Abstract
There are unique challenges to determining the epidemiology of rheumatoid arthritis (RA)-related lung disease stemming from differing case definitions and detection modalities in the literature. The prevalence of radiographic interstitial lung disease (ILD) is variable and ranges from 19 to 67%. Clinically significant disease, defined as contributing to death, is seen on 10% of patients. Risk factors have been identified and include smoking, male gender, and age. RA-related small airways disease is seen in 15–44% of all comers and 11–30% of never smokers. Large airways disease in the form of bronchiectasis is seen in 17% of patients and is associated with an increase in mortality. Pleural involvement is common in the form of pleural thickening and effusions, though a high percentage of these are asymptomatic.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Rheumatoid arthritis
- Interstitial lung disease
- Airways disease
- Prevalence
- Incidence
- Bronchiectasis
- Risk factors
- Pleural disease
Introduction
Rheumatoid arthritis (RA), characterized by inflammatory joint destruction , is the most common connective tissue disease, affecting 1% of the US population [1]. The global prevalence of RA is 0.24% (or 16 million people), and it ranks as the 42nd highest contributor to global disability [2]. The annual excess health cost related to RA in the USA is estimated at $19.3 billion [3].
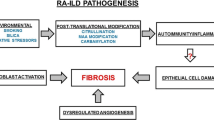
Extra-articular manifestations (ExRA) are common in RA [4], may involve nearly any organ, and lead to excess mortality [5, 6]. Lung involvement occurs in 60–80% [7,8,9] of RA patients, many of whom (29–68%) are asymptomatic [7, 9, 10]. Although any component of the respiratory tract may be affected [11], the parenchyma, airways, and pleura are the most common sites of involvement [12]. In addition to direct pulmonary involvement from RA, patients are at risk for secondary pulmonary complications such as drug-induced lung disease and opportunistic infections.
Challenges of Epidemiologic Studies in RA Lung Disease
There are unique challenges in determining the epidemiology of RA-related lung disease. A three-patient case series of RA patients with “reticulation” on chest radiograph was published in 1948 [13], but the first well-described case of “rheumatoid lung” wasn’t published for another decade [14]. In the subsequent years, authors tried to link RA and interstitial lung disease (ILD), but they remained limited by available detection methods [15, 16]. Since the 1970s, the connection between RA and lung disease has become well-established and, more recently, strongly confirmed with the advent of chest computed tomography (CT). Without CT, it was nearly impossible for investigators to detect subtle changes of ILD and extremely difficult for them to distinguish bronchiectasis and small airways disease. Thus, in the pre-CT era, epidemiological estimates of RA-related lung disease must be viewed as unreliable.
More recently, studies of the epidemiology of RA-related lung disease have yielded inconsistent results because of differing case definitions and case detection modalities. For example, studies using respiratory symptoms as a trigger for screening may underestimate prevalence, particularly in RA patients with severe articular disease and limited mobility. Evaluation for secondary causes of respiratory symptoms or lung disease (e.g., drug-related lung toxicity) has not been standard among epidemiologic studies. Studies that rely on health-care databases to determine incidence and prevalence are subject to issues related to diagnostic coding and classification bias. Tackling these issues will help investigators develop systematic approaches to identifying the true epidemiological burden of this disease.
Rheumatoid Arthritis-Associated Interstitial Lung Disease
Prevalence and Cumulative Incidence of RA-ILD in Patients Known to Have RA (Table 4.1)
Population-based studies that rely on medical records data to calculate incidence and prevalence estimates of RA-ILD are hindered by reporting bias and typically only include people with clinically significant disease. Studies of the US RA population have yielded cumulative incidence estimates of clinically significant RA-ILD in 5% of patients at 10 years [17], 6.3% at 15 years [18], and 6.8% over 30 years of follow-up [4]. Investigators from the Mayo Clinic reviewed data from 582 RA patients captured by the Rochester Epidemiology Project and estimated a lifetime risk of developing clinically significant ILD of 7.7% [19]. A large study of over 40 million US death certificates identified 160,000 records of decedents with RA; investigators found clinically significant ILD (defined as a contributor to death) in 6.8% of women and 9.8% of men [20]. Additional results from this study suggest that death from ILD among decedents with RA is on the rise [20]. However, these data should not be interpreted as incidence estimates.
A large study looking at the changing prevalence of severe ExRA from 1985 to 2006 in over 35,000 RA patients in the US Department of Veterans Affairs (VA) system found a decline in all ExRA (Felty syndrome, vasculitis, carditis) with the exception of rheumatoid lung disease, defined as ILD and pleurisy, which was increasing over this time in both outpatients and hospitalized patients [21]. These investigators hypothesized that these trends may be due to the fact that newer therapies have improved the treatment of joint disease in RA thus decreasing extrapulmonary-related death, while treatments for ExRa, in particular fibrotic lung disease, are lacking. In addition, the increased awareness of RA-associated ILD, as well as the increasing use of high-resolution computed tomography (HRCT) over time, is likely also contributing to an increase in the prevalence of disease.
Prevalence rates of RA-ILD have been reported for certain ethnic groups. Researchers from the University of California San Francisco (UCSF) analyzed medical records from Hispanic and Asian outpatients in a rheumatology clinic and calculated a prevalence of RA-ILD of 3.6% [22]. Prevalence of RA-ILD in other ethnic subgroups has also been calculated: 3% in Koreans [23], 4.2% in Italians [24], 3.7% in Spaniards [25], and 4.8% in Turks [26].
Prevalence of RA-ILD in Patients with Respiratory Symptoms
As mentioned, when different imaging modalities and screening criteria are used for ILD screening, disease rates can vary markedly. For example, prevalence rates of RA-ILD are typically higher when using highly sensitive imaging techniques (i.e., HRCT) as compared to lower-sensitivity studies (i.e., chest radiograph). In fact, due to its sensitivity, HRCT is the preferred method of screening for ILD. RA-ILD prevalence rates are also typically lower when screening studies are applied broadly to any RA patient rather than only to RA patients with respiratory symptoms. For instance, a study looking at patients with a documented lack of pulmonary symptoms (cough or dyspnea) found “subclinical” ILD in 33% [27]. Conversely, a screening study looking at selected patients at high risk for ILD (with either symptoms, impaired lung function or suspicious finding on chest X-ray (CXR)) identified ILD in as many as 91% [28] of patients.
Symptoms suggestive of lung disease are common in patients with RA. In 54 consecutive patients coming into a rheumatology clinic, 41% were found to have respiratory symptoms upon questioning, though symptoms did not correlate with either HRCT or PFT abnormalities [10]. Clinically significant ILD, defined as radiographic or physiologic changes in the setting of respiratory-related symptoms, occurs in approximately 10% of patients with RA [29,30,31]. A study of 252 RA patients found respiratory symptoms in 23% of patients; further investigation of these symptoms found ILD found in 9.1% of the entire cohort [31]. Clinically significant ILD was found to be associated with a 2.8-fold increased risk of death [5].
Prevalence of RA-ILD in Unselected Patients
Those with RA who undergo screening regardless of symptoms commonly have abnormalities on HRCT. The prevalence of radiographic ILD is variable and ranges from 19 to 67% depending on the population screened [8, 10, 29, 31,32,33,34]. A screening study of 150 RA patients irrespective of symptoms found interstitial changes (defined as reticular lines or ground glass) in 19% (with changes on CXR noted in less than 3%) [32], and these interstitial abnormalities correlated with reductions in the diffusing capacity for carbon monoxide (DLCO) and elevations in the forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) (suggestive of restrictive disease). Another study screening unselected RA patients with HRCT found findings consistent with ILD (defined as a reticulonodular pattern) in 42% [8]. The large differences in the prevalence of imaging abnormalities between these studies may be due in part to differences in RA disease duration or severity or differences in smoking history. Investigators looking at early RA (defined as a joint disease duration of <2 years) found HRCT changes consistent with ILD in 27–33% with a majority of those being defined as “mild” (with only 10–14% defined as clinically significant disease) [29, 30]. When lifelong non-smokers with RA are screened, ILD is less common, found in only 5% in one study [35]. In another unselected group of hospitalized patients with RA, 49% of patients had HRCT abnormalities with the majority of these being ILD [34].
Prevalence of RA-ILD Using CXR, Pulmonary Function Tests (PFTs), and Multimodality Approach
Early studies looking at the prevalence of lung disease on CXR found interstitial abnormalities in 1–12% of unselected RA patients [14, 29, 32, 33, 36, 37]. However, CXR is a notably insensitive tool for identifying ILD. For example, in a study screening hospitalized patients with HRCT, 48% of patients with an abnormal HRCT had a normal CXR, highlighting its insensitivity [34, 38].
PFTs can also be used to screen for ILD but, similar to CXR, are a less sensitive indicator of ILD compared to HRCT. In one study, PFTs were normal in 37% of people with HRCT evidence of ILD [8]. DLCO seems to be the best indicator of disease with reductions in DLCO correlating with the presence of interstitial abnormalities across a number of studies [28,29,30, 32]. In one study, reductions in DLCO were seen in 82% of patients with ILD on HRCT, and DLCO was the only PFT variable associated with ILD [32]. FVC is associated with ILD [34] though it has a weaker correlation compared to DLCO [28]. This may be contributed by the prevalence of smokers with obstructive physiology in this group (in one study of HRCT scans in 150 consecutive patients with RA, 43% of those with ILD had concomitant emphysematous changes [32]).
Overall, screening with multiple modalities finds a higher incidence of lung abnormalities in RA patients. A study looking at a combination of clinical symptoms, lung physiology, radiology (HRCT and CXR), bronchoalveolar lavage (BAL), and 99mTc-DTPA nuclear scan found abnormalities suggestive of ILD in 58% of patients, with 14% of those patients having clinically significant ILD [29].
Risk Factors for RA-ILD (Table 4.2)
RA is clearly a risk factor for ILD, conferring an increased risk of ILD with an odds ratio of 8.96 [19]. There are many reported risk factors for the development of ILD in RA, though few have strong supporting data.
Smoking
Smoking is a well-documented risk factor for RA in general [39, 40]—as well as RA-ILD [27, 33, 35, 41]. A multivariate analysis of risk factors for physiologic or radiographic abnormalities suggestive of ILD in patients with RA found that pack- years of smoking was associated with reductions in FVC and DLCO (even after exclusion of those with airflow obstruction as defined by an FEV1/FVC ratio <0.65) as well as interstitial changes on CXR [33]. A dose response was noted, with a higher incidence of ILD in those with ≥25 pack-years of smoking. A study of patients with RA but without pulmonary symptoms also found a strong association between longer smoking histories and the presence of subclinical ILD [27]. A much lower incidence of ILD is found when HRCT screening of RA patients is limited to lifelong non-smokers (5% in non-smokers [35] compared to 19–67% in all comers [8, 10, 29, 31,32,33,34]). In spite of these suggestive data, smoking has not been associated with ILD in all studies [8, 32, 42, 43], and smoking may not influence the pattern of ILD [28].
Sex
Although the prevalence of RA is more common in women [1, 44], male sex has been associated with the development of RA-ILD in multiple studies [19, 29, 31, 33, 45]. A study that longitudinally followed RA patients for a mean of 16 years found that males were more likely to develop ILD with a HR of 4.37 (95% CI 2.43–7.88) [19], although these estimates were not adjusted for smoking. A cross-sectional analysis of 252 RA patients found male sex was associated with the presence of clinically apparent ILD on multivariate analysis (OR 3.29, 95% CI 1.59–6.80, p = 0.0013) [31]. The association between male sex and ILD applies also to early RA [29]. However, not all studies find an association between sex and ILD [18, 43].
Age
Advanced age has been associated with RA-ILD in a number of studies [8, 18, 19, 33, 34, 41, 43]. Indeed, the average age across multiple studies ranges from 57 to 74 [4, 18, 28, 41, 46]. A population-based study following 582 patients over a mean of 16 years found age associated with the development of ILD with a HR of 1.41(95% CI 1.11–1.79) for every 10-year increase in age [19]. The analysis of an inception cohort with a 20-year follow-up found that every 10-year increase in age increases the likelihood of ILD by 64% [18]. Age is also predictive of ILD in studies utilizing multivariate analysis of other cofounders [8, 33].
RA Disease Activity
Disease activity , as measured by higher scores on the Health Assessment Questionnaire (HAQ) [18] and/or higher scores on the disease activity score 28 (DAS28) [30], has been associated with the presence of ILD, the HAQ showing further association with declines over time in the FVC and DLCO [33]. Erythrocyte sedimentation rate (ESR), as a measure of RA disease activity, has been associated with the presence of ILD and declines in DLCO over time [8, 18, 19] with one study showing an increased likelihood of ILD by 11% for every 10-unit increase in ESR [18]. Other measures of disease activity such as rheumatoid factor (RF) [8], erosions or destructive joint changes, and rheumatoid nodules [19] have been associated with the development of ILD [19] as well.
RA Disease Duration
Multiple studies suggest that duration of RA is not a risk factor for ILD [8, 27, 34, 47]. One group looked at HRCT screening in patients with early (<1 year) and long-standing (>3 years) RA and found no difference in the incidence of ILD [47]. The duration of RA, however, may influence the presence of clinical versus subclinical disease [27] or the subtype of ILD in these patients [28].
RA Treatment
Medications have been implicated as risk factors for the development of ILD. Methotrexate (MTX) is well known to cause lung disease [48], but its effect on the development of ILD in RA is not clear, with some studies concluding no association [18, 32] and others finding associations with decline in lung function [33], the presence of honeycombing [41], or the development of ILD over time [19]. There is no current consensus on the association between biologic agents and ILD. There have been case reports of exacerbations of existing ILD or new interstitial pneumonitis in patients with RA taking infliximab [49,50,51]. Etanercept has been linked to granulomatous lung disease and exacerbation of preexisting lung disease in patients with RA [52, 53]. In spite of these reports, a recent review of 367 patients with RA-ILD treated with either anti-TNF agents or traditional RA treatments found no difference in mortality [54], and a report using a databank to evaluate the associations between therapies for RA and ILD found no increased risk of hospitalization with the use of TNF inhibitors [55].
Genetics
There is limited data to determine whether genetics play a role in the development of RA-ILD. The presence of the HLA shared epitope (SE) is a known risk factor for RA in general, but older studies did not find associations between presence of SE alleles and ILD [8, 18, 56]. However, a recent study looking at 450 Japanese RA patients found associations between HLA-DR2 and ILD [57].
RA-Related Antibodies
Studies have suggested that RF and anti-cyclic citrullinated peptide (CCP) antibodies may be a risk factor for RA-ILD [43]. One study found that elevated levels of anti-CCP2 were associated with ILD (OR 1.49, 95% CI 1.25–1.78, p < 0.0001). In addition, a retrospective evaluation of 220 Greek patients with ILD found an association between anti-CCP2 levels and the presence of all ExRA manifestations, including pulmonary fibrosis (p = 0.004) [58]. Furthermore, an expanded repertoire of antibodies to citrullinated protein/peptide antigens (ACPA) that was defined as reactivity to multiple citrullinated targets was associated with an increased risk of ILD [59]. However, these associations may be confounded by the presence of smoking that has been linked to both anti-CCP positivity and RA-ILD.
Rheumatoid Arthritis-Associated Airways Disease
Prevalence and Cumulative Incidence of Airways Disease in RA (Table 4.3)
In addition to parenchymal lung disease, the prevalence of airways disease is also elevated in RA patients. While RA-ILD is associated with significant mortality, it is important to consider airways disease in RA as well because it can also lead to increased morbidity and even increased mortality [60, 61].
Airways disease in RA can involve the large airways (e.g., bronchiectasis and arthritis of the cricoarytenoid joint) or the small airways (e.g., asthma, chronic obstructive pulmonary disease (COPD), bronchiolitis). Airways abnormalities can be identified using PFTs and/or HRCT, and, as in RA-ILD, many RA patients display airways abnormalities in the absence of respiratory symptoms. Similar to RA-ILD, the prevalence rates of airways disease in RA can vary widely depending on the screening criteria used (e.g., all patients with RA, those with early RA, those with respiratory symptoms), the definition of airways disease (e.g., decreases in FEV1/FVC, decreases in forced expiratory flow25–75), and the level of dysfunction on PFTs that is considered to be abnormal. Another complicating factor in establishing the prevalence of airways disease in RA is the differences in patient populations studied, particularly related to smoking histories, as smoking is a known risk factor for RA, RA-ILD, and airways disease.
The prevalence of obstructive airways abnormalities in RA is reported to be in the range of 15–44% [10, 62,63,64]. In a study of 100 consecutive RA patients who had normal CXRs, screening PFTs found 32% of patients had airflow obstruction as measured by decreased FEV1/FVC and/or forced expiratory flow at 25%-75% of the FVC [62]. The prevalence of these abnormalities is higher in RA patients than in matched controls. A population-based cohort that included 603 RA patients from the Rochester Epidemiology Project and based on chart review found COPD to be more common in RA patients, even after adjusting for smoking [61]—suggesting that RA patients may be more susceptible to the effects from smoking.
Multiple studies demonstrate an association between smoking and the presence of airways disease in RA patients [61, 62, 65]. One study found that the hazard ratio for symptomatic obstructive lung disease in RA ever smokers was 4.38 (95% CI 2.14–8.99). However, it is important to note that even RA patients who are never smokers are also reported to have higher rates of airways disease, seen in 14–30% [62, 63]. In addition to the strong association of airways disease and smoking, airways disease has also been associated with longer RA disease duration [65]. One study found that the 10-, 20-, and 30-year cumulative incidence of symptomatic obstructive airways disease was 4, 7, and 10%, respectively. This incidence was significantly higher than in matched controls whose 10-, 20-, and 30-year incidence was 3, 5, and 6% [61]. Another study found a 13% incidence of airways abnormalities at 5-year follow-up in RA patients [66]. However, it is important to note that not all studies have found an association between RA disease duration and increased prevalence or incidence of airways disease [67] . In addition, airways abnormalities are reported at high rates in early RA as well based on HRCT imaging. Specifically, 66–92% in RA subjects with <1 year disease duration demonstrated airways abnormalities on HRCT [68, 69], although many of these patients were asymptomatic. Airways disease does have an impact on mortality in RA. In a study looking at women followed prospectively over 36 years in the Nurses’ Health Study, there was an increased risk of death in those with RA and respiratory disease with the majority of those deaths being attributable to COPD [60].
Bronchiectasis or bronchial dilation is also prevalent in RA, although studies dedicated to determining the epidemiology of bronchiectasis in RA are limited. Clinically significant bronchiectasis is estimated to have a prevalence of approximately 3% of RA patients [70]; however the overall prevalence of bronchiectasis in RA is likely much higher. For example, a study of 105 consecutive early RA patients found that 17% demonstrated evidence of bronchiectasis on HRCT imaging [69]. Importantly, bronchiectasis in RA patients has been linked with an increased risk of mortality [71, 72] supporting the need for additional research in this area. One study of 32 patients with RA and bronchiectasis found a 7.3-fold higher mortality compared to the general population and a fivefold higher mortality compared to an age- and sex-matched control group with RA alone [71].
While the prevalence of airways disease is consistently higher in RA patients, the pathogenesis of this link is not entirely clear. Inflammatory airways abnormalities have been suggested to play a role in the generation of RA-related autoantibodies [68]. In addition, while airways abnormalities are seen with higher frequency in RA non-smokers [67], studies have suggested that perhaps smoking can potentiate or exacerbate RA-associated airways disease [65]. Another factor at play may be infections; RA patients often get respiratory infections related to immunosuppressing medications, and airways disease including bronchiectasis can result from recurrent infections.
Rheumatoid Arthritis-Associated Pleural Disease
In the 1940s and 1950s, it was recognized that patients with RA had a higher incidence of pleural adhesions at biopsy, though a direct relationship between RA and pleural disease was not established until the 1960s [73]. Since then, pleural disease has been recognized as one of the most common intrathoracic manifestations of RA. In postmortem studies, pleural involvement is described in up to 73% of patients with RA [16, 74, 75]. In an earlier study utilizing chest radiographs, pleural thickening and/or effusion was reported in 24% of men and 16% of women [37]. Pleural effusions are more common in men over the age of 35 with rheumatoid nodules, have been associated with HLA-B8 [76, 77], and have an annual incidence of 1.54% in males and 0.34% in females [78]. Though the prevalence of pleural disease is high, symptomatic disease is less common. Only 20% of patients with RA have pleurisy [73], and clinical pleural disease, including symptomatic effusions, is seen in less than 5% [36, 76, 79].
Summary
In summary, the epidemiology of RA-associated ILD, airways disease, and pleural disease is complex. The prevalence and cumulative incidence depend on the mode of detection, the definition used to define disease, the duration of disease, and exposure to cigarette smoke. Clinically significant ILD appears to occur in approximately 10% of patients and is associated with a worse outcome. RA-ILD has been associated with a number of risk factors; further studies are warranted to determine more precisely how these risk factors confer disease. Clinically significant airways disease appears to occur in approximately 10% of patients. And while symptomatic pleural disease is uncommon, RA commonly affects the pleura. The role of not only smoking but also autoantibodies in the initiation and progression of RA lung disease needs further investigation. With a better understanding of the underlying mechanisms—for RA-ILD, RA-associated airways disease, and RA-associated pleural disease—ultimately, we may be able to reduce the incidence and impact of the pulmonary complications of this disease.
References
Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum. 1999;42(3):415–20.
Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22.
Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90.
Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722–7.
Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29(1):62–7.
Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford). 1999;38(7):668–74.
Cortet B, Perez T, Roux N, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56(10):596–600.
Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int. 2005;25(6):429–35.
Demir R, Bodur H, Tokoglu F, Olcay I, Ucan H, Borman P. High resolution computed tomography of the lungs in patients with rheumatoid arthritis. Rheumatol Int. 1999;19(1–2):19–22.
Kanat F, Levendoglu F, Teke T. Radiological and functional assessment of pulmonary involvement in the rheumatoid arthritis patients. Rheumatol Int. 2007;27(5):459–66.
Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443–8.
Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin N Am. 2015;41(2):225–36.
Ellman P, Ball RE. Rheumatoid disease with joint and pulmonary manifestations. Br Med J. 1948;2(4583):816–20.
Cudkowicz L, Madoff IM, Abelmann WH. Rheumatoid lung disease. A case report which includes respiratory function studies and a lung biopsy. Br J Dis Chest. 1961;55:35–40.
Aronoff A, Bywaters EG, Fearnley GR. Lung lesions in rheumatoid arthritis. Br Med J. 1955;2(4933):228–32.
Talbott JA, Calkins E. Pulmonary involvement in rheumatoid arthritis. JAMA. 1964;189:911–3.
Myasoedova E, Crowson CS, Turesson C, Gabriel SE, Matteson EL. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995-2007 versus 1985-1994: a population-based study. J Rheumatol. 2011;38(6):983–9.
Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford). 2010;49(8):1483–9.
Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91.
Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–8.
Bartels CM, Bell CL, Shinki K, Rosenthal A, Bridges AJ. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United State veterans over 20 years. Rheumatology (Oxford). 2010;49(9):1670–5.
Richman NC, Yazdany J, Graf J, Chernitskiy V, Imboden JB. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore). 2013;92(2):92–7.
Kim SK, Park SH, Shin IH, Choe JY. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J Rheumatol. 2008;35(6):995–1001.
Cimmino MA, Salvarani C, Macchioni P, et al. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol Int. 2000;19(6):213–7.
Carmona L, Gonzalez-Alvaro I, Balsa A, Angel Belmonte M, Tena X, Sanmarti R. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis. 2003;62(9):897–900.
Calguneri M, Ureten K, Akif Ozturk M, et al. Extra-articular manifestations of rheumatoid arthritis: results of a university hospital of 526 patients in Turkey. Clin Exp Rheumatol. 2006;24(3):305–8.
Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168(2):159–66.
Biederer J, Schnabel A, Muhle C, Gross WL, Heller M, Reuter M. Correlation between HRCT findings, pulmonary function tests and bronchoalveolar lavage cytology in interstitial lung disease associated with rheumatoid arthritis. Eur Radiol. 2004;14(2):272–80.
Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):528–35.
Habib HM, Eisa AA, Arafat WR, Marie MA. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol. 2011;30(2):217–21.
Aubart F, Crestani B, Nicaise-Roland P, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol. 2011;38(6):979–82.
Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56(8):622–7.
Saag KG, Kolluri S, Koehnke RK, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum. 1996;39(10):1711–9.
Zrour SH, Touzi M, Bejia I, et al. Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis. Prospective study in 75 patients. Joint Bone Spine. 2005;72(1):41–7.
Hassan WU, Keaney NP, Holland CD, Kelly CA. High resolution computed tomography of the lung in lifelong non-smoking patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54(4):308–10.
Hyland RH, Gordon DA, Broder I, et al. A systematic controlled study of pulmonary abnormalities in rheumatoid arthritis. J Rheumatol. 1983;10(3):395–405.
Jurik AG, Davidsen D, Graudal H. Prevalence of pulmonary involvement in rheumatoid arthritis and its relationship to some characteristics of the patients. A radiological and clinical study. Scand J Rheumatol. 1982;11(4):217–24.
Cervantes-Perez P, Toro-Perez AH, Rodriguez-Jurado P. Pulmonary involvement in rheumatoid arthritis. JAMA. 1980;243(17):1715–9.
Hoovestol RA, Mikuls TR. Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep. 2011;13(5):431–9.
Bergstrom U, Jacobsson LT, Nilsson JA, Berglund G, Turesson C. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50(11):2005–13.
Zou YQ, Li YS, Ding XN, Ying ZH. The clinical significance of HRCT in evaluation of patients with rheumatoid arthritis-associated interstitial lung disease: a report from China. Rheumatol Int. 2012;32(3):669–73.
McDonagh J, Greaves M, Wright AR, Heycock C, Owen JP, Kelly C. High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. Br J Rheumatol. 1994;33(2):118–22.
Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106(11):1591–9.
Symmons D, Turner G, Webb R, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford). 2002;41(7):793–800.
Rajasekaran A, Shovlin D, Saravanan V, Lord P, Kelly C. Interstitial lung disease in patients with rheumatoid arthritis: comparison with cryptogenic fibrosing alveolitis over 5 years. J Rheumatol. 2006;33(7):1250–3.
Remy-Jardin M, Remy J, Cortet B, Mauri F, Delcambre B. Lung changes in rheumatoid arthritis: CT findings. Radiology. 1994;193(2):375–82.
Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. 2008;35(8):1513–21.
Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf. 2005;4(4):723–30.
Ostor AJ, Chilvers ER, Somerville MF, et al. Pulmonary complications of infliximab therapy in patients with rheumatoid arthritis. J Rheumatol. 2006;33(3):622–8.
Ostor AJ, Crisp AJ, Somerville MF, Scott DG. Fatal exacerbation of rheumatoid arthritis associated fibrosing alveolitis in patients given infliximab. BMJ. 2004;329(7477):1266.
Villeneuve E, St-Pierre A, Haraoui B. Interstitial pneumonitis associated with infliximab therapy. J Rheumatol. 2006;33(6):1189–93.
Peno-Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest. 2002;122(5):1858–60.
Hagiwara K, Sato T, Takagi-Kobayashi S, Hasegawa S, Shigihara N, Akiyama O. Acute exacerbation of preexisting interstitial lung disease after administration of etanercept for rheumatoid arthritis. J Rheumatol. 2007;34(5):1151–4.
Dixon WG, Hyrich KL, Watson KD, Lunt M, Symmons DP. Influence of anti-TNF therapy on mortality in patients with rheumatoid arthritis-associated interstitial lung disease: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2010;69(6):1086–91.
Wolfe F, Caplan L, Michaud K. Rheumatoid arthritis treatment and the risk of severe interstitial lung disease. Scand J Rheumatol. 2007;36(3):172–8.
Hillarby MC, McMahon MJ, Grennan DM, et al. HLA associations in subjects with rheumatoid arthritis and bronchiectasis but not with other pulmonary complications of rheumatoid disease. Br J Rheumatol. 1993;32(9):794–7.
Furukawa H, Oka S, Shimada K, et al. Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PLoS One. 2012;7(5):e33133.
Alexiou I, Germenis A, Koutroumpas A, Kontogianni A, Theodoridou K, Sakkas LI. Anti-cyclic citrullinated peptide-2 (CCP2) autoantibodies and extra-articular manifestations in Greek patients with rheumatoid arthritis. Clin Rheumatol. 2008;27(4):511–3.
Giles JT, Danoff SK, Sokolove J, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2014;73(8):1487–94.
Sparks JA, Chang SC, Liao KP, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the Nurses’ health study. Arthritis Care Res (Hoboken). 2016;68(6):753–62.
Nannini C, Medina-Velasquez YF, Achenbach SJ, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken). 2013;65(8):1243–50.
Geddes DM, Webley M, Emerson PA. Airways obstruction in rheumatoid arthritis. Ann Rheum Dis. 1979;38(3):222–5.
Vergnenegre A, Pugnere N, Antonini MT, et al. Airway obstruction and rheumatoid arthritis. Eur Respir J. 1997;10(5):1072–8.
Perez T, Remy-Jardin M, Cortet B. Airways involvement in rheumatoid arthritis: clinical, functional, and HRCT findings. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1658–65.
Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with rheumatoid arthritis. Mod Rheumatol. 2011;21(2):164–73.
Avnon LS, Manzur F, Bolotin A, et al. Pulmonary functions testing in patients with rheumatoid arthritis. Isr Med Assoc J. 2009;11(2):83–7.
Fuld JP, Johnson MK, Cotton MM, et al. A longitudinal study of lung function in nonsmoking patients with rheumatoid arthritis. Chest. 2003;124(4):1224–31.
Demoruelle MK, Weisman MH, Simonian PL, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61.
Reynisdottir G, Karimi R, Joshua V, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66(1):31–9.
Allain J, Saraux A, Guedes C, Valls I, Devauchelle V, Le Goff P. Prevalence of symptomatic bronchiectasis in patients with rheumatoid arthritis. Rev Rhum Engl Ed. 1997;64(10):531–7.
Swinson DR, Symmons D, Suresh U, Jones M, Booth J. Decreased survival in patients with co-existent rheumatoid arthritis and bronchiectasis. Br J Rheumatol. 1997;36(6):689–91.
Puechal X, Genin E, Bienvenu T, Le Jeunne C, Dusser DJ. Poor survival in rheumatoid arthritis associated with bronchiectasis: a family-based cohort study. PLoS One. 2014;9(10):e110066.
Walker WC, Wright V. Rheumatoid pleuritis. Ann Rheum Dis. 1967;26(6):467–74.
Fingerman DL, Andrus FC. Visceral Lesions Associated with rheumatoid arthritis. Ann Rheum Dis. 1943;3(3):168–81.
Corcoran JP, Ahmad M, Mukherjee R, Redmond KC. Pleuro-pulmonary complications of rheumatoid arthritis. Respir Care. 2014;59(4):e55–9.
Balbir-Gurman A, Yigla M, Nahir AM, Braun-Moscovici Y. Rheumatoid pleural effusion. Semin Arthritis Rheum. 2006;35(6):368–78.
Hakala M, Tiilikainen A, Hameenkorpi R, et al. Rheumatoid arthritis with pleural effusion includes a subgroup with autoimmune features and HLA-B8, Dw3 association. Scand J Rheumatol. 1986;15(3):290–6.
Jurik AG, Graudal H. Pleurisy in rheumatoid arthritis. Scand J Rheumatol. 1983;12(2):75–80.
Horler AR, Thompson M. The pleural and pulmonary complications of rheumatoid arthritis. Ann Intern Med. 1959;51:1179–203.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Kristen Demoruelle, M., Olson, A.L., Solomon, J.J. (2018). The Epidemiology of Rheumatoid Arthritis-Associated Lung Disease. In: Fischer, A., Lee, J. (eds) Lung Disease in Rheumatoid Arthritis. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-68888-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-68888-6_4
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-68887-9
Online ISBN: 978-3-319-68888-6
eBook Packages: MedicineMedicine (R0)