
Rasmussen TE, Clouse WD, Jenkins DH, Peck MA, Eliason JL, Smith DL. The use of temporary vascular shunts as a damage control adjunct in the management of wartime vascular injury. J Trauma. 2006;61(1):8–12; discussion 12–5.
Chambers LW, Green DJ, Sample K, Gillingham BL, Rhee P, Brown C, Narine N, Uecker JM, Bohman HR. Tactical surgical intervention with temporary shunting of peripheral vascular trauma sustained during operation Iraqi freedom: one unit's experience. J Trauma. 2006;61(4):824–30.
Taller J, Kamdar JP, Greene JA, Morgan RA, Blankenship CL, Dabrowski P, Sharpe RP. Temporary vascular shunts as initial treatment of proximal extremity vascular injuries during combat operations: the new standard of care at echelon II facilities? J Trauma. 2008;65(3):595–603.
Gifford SM, Eliason JL, Clouse WD, Spencer JR, Burkhardt GE, Propper BW, Dixon PS, Zarzabal LA, Gelfond JA, Rasmussen TE. Early versus delayed restoration of flow with temporary vascular shunt reduces circulating markers of injury in a porcine model. J Trauma. 2009 Aug;67(2):259–65.
Gifford SM, Aidinian G, Clouse WD, Fox CJ, Porras CA, Jones WT, Zarzabal LA, Michalek JE, Propper BW, Burkhardt GE, Rasmussen TE. Effect of temporary shunting on extremity vascular injury: an outcome analysis from the global war on terror vascular injury initiative. J Vasc Surg. 2009;50(3):549–55; discussion 555–6.
Gifford SM, Eliason JL, Clouse WD, Spencer JR, Burkhardt GE, Propper BW, Dixon PS, Zarzabal LA, Gelfond JA, Rasmussen TE. Early versus delayed restoration of flow with temporary vascular shunt reduces circulating markers of injury in a porcine model. J Trauma. 2009;67(2):259–65.
Subramanian A, Vercruysse G, Dente C, Wyrzykowski A, King E, Feliciano DV. A decade’s experience with temporary intravascular shunts at a civilian level 1 trauma center. J Trauma. 2008;65:316–26.
Inaba K, Aksoy H, Seamon MJ, Marks JA, Duchesne J, Schroll R, Fox CJ, Pieracci FM, Moore EE, Joseph B, Haider AA, Harvin JA, Lawless RA, Cannon J, Holland SR, Demetriades D, Multicenter Shunt Study Group. Multicenter evaluation of temporary intravascular shunt use in vascular trauma. J Trauma Acute Care Surg. 2016;80(3):359–64; discussion 364–5.
Granchi T, Schmittling Z, Vasquez J, Schreiber M, Wall M. Prolonged use of intraluminal arterial shunts without systemic anticoagulation. Am J Surg. 2000;180(6):493–6; discussion 496–7
Feliciano DV, Mattox KL, Graham JM, Bitondo CG. Five-year experience with PTFE grafts in vascular wounds. J Trauma. 1985;25(1):71–82.
Vertrees A, Fox CJ, Quan RW, Cox MW, Adams ED, Gillespie DL. The use of prosthetic grafts in complex military vascular trauma: a limb salvage strategy for patients with severely limited autologous conduit. J Trauma. 2009;66(4):980–3.
Greer LT, Patel B, Via KC, Bowman JN, Weber MA, Fox CJ. Management of secondary hemorrhage from early graft failure in military extremity wounds. J Trauma Acute Care Surg. 2012;73(4):818–24.
Sohn VY, Arthurs ZM, Herbert GS, Beekley AC, Sebesta JA. Demographics, treatment, and early outcomes in penetrating vascular combat trauma. Arch Surg. 2008;143(8):783–7.
Watson JD, Houston R, Morrison JJ, Gifford SM, Rasmussen TE. A retrospective cohort comparison of expanded polytetrafluorethylene to autologous vein for vascular reconstruction in modern combat casualty care. Ann Vasc Surg. 2015;29(4):822–9.
Fox CJ, Patel B, Clouse WD. Update on wartime vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(1):13–25.
Chambers RW, Rhee P, Baker BC, et al. Initial experience of US marine corps forward resuscitative surgical system during operation Iraqi freedom. Arch Surg. 2005;140:26–32.
Fox CJ, Gillespie DL, Cox ED, Kragh JF Jr, Mehta SG, Salinas J, Holcomb JB. Damage control resuscitation for vascular surgery in a combat support hospital. J Trauma. 2008;65(1):1–9.
Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119–24.
Kauvar DS, White JM, Johnson CA, Jones WT, Rasmussen TE, Clouse DW. Endovascular versus open management of blunt traumatic aortic disruption at two military trauma centers: comparison of in-hospital variables. Mil Med. 2009;174(8):869–73.
Sullivan PS, Dente CJ, Patel S, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg. 2009;199:500–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
White, J.M., Rasmussen, T.E. (2018). Open Damage Control Vascular Surgery. In: Duchesne, J., Inaba, K., Khan, M. (eds) Damage Control in Trauma Care. Springer, Cham. https://doi.org/10.1007/978-3-319-72607-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-72607-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72606-9
Online ISBN: 978-3-319-72607-6
eBook Packages: MedicineMedicine (R0)