Abstract
This study aims to explore the clinical characteristics and risk factors of rheumatoid arthritis (RA)-associated interstitial lung disease (ILD). This is a retrospective study of 550 patients with RA. All patients underwent chest high-resolution computed tomography (HRCT) scanning. (1) Two hundred thirty-seven out of five hundred fifty (43.1%) patients with RA were diagnose with ILD. 13.5% ILD occurred before RA onset, 69.6% ILD occurred within 10 years of RA onset, and 16.9% ILD occurred more than 10 years after RA onset. (2) The most common chest CT characteristics of RA-ILD included reticular patterns (57.8%), pleural thickening (57%), ground-glass attenuation (53.2%), followed by interlobular septum thickening, nodules, emphysematous bullae, honeycombing, and bronchiectasis. The proportion of the UIP pattern and NSIP on HRCT was 18.6% and 57.8%. (3) RA-ILD was often associated with other lung lesions, including pleural disease, bronchiectasis, and pulmonary hypertension. (4) the comparisons between RA with ILD and RA without ILD showed that male, smoking, age, disease duration, number of swelling joints, globulin levels, erythrocyte sedimentation rate, C-reactive protein levels, lactate dehydrogenase, the positive rate of rheumatoid factor (RF) and the absolute value of RF, forced vital capacity, forced expiratory volume in 1 s, and carbon monoxide diffusion rate, were statistically different (P < 0.05). Logistic regression analysis showed that age, smoking, elevated lactate dehydrogenase, and RF positive were closely correlated to RA-ILD. RA-ILD occurs more often within 10 years of RA onset and coexists with other lung lesions. The elevated lactate dehydrogenase, RF positive, smoking, and advanced age are closely correlated with RA-ILD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that manifests as symmetric polyarthritis and affects approximately 0.5–1% of the population [1]. In addition to articular manifestations, 50% of RA patients present extra-articular manifestations involving the skin, eyes, heart, lungs, and kidneys. Interstitial lung disease (ILD) is the most common pulmonary manifestation [2–4] and an important early feature of RA. RA-ILD manifestation varied from subclinical inflammation to end-stage pulmonary fibrosis. Approximately 50% of the RA-ILD cases present either at baseline or within 3 years of RA onset [5]. It is difficult to diagnose ILD at an early stage due to the absence of overt symptoms. The incidence of ILD varies from 3.7 to 80%, which can be attributed to study population, diagnostic criteria, and detection techniques [6]. A recent study reported that the median survival following diagnosis of ILD was 2.6 years. Patients with RA-ILD have a 3-fold increased risk of death compared with those without ILD [7]. Thus, ILD does not only seriously impair quality of life but also shortens survival of patients with RA.
Meanwhile, RA causes disability in patients, which does not only produce high economic costs for the patient but also increases the social burden. RA-ILD causes even more significant increases in the burden for the patients and the society. Although ILD is a well-known complication of RA, in-depth understanding of the etiology and risk factors is still limited. Accurate diagnosis of RA-ILD is complicated by the absence of overt symptoms in the early stage of the disease and the lack of specific biomarkers.
In this study, all 550 patients underwent lung high-resolution CT (HRCT) scans. The characteristics of RA-ILD were assessed by clinical symptoms, physical examination, pulmonary imaging features, and a comprehensive analysis of lung function. We further analyzed the risk factors in patients with RA-ILD. These findings will be beneficial in identifying patients at risk and with early stage of ILD, as well as providing the rationale for identification and development of therapeutic strategies.
Materials and methods
Patients
This is a retrospective analysis performed in 550 patients who were diagnosed with RA at the Capital Medical University, Beijing Chaoyang Hospital, between January 2008 and June 2013. All patients underwent lung HRCT scans. Diagnosis criteria for RA were those from the American Rheumatism Association 1987 revised criteria or 2009 ACR, and the European League Against Rheumatism proposed new RA classification criteria [8, 9]. Exclusion criteria were (1) history of tuberculosis or TB infection; (2) history of chronic bronchitis, emphysema, pulmonary heart disease, bronchial asthma, bronchiectasis, pulmonary infarction, and other respiratory diseases; (3) nodular disease and lung tumors; (4) chronic liver and kidney dysfunction and other tumors; and (5) history of rheumatic heart disease, myocardial infarction, and other diseases. This study was approved by the ethics committee.
Data collection
Patient clinical data were carefully collected, including age, gender, race, RA duration, smoking history, RA family history, clinical manifestations (articular manifestations, respiratory manifestations, and other extra-articular manifestations), biochemical and immunological parameters, lung HRCT, lung function, and echocardiography. Patients were divided into two groups: RA-ILD-positive and RA-ILD-negative, according to lung HRCT. The clinical data of these two groups were compared.
Statistical analysis
SPSS17.0 software was used for statistical analysis. Normal or near-normal distribution data were expressed as \( \overline{x} \) ± s. Non-normal distribution data were expressed as median and interquartile range. Quantitative data were analyzed using t test, rank sum test, and one-way ANOVA; Chi-square test was used to compare frequencies. Risk factor analysis was performed using logistic regression. P < 0.05 was considered statistically significant.
Results
Characteristics of patients
This study included 550 patients with RA, 165 males (30%) and 385 females (70%). The male-to-female ratio was 1:2.3. The mean age was 61 ± 13 years, ranging from 17 to 89 years. The mean age of disease onset was 53 ± 14 years, ranging from 11 to 86 years. The mean disease duration was 8 ± 9 years, ranging from 2 weeks to 40 years.
ILD were detected in 237 of 550 (43.1%) patients with RA. The age of the disease onset in 125 of 237 (52.8%) RA-ILD-positive cases were 50 to 69 years. Thirty-two cases (13.5%) had ILD before RA onset, 165 cases (69.6%) had ILD within 10 years of RA onset, and 40 cases (16.9%) had ILD of more than 10 years after RA onset. The RA-ILD-positive group had 86 male patients (36.3%). Compared with the RA-ILD-negative group, the RA-ILD-positive group showed greater mean age (57.6 ± 13.2 vs. 47.7 ± 14.5), shorter mean disease duration (24 months vs. 48 months), higher smoking rate (40.9% vs. 4.8%), and higher male ratio (36.3% vs. 35.2%). The differences were statistically significant (P < 0.001). Family history of RA did not show a significant difference (P = 0.562).
Articular manifestations
The number of swelling joints in the RA-ILD-positive group was significantly higher than the RA-ILD-negative group (P = 0.000). There was no significant difference in joint pain and joint deformity (P > 0.05).
Pulmonary manifestations
Respiratory symptoms and signs
Ninety-seven of two hundred thirty-seven (41%) RA-ILD-positive patients showed respiratory symptoms, including post-activity shortness of breath (76%, 74/97), followed by cough (62%, 60/97) and fever (25% 24/97). Moreover, 15 cases had clubbing, and 97 cases had Velcro tone. Among the 313 RA-ILD-negative cases, 15 cases (4.7%) had respiratory symptoms, 7 cases had fever, 3 cases had cough, 5 cases had shortness of breath, 1 case had clubbing, and 5 cases had Velcro tone. Compared with the RA-ILD-negative group, the RA-ILD-positive group showed a significant higher incidence of upper respiratory tract symptoms and signs (P = 0.000).
Lung HRCT
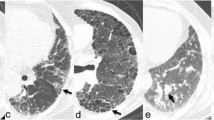
The most common mesenchymal lesions in lung HRCT in the 237 RA-ILD-positive patients were reticular patterns (Table 1). Compared with the RA-ILD-negative group, the RA-ILD-positive group showed significantly higher incidence of pleural thickening (57.0% vs. 28.1%), bronchiectasis (18.1% vs. 10.5%), bronchovascular bundle thickening (35.4% vs. 9.6%), and pulmonary hypertension (9.4% vs. 3.6%) (P = 0.000, P = 0.011, P = 0.000, and P = 0.009, respectively). There was no significant difference in emphysematous bullae, lung nodules, and subpleural nodules (P > 0.05). The proportion of the usual interstitial pneumonia (UIP) pattern and non-specific interstitial pneumonia (NSIP) pattern on HRCT was 18.6% and 57.8%.
Lung function
One hundred sixty-one patients underwent pulmonary function tests. Ninety-seven of ninety-nine (98%) RA-ILD-positive patients showed pulmonary function abnormalities: restrictive ventilatory dysfunction in 28 cases (28.3%), obstructive ventilatory dysfunction in 26 cases (26.3%), and diffuse dysfunction in 91 cases (91.9%). Fifty-five of sixty-two (88.7%) RA-ILD-negative patients showed pulmonary function abnormalities: restrictive ventilatory dysfunction in 3 cases (4.8%) and diffuse dysfunction in 47 cases (75.8%). Compared with the RA-ILD-negative group, the RA-ILD-positive group showed a significantly higher incidence of pulmonary function abnormalities, restrictive ventilatory dysfunction, and diffuse dysfunction, accompanied with a significantly lower forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and rate of diffusion of carbon monoxide (DLCO %) (P = 0.013, P = 0.003, P = 0.004, P = 0.000, P = 0.008, and P = 0.000, respectively). There was no significant difference in obstructive ventilatory dysfunction and small airway dysfunction (P > 0.05).
Other extra-articular manifestations
There was no significant difference in the incidence of rheumatoid nodules (5.9% vs. 5.1%), pericardial effusion (0.8% vs. 0.3%), and secondary Sjogren’s syndrome (28.7% vs. 32.3%) between the RA-ILD-positive and RA-ILD-negative groups (P > 0.05).
Laboratory tests
The biochemical index (GLB, LDH, and GGT), ESR, C-reactive protein, RF-positive rate, and absolute value were significantly higher in the RA-ILD-positive group than in the RA-ILD-negative group (P < 0.05). There was no significant difference in the total protein, immunoglobulins, ANA, anti-SSA antibodies, complement, AKA, APF-positive rate, anti-CCP antibody-positive rate, and absolute value (P > 0.05) (Table 2).
Logistic regression analysis
To perform a logistic regression analysis, an RA-ILD positive was used as the dependent variable. Variables that were significantly associated with ILD in univariate analysis (P < 0.2) were chosen as independent variables. The final regression model included five factors: age (OR value of 1.599), RA disease duration (OR value of 0.368), smoking (OR value of 2.116), LDH (OR value of 7.369), and RF (OR value of 1.693). The risk of ILD increased to 59.9% in the RA patients with each additional 10 years of age. There was no significant difference in the risk of ILD in the RA patients with a disease duration of 1–10 years or less than 1 year; whereas, the risk of ILD increased to 63% in the RA patients with a disease duration of 1–10 years, compared with the RA patients with more than 10 years of disease duration (see Table 3).
Discussion
RA is a common systematic autoimmune disease, and ILD is the common pulmonary complications in RA. To date, the etiology of RA-ILD is still unclear. Most patients carry fibrosis susceptibility gene, which can be triggered by certain injuries. For example, certain polymorphisms of the human leukocyte antigen (HLA)-DRB-shared epitope have been associated with an increased risk of ILD [10]. One study has investigated the association between the MUC5B polymorphism, a polymorphism associated with idiopathic pulmonary fibrosis (IPF), and the presence of RA-ILD but did not find any significant association [11].
In this study, we demonstrate that RA-ILD often occurs within 10 years of RA onset and the incidence of ILD is associated with RA disease duration. We found that 69.6% ILD occurred within 10 years of RA onset, and most of them occurred within 2 years of RA onset, while 16.9% ILD occurred more than 10 years after RA onset. Logistic regression analysis indicates that the incidence of ILD is 63% higher in the RA patients with less than 10 years of disease duration than the RA patients with over 10 years of disease duration. RA-ILD-positive group had a higher number of swelling joints than the RA-ILD-negative group, suggesting ILD occurrence is related to RA disease activity. The majority of the patients had higher disease activity within 10 years of RA onset; therefore, ILD is often seen in the early and active stages of RA. Evidence shows that lung lesions observed using HRCT are associated with RA disease activity [12]. A variety of activated immune cells, antibodies, and inflammatory cytokines are involved in the early and active stages of RA pathogenesis and associated with the incidence of ILD. Thus, at the early stage of RA, the higher disease activity is related to the higher ILD incidence, the faster disease progression, and the worse prognosis.
Meanwhile, in this study, elevated lactate dehydrogenase, RF positive, smoking, and advanced age are closely related to RA-ILD. Several studies have shown that high titer RF, smoking, male, advanced age, and HLA-DR4 positive are risk factors of RA-ILD [13–15]. Moreover, anti-CCP antibody titer and RF titer are closely related to the joint damage and RA airway lesions [16–18]. This study shows that RA-ILD-positive group had more elderly, male, and smokers than RA-ILD-negative group, which is consistent with the previous studies. Logistic regression analysis indicates that RF-positive, smoking and advanced age are risk factors for ILD. Smoking can cause lung abnormal secretion of cytokines and enzymes, damage airway epithelial cells and endothelial cells, promote fibroblast proliferation and reduce respiratory reserve capacity. Saag et al. have reported that the progression of RA-ILD is correlated with smoking [19]. Hoovestol et al. found that the incidence of ILD is increased with the increased quantity of smoking [20]. In this study, the risk of ILD doubled in smoking patients compared with non-smoking patients; however, we did not detect the correlation of the quantity of smoking and the incidence of ILD. Advanced age is a risk factor of ILD. In this study, we found that the age of ILD onset is more common between 50 and 69 years, and with each additional 10 years of age, the risk of ILD increased by 59.9%. These results are similar to Koduri and other studies [5]. Another study demonstrates that the incidence of ILD increased more than 4-fold in patients older than 65 years. Hence, it is important to perform ILD screening in elderly RA patients [21]. Other factors, such as male, erythrocyte sedimentation rate, and C-reactive protein, were not used in the regression model. This is because most of the smoking patients were male, while erythrocyte sedimentation rate and C-reactive protein are non-specific inflammatory markers that can be affected by many other factors.
LDH catalyzes the conversion of lactate to pyruvate. In this study, we found that elevated LDH is correlated with six times higher risk of ILD. LDH is widely present in human cells and body fluids. There are five isoenzymes of LDH, namely LDH1, LDH2, LDH3, LDH4, and LDH5. LDH3 is mainly expressed in the lungs. The synthesis of LDH increases in alveolar type II epithelial cells, endothelial cells and neutrophils during lung injury. The release of LDH into the bloodstream causes increased serum LDH levels. However, other studies suggest that LDH can be used as a diagnostic serum marker for interstitial pneumonia, but LDH lacks specificity in the diagnosis of lung injury [22–24].
The prevalence of ILD in RA patients varies greatly due to the inconsistency in the diagnostic criteria, diagnostic techniques, and study population. Clinical features, HRCT imaging scan, and lung physiology (especially pulmonary function test parameter DLCO) are often used to assess whether a RA patient has or does not have ILD. An early study using the diffusion capacity of the lung for DLCO estimated that the incidence of ILD in RA was 41.4% [25]. Recently, more population-based studies have attempted to clarify the definition and incidence of RA-ILD. The cumulative incidence of ILD among RA patients reported by Bongartz was 7.7% [6]. However, the incidence of ILD among RA patients reported by Olson was near 10% [26]. Advances in biomarker technologies and diagnostic techniques are required to improve the diagnosis and management of RA-ILD. In this study, the incidence of RA–ILD was 43.1% [27, 28].
Several histopathologic subtypes of ILD can be observed in patients with RA. The most common subtypes are the UIP and the NSIP. The UIP pattern was more predominant and a history of smoking was significantly associated with UIP [29–31]. In this study, the prevalence on NSIP-like pattern is higher to what has been reported in other cohorts. McDonagh et al. reported that the ground-glass-like attenuation is the most common feature in RA-ILD patients [32]. In our RA-ILD cohorts, the proportion of females is higher than males and most of them were non-smoking. Meanwhile, in our RA-ILD subjects, the time of onset with 50% RA-ILD patients was less than 1 year. The late-stage manifestation is honeycombing. Therefore, RA patients should have a lung HRCT at the time of diagnosis, especially the males.
The present study aimed to investigate the clinical characteristics of RA-ILD in a population of 550 Chinese patients with RA. All of the patients underwent HRCT. We analyzed the associations between patients’ clinical and laboratory parameters as well as patients’ demographics to evaluate the factors associated with ILD in this population of RA patients. In the present study, pulmonary abnormalities were detected by HRCT in 43.1% of RA patients. Another study of Chinese patients with RA reported 15% of pulmonary abnormalities [16]. Our result was similar to those reported by Dawson et al. (47%) [33], Youssef et al. (48%) [34], and Yilmazer et al. (46%) [35] but lower than those reported by Karazincir et al. (63%) [36] and Bilgici et al. (67%) [37]. HRCT is a valuable non-invasive method for the detection of the presence, type, and extent of pulmonary abnormalities. HRCT can also be used to determine prognosis and evaluate the response to treatment [38]. Moreover, a significant association was shown between HRCT findings and histological abnormalities [39]. The majority of RA patients have no evident respiratory symptoms at early stage of RA-ILD. In our study, 59% of patients with RA-ILD were asymptomatic. Therefore, HRCT provides a sensitive approach for identification of pulmonary abnormalities during the early stages, such as reticular patterns, which was the most common abnormality detected in our study. Patients with RA-ILD have a poor prognosis; therefore, HRCT scans should be obtained in all patients with suspected ILD. Lung biopsy remains the criterion standard for the diagnosis of ILD. However, it is an invasive method and should not be used in asymptomatic patients.
Diffusing capacity impairment is the most common pathophysiological change in ILD, which might provide an alternative method for detecting ILD in RA patients. In our study, diffusing capacity abnormalities were observed in 92% RA-ILD-positive patients and in 76% RA-ILD-negative patients. Similarly, Dawson et al. found that 82% of RA patients had a reduced DLCO2 [33]. Pulmonary function test, especially the DLCO, appears to be more sensitive in screening RA-ILD because it could detect the pulmonary diffusing function change at the early stage of the disease when pulmonary HRCT does show any abnormity. Impaired gas exchange is shown as lower DLCO percentage of predicted values in subclinical RA-ILD. Therefore, RA patients without respiratory symptoms and with normal lung HRCT should also complete the lung function tests to detect early lung damage.
In conclusion, we have demonstrated that the incidence of ILD is 43% in RA patients. We also identified several factors that are associated with ILD in RA patients, such as age of RA onset, smoking, and positive RF. However, the pathophysiological links between these factors and pulmonary parenchymal changes remain to be elucidated. We suggest that HRCT and pulmonary function tests should be carried out in RA patients to detect the abnormalities at early disease stage in order to make proper treatment.
The sample size in this study is large, and all patients underwent HRCT. However, this study has some limitations. Firstly, it is a single-center study; therefore, a multicenter study should be planned. Secondly, we did not screen biomarkers of RA; therefore, we might have missed peripheral blood biomarkers that could be stronger predictors of ILD presence in RA patients. Exploratory studies should be performed to discover new potential biomarkers of the presence of ILD in RA patients. In addition, the conclusions of the present study are limited by its retrospective design.
References
Gabriel SE (2001) The epidemiology of rheumatoid arthritis. Rheum Dis Clin N Am 27(2):269–281
Kapetanovic MC, Lindqvist E, Geborek P, Saxne T, Eberhard K (2011) Long-term mortality rate in rheumatoid arthritis patients with disease onset in the 1980s. Scand J Rheumatol 40(6):433–438
Kuo CF, Luo SF, See LC, Chou IJ, Chang HC, Yu KH (2013) Rheumatoid arthritis prevalence, incidence, and mortality rates: a nationwide population study in Taiwan. Rheumatol Int 33(2):355–360
Zayeni H, Haji-Abbasi A, Foumani SA et al (2016) Pulmonary involvement in rheumatoid arthritis: a cross-sectional study in Iran. Lung India 33(1):49–52
Koduri G, Norton S, Young A et al (2010) Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 49(8):1483–1489
Bongartz T, Nannini C, Medina-Velasquez YF et al (2010) Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 62(6):1583–1591
Assayag D, Lee JS, King TE Jr (2014) Rheumatoid arthritis associated interstitial lung disease: a review. Medicina (B Aires) 74(2):158–165
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Aletaha D, Neogi T, Silman AJ et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581
Furukawa H, Oka S, Shimada K et al (2012) Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PLoS One 7(5):e33133
C J (2013) Exploration of the MUC5B polymorphism frequency in rheumatoid arthritis interstitial lung disease. Am J Respir Crit Care Med 187:A5982
Perez-Dorame R, Mejia M, Mateos-Toledo H, Rojas-Serrano J (2015) Rheumatoid arthritis-associated interstitial lung disease: lung inflammation evaluated with high resolution computed tomography scan is correlated to rheumatoid arthritis disease activity. Reumatol Clin 11(1):12–16
Gabbay E, Tarala R, Will R et al (1997) Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 156(2 Pt 1):528–535
Ayhan-Ardie FFOO, Yorgancioglu ZR, Ustun N, Gokharman FD (2006) Pulmonary involvement in life long non-smoking patients with rheumatoid arthritis and ankylosing spondylitis without respiratory symptoms. Clin Rheumatol 25(2):213–218
Sebastiani M, Manfredi A, Cerri S, Della Casa G, Luppi F, Ferri C (2016) Radiologic classification of usual interstitial pneumonia in rheumatoid arthritis-related interstitial lung disease: correlations with clinical, serological and demographic features of disease. Clin Exp Rheumatol 34(3):564–565
Wang JX, Du CG (2015) A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in Chinese patients. Med Sci Monit 21:708–715
Turesson C, Jacobsson LT, Sturfelt G, Matteson EL, Mathsson L, Ronnelid J (2007) Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann Rheum Dis 66(1):59–64
Zrour SH, Touzi M, Bejia I et al (2005) Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis. Prospective study in 75 patients. Joint Bone Spine 72(1):41–47
Saag KG, Kolluri S, Koehnke RK et al (1996) Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum 39(10):1711–1719
Hoovestol RA, Mikuls TR (2011) Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep 13(5):431–439
Mori S, Koga Y, Sugimoto M (2012) Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med 106(11):1591–1599
Zou J, Guo Q, Chi J, Wu H, Bao C (2015) HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin Rheumatol 34(4):707–714
Kobayashi N, Takezaki S, Kobayashi I et al (2015) Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology (Oxford) 54(5):784–791
Aggarwal BD (2014) Lactate dehydrogenase as a biomarker for silica exposure-induced toxicity in agate workers. Occup Environ Med 71(8):578–582
Frank ST, Weg JG, Harkleroad LE, Fitch RF (1973) Pulmonary dysfunction in rheumatoid disease. Chest 63(1):27–34
Olson AL, Swigris JJ, Sprunger DB et al (2011) Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 183(3):372–378
Norton S, Koduri G, Nikiphorou E, Dixey J, Williams P, Young A (2013) A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford) 52(1):99–110
Richman NC, Yazdany J, Graf J, Chernitskiy V, Imboden JB (2013) Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore) 92(2):92–97
Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, Young A (2014) Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiologicaland radiological characteristics—a large multicentre UK study. Rheumatology (Oxford) 53(9):1676–1682
Solomon JJ, Ryu JH, Tazelaar HD, Myers JL, Tuder R, Cool CD, Curran-Everett D, Fischer A, Swigris JJ, Brown KK. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med 107(8):1247–1252.
Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, Lee JS, King TE Jr, Collard HR (2010) Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 35(6):1322–1328
McDonagh J, Greaves M, Wright AR, Heycock C, Owen JP, Kelly C (1994) High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. Br J Rheumatol 33(2):118–122
Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR (2001) Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 56(8):622–627
Youssef AA, Machaly SA, El-Dosoky ME, El-Maghraby NM (2012) Respiratory symptoms in rheumatoid arthritis: relation to pulmonary abnormalities detected by high-resolution CT and pulmonary functional testing. Rheumatol Int 32(7):1985–1995
Yilmazer B, Gumustas S, Cosan F et al (2016) High-resolution computed tomography and rheumatoid arthritis: semi-quantitative evaluation of lung damage and its correlation with clinical and functional abnormalities. Radiol Med 121(3):181–189
Karazincir S, Akoglu S, Guler H, Balci A, Babayigit C, Egilmez E (2009) The evaluation of early pulmonary involvement with high resolution computerized tomography in asymptomatic and non-smoker patients with rheumatoid arthritis. Tuberk Toraks 57(1):14–21
Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M (2005) Pulmonary involvement in rheumatoid arthritis. Rheumatol Int 25(6):429–435
Akira MSM, Hara H (1999) Thin-section CT findings in rheumatoid arthritis-associated lung disease: CT patterns and their courses. J Comput Assist Tomogr 23(6):941–948
Lee JS, Im JG, Ahn JM, Kim YM, Han MC (1992) Fibrosing alveolitis: prognostic implication of ground-glass attenuation at high-resolution CT. Radiology 184(2):451–454
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Yongfeng Zhang and Hongbin Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, H., Wu, N. et al. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 36, 817–823 (2017). https://doi.org/10.1007/s10067-017-3561-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3561-5