Abstract
Curcumin is a hydrophobic and lipophilic molecule which is the most active ingredient of Curcuma longa. Curcumin is used as a herbal drug in the treatment of many diseases. Even though curcumin is known for its therapeutic activity, various studies have shown that there are certain drawbacks, which minimize the usage of curcumin in various therapies. Curcumin has low solubility and less distribution property, which does not allow its proper absorption in the body. The recent investigation by researchers established several approaches which can tackle the problem of bioavailability of curcumin. The present chapter focuses on the different types of nanoparticles that can be used in the delivery of curcumin to enhance its bioavailability. Different nanoparticles such as polymeric nanoparticles, dendrimers and nanogels are emerging vehicle for the delivery of curcumin. Nanoparticles-loaded curcumin can be used in the treatment of cancer and also in the therapy of several neurological diseases. The chapter also discusses the role of curcumin in tissue engineering. In addition, antimicrobial activity of curcumin nanoparticles and its mechanism has been discussed in the present chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keyword
1 Introduction
Curcumin is a polyphenolic, yellowish-orange coloured crystalline powder, obtained from the herb Curcuma longa which is commonly known as turmeric and belongs to family Zingiberaceae. It is mainly cultivated in India and eastern Asia (Bayet-Robert and Morvan 2013; Prasad et al. 2014). Rhizome of turmeric contains 3–5% curcumin which is the major component of turmeric . Along with curcumin, some other compounds are also present in turmeric , which are known as curcuminoids. Curcuminoids are polyphenolic in nature which are responsible for yellow colouration of turmeric ; complexes of all curcuminoids are known as kacha haldi or yellow ginger or Indian saffron (Chattopadhyay et al. 2004; Akram et al. 2010; Nawaz et al. 2011). Commercially available curcumin possesses three major components such as Curcumin I (77% diferuloylmethane), Curcumin II (17% demethoxycurcumin) and Curcumin III (6% bisdemethoxycurcumin). Structure of different form of curcumin has been provided in Figs. 6.1, 6.2, 6.3. Traditionally, curcumin is only used as a spice, condiment, in cosmetics, textile and medicine (Saikia et al. 2015). In USA, curcumin is used as colouring agent in ice creams, pickles, cheese, soups and mustard. Medicinal uses of curcumin are described in Ayurveda. In ancient Indian and Chinese medicine, curcumin is used in the treatment of cough, sore throat, sinusitis, diabetic wounds, rheumatism and biliary diseases (Rahman et al. 2006; Leung and Foster 2009). In India, combination of turmeric and slaked lime is the most common remedy for curing inflammation and swelling. It has been found that curcumin acts as antioxidant , antiviral, antibacterial, antifungal and anti-inflammatory agent. Curcumin has potential application in the treatment of psoriasis, diabetes, asthma, cataract formation. It is also used in the treatment of neurodegenerative disorder such as Alzheimer, arthritis, cardiovascular diseases like atherosclerosis and inflammatory bowel disease (Saikia et al. 2015; Liu et al. 2017).
In 1987, Kuttan and co-workers for the first time reported anticancerous activity of curcumin. Later, curcumin has been nominated as one of the anticancerous drugs by National Institute of Cancer. Curcumin alone or in combination with other anticancer agents can be used in the therapy of different types of cancer such as breast cancer, pancreatic cancer, oral cancer, lung cancer and colorectal cancer (Lin et al. 2010; Saikia et al. 2015). Studies revealed that curcumin has capacity of inhibiting cancer in three stages, such as tumour growth, angiogenesis and tumour progression (Bar-Sela et al. 2010). From numerous in vivo and in vitro studies, it was revealed that curcumin has significant activity in the suppression of cancer by regulating the expression of tumour suppressor genes such as genes p53, PTEN and RB1 (http://www.herbaleducation.net/tumeric-monograph, Shankar and Srivastava 2007). Most significant and potential application of curcumin is the therapy of cancer. Most promising application of curcumin is the field of clinical research as a drug molecule with minimum side effects. Researchers reported antioxidant property of curcumin and demonstrated that curcumin has excellent antioxidant capacity and free radical scavenging activity. Curcumin is used as an antioxidant agent in the treatment of Parkinson’s disease and in therapy of other autoimmune diseases. It acts as an active antioxidant agent, which can protect the oxidative damage of DNA, proteins and prevent chronic neurodegenerative diseases and ageing (Rai et al. 2015). Curcumin is generally recognized as safe (GRAS) by Food and Drug Adminstration (FDA) (López-Lázaro 2008).
Despite all the potential applications of curcumin in the field of medicine, there are several drawbacks evidenced from pharmacological studies. It was found that curcumin has low absorption, poor solubility and relatively less distribution property. Hence, curcumin is not absorbed properly in the body and cannot be utilized for the treatment of diseases (Rai et al. 2015; Saikia et al. 2015). Curcumin possesses low bioavailability due to rapid metabolism, rapid elimination, poor distribution in tissue and low serum level (http://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-bioavailability; Tomren et al. 2007; Saikia et al. 2015). Studies have shown that the bioavailability of curcumin also depends on the route of administration. In vivo, study on mouse has demonstrated that when curcumin was injected intravenously its absorption was maximum in the serum, whereas when curcumin was given orally its absorption was very poor (Yang et al. 2007). From the last three decades, researchers are working to improve the bioavailability of curcumin. Various strategies have been developed by the researchers to enhance the therapeutic usage of curcumin. Different types of formulations have been evaluated to overcome the problem of curcumin (Shutava et al. 2009; Saikia et al. 2015). In the present chapter, we have focused on the nanotechnology-based strategies which can help in the delivery of curcumin and thereby solving its drawbacks.
2 Different Nanoparticles for Curcumin Delivery
Nanotechnology has gained importance in the field of medicine, and it is the most flourishing field which can be used in the prevention, treatment and diagnosis of the diseases. Nanoparticles have very small size and possess high surface to volume ratio, because of this property curcumin can easily enter into the biological membranes, cells, tissues and organ which may not be possible in case of larger molecules (Yun et al. 2013). The size, shape and surface of nanoparticles need to be manipulated for active and passive targeting of drug s. Hence, it is necessary to understand the interaction of nanoparticles with biological molecules which can help in the development of novel nanomaterials, which can help in the delivery of hydrophobic drugs like curcumin. The size, shape and surface of the nanoparticles need to be manipulated for active and passive targeting of curcumin (Jiang et al. 2008; Rao et al. 2010).
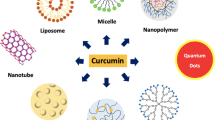
Nanomaterials have capacity to improve and alter pharmacodynamics properties of the drug molecule like curcumin which have solubility problem. Nanoparticles-based drug delivery can improve the safety, solubility and bioavailability of curcumin. Drugs are either adsorbed on the surface of the nanoparticles by physical entrapment, adsorption and chemical conjugation method (Jiang et al. 2008; Shutava et al. 2009; Saikia et al. 2015). From the previous research, it has been found that different types of nanomaterials such as liposomes, proteins, nanoemulsion, micelles, solid lipid nanoparticles and polymeric nanoparticles were used as promising carrier for the delivery of curcumin. Among all nanoparticles-based delivery approaches, polymeric nanoparticles are supposed to be the most significant and currently studied for the delivery of curcumin (Chirio et al. 2011; Basnet et al. 2012; Dhule et al. 2012).
Polymer nanoparticles include polymeric micelle, nanospheres and nanocapsules. Polymeric micelle possesses hydrophobic core which helps in the delivery of hydrophobic drugs like curcumin. Hydrophilic covering is present above the core which helps in the stabilization. It was reviewed that the solubility of curcumin increased by encapsulating curcumin into micelle. Nanosphere is the matrix system which enables uniform distribution in the polymer matrix. Hence, it is one of the efficient approaches for the encapsulation of water insoluble drugs like curcumin (Bisht et al. 2007; Ganta and Amiji 2009; Basnet et al. 2012). Benefits of using polymeric nanoparticles are that they are very stable in nature, and its size, shape, zeta potential and drug release profile can be manipulated by selecting the desired length of polymer, suitable stabilizing agent, filler, cross-linking agent and solvent The functional group present on the surface of polymeric nanoparticles can be modified by the addition of ligand molecule at the time of synthesis. It was reported that polymeric nanoparticles act as the carrier of curcumin and enhance the efficacy by targeting specific cells or tissues (Alizadeh et al. 2015; Saikia et al. 2015). Polymeric nanoparticles-based approach is used for efficient stabilization, solubility and controlled release of curcumin which can be used in cancer therapy.
3 Pharmaceutical Applications of Curcumin Nanoparticles
3.1 Curcumin in Drug Delivery
The method in which a carrier molecule is used to achieve efficient therapeutic activity of a pharmaceutical compound is called drug delivery (Sun et al. 2012). To overcome the solubility issues of curcumin, nanotechnology-based drug delivery approach has been used (Sun et al. 2012). Nanodelivery vehicles composed of phospholipid vesicles (liposomes) have been proved to be efficient for enhancing the bioavailability of curcumin. This is the second most widely used vehicle to encapsulate curcumin. A study led by Thangapazham and co-workers (2008) proved the antiproliferative activity of liposomal curcumin on two human prostate cancer cell lines (LNCaP and C4-2B). Their study has shown 70–80% inhibition of cellular proliferation without affecting their viability.
Nanogels are hydrogel nanoparticles of swollen cross-linked networks composed of hydrophilic and amphiphilic polymer chains. They have biocompatible and biodegradable properties. These vehicles can be designed to carry curcumin nanoparticles. It mimics human tissues due to higher hydrophilicity in the system due to swollen nature (Yellapu et al. 2015). In vitro, studies on medulloblastoma cell line have shown that encapsulation of curcumin within the β-hairpin hydrogel, curcumin released from hydrogel, induces caspase 3-mediated programmed cell death and it did not show any adverse effect on its bioactivity . Moreover, the rate of curcumin release and its therapeutic activity can be conveniently modulated (Altunbas et al. 2011). Bisht et al. (2007) studied the effects of nanocurcumin hydrogel on pancreatic cancer cell lines. Nanocurcumin efficiently inhibited the activation of NF-kB, downregulated steady-state transcripts of multiple pro-inflammatory cytokines and interleukin-6 synthesis.
Dendrimers are capable, highly selective and safe drug delivery agents having the ability to target the desired tissue. These are composed of highly branched networks of macromolecules and are three-dimensionally spherical in morphology (Bhosale et al. 2016). These drug delivery agents are highly suitable for loading of curcumin (Yellapu et al. 2015).
Other nano-based drug delivery vehicles for release of curcumin are various metal nanoparticles. Inert materials like gold and titanium have been used for the controlled release of curcumin which can help in the treatment of cancer. One of the outstanding properties of gold nanoparticles is its compatibility to combine with macromolecules. They have been studied as crucial drug delivery vectors due to some of their characteristic aspects, such as low toxicity, stability and large surface area. Nanoparticles-loaded curcumin has been shown in Fig. 6.4. Singh et al. (2013) reported the binding of the curcumin on gold nanoparticles which were synthesized by direct reduction of HAuCl4 using curcumin in the aqueous phase where curcumin acted as both reducing and capping agent. These curcumin-capped gold nanoparticles showed good antioxidant activity which was confirmed by the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical test. Therefore, these surface-functionalized gold nanoparticles with curcumin may lead towards a new way of using curcumin as possible drug delivery and therapeutic agent. In another study, Manju and Sreenivasan (2012) formulated a simple method for the fabrication of water soluble curcumin-conjugated gold nanoparticles to target various cancer cell lines. They prepared a water soluble conjugate of curcumin with hyaluronic acid (HA-Cur) and synthesized gold nanoparticles by reducing chloroauric acid using HA-Cur, which played a dual role as reducing and stabilizing agents and subsequently anchored folate-conjugated PEG. They probed these curcumin-conjugated gold nanoparticles by using different analytical techniques and assayed the blood compatibility and cytotoxicity. Blood materials interaction studies showed that these nanoparticles are highly hemocompatible. They checked its effects on cancer cell lines (Hela cells, glioma cells and Caco cells). The flow cytometry and confocal microscopy results showed significant cellular uptake and internalization of the nanoparticles by cells. So, HA-Cur gold nanoparticles showed significant cytotoxicity which resulted in the formation of blood compatible cur-conjugated gold nanoparticles with enhanced targeting and improved efficacy.
Other nano-based curcumin delivery agents include polymeric lipid micelles (Liu et al. 2013), chitosan (Zhang et al. 2013), cytodextrins (Tønnesen et al. 2002) and silver (Varaprasad et al. 2011).
3.2 Cancer Therapy
Cancer is the second most leading cause of human death following heart diseases. Commonly used treatments include chemotherapy, surgery, radiation therapy which increases the cost of cancer treatment. However, these treatments are associated with side effects on normal tissues and organs. Thus, curcumin and its nanoformulations play a vital role in enhancing chemo/radiosensitization with targeted treatment and lesser chances of side effects. The therapeutic effects of curcumin nanoparticles have been studied on various cancer cell lines and nowadays gaining much attention. Curcumin nanoparticles significantly internalize within cancer cells by the process of endocytosis and receptor-mediated pathways in the presence of endocytosis inhibitors in order to induce its biological effects (Yallapu et al. 2014).
There are several reports which have proved that curcumin inhibits the growth of cancer cells at concentration of 5–30 μM (Treasure 2005; Bar-sela et al. 2010; Basnet and Skalko-Basnet 2011). Up till now, the preclinical and clinical results from oral administration of curcumin have shown very poor bioavailability even at nanomolar concentrations (Maheshwari et al. 2006; Hatcher et al. 2008; Jurenka 2009). Thus, curcumin nanoparticles may be formulated which can overcome the drawbacks of curcumin. Various nanoparticles such as polymeric micelles, liposome, nanoemulsions, polymeric nanoparticles, its conjugates and nanogels were found to be suitable for the delivery of active form of curcumin (Muqbil et al. 2011).
Polymeric-drug conjugates are the useful alternative therapeutics from nanoscale family. The potential sites for conjugating curcumin biomacromolecules are two phenolic rings and active methylene groups. Kumar et al. (2000) designed nucleoside–curcumin bioconjugates to gain high levels of glucuronide and sulphate curcumin conjugates in healthy human volunteers. PEGylated curcumin conjugates have shown inhibitory effects on human pancreatic cancer cell lines. PEGylated curcumin blunted the cell proliferation in pancreatic cancer cells with Jab1/CSN activity (Li et al. 2009). In another study, Li et al. (2005) evaluated the effects of lipid curcumin ratio (10:1 wt/wt) on various pancreatic carcinoma cell lines such as ASPC-1, BxPC-3, MiapaCa2, Capan-1, Capan-2.
Anitha et al. (2011) quantified the cellular uptake of curcumin encapsulated in dextran sulphate-chitosan nanoparticles using a spectrophotometric method in MG 63, PC3, L929, MCF-7 cells. A cytotoxicity assay and FACS study showed that anticancer activity of this nanoformulation is high for MCF-7 as compared to other cancer cells. Kim et al. (2011) prepared novel curcumin (CCM)-loaded human serum albumin (HSA) nanoparticles (CCM-HAS-NPs) and administered intravenously using albumin-bound technology. In vivo, antitumour tests showed that these CCM-HAS-NPs in 10-or 20 mg/kg concentration had a greater 50–66% tumour growth inhibiting effect. These CCM-HAS-NPs showed potent antitumour activity and have enhanced water solubility and ability to traverse vascular endothelial cell. Tsai et al. (2011) used polymeric nanoparticles with co-encapsulation of curcumin and doxorubicin which showed significant therapeutic effects on multidrug resistant cancer cells (K-562 cells).
3.3 CNS Disorder
In recent years, nanotechnology has been employed to enhance efficacy of therapies associated with central nervous system (CNS) disorders for targeted drug delivery. The main advantage of using nanoparticles for drug delivery is specific action at targeted site along with better bioavailability and therapeutic efficacy for treatment of CNS disorders (Cheng et al. 2013; Patel and Patel 2017). Curcumin exerts neuroprotection due to its antioxidant properties for treating neurological disorders including Alzheimer’s disease (Mishra and Palanivelu 2008), Parkinson’s disease (Mythri and Bharath 2012), Huntington’s disease. (Darvesh et al. 2012; Tiwari et al. 2014). Curcumin has neuroprospective properties, but it is rapidly eliminated from the body (Tsai et al. 2011). Curcumin also plays potential neuroprotective role but its neuroprotectivity is limited by its poor brain availability due to limited blood-brain barrier permeability, poor absorption, systemic elimination and rapid metabolism (Kang et al. 2006; Tsai et al. 2011). The application of curcumin is very limited due to its non-solubility in water and to overcome this limitation, curcumin nanoparticles were prepared which were water soluble (Mathew et al. 2012).
Various formulation techniques have been applied to study, how to increase retention time of curcumin in the body. Cheng et al. (2013) reported novel nanoformulation of curcumin for the efficient delivery and bioavailability of curcumin in treating Alzheimer’s disease. But, several reports on curcumin-loaded adjuvants like piperine, liposome, phospholipid complexes and biodegradable polymers have been widely explored to increase bioavailability of curcumin for management of CNS disorders (Vergoni et al. 2009; Tsai et al. 2011; Tiwari et al. 2014).
Moreover, curcumin-PLGA (poly lactic-co-glycolic acid)-conjugated amino acid Tet-1 peptide can be used as potential therapeutic tool for treating Alzheimer’s disease (Mathew et al. 2012). Curcumin-PLGA conjugate can interact specifically with neurons and increase neuronal targeting efficacy. Similarly, Puglia et al. (2012) reported the study on nanostructured lipid carrier-loaded curcumin for potential administration and drug bioavailability . Nanoliposomal incorporation of curcumin improves bioavailability along with potential inhibition of Aβ aggregation (Taylor et al. 2011).
Szymusiak et al. (2016) reported the efficacy of nanocurcumin in prevention at relatively low oral dose with reversal of morphine tolerance. The pharmacokinetic study showed that concentration of curcumin in oral dosage of nanocurcumin was found to be 20 times lower than that of unformulated curcumin. The essence of evolving a successful nanocurcumin delivery methods lies not only in effective brain targeting formulations but also less toxic therapies for the healthy future of patients.
4 Curcumin Nanoparticles as Antimicrobial Agent
Since, ancient times curcumin is being used against various bacteria, fungi and parasites (Schraufstätter and Bernt 1949). Curcumin has demonstrated antimicrobial activity against Staphylococcus aureus, Trichophyton gypseum, Salmonella paratyphi and Mycobacterium tuberculosis (Schraufstatter and Bernt 1949).
It was found that curcumin nanoparticles inhibit the growth of both Gram-positive and Gram-negative bacteria. For example, Adahoun et al. (2015) tested NPs against Gram-positive (Staphylococcus aureus ATCC 29213, Micrococcus luteus ATCC 9341) and two Gram-negatives (Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922). The study reported that Gram-positive bacteria were highly sensitive to the nanocurcumin than Gram-negative bacteria (Adahoun et al. 2015). In another study on antimicrobial activities of nanocurcumin, Bhawana et al. (2011) reported antibacterial and antifungal activity of curcumin NPs. The antifungal activity of NPs against Aspergillus niger and Penicillium notatum was evident at the minimum concentration 350 μg/mL for nanocurcumin . The study revealed that by anchoring the cell wall of the bacteria, by damaging it, by entering into the cell followed by subsequent destruction of cell organelles, nanocurcumin inhibits the growth of bacteria (Bhawana et al. 2011). In contrary to the above studies, curcumin NPs were found to have more growth inhibitory activity against Gram-negative P. aeruginosa as compared to Gram-positive S. aureus (Pandit et al. 2015). No et al. (2017) tested antibacterial activity of curcumin NPs against one of the major food spoiling bacteria, Listeria monocytogenes. The results demonstrated that NPs positively surface charged with cetrimonium bromide (CTAB) exhibited highest antibacterial activity against L. monocytogenes. The study hinted towards the strong relationship between the surface charge and antimicrobial effect of curcumin nanoparticles. The study concluded that enhanced antibacterial effect of curcumin NPs stabilized with CTAB was due to increased cellular penetration of curcumin, its nanosize and enhanced cell–antimicrobial interaction resultant from opposite electric charges between curcumin NPs-CTAB and cells of L. monocytogenes (No et al. 2017).
In study reported by Shailendiran et al. (2011), nanocurcumin showed inhibition capacity (IC50) value 728.48 µg/mL against Gram-negative E. coli and 542.79 µg/mL against Gram-positive cocci. The authors reported time-dependent variation in zone of inhibition, and it was found that though after 10 h, a clear inhibition zone appears for both bulk curcumin and nanocurcumin, and the zone becomes unclear after 24 h. This indicates that nanocurcumin does not show bactericidal activity but demonstrated bacteriostatic activity against tested bacteria. Further, the study also revealed that nanocurcumin did not show any effect on degradation of bacterial genome (Shailendiran et al. 2011). The study reported by Gopal et al. (2016) revealed maximum antibacterial activity of nanocurcumin against Streptococcus mutans, followed by S. aureus, Salmonella spp. and E. coli. Moreover, curcumin NPs have also shown antiprotozoan parasitic activity. For example, curcumin NPs were successfully employed to inhibit the growth of protozoan parasite Giardia lamblia (Said et al. 2012). Taking into consideration, the antimicrobial potential of nanocurcumin, the commercial use of the curcumin NPs for development of antimicrobial ointments and formulations is of prime importance. In this context, the initiatives have already been taken by Pandit et al. (2015) who have formulated nano-antibacterial cream effective against human disease causing bacterial pathogens.
5 Tissue Engineering
The main objective of tissue engineering is to repair and regenerate damaged tissues and organs of the human body. In tissue engineering, porous scaffolds are used for cell regeneration, adhesion, proliferation and extra cellular matrix development (Han et al. 1998). The scaffolds have ability to load and release bioactive compounds to defected site in controlled manner.
As discussed earlier, curcumin exhibited antioxidant , anti-inflammatory, antibacterial, antifungal, antiviral, anticancerous and anticoagulant activities (Chattopadhyay et al. 2004). However, regardless of such bioactivities of curcumin, it suffers from poor solubility, stability and bioavailability . Therefore, formulation s with sustainable release of curcumin are needed. Karri et al. (2016) studied effect of curcumin-loaded chitosan nanoparticles impregnated into collagens alginate scaffolds on wound healing activity in diabetes. In this work, nanohybrid scaffolds were prepared by incorporating curcumin into chitosan for better stability and sustainable delivery of curcumin to control inflammation. Curcumin micro-complex-loaded chitosan scaffold can be efficiently used for delivering curcumin in wound healing application as delivery system (Amirthalingam et al. 2015). Chitosan scaffold-containing chitosan-curcumin micro-complex serves as carrier for curcumin delivery as well as supports the growth of normal cells.
A new study reported that curcumin released from polymeric nanofiber can enhance bone tissue regeneration over a period of time (Jain et al. 2016). The study showed the controlled release of curcumin and its effect on tissue regeneration. Gene and protein analysis confirmed the presence of curcumin in bone tissue regeneration. Curcumin-loaded polyaniline-conjugated poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) electro-conductive scaffold may lead to reaeration of damaged tissue (Pramanik et al. 2016). Curcumin-loaded polyaniline-conjugated poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) composite represents new kind of biodegradable, bioactive, antimicrobial material for tissue engineering applications.
The formulated curcumin–gelatin nanofibrous mats adequately enhance the bioavailability of curcumin for wound healing (Dai et al. 2017). The curcumin–gelatin nanofibrous mats increase regeneration process in rat model of acute wound. The potential bioactivity of curcumin accelerates wound healing. Engineering a smart integrated system that can deliver bioactive curcumin to wound and thereby improve wound healing is the need of the hour. Nanofibers of poly (hydroxybutyrate-co-hydroxyvalerate) and polycaprolactone can be used as scaffold for tissue engineering. The addition of bioactive compounds like curcumin enhances the antioxidant activity of polymeric scaffold (Uebel et al. 2016). Similarly, curcumin incorporated into silk fibroin (SF)/poly (L-lactic acid-co-e-caprolactone) (P(LLA-CL)) nanofibrous scaffolds has potential application in tissue engineering and wound dressings (Lian et al. 2014). Encapsulation of curcumin on scaffold prominently enhances antibacterial and antioxidant activity of nanofibrous scaffolds.
6 Mechanism of Action Against Microbes
The mechanism of curcumin’s action on human cells is related to curcumin’s capacity to modulate cell signalling molecules such as pro-inflammatory cytokines, apoptotic proteins, NF–κB, cyclooxygenase, endothelin, malondi aldehyde, creactive protein prostaglandin E2, glutathione, pepsinogen, phosphorylase kinase, transferrin receptor, total cholesterol, transforming growth factor, triglyceride, creatinine antioxidants (Gupta et al. 2013).
In bacterial cells, the mechanism related to antibacterial activity seems to be modulated by FtsZ protofilaments, which is important for functioning of the Z-ring. This filament is critical in bacterial cytokinesis. Curcumin inhibited the assembly of FtsZ protofilaments and also increased the GTPase activity of FtsZ. GTPase activity of FtsZ is lethal to bacteria and suggests that curcumin inhibits bacterial cell proliferation by inhibiting the assembly dynamics of FtsZ in the Z-ring (Rai et al. 2008). Bhawana et al. (2011) reported that in bacteria, the mechanism of antibacterial action of curcumin nanoparticles revealed that these particles enter inside the bacterial cell by breaking the cell wall, leading to cell death.
Antiviral activity of curcumin against virus like HIV showed that inhibition of viruses takes place by acetylation of Tat protein, which is responsible for suppression of HIV-1 multiplication. Tat protein transactivation of HIV-1 LTR can be measured by β-galactosidase activities. In fungus, probable effect was found to be downregulation of Δ 5,6 desaturase (ERG3) related to reduction in ergosterol of fungal cell. The reduction in production of ergosterol results into accumulation of biosynthetic precursors, which leads to cell death. Reduction in proteinase secretion and alteration of membrane-associated properties of ATPase activity are other possible critical factors for antifungal activity of curcumin. (Moghadamtousi et al. 2014). Antioxidant properties show that administration of curcumin significantly attenuated oxidative stress and lipid peroxidation, prevented apoptosis and increased antioxidant defence mechanism activity (Samarghandian et al. 2017).
7 Conclusions
Curcumin has been known for its therapeutic application in the treatment of various diseases since ancient times. There are few limitations, which restrict the usage of curcumin in the drug delivery and in the treatment of cancer. Nanoparticles-based approach can be used to enhance the bioavailability of curcumin which has helped in its delivery for the treatment of various diseases such as cancer, various neurological disorders such as Parkinson’s disease, Alzheimer’s disease. Cancer is the most dreadful disease all over the world; nanoparticles-loaded curcumin has many advantages as it can minimize the side effects of chemotherapy and other therapies of cancer. Nanotechnology-based approach is also useful in the regeneration of damaged tissues, bones and organs. Encapsulation of curcumin on the scaffolds can enhance the antioxidant and antibacterial activity of nanofibrous scaffolds . Curcumin possesses excellent antimicrobial activity, and hence researchers have started formulation of curcumin nanoparticles. As nanoparticles have smaller size and high surface area, curcumin nanoparticles have larger surface area and water solubility which can replace the usage of bulk curcumin.
References
Adahoun MA, Al-Akhras M-AH, Jaffar MS, Bououdina M (2015) Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif Cells Nanomed Biotechnol 45(1):98–107
Akram M, Uddin S, Ahmed A, Khan U, Hannan A, Mohihuddin E, Asif M (2010) Curcuma longa and curcumin. Rom J Biol Plant Biol 55(2):65–70
Alizadeh AM, Sadeghizadeh M, Najafi F, Ardestani SK, Erfani-Moghadam V, Khaniki M, Rezaei A, Zamani M, Khodayari S, Khodayari H, Mohagheghi MA (2015) Encapsulation of curcumin in diblock copolymer micelles for cancer therapy. BioMed Res Int (824746)
Altunbas A, Lee S, Rajasekaran S, Schneider J, Pochan D (2011) Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 32:5906–5914
Amirthalingam M, Kasinathan N, Mutalik S, Udupa N (2015) In vitro biocompatibility and release of curcumin from curcumin microcomplex-loaded chitosan scaffold. J Microencapsul 32(4):364–371
Anitha A, Deepagan V, Rani V, Menon D, Nair S, Jayakumar R (2011) Preparation, characterization in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr Polym 84:1158–1164
Bar-Sela G, Epelbaum R, Schaffer M (2010) Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem 17(3):190–197
Basnet P, Skalko-Basnet N (2011) Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 16:4567–4598
Basnet P, Hussain H, Tho I, Skalko-Basnet N (2012) Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharm Sci 101(2):598–609
Bayet-Robert M, Morvan D (2013) Metabolomics reveals metabolic targets and biphasic responses in breast cancer cells treated by curcumin alone and in association with docetaxel. PLoS ONE 8(3):1–17
Basniwal RK, Buttar HS, Jain VK, Jain N (2011) Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem 59:2056–2061
Bhosale R, Osmani R, Vaghela R, Deb T, Gangadharappa H, Afrasim M (2016) Dendrimers: inimitable nanoparticulate drug carriers—A comprehensive review. Adv Sci Eng Med 8(4):251–270
Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A (2007) Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 5(3):1–18
Chattopadhyay I, Biswas K, Bandopadhyay U, Banerjee RK (2004) Turmeric and curcumin: biological actions and medicinal applications. Curr Sci 87(1):44–53
Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AHL, Baum L (2013) Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in Alzheimer’s disease Tg2576 mice. J Am Assoc Pharm Sci 15(2):324–336
Chirio D, Gallarate M, Peira E, Battaglia L, Serpe L, Trotta M (2011) Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J Microencapsul 28(6):537–548
Dai X, Liu J, Zheng H, Wichmann J, Hopfner U, Sudhop S, Prein C, Shen Y, Hans-Günther M, Schilling AF (2017) Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater 9:368
Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van der Schyf CJ (2012) Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs 21:1123–1140
Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, He J, Alb A, John V, Pochampally R (2012) Curcumin-loaded -cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed Nanotechnol Biol Med 8(4):440–451
Ganta S, Amiji M (2009) Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm 6(3):928–939
Gopal J, Muthu M, Chun S (2016) Bactericidal property of macro-, micro- and nanocurcumin: an assessment. Arab J Sci Eng 41:2087–2093
Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15(1):195–218
Han DK, Park KD, Hubbell JA, Kim YH (1998) Surface characteristics and biocompatibility of lactide-based poly (ethylene glycol) scaffolds for tissue engineering. J Biomater Sci Polym Ed 9:667–680
Hatcher H, Planalp R, Cho J, Torti F, Torti S (2008) Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65:1631–1652
http://www.herbaleducation.net/tumeric-monograph. Accessed 6 Dec 2016
http://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-bioavailability. Accessed 6 Dec 2015
Jain S, Meka SRK, Chatterjee K (2016) Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed Mater 11(5)
Jiang W, Kim BYS, Rutka JT, Chan WCW (2008) Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3:145–150
Jurenka J (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14:141–153
Kang SK, Cha SH, Jeon HG (2006) Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev 15:165–174
Karri VV, Kuppusamy G, Talluri SV, Mannemala SS, Kollipara R, Wadhwani AD, Mulukutla S, Raju KR, Malayandi R (2016) Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol 93:1519–1529
Kim T, Jiang H, Youn Y, Park C, Taka KK, Lee S, Kim H, Jon SY, Chen X, Lee KC (2011) Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm 403:285–291
Kumar S, Dubey KK, Tripathi S, Fujii M, Misra K (2000) Design and synthesis of curcumin-bioconjugates to improve systemic delivery. Nucleic Acids Symp Ser 44:75–76
Kuttan R, Sudheeran PC, Josph CD (1987) Turmeric and curcumin as topical agents in cancer therapy. Tumori J 73(1):29–31
Leung AY, Foster S (2009) Encyclopedia of common natural ingredients used in food, drugs, and cosmetics, 3rd. Wiley, Hoboken, NJ
Li J, Wang Y, Yang C, Wang P, Oelschlager D, Zheng Y, Tian D, Grizzle W, Buchbaum D, Wan M (2009) Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol Pharmacol 76(1):81–90
Li L, Braiteh F, Kurzrock R (2005) Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 104:1322–1331
Lian Y, Zhang Zhan JC, Zhang KH, Mo XM (2014) Fabrication and characterization of curcumin-loaded silk fibroin/P(LLA-CL) nanofibrous scaffold. Front Mater Sci 8:354–362
Lin YC, Chen HW, Kuo YC, Chang YF, Lee YJ, Hwang JJ (2010) Therapeutic efficacy evaluation of curcumin on human oral squamous cell carcinoma xenograft using multimodalities of molecular imaging. Am J Chin Med 38(2):343–358
Liu L, Sun L, Wu Q (2013) Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int J Pharm 443(1–2):175–182
Liu XF, Hao JL, Xie T, Mukhtar NJ, Zhang W, Malik TH, Lu CW, Zhou DD (2017) Curcumin, a potential therapeutic candidate for anterior segment eye diseases: a review. Front Pharmacol 8:66
López-Lázaro M (2008) Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res 52(1):103–127
Maheshwari R, Singh A, Gaddipati J, Srimal R (2006) Multiple biological activities of curcumin: a short review. Life Sci 78:2081–2087
Manju S, Sreenivasan K (2012) Gold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: blood compatibility evaluation and targeted drug delivery onto cancer cells. J Colloid Interface Sci 368(1):144–151
Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS (2012) Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 7(3):e32616
Mishra S, Palanivelu K (2008) The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Annals Indian Acadamy Neurol 11:13–19
Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K (2014) A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int 186864
Muqbil I, Masood A, Sarkar F, Mohammad R, Azmi A (2011) Progress in nanotechnology based approaches to enhance the potential of chemopreventive agents. Cancer 3:428–445
Mythri RB, Bharath MM (2012) Curcumin: a potential neuroprotective agent in Parkinson’s disease. Curr Pharm Designs 18:91–99
Nawaz A, Khan G, Hussain A, Ahmad A, Khan A, Safdar M (2011) Curcumin: a natural product of biological importance. Gomal Univ J Res 27(1):07–14
No DA, Algburi A, Huynh P, Moret A, Ringard M, Comito N, Drider D, Takhistov P, Chikindas ML (2017) Antimicrobial efficacy of curcumin nanoparticles against Listeria monocytogenes is mediated by surface charge. J Food Saf. doi:10.1111/jfs.12353
Pandit R, Gaikwad S, Agarkar G, Gade A, Rai M (2015) Curcumin nanoparticles: physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 5(6):991–997
Patel MM, Patel BM (2017) Crossing the blood–brain barrier: recent advances in drug delivery to the brain. CNS Drugs 31:109
Pramanik N, Dutta K, Basu RK, Kundu P (2016) Aromatic π-conjugated curcumin on surface modified polyaniline/polyhydroxyalkanoate based 3d porous scaffolds for tissue engineering applications. ACS Biomater Sci Eng 2(12):2365–2377
Prasad S, Gupta SC, Tyagi AK, Aggarwal BB (2014) Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv 32:1053–1064
Puglia C, Frasca G, Musumeci T, Rizza L, Puglisi G, Bonina F, Chiechio S (2012) Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice. Europian J Pharm Biopharm 81(2):288–293
Rahman I, Biswas SK, Kirkham PA (2006) Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 72:1439–1452
Rai D, Singh KJ, Roy N, Panda D (2008) Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem J 410:147–155
Rai M, Pandit R, Gaikwad S, Yadav A, Gade A (2015) Potential applications of curcumin and curcumin nanoparticles: from traditional therapeutics to modern nanomedicine. Nanotechnol Rev 4(2):161–172
Rao DP, Srivastav SK, Prasad C, Saxena R, Asthana S (2010) Role of nanopar-ticles in drug delivery. Int J Nanotechnol 4(1):45–49
Said D, Elsammad M, Gohar Y (2012) Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents. Parasitol Res 111:545–554
Saikia C, Sarmah M, Das MK, Ramteke A, Maji TK (2015) Biodegradable polymeric nanocomposites: advances in biomedical applications, Chap. 8. Taylor and Francis. Boca Raton
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229
Schraufstätter E, Bernt H (1949) Antibacterial action of curcumin and related compounds. Nature 164(4167):456–457
Shailendiran D, Pawar N, Chanchal A, Pandey RP, Bohidar HB, Verma AK (2011) Characterization and antimicrobial activity of nanocurcumin and curcumin. In: Proceedings of nanoscience, technology and societal implications, 1–7
Shankar S, Srivastava RK (2007) Involvement of Bcl-2 family members, phospha-tidylinositol 3-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol 30(4):905–918
Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Biqelow LR, Cardelli JA, Patrick O’Neal D, Lvov YM (2009) Layer-by-layercoated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 3(7):1877–1885
Singh D, Jagannathan P, Khandelwal P, Abraham M, Poddar P (2013) In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: an experimental and density functional theory. Nanoscale 5(5):1882–1893
Sun M, Su X, Ding B, He X, Liu X, Yu A, Lou H, Zhai G (2012) Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 7(7):1085–1100
Szymusiak M, Hu X, Plata PAL, Ciupinski P, Wang ZJ, Liu Y (2016) Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int J Pharm 511:415–423
Taylor M, Moore S, Mourtas S, Niarakis A, Re F, Zona C, La Ferla B, Nicotra F, Masserini M, Antimisiaris SG, Gregori M, Allsop D (2011) Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Aβ peptide. Nanomedicine 7:541–550
Thangapazham R, Puri A, Blumenthal R, Maheshwari R (2008) Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol 32:1119–1123
Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LK, Patel DK, Srivastava V, Singh D, Gupta SK, Tripathi A, Chaturvedi RK, Gupta KC (2014) Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 8(1):76–103
Tomren MA, Masson LM, Hjorth TH (2007) Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int J Pharm 338:27–34
Tønnesen H, Másson M, Loftsson T (2002) Studies of curcumin and curcuminoids. XXVII cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm 244(1–2):127–135
Treasure J (2005) Herbal medicine and cancer: an introductory overview. Semin Oncol Nurs 21:177–183
Tsai YM, Chien CF, Lin LC, Tsai TH (2011) Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm 416(1):331–338
Uebel LS, Schmatz DA, Kuntzler SG, Dora CL, Muccillo-Baisch AL, Costa JAV, de Morais MG (2016) Quercetin and curcumin in nanofibers of polycaprolactone and poly(hydroxybutyrate-co-hydroxyvalerate): assessment of in vitro antioxidant activity. J Appl Polym Sci. doi:10.1002/app.43712
Varaprasad K, Murali M, Vimala K, Raju K (2011) Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J Appl Polym Sci 121(2):784–796
Vergoni AV, Tosi G, Tacchi R, Vandelli MA, Bertolini A, Costantino L (2009) Nanoparticles as drug delivery agents specific for CNS: in vivo biodistribution. Nanomedicine 5:369–377
Yallapu M, Khan S, Maher D, Ebeling M, Sundram V, Chauhan N (2014) Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 35(30):8635–8648
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH (2007) Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 853(1–2):183–189
Yellapu M, Bhusetty P, Jaggi M, Chauhan S (2015) Therapeutic applications of curcumin nanoformulations. AAP J 17(6):1341–1356
Yun Y, Cho YW, Park K (2013) Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Delivery Rev 65(6):822–832
Zhang J, Tang Q, Xu X, Li N (2013) Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int J Pharm 448(1):168–174
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Rai, M., Pandit, R., Paralikar, P., Nagaonkar, D., Rehman, F., Alves dos Santos, C. (2017). Pharmaceutical Applications of Curcumin-Loaded Nanoparticles. In: Rai, M., Alves dos Santos, C. (eds) Nanotechnology Applied To Pharmaceutical Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-70299-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-70299-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70298-8
Online ISBN: 978-3-319-70299-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)