Abstract
Physical examination plays a very important role in the evaluation of critically ill patients. Certain features such as skin color, capillary refill, mentation, urine output, and pulse quality can tell us a lot about the patient’s hemodynamic status. However, some very important features remain hidden even from the most experienced observer or become obvious only at their extremes. These are bicarbonate and lactate levels, hydrogen ion concentrations (i.e., pH), and the balance between oxygen delivery and consumption. Although for detailed monitoring invasive hemodynamic measurements are required, these are not available in every patient. However, arterial and central venous catheters are part of routine monitoring of the intensive care patient, and a simple blood gas measurement can reveal important physiological processes, which cannot be detected otherwise. In the coming chapter, we are going to discuss the rationale and clinical implication of the venous oxygen saturation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
The primary goal of hemodynamic optimization is to restore and maintain the balance between oxygen supply (DO2) and consumption (VO2) in critically ill patients. There is increasing evidence that patients may benefit from a multimodal individualized approach as compared to protocolized therapy, when predefined hemodynamic goal or goals are targeted. For this purpose, monitoring actual tissue oxygenation/metabolism of a given patient is a very important piece in this hemodynamic puzzle.
-
Mixed venous oxygen saturation (SvO2) and its surrogate, central venous oxygen saturation (ScvO2), are two easily determined blood gas-driven parameters that can mirror changes of the relationship between DO2 and VO2.
-
This article summarizes the physiological rationale, current knowledge, and some aspects of the clinical applications of SvO2/ScvO2 and also highlights some of the most important pitfalls of their interpretation at the bedside.
1 Introduction
Physical examination plays a very important role in the evaluation of critically ill patients. Certain features such as skin color, capillary refill, mentation, urine output, and pulse quality can tell us a lot about the patient’s hemodynamic status. However, some very important features remain hidden even from the most experienced observer or become obvious only at their extremes. These are bicarbonate and lactate levels, hydrogen ion concentrations (i.e., pH), and the balance between oxygen delivery and consumption. Although for detailed monitoring invasive hemodynamic measurements are required, these are not available in every patient. However, arterial and central venous catheters are part of routine monitoring of the intensive care patient, and a simple blood gas measurement can reveal important physiological processes, which cannot be detected otherwise. In the coming chapter, we are going to discuss the rationale and clinical implication of the venous oxygen saturation.
2 Physiological Notes
Tissue oxygenation is the net product of oxygen delivery and oxygen consumption, which can be described by the following formulae:
If SaO2 is taken as 1, as under normal circumstances the hemoglobin is almost fully saturated with oxygen, and the other hemodynamic variables are kept constant, then:
where DO2 is oxygen delivery; C, cardiac output; Hb, hemoglobin; SaO2, arterial oxygen saturation; PaO2, partial pressure of oxygen in the arterial blood; CaO2, arterial oxygen content; VO2, oxygen consumption; SvO2, mixed venous oxygen saturation; and CvO2, mixed venous oxygen content.
Taking a 75 kg healthy adult man when resting, the relationship between DO2 and VO2 can be estimated as:
The main difference between the equations of DO2 and VO2 is the oxygen content (CaO2 vs. CvO2), especially the venous oxygen saturation (this can either be mixed venous, SvO2, or central venous, ScvO2). Therefore, it can be useful to assess the imbalance between DO2 and VO2 in the critically ill. The potential causes of an imbalance between DO2 and VO2 and the basic therapeutic interventions are summarized in ◘ Fig. 15.1.
The relationship between venous saturations and DO2 and VO2. DO2 oxygen delivery, VO2 oxygen consumption, OER oxygen extraction ratio. ∗ − Although sedation can decrease VO2, however, this should be a delicate option as this may also cause decreased cardiac output; hence, it may worsen the situation by decreasing DO2. For further explanation, see main text
3 Interpreting Venous Saturations
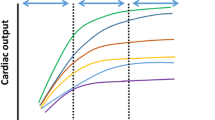
When DO2 is decreasing, oxygen consumption can be maintained – due to an increase in O2ER – for a considerable period of time. However, without intervention, compensatory mechanisms will become exhausted, and beyond that critical point, VO2 becomes DO2 dependent (◘ Fig. 15.2). Till this critical point, venous saturations should decrease proportionally to that of DO2. On the steep part of the curve, cells switch to anaerobic metabolism; hence, lactate production increases. If urgent interventions are delayed, tissue hypoxia and organ dysfunction can develop.
Relationship between oxygen delivery and consumption. DO2 oxygen delivery, VO2 oxygen consumption, ScvO2 central venous oxygen saturation. For details, see main text. Of note, this is a simplified diagram to show the rough tendency how these parameters are related. However, due to the irregular redistribution of blood flow as a compensatory mechanism to centralize circulation, certain organs may start anaerobic metabolism earlier than others; therefore, lactate may increase sooner and can be detected in the serum as compared to what is indicated in this figure as the “critical point.” Regarding ScvO2, its decrease and increase during resuscitation may not be that dramatic, as it depends on the relationship between VO2 and DO2. If VO2 increases parallel with DO2, this should cause hardly any change in ScvO2 during resuscitation. However, if DO2 increases faster than VO2, then ScvO2 will also increase rapidly
It is important to note that during resuscitation – i.e., on the steep or DO2-dependent part of the curve – when interventions are applied to increase DO2, there is also an increase of VO2; hence, there is little if any change in venous oxygen saturations, which may remain “low” and will only increase dramatically when VO2 becomes DO2 independent (i.e., when the patient reaches the flat part of the curve shown in ◘ Fig. 15.2).
Another problem when interpreting venous saturations is that “high” values can indicate improvement but may also indicate inadequate oxygen uptake [1]. Similar to fluid therapy, this is also reflected in morbidity and mortality, as both high and low venous saturations are accompanied by increased morbidity and mortality (◘ Fig. 15.3). Therefore, despite the high values, further interventions may be required (fluid resuscitation, positive inotropic agents, etc.).
The relationship between ScvO2 and morbidity and mortality. DO2 oxygen delivery, VO2 oxygen consumption, ScvO2 central venous oxygen saturation, Pcv-aCO2 central venous-to-arterial CO2 gap, C(a-v)O2 arterial and venous oxygen content difference. This figure indicates that regardless of the actual value of ScvO2, whether it is considered low, normal, or high, careful assessment of the full clinical picture is necessary to best interpret results and to commence appropriate interventions in time
Under these circumstances, when venous oxygen saturations are difficult to interpret, the central venous-to-arterial pCO2 gap [2] and/or detailed invasive hemodynamic monitoring may serve as complementary tools to assess the hemodynamic status [3]. These will be discussed in other chapters.
4 SvO2 or ScvO2?
Nowadays, measurement of SvO2 has become a rarity in the everyday clinical practice, because for sampling, a pulmonary artery catheter must be placed, which is a time-consuming, complicated procedure with significant risks [4]. On the contrary, central venous catheters are part of routine monitoring; hence, central venous oxygen saturation (ScvO2) measurement is readily available. It has been shown that oxygen saturation measured in the superior vena cava is a good alternative of SvO2 [5].
Accurate measurement requires that the tip of the catheter is positioned at the superior vena cava a couple of centimeters above the right atrium. The normal value of ScvO2 ranges between 67% and 77% which is 5–8% higher compared to SvO2 [6]. Although the absolute values are not interchangeable, their trends show good correlation in various disease states [7].
However, as ScvO2 reflects the oxygen consumption mainly of organs draining blood into the superior vena cava, one has to take into account that the biggest consumer of those is the brain. Therefore, during circumstances when brain oxygen uptake is affected (i.e., anesthesia, diffuse brain damage, etc.), ScvO2 may be misleading or at least difficult to interpret.
Nevertheless, by and large these two parameters can be discussed in a similar manner; therefore, to avoid unnecessary citations of both, in the coming paragraphs, we will mainly quote ScvO2, which is the most readily available of the two, unless indicated otherwise.
5 The Current Place of ScvO2 in Clinical Practice
5.1 ScvO2 in Sepsis and Septic Shock
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [8]. Organ dysfunction is most likely the result of inadequate tissue perfusion causing cellular hypoxia. Therefore, treatment strategies that are aimed to restore tissue perfusion by improving the balance between DO2 and VO2 may prevent the development of organ dysfunction syndrome and thus improve the outcome of septic patients.
Rivers and colleagues reported in a landmark paper that in patients with severe sepsis, early goal-directed intervention guided by continuous monitoring of ScvO2, central venous pressure, and mean arterial pressure (MAP), with target values of CVP 8–12 mmHg, MAP > 65 mmHg, and ScvO2 > 70%, reduced mortality from 46.5% to 30.5% at the 28th day [9].
Consequent studies applying early goal-directed therapy (EGDT) with these clinical endpoints suggested that incorporation of ScvO2 in the treatment algorithm and compliance with the algorithm are beneficial in septic patients [10,11,12]. On the contrary, two large randomized trials, the ProCESS and the ARISE trials, could not show any benefit of the “protocol-based standard therapy” and “usual care” groups. They found no significant difference in 90-day mortality, 1-year mortality, or the need for organ support [13, 14].
The controversy around the usefulness of the “Rivers’ EGDT protocol” has been going on for years. Detailed evaluation of these studies is well beyond the scope of this chapter. However, there are some other issues worth discussing in this context.
During the aforementioned studies, “low” ScvO2 was a warning sign that intervention is needed; however, recent data suggest that high ScvO2 values may also have adverse outcomes in septic patients [15]. Due to impaired oxygen utilization, normal or supraphysiological ScvO2 values may thus represent an inability of the cells to extract oxygen or microcirculatory shunting in sepsis [16]. This underscores that some of these patients can be fluid responsive; in other words, their DO2 can be further increased despite high ScvO2 [1]. In patients with ScvO2 > 70% complimentary parameters, such as elevated venous-to-arterial CO2 gap (dCO2) (>6 mmHg), serum lactate levels could help the clinicians to identify tissue hypoxia. In a retrospective analysis, septic patients with physiological ScvO2 and abnormal dCO2 mortality were significantly higher compared to patients with physiological values (56.1% vs. 16.1%; p < 0.001) [17].
5.2 ScvO2 in Cardiogenic Shock
Based on the previous physiological notes, it follows a simple logic that acute heart failure which caused low cardiac output, irrespective from the underlying pathophysiology, can cause VO2/DO2 imbalance that could be detected by low ScvO2 [18].
Indeed, it has been shown in one of the earliest papers in this field that after myocardial infarction in patients with heart failure and cardiogenic shock, SvO2 was 43%, while in patients with heart failure without shock, it was 56% compared to patients without heart failure with an SvO2 of 70% [19].
Treatment effectiveness may also be supported by changes in ScvO2. When cardiogenic shock patients were treated with fluids and inotropes, improvement of DO2 resulted in an increase in SvO2 suggesting better tissue oxygenation [20]. It may also be useful in patients with cardiogenic shock requiring the support by intra-aortic balloon counter pulsation. In a study, intra-aortic balloon pump assist ratio was decreased gradually from 1:1 to 1:3. In the weaning failure group decreased support was accompanied by a drop in ScvO2, while it remained constant in the successful group [21].
Even in patients with chronic heart failure, ScvO2 has important predictive values. In these patients, the ScvO2 can be chronically low. However, during acute decompensation, major cardiac events were observed in 81% of patients with ScvO2 ≤ 60% at 24 h, while it was only 13% in patients with higher ScvO2 [22].
5.3 ScvO2 to Predict Successful Extubation
During the weaning procedure, there can be an increase of VO2 due to the increased respiratory muscle activity and increased alertness. If DO2 is inadequate, then an imbalance can occur between the VO2/DO2. Theoretically, this can be picked up by low or at least decreasing ScvO2 values. In a recent clinical trial, a > 4% drop in ScvO2 after a 30-min spontaneous breathing trial indicated extubation failure with high sensitivity and specificity [23].
5.4 ScvO2 as a Physiological Transfusion Trigger
One of the most common causes of impaired DO2 in critically ill patients is anemia requiring red blood cell transfusions [24]. Large multicenter trials (TRICC, TRISS) suggest that patients with hemoglobin levels above 10 mg/dl usually do not require transfusion, while red blood cell administration is usually beneficial if the hemoglobin level is below 7 mg/dl [25, 26]. However, there is a gray zone between 7 and 9.5 mg/dl where physicians have to rely on clinical signs like mental status, tachycardia, tachypnea, blood pressure, and diuresis.
In this gray zone, ScvO2 may offer an easily obtainable tool to detect a low hemoglobin-related altered O2ER and hence may serve as a physiological trigger for blood transfusion [27]. It was found during hemorrhage in animal and human experimental models that ScvO2 may be useful for the identification of patients with occult or ongoing clinically significant blood loss [28]. In a human study, acute isovolemic anemia of hemoglobin of 50 g/l in conscious healthy resting humans did not produce hemodynamic instability, but oxygen imbalance was accompanied by a significant drop in SvO2 [29]. These results were reinforced by a retrospective analysis of a prospective observational study in which ScvO2 was found to be a good indicator of transfusion [30]. The results of our animal study on isovolemic hemodilution gave further evidence that anemia-induced change in VO2/DO2 showed significant negative correlation with changes of ScvO2 [31].
6 ScvO2 and Major/High-Risk Surgery
In addition to the acutely ill, the high-risk surgical patients may also develop an imbalance between VO2 and DO2 in the perioperative period. Therefore, monitoring ScvO2 may have a rationale during both the intraoperative and postoperative management.
It has been shown that low ScvO2 values are good indicators of complications and poor prognosis in the postoperative period [27]. We reported in a small, single-center prospective randomized study that an ScvO2-assisted intraoperative hemodynamic optimization resulted in less organ dysfunction and better outcome after major abdominal surgery [32]. This was in accord with the results of an earlier single-center study, where patients in the ScvO2-directed group had fewer postoperative complications and had shorter length of hospital stay compared to patients in the control group [28].
However, there are some special considerations when interpreting ScvO2 in the perioperative setting. Firstly, in an anesthetized, mechanically ventilated patient, “normal” values of ScvO2 are 5–10% higher (i.e., 75–80%) than in an awake or sedated intensive care patient or in a normal subject. Secondly, it is important to note that while fluid therapy on the one hand improves cardiac output, on the other hand, it can also cause hemodilution. In our experimental stroke volume-guided hemorrhage and fluid resuscitation animal model, ScvO2 normalized at the end of resuscitation but returned to a significantly lower level (with a mean of 5%) due to the hemodilution which caused significant drop in hemoglobin levels [33].
Goal-directed therapy is also a controversial issue in surgical patients. However, according to a recent meta-analysis, while goal-directed therapy had no significant effect in the low-risk surgical population, both mortality and morbidity were significantly better in the goal-directed group among the high-risk subgroups [34]. In our view, ScvO2 is an important element of this complex perioperative multimodal monitoring-based concept, including advanced hemodynamic monitoring and assessment of VO2/DO2, what we call the individualized, multimodal approach [35].
7 Pitfalls of ScvO2
ScvO2 is the net result of the complex physiological and pathophysiological interactions of DO2 and tissue VO2. Low values strongly suggest inadequate DO2; however, in patients with chronic heart failure, chronic anemia, etc., with a “compensated” state, low levels should be considered as “normal” but at least accepted. Not acknowledging this may result in unnecessary and potentially harmful interventions like overzealous fluid resuscitation.
The interpretation of “high” values of ScvO2 is even more challenging. Under physiological circumstances, dissolved oxygen is negligible in DO2. In an elegant trial on mechanically ventilated ICU patients, after increasing FiO2 from 40% to 100%, PaO2 increased from 100 mmHg to almost 400 mmHg: Without any change in cardiac output or hemoglobin, ScvO2 rose from 71% to 84% [36]. Therefore, and this holds true for all the above mentioned examples, relatively stable conditions are desirable for the appropriate assessment. When there are too many changes occurring within a relatively short period time, this can make interpretation of ScvO2 even more difficult.
During circumstances when brain oxygen uptake is affected (i.e., anesthesia, diffuse brain damage, etc.), ScvO2 may be misleading or at least difficult to interpret. Data are lacking, but for these special situations, multimodal monitoring of depth of anesthesia (bispectral index, entropy) and brain oxygen consumption (near-infrared spectroscopy) may be useful and also another step to individualize our treatment for the given patient’s actual needs..
Practical Implications
Venous oxygen saturation can be determined from either obtaining blood from the pulmonary artery (SvO2) or from the superior vena cava (ScvO2). Both can provide useful information about the balance between VO2 and DO2 and may also help monitoring the effectiveness of hemodynamic stabilization.
-
1.
In sepsis, impaired oxygen utilization can result in normal or supraphysiological ScvO2 values, which may represent the inability of cells to extract oxygen most likely due to microcirculatory shunting [16]. In the complex pathology of sepsis, treating one single parameter – Let it be ScvO2, lactate, MAP, cardiac output, or else – Can certainly be misleading. Putting easily obtainable clinical and laboratory data including arterial and venous blood gas-driven parameters into context may help to recognize oxygen debt early and may also help to identify those patients who will require advanced invasive hemodynamic monitoring [3]. This also forms the basis of multimodal, individualized patient management.
-
2.
It has been shown by several studies that in acute left ventricular failure, low SvO2/ScvO2 is an important sign of severe imbalance in the VO2/DO2 relationship, and this parameter also has an important prognostic value [19, 22].
-
3.
Following the changes of SvO2/ScvO2 over time may be used for weaning patients from cardiac support both pharmacological and assist devices [21], and during spontaneous breathing trials, changes may also provide a good prognosticating factor for extubation success or failure [23].
-
4.
In otherwise stable but anemic patients, SvO2/ScvO2 may serve as physiologic transfusion trigger [30, 31], although no precise recommendation can be made.
-
5.
In high-risk surgical patients, intraoperative evaluation of ScvO2 can be a very useful tool both for diagnosing and monitoring VO2/DO2 imbalance as described in other clinical scenarios, as part of the multimodal monitoring approach [35].
Take-Home Messages
-
Venous oxygen saturations are important tools to assess VO2/DO2 at the bedside.
-
ScvO2 is an easily obtainable and useful alternative of SvO2.
-
Low venous saturations should be considered as an important alarming signal of VO2/DO2 imbalance, and causes of low DO2 – Such as hypovolemia, heart failure, bleeding, anemia, and hypoxemia – Should be looked for.
-
High or even normal venous saturations should be interpreted with caution especially in patients who require moderate or high level of hemodynamic support, as they may indicate impaired oxygen uptake.
-
In general, but especially under circumstances when interpretation of venous saturation is not straightforward, instead of targeting a given value of SvO2/ScvO2 (i.e., 65–70%), complimentary parameters, such as venous-to-arterial CO2 gap, lactate levels, echocardiography, and/or invasive hemodynamic monitoring provided indices, should be put into context in order to individualize hemodynamic support.
Conclusion
Assessing oxygen consumption requires detailed hemodynamic assessment, which is not always feasible. Measurement of venous oxygen saturations – especially ScvO2 – may serve as a simple, easily and readily available tool for assessing oxygen debt at the bedside. When interpreting the cellular well-being of the high-risk intensive care or surgical patient, ScvO2 can play a very useful role. On its own it can be an important alarming signal of inadequate oxygen delivery, but to see the full picture, it should be incorporated into the complex of the hemodynamic puzzle.
8 Case Studies
8 Clinical Case 1
A 35-year-old man suffered acute myocardial infarction. During percutaneous coronary angioplasty, he developed cardiogenic shock and required continuous infusion of norepinephrine (NE) and endotracheal intubation. At the end of the intervention, due to the persistent shock, intra-aortic balloon pump (IABP) was placed to support coronary flow. On arrival to the ICU, he required 75 μg/min NE to maintain a blood pressure of 98/51(73) mmHg. He was ventilated at 60% FiO2, 10 of PEEP, in BiPAP mode.
The IABP was set to a 1:1 support mode and a control arterial and central venous blood gases were taken.
Arterial blood gas | Central venous blood gas | |
|---|---|---|
pH | 7.41 | 7.35 |
pCO2 (mmHg) | 42 | 53 (Pcv-aCO2-gap: 11) |
pO2 (mmHg) | 103 | 46 |
BE (mmol/L) | 1.3 | – |
HCO3 (mmol/L) | 26.0 | – |
SO2 (%) | 98 | 77 |
Lactate (mmol/L) | 1.4 | 1.3 |
These results indicate remarkable oxygenation, ventilation, and acid-base homeostasis, as far as pH, HCO3, and lactate are concerned. However, central venous blood gas results, taken at the same time, revealed a completely different picture.
ScvO2 could be considered as “normal” or “high.” However, the elevated CO2 gap suggests that cardiac output may be low. An echocardiography was performed, which revealed poor left ventricular function (EF, 35%) with dilated ventricles (135 mL). The IABP was then stopped for 5 min and blood gases were repeated.
Arterial blood gas | Central venous blood gas | |
|---|---|---|
pH | 7.39 | 7.36 |
pCO2 (mmHg) | 44 | 51 (Pcv-aCO2-gap: 7) |
pO2 (mmHg) | 87 | 46 |
BE (mmol/L) | 10.8 | – |
HCO3 (mmol/L) | 26.0 | – |
SO2 (%) | 97 | 81 |
Lactate (mmol/L) | 1.3 | 1.3 |
8 Interpretation
Stopping the IABP for 5 min caused an increase in ScvO2 by 4% and a decrease in CO2 gap to 7 mmHg, indicating a possible improvement in cardiac output. For more information, invasive hemodynamic monitoring was commenced with transpulmonary thermodilution, which revealed elevated end-diastolic volume (GEDVI) of 1043 ml/m2 (normal, 600–800 ml/m2) and increased extravascular lung water (EVLWI) of 21 ml/kg (normal, less than 10 ml/kg), indicating gross fluid overload; hence, fluid removal was decided, initially with furosemide, and then later with continuous veno-venous hemofiltration.
8 Conclusion
Arterial blood gas analysis on its own is not enough to assess the hemodynamic situation – in fact it may show a false-positive picture – unless there is already severe metabolic acidosis with low pH, HCO3, and high lactate levels. Including the central venous blood gas results in the assessment, an early warning sign was revealed indicating that the patient is still unstable, and further information and intervention may be required.
8 Clinical Case 2
An 83-year-old woman with urinary tract infection was treated on a medical ward and was asked to be reviewed due to respiratory distress and hypotension. On assessment she looked frail, she was tachypneic (30/min), and her blood pressure was 90/40(57) mmHg. The attending ICU resident immediately started oxygen supplementation via face mask and after inserting a large bore (14G) peripheral venous catheter ordered a fluid bolus of 500 mL balanced crystalloid solution to be infused. At the same time, an arterial blood gas was sent to the ICU.
Arterial blood gas | |
|---|---|
pH | 7.19 |
pCO2 (mmHg) | 28 |
pO2 (mmHg) | 64 |
BE (mmol/L) | −16.4 |
HCO3 (mmol/L) | 10.5 |
SO2 (%) | 88 |
Lactate (mmol/L) | 6.9 |
Based on these results, the patient was immediately transferred to the ICU.
By the time of arrival, her blood pressure and oxygenation already improved, and she felt better in general. An indwelling arterial catheter was inserted into the left radial artery, and another blood gas was taken. In the meantime she received another bolus of 500 mL crystalloid.
Arterial blood gas | |
|---|---|
pH | 7.27 |
pCO2 (mmHg) | 27 |
pO2 (mmHg) | 92 |
BE (mmol/L) | −13.1 |
HCO3 (mmol/L) | 12.5 |
SO2 (%) | 96 |
Lactate (mmol/L) | 3.7 |
These results indicate improvement, but metabolic acidosis is still present; hence, a central venous catheter was inserted into the right internal jugular vein, and in the meantime a transthoracic echocardiography was also performed. The latter revealed good ventricular function and small ventricular diameters; therefore, fluid administration was continued, and another 500 mL of bolus crystalloid was administered. The patient’s blood pressure hasn’t changed and remained anuric; hence, norepinephrine was also commenced into a peripheral vein at a rate of 5 μg/min. After inserting the central venous catheter, arterial and central venous blood gases were taken at the same time.
Arterial blood gas | Central venous blood gas | |
|---|---|---|
pH | 7.38 | 7.34 |
pCO2 (mmHg) | 39 | 52 (Pcv-aCO2-gap: 13) |
pO2 (mmHg) | 130 | 25 |
BE (mmol/L) | −5.1 | – |
HCO3 (mmol/L) | 20.5 | – |
SO2 (%) | 98 | 49 |
Lactate (mmol/L) | 2.4 | 2.2 |
8 Interpretation
According to these results, there is still an imbalance between VO2 and DO2 as indicated by low ScvO2, and the grossly elevated CO2 gap also suggests the inadequacy of flow (cardiac output). Therefore, fluid resuscitation was continued, and after another two boluses of 500 mL of crystalloid, the patient’s condition eventually improved, and both macrohemodynamics (blood pressure, urine output) and blood gases normalized.
8 Conclusion
Despite dramatic improvement in arterial blood gases, lactate, respiratory, and macrohemodynamic indices, central venous blood gas results revealed that serious hemodynamic instability is still present indicated by very low ScvO2 and very high CO2 gap. Putting both blood gases into context helped the decision to continue fluid resuscitation, which ended with positive results; hence, advanced monitoring and further intervention became unnecessary.
8 Clinical Case 3
A 67-year-old man required acute surgery due to a perforated colon diverticulum. From his previous medical history, controlled hypertension and mild ischemic heart disease are worth mentioning. In the postoperative period, he required some vasopressor support for 24 h, but by day 3 his condition improved, he felt well, he was without any pain, all vital signs were stable, and he started eating and drinking the day before; hence, he was considered as ready to be discharged. The only abnormal finding was a hemoglobin of 7.2 g/dL. These were his blood gases:
Arterial blood gas | Central venous blood gas | |
|---|---|---|
pH | 7.34 | 7.32 |
pCO2 (mmHg) | 46 | 52 (Pcv-aCO2-gap: 6) |
pO2 (mmHg) | 84 | 43 |
BE (mmol/L) | −0.6 | – |
HCO3 (mmol/L) | 26.5 | – |
SO2 (%) | 98 | 73 |
Lactate (mmol/L) | 1.9 | 2.0 |
8 Interpretation
Based on the stable macrocirculation, well-established oral intake of food and drinks, the normal ScvO2, lactate, and CO2 gap, we decided not to transfuse this patient. He was then discharged, and following him up, his hemoglobin started to increase gradually and did not require blood transfusion during his hospital stay.
8 Conclusion
Although most transfusion guidelines would recommend transfusing an elderly patient with previous medical history of ischemic heart disease, especially in the early postoperative period with a hemoglobin of 7.2 g/dL, but putting all available data into context, there was no evidence that this degree of anemia caused any instability to this particular patient; therefore, transfusion had no physiological indication; hence, it was put on hold, and transfusion – with all its potential side effects – was eventually avoided.
References
Velissaris D, Pierrakos C, Scolletta S, Backer D, Vincent JL. High mixed venous oxygen saturation levels do not exclude fluid responsiveness in critically ill septic patients. Crit Care. 2011;15:R177.
Weil MH, Rackow EC, Trevino R, Grundler W, Falk JL, Griffel MI. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med. 1986;315:153–6.
Møller MH, Cecconi M. Venous-to-arterial carbon dioxide difference: an experimental model or a bedside clinical tool? Intensive Care Med. 2016;42:287–9.
Evans DC, Doraiswamy VA, Prosciak MP, Silviera M, Seamon MJ, Rodriguez Funes V, et al. Complications associated with pulmonary artery catheters: a comprehensive clinical review. Scand J Surg. 2009;98:199–208.
Dueck MH, Klimek M, Appenrodt S, Weigand C, Boerner U. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103:249–57.
Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30:1572–8.
Reinhart K, Rudolph T, Bredle DL, Hannemann L, Cain SM. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest. 1989;95:1216–21.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Trzeciak S, Dellinger RP, Abate NL, Cowan RM, Stauss M, Kilgannon JH, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129:225–32.
Jones AE, Shapiro NI, Roshon M. Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med. 2007;14:1072–8.
Rhodes A, Phillips G, Beale R, Cecconi M, Chiche JD, De Backer D, et al. The surviving sepsis campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med. 2015;41:1620–8.
ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93.
ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506.
Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI, Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of central venous oxygen saturation (ScvO2) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40–6.
Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–77.
Du W, Liu DW, Wang XT, Long Y, Chai WZ, Zhou X, et al. Combining central venous-to-arterial partial pressure of carbon dioxide difference and central venous oxygen saturation to guide resuscitation in septic shock. J Crit Care. 2013;28:1110.
Muir AL, Kirby BJ, King AJ, Miller HC. Mixed venous oxygen saturation in relation to cardiac output in myocardial infarction. Br Med J. 1970;4:276–8.
Goldman RH, Braniff B, Harrison DC, Spivack AP. The use of central venous oxygen saturation measurements in a coronary care unit. Ann Intern Med. 1968;68:1280–7.
Creamer JE, Edwards JD, Nightingale P. Hemodynamic and oxygen transport variables in cardiogenic shock secondary to acute myocardial infarction, and response to treatment. Am J Cardiol. 1990;65:1297–300.
Hsin HT, Chen LY, Lin PC, Shieh JS, Ao CV. Central venous oxygen saturation (ScVO2) facilitates the weaning of intra-aortic balloon pump in acute heart failure related to acute myocardial infarction. Int J Cardiol. 2013;168:4568–70.
Gallet R, Lellouche N, Mitchell-Heggs L, Bouhemad B, Bensaid A, Dubois-Randé JL, et al. Prognosis value of central venous oxygen saturation in acute decompensated heart failure. Arch Cardiovasc Dis. 2012;105:5–12.
Teixeira C, da Silva NB, Savi A, Vieira SR, Nasi LA, Friedman G, et al. Central venous saturation is a predictor of reintubation in difficult-to-wean patients. Crit Care Med. 2010;38:491–6.
Luciano Gattinoni MD, Davide Chiumello MD. Anemia in the intensive care unit: how big is the problem? Transfusion Alternatives Transfusion Med. 2002;4:118–20.
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17.
Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–91.
Collaborative Study Group on Perioperative ScvO2 Monitoring. Multicentre study on peri- and postoperative central venous oxygen saturation in high-risk surgical patients. Crit Care. 2006;10:R158.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Changes in central venous saturation after major surgery, and association with outcome. Crit Care. 2005;9:R694–9.
Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–21.
Kobayashi M, Ko M, Irinoda T, Meguro E, Hayakawa Y, et al. Clinical usefulness of continuous central venous oxygen saturation measurement for postoperative management of patients following transthoracic esophagectomy for carcinoma. Esophagus. 2011;8:53–8.
Kocsi S, Demeter G, Fogas J, Erces D, Kaszaki J, Molnar Z. Central venous oxygen saturation is a good indicator of altered oxygen balance in isovolemic anemia. Acta Anaesthesiol Scand. 2012;56:291–7.
Mikor A, Trasy D, Nemeth MF, Osztroluczki A, Kocsi S, Kovacs I, et al. Continuous central venous oxygen saturation assisted intraoperative hemodynamic management during major abdominal surgery: a randomized, controlled trial. BMC Anesthesiol. 2015;15:82.
Nemeth M, Tanczos K, Demeter G, Erces D, Kaszaki J, Mikor A, et al. Central venous oxygen saturation and carbon dioxide gap as resuscitation targets in a hemorrhagic shock. Acta Anaesthesiol Scand. 2014;58:611–9.
Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209.
Molnar Z, Szabo Z, Nemeth M. Multimodal individualized concept of hemodynamic monitoring. Curr Opin Anaesthesiol. 2017;30:171–7.
Legrand M, Vallée F, Mateo J, Payen D. Influence of arterial dissolved oxygen level on venous oxygen saturation: don’t forget the PaO2! Shock. 2014;41:510–3.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 European Society of Intensive Care Medicine
About this chapter
Cite this chapter
Molnar, Z., Nemeth, M. (2019). SvO2/ScvO2. In: Pinsky, M.R., Teboul, JL., Vincent, JL. (eds) Hemodynamic Monitoring. Lessons from the ICU. Springer, Cham. https://doi.org/10.1007/978-3-319-69269-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-69269-2_15
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69268-5
Online ISBN: 978-3-319-69269-2
eBook Packages: MedicineMedicine (R0)