Abstract
The cardiovascular system is a close loop system, where pressures and resistances of every part affect the flow through the whole system. Under steady conditions, cardiac output and venous return are equal, so the determinants of cardiac output are also determinants of venous return. However, in this chapter, we will examine the circulation starting from the peripheral tissues, which is where blood flow is finely controlled and where the oxygen consumption takes place. The metabolic demand of every tissue and organ is the main regulator of blood flow, particularly at the level of arterioles and meta-arterioles. Then, the resistance to blood flow between the peripheral vessels and the right atrium, the ability of the heart to maintain a low right atrial pressure and the degree of filling of the circulation are all factors that affect and somehow determine the venous return. The degree of filling of the circulation can be estimated by the mean systemic filling pressure (Pmsf), which can be measured at the bedside in critically ill patients. Knowing the Pmsf value will help clinicians to understand the haemodynamic situation and to interpret the changes that follow the therapeutic interventions. From this perspective, all those interventions are targeted to improve and maintain tissue perfusion, and not only to correct haemodynamic values.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiovascular system
- Cardiovascular failure
- Cardiac output
- Venous return
- Peripheral tissues
- Oxygen consumption
- Metabolic demand
- Resistance to blood flow
- Mean systemic filling pressure (Pmsf)
- Effective fluid challenge
- Metabolic level
- Availability of oxygen
- α1-adrenergic agonist
- Venoconstriction
- Anaesthetic drugs
- Vasodilation
After reading this chapter, the reader will learn:
-
1.
Which are the main determinants of venous return and cardiac output?
-
2.
What is the mean systemic filling pressure, what is its importance and what is the gradient of venous return?
-
3.
What is the meaning of central venous pressure in the context of venous return?
-
4.
What is an effective fluid challenge, using Pmsf?
1 Introduction
Cardiovascular failure is one of the most common reasons for admission to intensive care units. The main function of the cardiovascular system is the transport of oxygen (O2) to the tissues. If the concentration of haemoglobin is stable, the main determinant of O2 transport is the cardiac output (CO).
CO is total volume of blood mobilised by the heart per unit of time, and it is measured in units of flow (L/min). In stable conditions, the cardiovascular system is a close loop system, and the heart can only eject the amount of blood that it receives. Thus, the total volume ejected over a period of time would equal the total volume of blood returning from the venous system. Therefore, venous return equals cardiac output.
In this chapter we will analyse the determinants of venous return, their impact on the cardiovascular physiology and the practical implications that those factors may play a role in the treatment of critically ill patients. The main factors that determine the venous return to the heart from the systemic circulation are:
-
1.
The degree of filling of the circulation
-
2.
The ability of the heart to maintain a low right atrial pressure
-
3.
The resistance to blood flow between the peripheral vessels and the right atrium
-
4.
The resistance to blood flow between the heart and the capillaries
2 Blood Volume and Mean Systemic Filling Pressure
The venous system contains about 70% of the total blood volume, whereas the arterial system contains only 13–18% and the capillaries 7% [1, 2]. The venous system is a blood reservoir, able to adjust his capacity according to the haemodynamic conditions. The venous wall is much thinner than the arterial wall, as blood is circulating at low pressure, but it still contains smooth muscle fibres, able to contract and expand according to the haemodynamic situation. During hypovolaemia, sympathetic nervous reflexes cause venoconstriction, sending blood back to the central circulation, increasing preload and increasing cardiac output. Actually, even after 20% of the total blood volume has been lost, the circulatory system functions almost normally because of this variable reservoir function of veins [1].
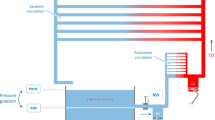
The heart pumps blood continuously into the aorta keeping the mean arterial pressure high, averaging 80–100 mmHg. This pressure decreases progressively as the blood flows into the systemic circulation, as low as the level of the right atrial pressure (Pra). This fall in pressure is mainly caused by the increasing total cross-sectional area in each level of the vascular tree (◘ Fig. 4.1). When the heart stops, the arterial pressure decreases, and the RAP progressively increases. At certain point, blood will not be flowing, and if the arterioles do not collapse trapping blood in the arterial space, the pressure will be the same in all territories of the circulatory system. This pressure is the mean systemic filling pressure (Pmsf). This pressure was described by Bayliss and Starling [3], and they figured that somewhere in the circulation, there must be a point where the pressure is not changing when the heart stops. Actually, during a cardiac arrest, the pressure in the small veins (<1 mm) and venules do not change substantially; they are the “pivoting point” of the system (◘ Fig. 4.1) [4]. This pressure is less than the capillary pressure, close to the portal venous pressure and greater than the RAP. Its anatomic location is not necessarily at the same venous branching level in every organ. The importance of this pressure, rather than its anatomical location, is that it provides a quantitative measurement of the intravascular filling status independent from cardiac function: its value is equal to the Pmsf.
Pressures and cross-sectional area across cardiovascular system. Pmsf mean systemic filling pressure. This is the pressure at all points in the cardiovascular system when the heart stops. During normal circulation, there is a point (pivot point) where the pressure equalises the Pmsf. At that point, the pressure is independent of flow and theoretically localises at the venule territory. The drop in pressure is mainly related to the increase in the total cross-sectional area and the compliance of the vascular wall
Let us imagine the “blood reservoir” as a distensible compartment. The volume required to fill a distensible tube, such as a tyre or a blood vessel, with no pressure rise is called the “unstressed” volume (Vo). Further volume expansion will imply necessarily a pressure rise and an elastic distension of the wall of the tube, which depends on the compliance (C) of the wall. This volume is the “stressed” volume (Vs) and is related to the pressure in the next equation:
3 The Right Atrial Pressure
In order to move a fluid across a tube or tubular system, the variables described in Poiseuille’s Law apply. Therefore, it is the gradient of pressure between two points in the system, not any single pressure at any particular point, which determines the rate of flow [5, 6]. Given that most of the blood is in the venous reservoir, the pressure at this point is particularly interesting. Following Poiseuille’s Law equation, Guyton pointed out that venous return could be defined by three parameters: the mean systemic filling pressure (Pmsf), Pra and the resistance to venous return (RVR). This can be also mathematically represented as follows:
Guyton [7] drew venous return curves in recently dead dogs. The heart was replaced with a pump, and the Pra was controlled by increasing or decreasing the minute capacity of the pump. Increasing or decreasing the total quantity of blood controlled the mean circulatory filling pressure. From these curves (◘ Fig. 4.2), one can see that for a given Pra, the greater the Pmsf, the greater the venous return is. Importantly, under isovolumetric conditions, the greater is the Pra, the lower is the venous return. Thus, given this linear relationship, if venous return and Pra can be measured and changed without changes in the volume status, the slope of the line could be calculated (the RVR), and the Pmsf could be estimated. Under isovolaemic conditions, Pra depends mainly on the right ventricular function and its ability to accommodate and pump blood to the pulmonary circulation. Any decrease in the heart function will increase Pra and therefore create congestion in the venous circulation.
Venous return and Frank-Starling curves. Pmsf mean systemic filling pressure, Pra right atrial pressure. Each venous return curve represents different levels of volume status. To move across a venous return curve, changes in cardiac function are required, without changes in the volume status. The black line represents the Frank-Starling curve for a particular level of cardiac function. In order to move from the blue to the red curve, Pmsf must change from a to c without changes in resistance
A second factor that affects the Pra is the intrathoracic and the pericardial pressure. Under normal circumstances, intrathoracic pressure is negative or equal to the atmospheric pressure. Any increase in this pressure that translates in an increase of Pra will result in a decrease of venous return. Similar phenomenon is observed if there is an increase in pericardial pressure, which will increase Pra, and will result in a decrease of venous return.
4 Arteriolar Resistance and Metabolic Demand
As the venous system can only transport the amount of volume that arrives from the capillary system, the regulation of the capillary circulation will also determine the venous return. Each tissue in the body has the ability to control its own local blood flow in proportion to its metabolic needs. Rapid changes in vasoconstriction or vasodilation of arterioles, meta-arterioles and precapillary sphincters may happen within seconds to provide adequate local tissue blood flow. The main factors involved in the acute control of local blood flow are:
-
1.
Tissue metabolism: this is by far the most powerful factor. The higher is the local oxygen demand, the higher is the blood flow.
-
2.
Availability of oxygen: whenever there is a deficit of oxygen (hypoxia), there is an increase in local blood flow. Oxygen deficit can occur in several ways, such as (1) failure in oxygenation of blood (ARDS, pneumonia, etc.), (2) failure in transport of oxygen by haemoglobin (CO poisoning) or (3) failure in the ability of tissue to use oxygen (septic shock, cyanide poisoning).
-
3.
Deficit of other nutrients: under special conditions, the lack of glucose, thiamine, niacin and riboflavin can cause vasodilation.
5 Control of Venous Tone: Resistance to Venous Return
Certain parts of the venous system are particularly compliant: these include the spleen, the liver, the large abdominal veins and the venous plexus beneath the skin. Splanchnic and cutaneous veins have a high population of α1- and α2-adrenergic receptors, so they are very sensitive to adrenergic stimulation, contrary to skeletal and muscle veins [8]. The control of the venous system has been extensively studied in animal models. There are nerve terminations in the proximity of many small vein smooth muscles [9] but not in the veins of the skeletal muscle [10]. However, circulating catecholamines can induce contraction of venules and veins of the skeletal muscle and mesentery [9, 10]. Thus, probably catecholamines released from the sympathetic nerve termination of the arterial side may pass through the capillary bed and affect the venous system.
Smooth muscles of the veins and arteries do not respond necessarily in the same way to chemical signals. Dihydroergotamine can activate the veins but not the arteries [11]. The venous system primarily has α-adrenergic receptors [12,13,14,15]. Stimulation of the β-adrenergic receptors of arterioles cause vasodilation but has little effect on the veins [16, 17]. Angiotensin can increase Pmsf [16, 18]. Isoproterenol, a β-adrenergic agonist, causes a decrease in Pmsf when veins are constricted with angiotensin. On the other hand, vasopressin has very little effect on Pmsf [19] or on vascular capacity once reflex blockade [20] and similar results were reported regarding natriuretic peptides [21].
Nitroglycerin and nitroprusside decrease Pmsf and increase unstressed blood volume but do not change vascular compliance in ganglion-blockade dogs [22]. Verapamil and nifedipine increase venous return by reducing the resistance to venous return without changing the Pmsf, whereas nitroglycerin in small doses can reduce Pmsf without changes in resistance to venous return [23]. Diltiazem reduces both resistance and Pmsf increasing CO [23].
Moderate hypercapnia and hypoxia have little direct non-reflex effect on CO and Pmsf [24]. Severe hypercapnia (PaCO2 to 114 mmHg (15.2 KPa)) caused an increase in Pmsf by 5.5 mmHg, whereas a PaO2 of 34 mmHg (4.5 KPa) caused an increase in Pmsf by 2.5 mmHg [25].
6 Measurement of Pmsf in Humans with Intact Circulation
The Pmsf is not easy to measure in patients with an intact circulation. Schipke et al. [26] performed a fibrillation-defibrillation sequence in 82 patients during cardioverter/defibrillator implantation to measure the Pmsf over 13 s. A true equilibrium pressure was not achieved, and the arterial-central venous pressure difference was 13.2 ± 6.2 mm Hg, and differences still persisted in sequences of 20 s.
Pinsky [27] proposed a model in animals with an intact circulation to construct venous return curves observing the relationship between isovolumetric changes in CO and Pra during intermittent positive pressure recruitment manoeuvres. Pmsf was estimated by calculation of the slope and extrapolation of the Pra value to zero CO. Pmsf calculated was found similar to Pmsf measured during circulatory arrest. Other studies [28,29,30] have confirmed this linear relationship between VR and Pra and derived Pmsf from the regression equation in animal models with intact circulation. Maas and colleagues [31] applied the same rationale to study the effect of a 12-second inspiratory hold manoeuvre to three different steady-state levels on central venous pressure (CVP), as an estimate of Pra, and blood flow (CO) measured via the pulse contour method during the last 3 s in mechanically ventilated postoperative cardiac patients. This study showed again a linear relationship between changes in CVP and CO, and importantly, Pmsf could be estimated at bedside in intensive care patients with an intact circulation. Obviously this technique is only feasible in fully sedated patients under mechanical ventilation. Keller and colleagues [32] used this method to assess the changes on venous return with passive leg raising (PLR) manoeuvre: they observed nine postoperative cardiac patients at baseline, during PLR and after volume expansion (500 ml of hydroxyethyl starch). They reported a Pmsf at baseline of 19.7 mmHg. This increased to 22 mmHg after PLR and to 26.9 mmHg after volume expansion (VE). Although CO increased after PLR and VE, the gradient of pressure of venous return (difference between Pmsf and CVP) increased by 2 mmHg after PLR and by 5.8 mmHg after VE. This could explain why a PLR test does not consistently increase CO in fluid-responsive patients [33], or even for a fluid challenge, the increase in Pmsf is an essential condition to effectively test the cardiac response.
Parkin and Wright [34] proposed a method for estimating a mean systemic filling pressure analogue (Pmsa) using the mean arterial pressure (MAP), Pra, CO and anthropometric data. Pmsa algorithm is fully described in other publications [35]. In essence, they build a mathematical model that uses the patient’s data as predictors of Pmsa. The clinical validity of this approach was tested in ten patients in acute renal failure receiving continuous vein-venous haemofiltration [36]. Fluid replacement therapy was electromechanically controlled to a target value of Pmsa. This method was also used to analyse haemodynamic changes after a fluid challenge (250 ml of colloids or crystalloids in 5 min) in patients admitted to intensive care [37]: Pmsa increased similarly in responders and nonresponders, as expected, but interestingly Pra increased more in nonresponders, neutralising the changes in the gradient of pressure of venous return as described by Guyton. Recently, Gupta et al. [38] used Pmsa to investigate the performance of cardiac power (defined as the product of arterial pressure and cardiac output) relative to Pmsa (CPvol). CPvol represents a measurement of cardiac performance adjusted to the vascular tone. According to the authors, values below 0.047 of CPvol have a high sensitivity (97%) and not so high specificity (57.5%) to predict fluid responsiveness.
Anderson [39] proposed a non-invasive technique to measure Pmsf by a rapid occlusion of the circulation in the arm (Pmsf-arm). Once the arterial (Pa) and venous pressures (Pv) in the arm equilibrate, the pressure measured would be Pmsf (◘ Fig. 4.3). Maas et al. [40] compared these three methods in 11 postoperative cardiac surgery patients. Bland-Altman analysis for the difference between Pmsf-arm and Pmsf showed a bias of −1.0 (±3.1) mmHg (p = 0.06) and a coefficient of variation (CV) of 15%. Although there was a statistically non-significant bias, one may think that this is actually quite significant considering the small sample size of this study. Regarding the difference between Pmsf and Pmsa, there was a bias of −6.0 (±3.1) mmHg (p < 0.001) and a CV of 17%. The three methods were useful to track changes after volume expansion.
The precision of the Pmsf-arm technique has been recently studied [41]. Four repeated measurements were performed in 20 patients after cardiac surgery. Pa and Pv equalised after 60 s of cuff inflation. For a single measurement, the coefficient error (CE) was 5% (±2%), and the least significant change (LSC) was 14% (±5%). Averaging two measurements, the CE improves to 4% (±1%), and the LSC was reduced to 10% (±4%).
Practical Implications
Although the measurement of the vascular tone in the venous side of the circulation may have a lot of potential applications, there is still very little evidence about the clinical impact of this information on the management of critically ill patients.
-
1.
Understanding venous return improves management of haemodynamically unstable patients. Rangappa et al. [42] investigated the potential of a computerised decision support system (Navigator™, Applied Physiology, Sidney, Australia) to improve the consistency of haemodynamic evaluation and treatment decisions by intensive care unit clinical staff with different levels of expertise and experience in 20 patients admitted after elective cardiac surgery. The authors concluded that this system improves consistency in decision-making. Sondergaard et al. [43] carried out a small pilot clinical trial in 27 postoperative patients requiring goal-directed therapy to evaluate the efficiency of the Navigator™ system in achieving haemodynamic targets (measuring the percentage time in target zone and the average standardised distance (ASD) from the centre of the target and time to achieve targets) and the level of concordance between the therapy suggested by the system and an expert clinician. The mean percentage time in the target zone was 36.7% for control and 36.5% for intervention, and the ASD was 1.5 in control and 1.6 in intervention (no p value was reported). There was a high level of concordance between decision support recommendation and anaesthetist action (84.3%). The authors concluded that the treatment recommended by the Navigator™ system mirrored that of a senior anaesthetist in the achievement of therapeutic goals. Unfortunately, this study is probably underpowered to show differences in the efficiency measurements, fluid balance or vasoactive medications.
-
2.
The changes in Pmsf can be used to assess systemic compliance and guide the choice between fluids or vasopressors. The current consensus on circulatory shock and haemodynamic monitoring recommends that even in the context of fluid-responsive patients, fluid management should be carefully titrated, especially in the presence of elevated intravascular filling pressures [44]. The similar principle applies to the Pmsf. A fluid challenge can be used to assess fluid responsiveness and also, as spotted by Maas and colleagues [45], to assess systemic compliance. In this study, systemic compliance is reported from 15 postoperative cardiac surgery patients around 64 mL/mmHg. Systemic venous compliance could be very useful information to prioritise treatment: a high compliance after a fluid challenge may indicate the early use of vasoconstrictors instead of infusion of a large amount of fluids. Another study [46] showed that administration of noradrenaline (an α1-adrenergic agonist) increased CO in preload-responsive patients. Noradrenaline increased Pmsf either by reducing venous compliance or by venoconstriction (reduction of venous capacity and shifting unstressed volume to stressed compartment; see ◘ Fig. 4.2). Unfortunately, the authors did not assess the effect of noradrenaline on venous compliance. In the rest of the patients, noradrenaline had predominantly an arterial vasoconstrictive effect, increasing cardiac afterload. This study stressed the importance of monitoring venous tone and CO when using vasopressors.
-
3.
Pmsf can also be used to assess the effect of IV fluid in the circulation, regardless the cardiac response and the efficacy of a fluid challenge. In a recent study about fluid challenges [47], the observation of the Pmsa along with other haemodynamic variables described the short living effect of this technique and pointed out that the maximal change in CO is about 1 min after the end of IV fluid infusion. Pmsf-arm has been also used to evaluate the minimal volume required to challenge the cardiovascular system. This is a quasi-randomised clinical trial with 80 patients who received between 1 and 4 ml/kg of IV fluids in 5 min. Pmsf-arm only increased significantly in the group of 4 ml/Kg, and the proportion of fluid responders increased significantly from 20% in the group of 1 mL/Kg to 65% in the group of 4 mL/Kg.
-
4.
Since venous return equals CO, in practice CO and CVP changes can provide most of the information about the Guytonian view of the circulation. However, without the understanding how the venous tone works, the values of CVP can be misunderstood. Proof of this is the number of studies that looked at the CVP as a fluid responsiveness predictor [48]. CVP preforms as the meeting point between venous return and the cardiac function: a high CVP can be related to a high Pmsf or a low cardiac function or both. Thus, knowing Pmsf would help clinicians to better understand the haemodynamic status of critically ill patients at bedside.
-
5.
Any cardiovascular intervention in critically ill patients should take into account that the main regulation of cardiac output occurs in the peripheral tissues. Therefore, therapy should also be guided by tissue perfusion signs, and not only by haemodynamic measurements.
Take-Home Message
-
Venous return, which is equivalent to cardiac output, is finely controlled at the microcirculatory level in the peripheral tissues. Always remember to put haemodynamics in that context.
-
The two main factors that influence the blood flow in peripheral tissues are the metabolic level and the availability of oxygen. These two are also determinants of venous return.
-
α1-Adrenergic agonist causes venoconstriction, increasing the availability of blood volume from the venous reservoir to increase cardiac output and blood pressure. This is very useful in the anaesthetic induction of unstable patients, as most anaesthetic drugs may cause profound vasodilation and a severe decrease in venous return.
-
In order to do an effective fluid challenge, it is necessary to infuse enough volume to increase Pmsf; otherwise, there is a possibility of a false-negative response. 4 mL/Kg is an adequate dose in most postoperative patients.
Conclusion
The venous system plays an important role in the haemodynamic stability. Most of blood volume is stored and regulated in the venous territory. The mean systemic filling pressure can be now measured, and it is the pressure of the pivot point of the circulation, where the pressure is independent of blood flow. This pressure is the driving pressure of the circulation and affects, along with the cardiac function, venous return. Three methods have been described to measure Pmsf at bedside, in patients with intact circulation. This variable can be now integrated as another piece of information that helps to understand patient’s conditions and to guide haemodynamic therapy in accordance to patient’s physiology.
References
Guyton AC. Textbook of medical physiology. 12th ed. Philadelphia: Elsevier Saunders; 2011.
Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev. 1983;63(4):1281–342.
Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;16(3–4):159–318 7.
Rothe CF. Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol (1985). 1993;74(2):499–509.
Guyton AC, Lindsey AW, Kaufmann BN, Abernathy JB. Effect of blood transfusion and hemorrhage on cardiac output and on the venous return curve. Am J Phys. 1958;194(2):263–7.
Guyton AC, Lindsey AW, Kaufmann BN. Effect of mean circulatory filling pressure and other peripheral circulatory factors on cardiac output. Am J Phys. 1955;180(3):463–8.
Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35(1):123–9.
Rowell LB. Human cardiovascular control. New York: Oxford University Press; 1993.
Furness JB, Marshall JM. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol. 1974;239(1):75–88.
Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982;332:169–86.
Mellander S, Nordenfelt I. Comparative effects of dihydroergotamine and noradrenaline on resistance, exchange and capacitance functions in the peripheral circulation. Clin Sci. 1970;39(2):183–201.
Appleton CP, Lee RW, Martin GV, Olajos M, Goldman S. Alpha 1- and alpha 2-adrenoceptor stimulation: changes in venous capacitance in intact dogs. Am J Phys. 1986;250(6 Pt 2):H1071–8.
Patel P, Bose D, Greenway C. Effects of prazosin and phenoxybenzamine on alpha- and beta-receptor-mediated responses in intestinal resistance and capacitance vessels. J Cardiovasc Pharmacol. 1981;3(5):1050–9.
Ruffolo RR Jr. Distribution and function of peripheral alpha-adrenoceptors in the cardiovascular system. Pharmacol Biochem Behav. 1985;22(5):827–33.
Shi AG, Ahmad S, Kwan CY, Daniel EE. Characterization of alpha-adrenoceptor subtypes by [3H]prazosin and [3H]rauwolscine binding to canine venous smooth muscle membranes. Can J Physiol Pharmacol. 1989;67(9):1067–73.
Hirakawa S, Itoh H, Kotoo Y, Abe C, Endo T, Takada N, et al. The role of alpha and beta adrenergic receptors in constriction and dilation of the systemic capacitance vessels: a study with measurements of the mean circulatory pressure in dogs. Jpn Circ J. 1984;48(7):620–32.
Rothe CF, Flanagan AD, Maass-Moreno R. Role of beta-adrenergic agonists in the control of vascular capacitance. Can J Physiol Pharmacol. 1990;68(5):575–85.
Lee RW, Lancaster LD, Buckley D, Goldman S. Peripheral circulatory control of preload-afterload mismatch with angiotensin in dogs. Am J Phys. 1987;253(1 Pt 2):H126–32.
Pang CC, Tabrizchi R. The effects of noradrenaline, B-HT 920, methoxamine, angiotensin II and vasopressin on mean circulatory filling pressure in conscious rats. Br J Pharmacol. 1986;89(2):389–94.
Martin DS, McNeill JR. Whole body vascular capacitance response to vasopressin is mediated by autonomic function. Am J Phys. 1991;261(2 Pt 2):H493–9.
Chien Y, Pegram BL, Kardon MB, Frohlich ED. ANF does not increase total body venous compliance in conscious rats with myocardial infarction. Am J Phys. 1992;262(2 Pt 2):H432–6.
Ogilvie RI, Zborowska-Sluis D. Effects of nitroglycerin and nitroprusside on vascular capacitance of anesthetized ganglion-blocked dogs. J Cardiovasc Pharmacol. 1991;18(4):574–80.
Ito H, Hirakawa S. Effects of vasodilators on the systemic capacitance vessels, a study with the measurement of the mean circulatory pressure in dogs. Jpn Circ J. 1984;48(4):388–404.
Rothe CF, Flanagan AD, Maass-Moreno R. Reflex control of vascular capacitance during hypoxia, hypercapnia, or hypoxic hypercapnia. Can J Physiol Pharmacol. 1990;68(3):384–91.
Rothe CF, Stein PM, MacAnespie CL, Gaddis ML. Vascular capacitance responses to severe systemic hypercapnia and hypoxia in dogs. Am J Phys. 1985;249(6 Pt 2):H1061–9.
Schipke JD, Heusch G, Sanii AP, Gams E, Winter J. Static filling pressure in patients during induced ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2003;285(6):H2510–5.
Pinsky MR. Instantaneous venous return curves in an intact canine preparation. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(3):765–71.
Versprille A, Jansen JR. Mean systemic filling pressure as a characteristic pressure for venous return. Pflugers Arch. 1985;405(3):226–33.
Den Hartog EA, Versprille A, Jansen JR. Systemic filling pressure in intact circulation determined on basis of aortic vs. central venous pressure relationships. Am J Phys. 1994;267(6 Pt 2):H2255–8.
Hiesmayr M, Jansen JR, Versprille A. Effects of endotoxin infusion on mean systemic filling pressure and flow resistance to venous return. Pflugers Arch. 1996;431(5):741–7.
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med. 2009;37(3):912–8.
Keller G, Desebbe O, Benard M, Bouchet JB, Lehot JJ. Bedside assessment of passive leg raising effects on venous return. J Clin Monit Comput. 2011;25(4):257–63.
Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38(9):1824–9.
Parkin WG, Wright CA. Three dimensional closed loop control of the human circulation. Int J Clin Monit Comput. 1991;8(1):35–42.
Parkin WG, Leaning MS. Therapeutic control of the circulation. J Clin Monit Comput. 2008;22(6):391–400.
Parkin G, Wright C, Bellomo R, Boyce N. Use of a mean systemic filling pressure analogue during the closed-loop control of fluid replacement in continuous hemodiafiltration. J Crit Care. 1994;9(2):124–33.
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39(7):1299–305.
Gupta K, Sondergaard S, Parkin G, Leaning M, Aneman A. Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med. 2015;41:265.
Anderson RM. The gross physiology of the cardiovascular system. 2012 ed. Tucson: Racquet Press; 1993.
Maas JJ, Pinsky MR, Geerts BF, de Wilde RB, Jansen JR. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med. 2012;38(9):1452–60.
Aya H, Rhodes A, Fletcher N, Grounds M, Cecconi M, editors. Transient stop-flow arm arterial-venous equilibrium pressure measurement: determination of precision of the technique. Annual Congress of the European Society of Intensive Care Medicine. Barcelona/New York: Springer; 2014.
Rangappa R, Sondergaard S, Aneman A. Improved consistency in interpretation and management of cardiovascular variables by intensive care staff using a computerised decision-support system. Crit Care Resusc. 2014;16(1):48–53.
Sondergaard S, Wall P, Cocks K, Parkin WG, Leaning MS. High concordance between expert anaesthetists’ actions and advice of decision support system in achieving oxygen delivery targets in high-risk surgery patients. Br J Anaesth. 2012;108(6):966–72.
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–815.
Maas JJ, Pinsky MR, Aarts LP, Jansen JR. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg. 2012;115(4):880–7.
Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1):143–50.
Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44(5):880–91.
Cecconi M, Aya HD. Central venous pressure cannot predict fluid-responsiveness. Evid Based Med. 2014;19(2):63.
Conflict of Interest
Hollmann D. Aya received financial support for educational programmes and for attending symposia from LiDCO. Maurizio Cecconi has received honoraria for speaking at symposia, financial support for educational programmes and honoraria for advisory board from Edwards Lifesciences, LiDCO, Deltex, Applied Physiology, Massimo, Bmeye, Cheetah, and Imacor.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 European Society of Intensive Care Medicine
About this chapter
Cite this chapter
Aya, H.D., Cecconi, M. (2019). Determinants of Venous Return. In: Pinsky, M.R., Teboul, JL., Vincent, JL. (eds) Hemodynamic Monitoring. Lessons from the ICU. Springer, Cham. https://doi.org/10.1007/978-3-319-69269-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-69269-2_4
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69268-5
Online ISBN: 978-3-319-69269-2
eBook Packages: MedicineMedicine (R0)