Abstract
This work investigated the effect of ZnO-Citrus sinensis nano-additive on the electrokinetic deposition of Zinc on mild steel in acid chloride. Fifty-four plates of (100 × 10 × 3) mm3 mild steel samples were cut, cleaned with dilute H2SO4 solution, rinsed in water and dried. The nano-additive was produced by infusing 30 ml Orange Juice extract in Zinc Oxide solution. The acid chloride electrolyte consisting of 71 g ZnCl, 207 g KCl and 35 g H3BO3 in 1 l of distilled water was divided into six portions. The nano-additive with different molar concentrations 0(0.2)1.0 was added to each portion of the acid chloride. Nine plates of mild steel samples were electroplated with zinc as the anode in each of the six prepared electrolyte solution and plated at different times (three plates each at 10, 15 and 20 min). The effects of electroplating on the average weights were measured and the results from the experiment showed the optimal nano-additive concentration and electroplating time.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Steel has found various applications in the industries because of their excellent properties. It is reasonable in cost, longer life, and variability in strength levels and also very adaptable to corrective rework [1]. These properties made steel to meet the ever increasing stringent engineering needs in the industries. Steel is a major material in automative and other sectors majorly because of corrosion resistance with zinc coatings, ease of joining, recyclability and good crash energy absorption [2,3,4].
In most manufacturing sectors and industries chloride solutions are used as cleaning agents and other function but steel being widely used in such industries are affected by the action of chloride solutions [5]. Electrodeposited steel can be made to withstand and reduce aggrieved strength of chloride as a medium [6, 7]. Different methods have been employed for corrosion protection but zinc coating is the major method used in industrial sectors as protective coating for large quantities of products and other fabricated ferrous metal parts [5, 8,9,10,11,12,13]. The addition of agents to aqueous electroplating baths plays an important role because of the important effects they produce on the growth, structure and glossiness of deposits [12, 13]. Additives have different benefits which include reduction in grain size and tendency to tree, improve mechanical and physical properties, reduces stress and pitting, increase current density range and promote levelling and brightening of deposit. In this investigation, the effect of orange nanoparticle additive on the surface morphology of the substrate performance was studied to determine the optimal nano-additive concentration and electroplating time.
Experimental Method
Samples of mild steel plates were cut into various pieces with the dimension 100 × 10 × 3 mm. Surface preparation was done using polishing machine with different grades of emery papers. The pickling of the mild steel was done for 15 min using dilute H2SO4 of 120 ml of H2SO4 in 1litre of water. The samples were rinsed, dried and stored in a desiccator. The percentage chemical composition of the mild steel substrate was analysed using Optical Emission Spectrometer as showed in Table 1.
Orange nanoparticle supernatant was used for this experiment. The additive was prepared by infusion of 30 ml of orange juice in zinc oxide solution of varying concentrations 0.2, 0.4, 0.6, 0.8 and 1 M. The mixtures were left to react for 48 h. The reacted mixture were centrifuged at 4500 rpm for 15 min. The supernant were then, decanted from the mixture. The nano particle suspension was transferred to watch glass, after which it was air dried and stored in sample bottles at room temperature. The acid chloride bath for this experiment contained zinc chloride, boric acid and potassium chloride. The acid chloride solution was prepared by dissolving 71 g of zinc chloride, 207 g of potassium chloride and 35 g of boric acid in 1 l of distilled water. This solution was filtered to remove any form of impurity.
The mild steel to be electroplated (cathode) and two zinc anodes were partially immersed in 150 ml of acid chloride bath. The cathode was connected to the negative terminal while the zinc anodes were connected to the positive terminal of the direct current (DC) power supply, current was set to 0.8 A. The additives concentrations and plating time were varied as shown in Table 2. The weight of the mild steel was measured before and after the plating to determine the mass deposited. The experiment was replicated to arrive at an average coherent value.
Results and Discussion
Effect of Electrodeposition Time
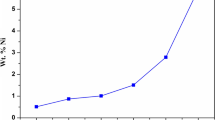
The result of the electro deposition experiment revealed the different mass addition of zinc on mild steel substrate in the bath for varying orange zinc oxide nanoparticle additive concentrations. The results obtained were for plating time of 10, 15 and 20 min. Figure 1 showed the graph of the change in mass against time at 0, 0.2, 0.4, 0.6, 0.8 and 1.0 M additive concentrations. A steady increase in total mass change was observed as the electrodeposition time increased for all the additive concentration used. Thus, irrespective of the additive concentration, the total mass change increases with electrodeposition time.
Effect of the ZnO-Citrus Sinensis Nano-additive
Figure 2 shows the effect of ZnO-Citrus sinensis nano-additive on the electrodeposition of Zinc on steel at different deposition time. For deposition done at 10 and 15 min, there was no significant impact of the additive concentration on the mass change. However, at 20 min electrodeposition time, a significant increase in total mass changed was observed at 1 M additive concentration.
Conclusion
Nanodeposition of zinc on steel in acid chloride environment with ZnO-Citrus sinensis nano-additive increases with electrodeposition time.
Moreover, physical examination of the zinc deposited mild steel shows a smoother surface finish with an increase in the concentration of ZnO-Citrus sinensis as nano-additive in the electrolyte solution.
Also, the study of the effect of ZnO-Citrus sinensis nano-additive on the mass change shows that optimum combination of factors to yield optimum deposition of zinc on mild steel occurred at the additive concentration of 1 M when electroplated for the period of 20 min.
References
Singh MK (2016) Application of Steel in Automotive Industry. Int J Emerg Technol Adv Eng 6(7):2250–2459
DeCicco JM (2005) Steel and iron technologies for automotive light weighting. Environ Defense
Ultralight Steel Auto Body (1998) Final report. American Iron and Steel Institute, Southfield, MI. March
Opbroek E, Weissert U (1998) Ultralight steel auto closures project. SAE Paper No. 982308
Popoola API, Fayomi OS (2011) Performance evaluation of zinc deposited mild steel in chloride medium. Int J Electrochem Sci 6(2011):3254–3263
Fang F, Brown B, Nesic S (2010) Corr Sci Sect 67:1–12
Abdullah M, Fouda AS, Shama SA, Afifi EA (2008) Africa J Pure Appl Chem 2(2008):83–91
Donald RA (1994) The science and engineering of materials. PWS Publishing Company, Washington, p 8
Joo YL, Joe WK, Min KL, Hyun TK, Su-moon P (2004) J Electrochem Soc 151:C25–C31
de Pedro N, Adriana N, Correia P, Walney SA (2007) J Braz Chem Soc 18:1164–1175
Shivakumara S, Manohar U, Arthoba Naik Y, Venkatesha Enkatesha TU (2007) Influence of additives on electrodeposition of bright Zn–Ni alloy on mild steel from acid sulphate bath. Bull Mater Sci 30(5):455–462. © Indian Academy of Sciences
Popoola API, Fayomi OSI (2011) Effect of some process variables on zinc coated low carbon steel substrates. Sci Res Essays 6(20):4264–4272, 19 Sept 2011. https://doi.org/10.5897/sre11.777. ISSN 1992-2248 ©2011 Academic Journals
Hague IU, Ahmad N, Akhan Jour A (2005) Chem Soc Pak 27:307–311
Field S, Weil AD (1951) Electroplating. Sir Isaac Pitman & Sons Ltd., London, p 136
Dini JW (1993) Electrodeposition—the materials science of coatings and substrates. Noyes Publications, New Jersey, USA, p 195
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Ajayi, O.O. et al. (2018). Experimental Investigation of the Effect of ZnO-Citrus sinensis Nano-additive on the Electrokinetic Deposition of Zinc on Mild Steel in Acid Chloride. In: & Materials Society, T. (eds) TMS 2018 147th Annual Meeting & Exhibition Supplemental Proceedings. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72526-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-72526-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72525-3
Online ISBN: 978-3-319-72526-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)