Abstract
In the present research the synthesis of gold nanoparticles (AuNPs) was established using the extract of Sedum praealtum. It should be pointed out that the conditions of synthesis directly influence the size, morphology, stability and physicochemical properties of the nanoparticles obtained. The extract of the plant was characterized by FTIR spectroscopy finding groups N-H, C-OH, who are credited with the reducing capacity of the ions AU+3. The AuNP’s obtained were characterized by UV-visible spectroscopy, observing plasmones absorbance between 530 and 550 nm characteristic of these nanoparticles, moreover they were characterized by SEM and was observed the nanometric sizes, the reduction capacity of the extract was evaluated by voltammetric study, observing the intervals of the processes of reducing and oxidation of the ionic species. Additionally, it was done the deposition of nanoparticles synthesized on a ceramic substrate in order to achieve its stabilization and then the nanocomposite was analyzed by SEM.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
There are several methods of synthesis of nanoparticles, however, most have negative effects on the environment, that is why they developed a discipline that is eco-friendly and economic, also has the same effectiveness as conventional physical and chemical methods; this is the green chemistry. This discipline has as main objective to promote the development and use of innovative chemical technologies that reduce or eliminate the use or generation of harmful substances in the design, manufacture and use of chemical products [1].
Through the control of biological molecules, green chemistry offers the synthesis of nanoparticles, several studies have reported the synthesis of metallic nanoparticles using plant species as reducing agents of ions. Nanoparticles of noble metals, and especially gold nanoparticles (AuNP’s), are of great interest because they possess optical, electrical and conductive properties, properties which are suitable for application in controlled release of drugs, in therapeutic macromolecules, in gene therapy, biosensors, etc. [2].
Recently interest in AuNPs has been strengthened because of the physical behaviors they exhibit intrinsic to size; among them are the enhancement of the Raman dispersion by surfaces, magnetization and Resonance of surface Plasmons. This property occurs when the particle is smaller than the incident wavelength in the sample; the oscillating electric field induces an electric dipole, thus generating a negative charge on one side of the particle [3].
Different biological means are known to synthesize nanoparticles through green chemistry, among which are viruses, bacteria, yeasts, fungi and plants; those that possess greater potential to accumulate heavy metals have better ability to synthesize nanoparticles. Plants have been considered as the most eco-friendly route for the synthesis of metallic nanoparticles; its extracts contain bioactive alkaloids, phenolic acids polyphenols, proteins, sugars and terpenoids that play an important role in the reduction of metal ions and subsequent stabilization of the nanoparticles [4].
Nanoparticles are synthesized from all parts of the plant such as seeds, stem, flowers, leaves and skin of fruits, although the properties of the particles vary due to the change in concentration of the reducing agents through the plant [5]. The size of AuNPs governs its properties and the applications for which they are used: small size (2–15 nm) has applications such as immunohistochemistry, microscopy and biomarkers; medium-sized (20–60 nm) are used in the detection and purification of the environment, drug delivery, biomarkers, chemical sensors, DNA detection; while the large ones (80–250 nm) are used in forensic science, electronic devices, manufacturing, etc. [6]. The AuNPs were tested in the first room for the color changes of the colloidal solutions, ranging from purple to brown and the appearance of absorbance bands in the range of 510–550 nm, a result obtained by UV- Vis; a shift of the bands towards larger wavelengths is associated with an increase in the size of the nanoparticles while the spreading indicates a greater distribution of such sizes and shapes.
In metallic colloids there is no well-defined metal-metal bond with a certain nuclearity, but they are agglomerates of atoms. An important aspect is the stabilization of NPs to maintain their size and shape as a function of time; in the absence of repulsive forces the particles tend to coagulate and subsequently precipitate. To counteract this problem, the use of stabilizing agents such as fatty acids, amines and polymers is used, which absorb on the surface of the newly formed nanoparticle and prevent aggregation [7]. Sometimes the plant species plays the role of stabilizer, however, there are other methods to achieve a more lasting stability, for example a deposition of the nanoparticles on a substrate; in this case a nanocomposite material is obtained.
Among the species reported with potential for the synthesis of nanoparticles is the species Sedum praealtum . It is native to Mexico and is distributed from the center of the country to Central America. It grows in semi-warm, semi-dry, temperate or cold climates, in humid stony soils. It tends to be associated with disturbed vegetation of xerophytic scrub, and oak and pine forest. The flowering period occurs between the periods from winter to spring. It resists temperatures of up to −3 °C and survives in full sunlight, for cultivation, can be watered once a week in spring and summer and once a month during the winter. This plant is multiplied by cuttings of leaves or stem in late spring or summer [8]. Herbaceous of long stems, reaches about 50 cm or more, the branch is basipeta and presents knots light brown color to reddish and white in terminal zones. Knots are associated to buds of leaf which have auxiliary yolks. The leaves emerge in clusters and later they become ramifications. The buds are arranged vertically in 6-leaf fascicles at an early stage, increasing the number of leaves as it grows. The leaves are green, fleshy, thick, spatulate with whole margin and reticulated venation in color light green, the apex is reddish, truncated when they are young and as they grow rounded. The base of the leaves is acuminate, sessile and without petiole. As they grow they leave the stem naked so that they are accumulated in the terminal part of it. The size of the leaves can reach up to 5 cm in length in the observed specimen. Yellow inflorescences have been observed in pinnate panicle cluster. Following the dichotomous key given by Rzedowski and Calderón, the described specimen was identified as Sedum praelatum belonging to the family Crassulaceae, considering the traits of shrub, glabra erecta with stems branched from the base, numerous leaves in basal rosette imbricated and flat, thick stems of woody appearance, yellow petals with non-exfoliating bark [9]. An image of the specimen used is shown in Fig. 1.
Experimental
Preparation of Sedum praealtum leaves extract
The extract was prepared from green leaves of the plant, washed with running water and then with distilled water. It weighed 1.25 g of the leaf and were maintained at 80 °C for 10 min in deionized water with Bath Maria. Once the extract was cold, it was leaked.
Characterization by of Sedum praealtum leaves extract
FTIR analysis
The leaves of Sedum praealtum were characterized by the technique Fourier-transform infrared spectroscopy (FTIR) to identify the main functional groups present. The sample was prepared by cutting the blade into small pieces.
Cyclic voltammetric study
As a working electrode (ET) a Pt electrode was used, as a counter electrode (CE) a type DSA mesh and as reference electrode (ER) a Calomel saturated electrode SCE. The sweep speed was 12 mVs−1. In the first place the extract was analyzed and later the solution after the synthesis . The analysis was conducted in a galvanostat potentiostat Princeton Applied Research model VersaSTAT4.
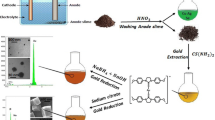
Synthesis of gold Nanoparticles
To obtain nanoparticles, an ion Au+3 solution was used as a precursor agent at different concentrations (5, 10, 20 and 30 mgL−1). The concentration of the extract used was 1.25%. The synthesis was made with Bath Maria at 80° C adding 10 mL of the precursor solution in aliquots of 2 ml every 10 min.
Characterization of gold nanoparticles
Characterization by UV-Vis
The samples obtained were analyzed in a UV-Visible spectrophotometer Perkin Elmer Lambda 35 double beam using 1 cm quartz cells of optical pathway in the range of 200–800 nm λ, in order to identify the resonance band of the plasmon surface characteristic of the gold nanoparticles.
Preparation of the substrate
A ceramic substrate was used, obtained from natural source. The ceramic was washed with running water and cleaned manually. It was subsequently washed with deionized water. For drying, it was left under sunlight for 5 h. Past this time they proceeded with grinding in an agate mortar. The dust obtained was then dried in a flask at 100° C for 1 h and then sieved to obtain grains with a diameter of less than 38 microns.
Deposition of gold nanoparticles
The synthesis was carried out using a concentration of 1.25% of the plant and the precursor 30 mg L−1, since under these conditions there was less precipitation. The ceramic matrix powder was added to the extract to make the deposition and later the synthesis was performed. After the 50 min of synthesis , the final solution was decanted and finally filtered using paper filtering, in this way, to separate from the solution the substrate with the nanoparticles. The paper filters obtained were introduced in a desiccator to later characterize the nanocomposite .
Characterization of the nanocomposite
SEM analysis:
The powder deposited on the filter paper was used for analysis by Scanning Electronic Microscope on an equipment JOEL model JSM-6701F.
X-ray Difraction :
The ceramic matrix with was deposited nanoparticles was dried with filter paper and subsequently analyzed by XRD.
Results and Discussion
Characterization of Sedum praealtum leaves extract
FTIR analysis
The characterization of the leaves of Sedum praealtum by FTIR was performed to know the main functional groups present in the extract of the plant. The Fig. 2 shows the obtained spectrum. The FTIR spectrum shows a pronounced peak in the 3400 cm−1 which is associated with the N-H band of amino groups or the hydroxyl group (−OH). The band in the 2908 cm−1 is attributed to alkanes groups (H-CH) or carboxylic acids (O-h). The band found in the 2843 cm−1 can be produced by alkanes groups or by the presence of acids (O-H). The band located at 1603 cm−1 denotes the presence of aldehydes (= C = O) or primary amides. The band at 1435 cm−1 may occur due to the presence of amino groups (N-H) or alcohols (C-OH). Likewise, the peaks in 1075 and 672 cm−1 denote the presence of amines and alquinos respectively. The reducing power of the ions Au+3 was attributed to the acid groups and OH.
Ciclic voltammetric study
In Fig. 3 is shown the Voltammogram obtained when analyzing (a) the extract of Sedum praealtum and (B) The solution of colloidal AuNPs after 50 min of synthesis . In the Voltamperograma (a) no peaks of oxidation and reduction are observed while in the Voltamperograma obtained from the synthesis of AuNPs is appreciated a peak reduction in the range of potential from −0.2 to −0.3 V which is attributed to the reduction of the ions Au+3 over Titanium electrode; Likewise, a second peak starts at −0.33 V, associated with the reduction of the medium. By compared both voltammograms is evidenced the ability of the species Sedum raealtum to reduce the ions Au+3 in Au0 and thus obtain nanoparticles; However, the reduction peak corresponds to Au+3 ions still present in the solution.
Visual observation
The synthesis of AuNPs can be evidenced by the change of colouring of the colloidal solutions obtained. By adding the precursor agent of the Au+3 ions to the extract of Sedum praealtum , an immediate color change was presented, obtaining solutions with violet pink tones as shown in Fig. 4. A change in pH value of 7–2 was presented when synthesis was performed.
Characterization of gold nanoparticles
Characterization by UV-Vis
Figure 5 shows the spectrogram obtained for different synthesis of nanoparticles using the extract at 1.25% but varying the concentration of the precursor. In all the studies, the extract was analyzed and it was found that no plasmons of absorbance were present in the area of interest; later, it was compared with the synthesis of AuNPs. In the spectrogram, a plasmon of absorbance is observed in the region of 480–720 nm, which denotes obtaining AuNPs. In all the graphs it is observed that the band of the Plasmon suffers a shift towards greater wavelengths as the time of synthesis elapses, as well as to increase the concentration of the precursor agent, which is associated to the increase of the size of the Nanoparticles. Similarly, the Plasmon resonance band is widened proportionately to the increase in the concentration of the precursor, due to an increase in the variety of nanoparticle sizes. Compared to the obtained spectra, it is observed that using the extract of the plant with concentration of 1.25% and the precursor to 30 mgL−1 the plasmon corresponding the highest absorbance is presented, i.e. the highest number of AuNPs synthesized.
Characterization of the nanocomposite
SEM analysis
The MEB image of Fig. 6 corresponds to the nanocomposite whose matrix was prepared from ceramic matrix. Micrographs were obtained at different magnifications. Observing the micrographs shown, it is observed that some of the synthesized nanoparticles show a tendency towards rounded shapes; however, the greatest number of them do not exhibit a defined form. The average size of nanoparticles have in a range of 13.8–71.9 nm.
X-ray Difraction
Figure 7a shows the diffractogram obtained from the characterization of the eggshell while the Diffractogram of (b) belongs to the same substrate with the deposited nanoparticles.
In the diffractrogram observed in Fig. 7 are presented peaks located at angles 27, 34, 36, 42, 46, 51, 55, 57, 68, 72 and 77 in 2 θ, which correspond to the planes (012), (104), (006), (110), (113), (202), (108), (116), (212), (214), (300), (0012), indicators of calcite, the main component of ceramic matrix. On the other hand the diffractogram corresponding to the Nanocompósito shows peaks at angles 45°, 52°, 77°, 94° and 99° in 2 θ representing the planes (111), (200), (202), (311) and (222), which indicate the presence of metallic gold in the sample. The remaining peaks belong to the planes (104), (110), (113), (108), (116), (212) and (214), which correspond to the composition of the Matrix (calcite), however, the intensity of these peaks is lower compared to the first diffractogram, which is because the structure of the calcite was modified to a lesser extent when depositing the nanoparticles.
Conclusions
According to the analyzes shown, it is established that the aqueous extract of the species Sedum praealtum has the ability to reduce the Au+3 ions to Au0 to synthesize nanoparticles due to the macromolecules present in the extract. Initially, the presence of AuNPs was estimated by visual analysis, with changes in the coloration of the solution during synthesis ; subsequently through UV-Vis spectroscopy its existence was corroborated due to the appearance of plasmas of absorbance in characteristic wavelengths of this metal.
It was taken into account that the AuNP’s synthesized have little stability, since they tend to precipitate, reason why a deposition on a ceramic substrate was realized, being effective. The AuNP’s powders were analyzed by XRD, SEM and EDS, observing through XRD the presence of Au in small amounts.
It was observed by SEM that the AuNPs synthesized have sizes that fluctuate within 13–70 nm and the micrographs of the obtained nanocomposite show that the AuNPs are deposited on the substrate, anchored to the grains.
References
Meléndez Pizarro CO, Camacho Dávila AA (2008) Química verde, la química del nuevo milenio. Facultad de Ciencias Químicas, Universidad Autónoma de Chihuahua, Aventuras del pensamiento
Dávila JL, Galeas VH, Pontón P, Rosas NM, Sotomayor V, Valdivieso C (2011) Nuevos materiales: Aplicaciones Estructurales e Industriales. imprefepp
Quintana MH (2008) Nanopartículas, principios y aplicaciones. http://www.cyd.conacyt.gob.mx/221/Articulos/Nanoparticulas/Nano1.html. Accessed 15 Apr 2017
Ahmed S, Saifullah Ahmad M, Lal Swami B, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 1–7. http://dx.doi.org/10.1016/j.jrras.2015.06.006
Saranyaadevi K, Subha V, Ernest RR, Renganathan S (2014) Synthesis and characterization of copper nanoparticle using Capparis Zeylanica leaf extract. Int J ChemTech Res 4533–4541
Nalawade P, Mukherjee T, Kapoor S (2013) Green synthesis of gold nanoparticles using glycerol as a reducing agent. Adv Nanopart 2:78–86. https://doi.org/10.4236/anp.2013.22014
Rodríguez Llamazares SM (2007) Obtención de nanopartículas y nano-ordenamientos metálicos empleando la química de los compuestos de inclusión. Universidad de Chila, Facultad de Ciencias Químicas y Farmacéuticas
Estrada E (2009) Biblioteca digital de la medicina tradicional mexicana. http://www.medicinatradicionalmexicana.unam.mx/monografia.php?l=3&t=&id=7495. Accessed 10 Jan 2017
Rzedowski GC, de Rzedowski y J (2005) Colaboradores, Flora fanerogámica del Valle de México 2a. ed., 1a reimp., Instituto de Ecología, A.C. y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Pátzcuaro (Michoacán), 1406 pp
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
García-Hernández, L., Aguilar-Pérez, B., Ramírez-Castro, J., Ramírez-Ortega, P.A., Flores-Guerrero, M.U., Arenas-Islas, D. (2018). Synthesis of Gold Nanoparticles Using the Extract of Sedum praealtum and Its Deposition on a Ceramic Substrate. In: & Materials Society, T. (eds) TMS 2018 147th Annual Meeting & Exhibition Supplemental Proceedings. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72526-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-72526-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72525-3
Online ISBN: 978-3-319-72526-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)