Summary
Carbon isotopes have long been used to dissect metabolic pathways. More recently, stable isotopes have become an important tool in modeling global fluxes in the biosphere, and notably CO2 isofluxes. The accuracy of these models relies partly on the knowledge of fractionations associated with each individual flux component. This has led to the observation that carbon isotope fractionation occurs during respiration in plants, and exhibits large temporal and spatial variations. Despite important advances in the area, metabolic features underlying such variability remain to be fully elucidated. The present chapter summarizes available data on plant respiratory fractionation, and presents a critical discussion about the metabolic origin of its variation, in the light of recent developments in understanding the compartmentation and plasticity of plant respiration. It emphasizes the need for refining existing frameworks, and points out knowledge gaps that need to be filled so as to achieve a more quantitative modeling of respiratory fractionation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory Fractionation
- Light-enhanced Dark Respiration (LEDR)
- PEPC Phosphoenolpyruvate Carboxylase
- Photosynthetic Discrimination
- Tcherkez
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Both stable and radioactive isotopes are used as tracers in a wide range of domains, either at natural abundance or using labeling approaches (e.g. hydrology, geophysics, geochemistry, forensics, criminology, medicine, and different fields of biology, ecology and agronomy). In particular, they have a wide range of applications related to respiration. For example, radioactive 14C can be used as a tracer to study carbon residence time in plants before it is released through respiration (see Chap. 12; e.g. Carbone et al. 2007). Also, electron partitioning between cytochrome and alternative pathways of mitochondrial respiration can be measured using the natural 18O abundance in oxygen exchange flux (Guy et al. 1989; Ribas-Carbo et al. 1995; McDonald et al. 2002). Furthermore, the combined effect of respiratory and photorespiratory discriminations against 18O is one of the major causes of the Dole effect (natural 18O-enrichment of atmospheric O2 relative to oxygen in seawater) and thus needs to be understood in order to explain isotopic fluxes (isofluxes) of oxygen at the global scale and use this knowledge for the reconstruction of past environments (Bender et al. 1994; Angert and Luz 2001).
In this chapter, we will focus on the use of stable carbon isotopes, 12C and 13C. Carbon forms the backbone of biomolecules and represents the major fraction of plant dry mass (generally around 40%). The natural 13C abundance in metabolites and CO2 exchange fluxes can thus be used to trace the fate of carbon within plants and ecosystems (Buchmann et al. 1998; Schnyder et al. 2003; Bowling et al. 2008), and to help disentangling the contribution of the different components of the carbon cycle in the biosphere (Ciais et al. 1995; Yakir and Wang 1996; Fung et al. 1997).
However, this requires a sufficient knowledge of isotopic fractionations associated with the different components of the CO2 exchange flux, and how they vary. Photosynthesis has been known for now more than 50 years to discriminate against 13C so that the organic matter is naturally 13C-depleted compared to atmospheric CO2 . Photosynthetic discrimination has been satisfactorily modeled and explains most of the isotopic signal of net fixed CO2 (as measured in organic matter). However, it is only relatively recently that it has been recognized that further fractionations do occur in the metabolism downstream carbon assimilation, especially during respiration. Ghashghaie and coworkers have thus shown that the CO2 evolved by leaves in the dark exhibited an isotopic composition systematically different from that in organic matter (Duranceau et al. 1999), providing evidence for the so called ‘apparent respiratory fractionation’. Since this pioneering work, respiratory fractionation has been shown to be widespread amongst plants, although being highly variable between taxa, organs and environmental conditions. This has facilitated the understanding of metabolic fluxes associated with respiration in different plant tissues, but to date, a complete understanding of the mechanism underlying these variations is still lacking.
In most cases, more than 25% of the carbon fixed by photosynthesis is subsequently released by respiration at the whole plant level (although it can vary widely throughout plant development; Thornley 2011). The annual ratio of carbon released through plant respiration relative to photosynthesis determined experimentally was 0.53 to 0.69 during boreal forest post-fire succession (Goulden et al. 2011), and varied between 0.17 and 0.78 as reviewed by DeLucia et al. (2007) for evergreen and deciduous forest stands. In the latter study, it was shown that the respiration/photosynthesis ratio varies with biome , stand age and leaf mass ratio. For sunflower, the ratio was 0.42 throughout the crop growth cycle (Cheng et al. 2000) and it varied between 0.23 and 0.38 in two Plantago species at a daily time scale depending on species and growth temperature (Atkin et al. 2007). When a respiratory fractionation exists, it must then influence the isotopic composition of plant organic matter , impairing its accuracy as a proxy for photosynthetic discrimination.
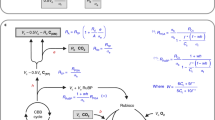
Part of the problem lies in the somewhat ambiguous nature of respiration, even at a cellular level. In the simplest case respiration consists in the stoichiometric oxidation of glucose in the presence of oxygen to yield CO2 and water through the action of glycolysis and the tricarboxylic acid (TCA) pathway (i.e. Krebs cycle when the pathway is cyclic). This definition is not easily applicable to the carbon isotope fractionation associated with respiration. In fact, these two central pathways are intimately interconnected to the other branches of metabolism (see also Chaps. 1 and 14), through intermediates that can engage in biosynthesis (e.g. amino acids, fatty acids, nucleotides…). Therefore, (i) other substrates than glucose can fuel respiration depending on the physiological status of cells, and (ii) additional carboxylating/decarboxylating steps can participate in the net respiratory CO2 flux measured experimentally. In other words, the knowledge of fluxes in anabolic and catabolic processes (within different plant cell types) is required to fully understand the metabolic origin of the observed isotope composition in CO2 evolved by plants (see Fig. 3.1 for metabolic pathways associated with respiration).
Simplified metabolic scheme of main respiratory pathways (pink boxes including glycolysis, translocation and decarboxylation of pyruvate and Krebs cycle ) and associated pathways (grey boxes including pentose phosphate pathway , anaplerotic pathway , biosynthesis of lipids and secondary metabolites, and green box including glyoxylate pathway). Biosynthesis of proteins linked to the tricarboxylic acid (TCA) pathway are shown in light brown. Metabolites involved in processes occurring in the cytosol, mitochondrion, and glyoxysomes are shown in blue, red and green, respectively. Anabolic pathways are shown with solid-line arrows, and catabolic ones with dashed-line arrows, i.e. degradation of lipids, β-oxidation of fatty acids, and gluconeogenesis [from the incorporation of succinate (Succ) coming from the glyoxylic cycle into TCA pathway, and transformation of resulting OAA to PEP and finally to sugars (dashed-line arrows in green)]. The degradation of proteins to amino acids is also indicated by dashed-line arrows. Evolved CO2 molecules are shown in ellipses. Pathways and enzymes are in bold letters: PPP pentose phosphate pathway, PEPC phosphoenolpyruvate carboxylase, PDH pyruvate dehydrogenase, PEPCK phosphoenolpyruvate carboxykinase, MDH malate dehydrogenase, ME malic enzyme , CA carbonic anhydrase
In the present Chapter, the current knowledge about the variability of respiratory apparent fractionation in plants will be reviewed. Besides, we will make an attempt to describe metabolic features (fluxes, isotope effects ) that underpin such variations as far as they are now understood. Finally, a rapid survey of areas where additional knowledge is needed to improve the understanding of respiratory carbon isotope fractionation will be provided.
2 Stable Carbon Isotopes and Photosynthesis
2.1 Carbon Isotopes, Abundance and Fractionation Definitions
The fundamental definitions required for the use of stable isotopes in biological or geochemical studies have already been extensively reviewed in previous contributions (e.g. Kendall and McDonnell 1998), which the unaccustomed reader can refer to for further details. In what follows, the main features are briefly addressed, and points particularly relevant to metabolic studies are underlined.
Chemical elements naturally occur in various forms named isotopes, which differ only in their mass number. Forms that do not decay with time (i.e. non-radioactive forms) are referred to as stable isotopes. The lightest forms are always the most abundant (Meija et al. 2016). The atomic mass difference between the heavy and light isotopes of a given element comes from the number of neutrons in the nucleus. Heavy isotopes have one, two or more additional neutrons compared with the light isotope. There are 15 known carbon isotopes with mass numbers ranging from 8 to 22 (https://en.wikipedia.org/wiki/Isotopes_of_carbon). Among them, only 12C and 13C are stable, with an average natural abundance of 98.9% and 1.1%, respectively. All other carbon isotopes are radioactive, and only 14C (half-life of 5730 years) is found in nature. The most stable artificial carbon isotope is 11C with a half-life of 20.334 min. Besides stable carbon isotopes, radioactive 14C (e.g. Dieuaide-Noubhani et al. 1995) and 11C (e.g. Bloemen et al. 2015) have also been applied as tracers in physiological studies.
The relative abundance of stable isotopes can be expressed as an isotope ratio (R), defined as the molar ratio of the heavy to light isotope, e.g. for carbon, R = 13C/12C. Because these ratios are very small, it is generally more practical to express them as a deviation relative to an international standard, the isotopic composition δ. For carbon:
where, R S and R PDB are the isotope ratios of the sample and the PDB standard, respectively. PDB is a belemnite fossil coming from the geological formation Pee Dee in South Carolina, USA. Since the PDB is only slightly 13C-enriched (R PDB = 0.0112372) compared to almost all organic and inorganic materials, the δ13C of biological samples are generally very small (expressed in per mil, ‰) negative values. Different combinations of light and heavy isotopes within a given molecule are defined as ‘isotopologues ’ (e.g. 12C16O16O, 13C16O16O, 12C18O16O are three isotopologues of CO2).
The atomic mass difference between the heavy and light isotopes of a given element leads to differences in physical (e.g. coefficients for diffusion, dissolution, evaporation, etc.) and chemical properties (e.g. rate constants of reaction) forming the basis for isotopic fractionation processes occurring in the biosphere and during metabolism (e.g. during enzymatic reactions involving bond formation or cleavage). Fractionating processes are at the origin of the observed natural differences in heavy-to-light isotope ratios between different compartments of the biosphere (both organic and inorganic compartments), between metabolites, but also between the atoms within molecules (see Fig. 3.2).
Fractionation processes that affect the carbon isotope composition of compounds within or between compartments of a biological system. Pink rectangles represent compartments that can range from organelles, through tissues, organs up to the whole organism. S1, S2, S3 denote compounds (metabolites or components of structural mass) that are involved in reactions or transport associated with fractionation processes and/or exchange with other compartments. Metabolic or transport fluxes denoted by blue arrows can proceed without fractionation or be associated with thermodynamic (αe), kinetic (αk) or fragmentation fractionation . The two columns on the right hand side indicate isotope effects on individual compounds and/or on bulk organic matter (OM) and thus leading to a difference in isotope composition between the compartments and the external environment. Note that only processes involving trans-boundary transport can have an effect on the bulk isotope composition of the compartment considered. Reactions limited to within compartments will increase or decrease the heterogeneity of isotopic signatures between compounds within the compartment. Thus, they may cause a change in the bulk isotope composition only if reactants are exchanged across the compartment boundaries
For a (bio)chemical reaction converting a substrate S into a product P, the isotope effect can be expressed as:
In the case of kinetic reactions (i.e. irreversible reactions), the isotope effect (αk) corresponds to the ratio of kinetic rate constants of the light to the heavy isotope (e.g. for carbon, αk = 12 k/13 k) and R S and R P are measured at the beginning of the reaction. The light isotopologue generally reacts faster than the heavy one, so that αk > 1 and the product is depleted in 13C compared with the substrate. For reversible reactions (i.e. operating close to the thermodynamic equilibrium), the isotope effect (αe) is then defined as a ratio of equilibrium constants (e.g. for carbon, αe = 12 K/ 13 K). Quite often for reactions involving the making of a C-C bond, αe < 1 (i.e. the product is 13C-enriched compared with the substrate).
Biologists use more frequently the isotope fractionation (or discrimination), defined as:
In fact, the isotope discrimination can be conveniently rearranged using δ values:
Because δ values are negligible compared to 1, Δ can be approximated by the difference in the isotopic composition between the source and the product (i.e. Δ = δs – δp). It is thus a convenient parameter to manipulate in biological and ecological systems where fractionating processes can be very diverse (Fig. 3.2).
When dealing with in vivo isotopic discrimination (Δ) in a metabolic context (like respiration), it is important to keep in mind that observed kinetic isotope effects will vary with the fraction of substrate that is consumed by the reaction under consideration. If all of the substrate is converted to the product, then Δ will be equal to zero. It is only when the substrate is partially consumed by the enzymatic reaction under consideration and/or another fraction of the substrate has an alternative fate (i.e. metabolic branching occurs) that the kinetic isotope effect will be expressed. The observed magnitude of the fractionation will depend on the relative fluxes in competing reactions (for a detailed analysis see Hayes 2001). A typical example is the photosynthetic discrimination against 13C.
2.2 Setting the Stage for Respiration: Photosynthetic Carbon Isotope Fractionation
Plants discriminate against 13C during photosynthetic CO2 assimilation. Therefore, plant organic matter (OM) is naturally 13C-depleted compared to atmospheric CO2 by on average around 20‰ in C3 and 4‰ in C4 plants. Net photosynthetic carbon isotope discrimination has been extensively studied and robust models have been developed and validated for many C3 and C4 species (Farquhar et al. 1982, 1989). It results mainly from the interplay between two contrasted fractionating processes: the diffusion of CO2 from the air into the leaves through stomata and its subsequent carboxylation by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco ), which discriminate against 13C by 4.4‰ and 29‰ (Roeske and O’Leary 1984; Guy et al. 1993; McNevin et al. 2006; Tcherkez et al. 2013), respectively. Diffusion occurs across the leaf boundary layer, stomata, intercellular air spaces, and cellular medium to the site of carboxylation. Hydration, facilitated by carbonic anhydrase, supplies HCO3 − for carboxylation by phosphoenolpyruvate carboxylase (PEPC ) and feeds the pool of CO2/HCO3 − present in a given compartment, functioning as a buffer for internal carbon dioxide.
Photosynthetic discrimination can be estimated using either the δ13C of bulk OM (integrated value during plant growth), δ13C of sugars (integrates about 2–3 days of photosynthetic discrimination) or measured on-line during leaf CO2 exchanges (instantaneous net photosynthetic discrimination under fixed conditions). However, the photosynthetic discrimination and thus the 13C content in photosynthetic products vary between plant species, plant developmental stages and environmental conditions. While the instantaneous discrimination value (net photosynthesis) is physiologically the most relevant, values obtained from organic materials should be viewed as average values. Thus, their biological significance is different since they also result from differences in residence times of different metabolites.
In addition, in C3 plants, there is a competition between CO2 and O2 at the active site of Rubisco , which can catalyze both the carboxylation and oxygenation of ribulose-1,5-bisphosphate (RuBP), so that the relative rates of photosynthesis versus photorespiration (carboxylation-to-oxygenation ratio) depend on the relative concentration of CO2/O2 at carboxylation sites. CO2 production by photorespiration has been shown to discriminate by up to 12‰ against 13C (i.e. with an isotopic difference between released CO2 and net fixed C of 12‰) thus blurring the on-line photosynthetic discrimination measurements (Lanigan et al. 2008). It is the combination of all these concomitant processes which determines the overall fractionation between CO2 and primary photosynthetic products. Note that net photosynthetic discrimination against 13C is also influenced by anaplerotic CO2 fixation (by PEPC ) as well as (photo)respiratory release of CO2 and as such can be impacted by high fluxes through PEPC, feeding malate into the TCA pathway that operates in a non-cyclic manner in the light (Tcherkez et al. 2012).
Variations in photosynthetic discrimination values in C3 plants have been shown to be mainly due to changes in stomatal closure caused by changes in environmental factors, thereby limiting the CO2 supply to Rubisco . In C4 plants, the lower discrimination value is due to the CO2 concentrating mechanism involving PEPC. Variations in the discrimination value of C4 plants are mainly driven by the proportion of CO2 leaking from the bundle sheath to mesophyll cells rather than by stomatal closure (for a review, see Brugnoli and Farquhar 2000). CAM plants can exhibit either a C3 or C4 photosynthetic metabolism depending on the environmental conditions. The integrated discrimination, as measured in the δ13C value of total organic matter , may thus vary in between the typical signatures of these two metabolic types, but quite usually, is observed to be intermediate between the characteristic values of C3 and C4. The isotopic composition of plant OM can thus be used to identify the prevalent photosynthetic pathway (C3, C4 or CAM).
3 Respiratory Carbon Isotope Fractionation
As recalled above, the δ13C of leaf bulk OM has been first considered to reflect net photosynthetic discrimination. However, inter-organ isotopic differences have been repeatedly observed in plants that cannot be accounted for by photosynthetic discrimination only (Badeck et al. 2005). In fact, leaves have been shown to be generally 13C-depleted compared to all other organs, suggesting that fractionating mechanisms do occur after CO2 fixation (reviewed by Cernusak et al. 2009). Such differences imply that (i) fractionating processes occur in the metabolism downstream photosynthesis, so that metabolic pools can have contrasted isotopic compositions, but also that (ii) some of these pools are either lost or transported differentially between organs. There are several processes in plants where carbon is lost to the environment such as volatile organic compounds (VOC) emission, ablation of waxes, exudation, etc. Among those, respiration is certainly the most important in terms of flux. In what follows, we will focus on the carbon isotope fractionation during respiratory processes, and how it can be explained by interactions in the metabolic network. Yet, to understand possible origins of the isotope composition in CO2, the end product of respiration, we must first have a deeper look into how carbon isotopes are distributed within the potential substrates, before they are oxidized.
3.1 Origin of Non-statistical Intramolecular Distribution of 13C in Carbohydrates
One of the first post-carboxylation fractionation steps occurs in the Calvin cycle during aldolase reaction (i.e. synthesis of fructose-1,6-bisphosphate from triose phosphates), enriching in 13C the C-3 and C-4 atom positions of hexoses while leaving behind the light triose-phosphates at the equilibrium (Gleixner and Schmidt 1997). Rossmann et al. (1991) experimentally showed that the C-3 and C-4 positions of glucose molecules extracted from both C3 (sugar beet syrup) and C4 (maize flour) plants are effectively heavier (13C-enriched) while other carbon atom positions (C-1, C-2, C-5 and mainly C-6) are lighter (13C-depleted) than the average of the molecule. A simple model developed by Tcherkez et al. (2004) based on the isotope effects of both aldolase reported by Gleixner and Schmidt (1997) and transketolase (estimated values) fits well the reproducible non-statistical 13C distribution in hexose molecules reported by Rossmann et al. (1991), although not properly accounting for the 13C-depletion in C-6. More recently, however, the refinement of quantitative 13C-NMR techniques has allowed the intramolecular isotopic distribution to be measured on natural glucose and sucrose without the need for prior chemical degradation (Gilbert et al. 2011). While globally confirming the isotopic pattern initially described by Rossmann et al. (1991) in glucose from beet sugar, this work clearly demonstrated that (i) the glucosyl and fructosyl moieties of sucrose have contrasted 13C distributions, especially in C-2 and C-3 atom positions, but also that (ii) the intramolecular pattern in glucose could exhibit significant variations depending on its origin (starch /sucrose from either source or sink tissues), that cannot be accounted for by the sole action of aldolase and transketolases. Interestingly, the addition in the model cited above of the isotope effects associated with (i) the interconversion of glucose-6-phosphate (G6P ) by phosphoglucose isomerase , and (ii) the breakdown of sucrose by invertase , have led to reasonably good predictions of the observed distributions, notably for the 13C-depletion in the C-6 atom position of glucose (Gilbert et al. 2012). Importantly, it means that the intramolecular 13C pattern of glucose, generally the main substrate for respiration, can be modified downstream the Calvin cycle by starch and sucrose metabolism, which are variable both spatially (among the different tissues of the plant) and temporally (day/night cycle, and seasonal cycle). Besides, it shows that different patterns can be found amongst carbohydrates .
Intramolecular isotopic heterogeneity has long been documented in other compounds such as amino acids (Abelson and Hoering 1961; Melzer and O’Leary 1987) and lipids (Monson and Hayes 1980, 1982), and is likely to be a general feature among all classes of metabolites (e.g. in alkaloids; Romek et al. 2015, 2016). Certainly then, the expected developments in that area in the near future will be of great help to improve our understanding of the spatial and seasonal variability in the isotopic composition of respired CO2 (see also Sect. III.C). In fact, the isotope composition in specific carbon atom positions that are decarboxylated during respiratory processes is crucial to anticipating the overall δ13C of evolved CO2, as discussed in what follows.
3.2 Differences in Isotopic Composition Among Metabolites
Enzymatic fractionations in metabolic pathways not only produce intramolecular heterogeneities, but also lead to substantial isotopic differences between metabolite classes. The updated survey of available data (Fig. 3.3) confirms the trend described by Schmidt and Gleixner (1998). Compared to OM, a large 13C-depletion is observed in lignin and lipids (around 3‰ and 5‰, respectively). Conversely, sugars, starch and cellulose are generally slightly 13C-enriched due to the equilibrium isotope effect of aldolase described above. The depletion in lipids have been attributed to the fractionation against 13C of chloroplastic pyruvate dehydrogenase complex (PDH) (Melzer and Schmidt 1987), while that in lignin has been proposed to originate from phenylalanine ammonia lyase activity (Butzenlechner et al. 1996). Interestingly, amino acids are also found to be substantially 13C-depleted (by about 3.5‰), but this isotopic signature does not seem to be conserved in proteins (Fig. 3.3).
Difference in δ13C value (in ‰) between compound classes (δ13Csubstance) within plant organs and total organic matter (δ13COM), assembled from literature data. Numbers on top indicate the number of measurements. Compounds that are significantly 13C-enriched compared to bulk OM are indicated in blue, those that are not significantly enriched or depleted in green, and significantly depleted compounds in white (amino acids with low sample number) or red. Note the higher variance for the compound classes of shorter residence time (e.g. sugars and amino acids), that is likely due to dynamic changes in the photosynthetic discrimination with fluctuating environmental conditions
It must also be noted that the isotope composition in metabolites can vary temporally, as found in starch . In fact, aldolase favors 13C in hexoses, thereby forming 13C-enriched transitory starch in chloroplasts, while leaving behind 13C-depleted triose phosphates , which are then converted to sucrose in the cytosol. Accordingly, phloem sugars are 13C-enriched during night-time (because they come from transitory starch degradation), while day-time sugars originating from trioses are 13C-depleted. Such a diel change in the 13C content of phloem sugars modeled by Tcherkez et al. (2004) was measured by Gessler et al. (2008) on Ricinus communis (castor bean) plants.
3.3 Metabolic Branching and General Causes for Respiratory Fractionation
There is now a strong body of evidence that the isotope composition in CO2 respired by different organs of the plant often differ from that in either bulk organic matter or carbohydrates (Fig. 3.4). This clearly illustrates the fact that respiratory metabolism and anabolic pathways are strongly interconnected, so that not all glucose molecules entering glycolysis are eventually fully oxidized in the mitochondria (therefore, isotope fractionations can be expressed), and/or other substrates (with contrasted isotope compositions) can be oxidized. However, data that have accumulated in recent years also show a large variability of the apparent respiratory fractionation in plants (more than 10‰; Fig. 3.4). Understanding the origin of such a large range is a challenge that has triggered a renewed interest in the plasticity of respiration. Specifically, it has highlighted the need for a better understanding of metabolic fluxes, which ultimately determine the extent to which potential fractionations are expressed throughout the metabolic network (Hayes 2001).
Frequency distribution of apparent respiratory fractionation values (ΔR) in leaves (a, c) and roots (b, d) of C3 herbs, C3 woody plants, and C4 herbs under varying growth conditions and measured in the dark at different day or night time using different methods. For leaves, only data under non-LEDR conditions are presented (measured during either the night or the day but after at least 15 min darkness). Leaf data from the literature are from 32 herbaceous C3, 29 woody C3 (including 7 coniferous) and 12 herbaceous C4 species, and root data are from 20 herbaceous C3 species, 9 woody C3 species (including 3 coniferous) and 5 herbaceous C4 species. ΔR is calculated as the difference between the carbon isotope composition of leaf or root material available in the literature [bulk organic matter (OM, left side panels) or water soluble fraction (WSOM, right side panels), considered as respiratory substrates], and that in leaf- or root-respired CO2 (δ13CR) as the product of respiration. Negative ΔR values correspond to a 13C-enrichment and positive ΔR values to a 13C-depletion in respired CO2 compared to the substrate. Vertical dashed lines indicate no respiratory fractionation (i.e. ΔR = 0)
Some important features driving variations in respiratory fractionation have been put forward. In what follows, we will try to present them based on specific examples, but also highlight their limits based on experimental evidence currently available, so as to point out where, in our view, further research efforts are needed.
3.3.1 Apparent Respiratory Fractionation
Because the mixture of substrates effectively sustaining respiration (and its isotope composition) is in general not known when measuring the isotope composition of respired CO2 in gas exchange systems, respiratory fractionation has to be expressed relative to a somewhat arbitrary reference, and thus the term ‘apparent fractionation’ is used. One can choose to use the isotope composition of total organic matter , or that of a subset representative of a putative respiratory substrate, when available.
In typical conditions, dark respiration in plants exhibits a respiratory quotient (RQ) close to 1, suggesting that the main substrate is generally carbohydrates . Although the RQ may be different from 1 or an RQ of 1 may result from a mixture of respiratory substrates of different oxidation level (as will be discussed later), in this section we chose to refer to the apparent fractionation defined against the isotope composition of the carbohydrate pool (generally measured as the water soluble fraction, WSOM) as a reference point. Apparent respiratory fractionation may be expressed relative to the whole organic matter when assessing the role of respiration in driving the observed differences in isotopic composition between autotrophic and heterotrophic organs (see Ghashghaie and Badeck 2014 for a discussion). However, when looking into the variability of respiratory fractionation from a metabolic point of view, anchoring the discussion to the most probable main substrate is preferable. WSOM is generally slightly heavier than OM (1–3‰), so that the 13C-enrichment in respired CO2 is artificially exaggerated when compared to OM. Besides, the isotope composition of WSOM can exhibit variations on short time scales (diel range of up to 3‰, Werner and Gessler 2011) that are not apparent in OM. Also, it must be emphasized that WSOM is not only composed of carbohydrates , so that large variations of other compounds, (e.g. organic acids or sugar alcohols) can influence its isotopic composition. Ideally, the isotope composition in sucrose (which is generally found to be close to that of WSOM) would be a better reference to express respiratory apparent fractionation in this context, but it is not available in many of the studies reviewed here.
3.3.2 Dark Respiration in Leaves: PDH -TCA Imbalance and Beyond
First systematic investigations of the isotopic composition of respired CO2 in darkened mature leaves of herbaceous C3 plants have shown that released CO2 is on average heavier than sucrose by about 3–6‰ (Duranceau et al. 1999; Ghashghaie et al. 2001). Subsequent studies suggested that the systematic enrichment in leaf respired CO2 compared to WSOM is a widely distributed feature in plants, but also demonstrate that, as pointed out earlier, the range of variation can be quite large (Fig. 3.4).
The 13C-enrichment in leaf respiratory CO2 has been attributed to the so called ‘fragmentation fractionation ’ (see Tcherkez et al. 2004). Glycolysis produces pyruvate, which can enter mitochondria and subsequently be decarboxylated by PDH to produce acetyl-CoA . The C-1 atom position of pyruvate decarboxylated in the process originates from positions C-3 and C-4 of glucose that are 13C-enriched by about 4‰ compared to the average isotopic composition of the molecule. Accordingly, acetyl-CoA carries the four other carbon atoms of glucose into the TCA (C-1, C-2, C-5, and C-6), which are relatively 13C-depleted (see Sect. III.A). If a large amount of acetyl-CoA is diverted to various anabolic pathways (e.g. fatty acid biosynthesis, secondary metabolites…) instead of fueling the TCA cycle, the respiratory CO2 efflux will be dominated by PDH activity, and therefore substantially 13C-enriched. It is thus generally accepted that the degree of imbalance between PDH and TCA decarboxylations in the respiratory flux is an important driver of the variability in the isotopic composition of respired CO2 (Ghashghaie et al. 2003; Badeck et al. 2005; Cernusak et al. 2009; Werner and Gessler 2011; Ghashghaie and Badeck 2014). An important assumption here is that pyruvate is fully committed to acetyl-CoA , so that the isotope effect of PDH (1.023), which would deplete respired CO2 in 13C, is not expressed. This is probably valid in darkened leaves where pyruvate entering mitochondria does not have obvious alternative fates to decarboxylation .
In this framework, however, accounting for the commonly observed respiratory fractionation in leaves (around −4‰, the negative value showing that it is 13C-enriched) requires that the commitment of acetyl-CoA to TCA remains very low (around 5%, see Tcherkez 2010). This is because (i) for one fully oxidized pyruvate molecule, two CO2 are released by the TCA when only one arises from PDH , and (ii) citrate synthase (CS) exhibits a kinetic isotope effect of about 1.020 during the formation of citrate, further 13C-depleting TCA intermediates when acetyl-CoA is not fully committed.
Yet, such a large imbalance seems unlikely to occur in vivo, especially in the dark. Most biosynthetic processes probably occur predominantly in the light, and in particular, fatty acid synthesis has been shown to take place mainly in chloroplasts (Schmid and Ohlrogge 2002) during the day, when light energy can provide the required ATP and NADPH, and chloroplastic PDH is activated (Tovar-Mendez et al. 2003). It is still possible that acetyl-CoA in the cytosol can be involved in lipid chain elongation and biosynthesis of various secondary metabolites (isoprenoids, flavonoids, etc.…), even in the dark. However, these fluxes are unlikely to exceed 5–10% of the respiratory flux of PDH. In addition, the accepted view is rather that the cytosolic pool of acetyl-CoA is fueled by citrate exported from the mitochondria and cleaved by citrate lyase (Oliver et al. 2009). In other words, there is very little evidence that large fluxes of acetyl-CoA can escape the TCA pathway. Consistently, no acetyl-CoA mitochondrial membrane carrier has been identified in Arabidopsis thaliana (Lee and Millar 2016). In fact, if a very low proportion of acetyl-CoA were to enter the TCA pathway, one would expect CO2 production to largely exceed O2 consumption by mitochondria, as the latter is coupled to the TCA pathway through NADH dehydrogenases and succinate dehydrogenase (regardless of complications that would arise in imbalances in ATP/ADP and NAD/NADH pools). This does not seem to be the case since (i) the RQ of darkened leaves is generally close to 1 (Noguchi and Terashima 1997; Tcherkez et al. 2003), and (ii) their respiration rate matches reasonably well the estimated ATP demand for sucrose export and protein turn-over (Noguchi et al. 2001).
It is also worth noting that the observed range of leaf respiratory fractionation spans values up to at least −8‰ (Fig. 3.4), which largely exceeds the theoretical maximum that can be attained based on fragmentation fractionation . Other explanations thus need to be found to explain the general 13C-enrichment in dark-evolved CO2.
In fact, if both pyruvate and acetyl-CoA were nearly fully committed, one would expect very little respiratory fractionation to occur upon oxidation of carbohydrates (Werner et al. 2011). Interestingly, some clues to try and solve this conundrum can be sought in recent progresses toward an integrated view of respiratory metabolism and the plasticity of the TCA cycle (Sweetlove et al. 2010; Tcherkez et al. 2012). Thus, because organic acids of the TCA pathway contribute to several anabolic pathways, but also to redox and pH regulation within the cell, the cyclic nature of the pathway can vary considerably. This is clearly the case in illuminated leaves (as will be discussed later), where a non-cyclic flux mode of the TCA have been demonstrated using isotopic tracers (Tcherkez et al. 2009; Gauthier et al. 2010).
In the dark, cells largely rely on mitochondrial ATP production, so that such non-cyclic flux modes are less likely to occur. However, this does not preclude some intermediates to be withdrawn from the cycle for other metabolic purposes, as long as these leaks are compensated for in some ways. In fact, it is known that citrate , and to a lesser extent malate , can be accumulated in the vacuole of plant cells in the dark (Gout et al. 1993). Consistent with these observations, a recent diel flux balance model applied to C3 leaf metabolism has predicted that up to 15% of citrate produced by CS at night is stored in the vacuole, before being remobilized during the day to support glutamate synthesis (Cheung et al. 2014). This has consequences regarding the isotopic composition of the CO2 evolved in the TCA during the night.
To understand these implications, we first need to come back to the fate of each individual carbon atom position of glucose that is decarboxylated in the TCA pathway (positions C-1, C-2, C-5 and C-6), which, as pointed out earlier (Sect. III.A), are not isotopically equivalent. When glucose is converted into pyruvate by glycolysis, positions C-1 and C-6 get pooled in the C-3 atom of pyruvate (corresponding to C-2 in acetyl-CoA ), while positions C-2 and C-5 end up in the C-2 atom of pyruvate (corresponding to C-1 in acetyl-CoA). Complication then arises because the 2 carbon atoms of acetyl-CoA incorporated into citrate by CS are not immediately decarboxylated in the TCA pathway. None of them is decarboxylated upon first turn, and it is only during the second turn that the C-1 position of acetyl-CoA is lost. But from then on, because of the symmetry of the succinate molecule, the C-2 position of acetyl-CoA has only 50% chance to be decarboxylated at each subsequent turn. In other words, it means that in conditions where a substantial flux of organic acids (e.g. citrate) is withdrawn from the TCA, glucose atom positions C-1 and C-6 will be underrepresented in the CO2 efflux , so that any isotopic disequilibrium between these positions and positions C-2/C-5 will be expressed in respiratory fractionation. Glucose atoms C-2/C-5 are 13C-enriched in comparison to positions C-1/C-6. Intramolecular data suggest that the difference is generally around 2‰, but might vary with environmental conditions (Gilbert et al. 2012). However, it has been shown to be substantially larger in the fructosyl moiety of sucrose (8‰), where the C-2 atom is strongly 13C-enriched (Gilbert et al. 2011). If, as hypothesized by these authors, this enrichment is due to the equilibrium isotope effect of glucose-6-phosphate-isomerase, it is likely to be expressed in fructose-6-phosphate entering glycolysis in darkened leaves, where the glucose-6-phosphate pool is constantly utilized for sucrose export.
The explanation given just above can be summarized with a simple numerical example. As discussed, both pyruvate and acetyl-CoA are supposed to exhibit high commitments (>95%) to PDH and CS, respectively, so that the isotope effects of these enzymes are probably negligible in vivo. The isotope composition in respired CO2 can thus be calculated with an isotopic mass balance, summing the contribution of each pyruvate atom position to the total CO2 efflux (i.e. meaning that three different pools of glucose carbon atoms are considered: C-3/C-4, C-2/C-5, and C-1/C-6). The relative contribution of positions C-2/C-5, and C-1/C-6 to the overall efflux from the TCA pathway can then be varied in order to illustrate the effect of the C-1/C-6 trapping when intermediates of the TCA pathway are being withdrawn. Starting from the experimentally measured isotopic distribution in glucose carbon atoms, it yields slightly negative respiratory fractionations (i.e. 13C-enriched CO2 compared to glucose), ranging from 0 to −0.5‰, while the values of sucrose fructosyl extend that range to up to −2‰. This illustrates that the CO2 produced by the TCA pathway does not necessarily have to be 13C-depleted, as often assumed. We nevertheless recognize that the above calculation does not capture the whole complexity of the system. Notably, even relatively small leaks (between 5 and 15% of the flux) of pyruvate, acetyl-CoA , and/or 2-oxoglutarate could suppress this enrichment, and lead to slightly positive apparent fractionations (easily up to 5‰), because PDH , CS and 2-oxoglutarate dehydrogenase (2OGDΗ) can all discriminate against 13C by up to 20‰ or more (See Tcherkez et al. 2011a for details on isotope effects ). Overall, it suggests that in a respiratory system where all commitments are high (85% and above), the mere interplay of glycolysis and TCA pathway can only account for apparent fractionations roughly in between 5‰ and −2‰. Clearly then, other processes must occur in darkened leaves where the respiratory CO2 has commonly been found to be heavier than WSOM by 3‰ to 8‰ (Fig. 3.4).
In that perspective, we must consider how the organic acid leaks discussed previously can be compensated for in order to keep the cycle running. The first possibility is a sustained flux of cytosolic oxaloacetate (OAA), derived from PEP by the action of PEPC , into the mitochondria. The anaplerotic function of PEPC in leaves in the light has been known for some time (Melzer and O’Leary 1987), and it has been shown that it can carry substantial fluxes in heterotrophic organs (around 10% of the respiratory efflux, Dieuaide-Noubhani et al. 1995; Bathellier et al. 2009). Yet, its quantitative contribution in darkened leaves remains poorly documented, despite early reports of 14CO2 incorporation into organic acids in tobacco and avocado leaves in the dark (Kunitake et al. 1959; Clark et al. 1961) and in other species (Nalborczyk 1978). Accounting for the isotope effect of the equilibrium between CO2 and HCO3 −, carbon fixation by PEPC discriminates in favor of 13C by about 5.7‰ (Farquhar 1983). When operating during the day, it would fix mostly atmospheric CO2 , thus strongly enriching OAA and its derivatives (malate , fumarate, aspartate ; Melzer and O’Leary 1987; Tcherkez et al. 2011b; Lehmann et al. 2016). Nevertheless, at night, when stomata are closed, PEPC will merely refix CO2 from respiration. No matter how large the flux is, its net isotope contribution will thus be negligible, unless a large proportion of the OAA produced is not subsequently decarboxylated, in which case it should slightly deplete respired CO2 in 13C.
Alternatively, TCA cycle intermediates could be fed by stored pools of organic acids. In Arabidospsis, both malate and fumarate have been shown to exhibit large diel variations, accumulating to substantial amounts (up to 10 μmol g FW−1) during the day, while decreasing sharply during the night (Chia et al. 2000; Pracharoenwattana et al. 2010). As noted above, accumulation of organic acids in the light is strongly supported by PEPC activity, so that they can be quite substantially enriched in 13C, especially in carbon atom positions C-4 and C-1, which can be decarboxylated by the TCA cycle. Considering the enrichment of about 20‰ observed in the C-4 of aspartate (Melzer and O’Leary 1987), it follows that even a relatively modest contribution of these pools to the respiratory CO2 efflux (i.e. 10–20%) could suffice to account for the most frequently observed fractionations (−3‰ to −6‰) during dark respiration.
Currently, no direct experimental evidence is available to confirm such a hypothesis, and further work is clearly needed so as to clarify the contribution of organic acids to dark respiration. However, if correct, it implies that the extent of the 13C-enrichment in leaf respired CO2 in the dark will largely depend on (i) the degree of accumulation of organic acids (such as malate or fumarate) during the day, which should in turn change their contribution throughout the night, and (ii) the degree of enrichment that they will exhibit. A recent model of Arabidopsis leaves predicts that the partitioning of assimilates between carbohydrate and organic acids should exhibit a tight trade-off that will be modulated by the energy and redox status of photosynthesizing cells (Cheung et al. 2015). In the studies cited above (Chia et al. 2000; Pracharoenwattana et al. 2010), fumarate accumulation in Arabidopsis leaves was tightly linked to nitrogen availability. Besides, one can expect that varying physiological conditions will influence the relative contribution of PEPC to organic acid synthesis in the light, but also the isotope composition of its substrate, HCO3 −. Overall, it could thus offer a promising framework toward a better understanding of the variability of respiratory fractionation in leaves during the night.
An interesting observation in that context is the general trend for a reduction of the 13C-enrichment in respiratory CO2 between the beginning and the end of the night illustrated in Fig. 3.5. It is indeed possible that the contribution of organic acids would decrease throughout the night as the vacuolar pool shrinks. The direction of malate flux, to or from the vacuole in protoplasts, has been shown to be dictated by a threshold value of the cytosolic pool (Gout et al. 1993). Note however that other processes are probably involved, such as the increasing contribution of recycled proteins and/or lipids, as suggested by the slight decrease that has been observed in the RQ during the night (Noguchi and Terashima 1997, see also Sect. III.D).
Apparent respiratory fractionation (ΔR) of leaves from C3 herbs (a, d), C3 woody species (b, e) and C4 herbs (c, f) measured at the end of the night versus ΔR values determined at the end of the day (or beginning of the night) from literature when both day and night data were available. Data reported under LEDR conditions are not used. ΔR is calculated as the difference between carbon isotope composition in leaf bulk organic matter (OM, circles) or water soluble fraction (WSOM, triangles) considered as potential respiratory substrates and that of leaf-respired CO2 (δ13CR). Negative ΔR values correspond to a 13C-enrichment and positive ΔR values to a 13C-depletion in respired CO2 compared to plant material, shown by black and white arrows, respectively. Solid lines correspond to 1:1 relationships and dashed lines indicate no respiratory fractionation (i.e. ΔR = 0)
3.3.3 The Special Case of Light-Enhanced Dark Respiration (LEDR)
A phenomenon which seems to unequivocally illustrate the impact of organic acids on respiratory fractionation is LEDR . LEDR corresponds to a transient increase in respiration rates (both CO2 release and O2 consumption) that occurs within the first 15–25 min after darkening. It needs to be distinguished from the post-illumination burst (PIB). The latter occurs within the first minute after darkening and is presumably related to the decrease in the photorespiratory glycine pool, while LEDR sets on after the end of PIB and attains maximal rates around 3 –4 min after darkening (Atkin et al. 1998, and earlier work cited therein). During that transient time, respired CO2 has been found to exhibit large 13C-enrichments that decreased exponentially to reach a steady state when respiration rate stabilizes (reviewed in Werner and Gessler 2011). LEDR has been attributed to the rapid consumption of an excess malate pool upon darkening, which triggers the activity of light inhibited NAD-dependent malic enzyme (NAD-ME) and PDH in the mitochondria. Consistently in Ricinus, the transient enrichment in respired CO2 could be satisfactorily explained by a 22% contribution of the decarboxylation of the strongly enriched C-4 atom of malate (see above) by NAD-ME, which matched the reduction of the malate pool (Gessler et al. 2009).
Yet, it is worth noting that LEDR time frame is probably rather loosely defined. Most studies of the phenomenon, done on dark adapted protoplasts or leaves subjected to very short pre-illumination periods (10–15 min, Heichel 1971; Reddy et al. 1991; Igamberdiev et al. 1997; Atkin et al. 1998), show a rapid bell shaped transient (within 25 min or less). However, when measured on leaves taken after a longer photoperiod (>6 h), the pattern is slower (up to 1 h), and strongly temperature dependent (Azcon-Bieto and Osmond 1983). Besides, the bell shape only appears when non-photorespiratory conditions are applied 20 min before darkening. When continuously recorded overnight, after a full photoperiod, leaf respiration decrease looks more like a single exponential decay, except for C4 plants (Byrd et al. 1992). A clear-cut distinction between LEDR and steady state respiration might thus be difficult to achieve in practice, and in fact, the isotope composition in respired CO2 during LEDR , and after 1 h in the dark, exhibits a rather good correlation, with a consistent offset (Fig. 3.6).
Apparent respiratory fractionation (ΔR) of leaves from C3 herbs (dark symbols) and C3 woody species (light green symbols) from the literature, determined after 1 h in darkness (i.e. non-LEDR conditions) versus values obtained at the end of the day after a few minutes in darkness (under LEDR conditions). ΔR is calculated as difference between the carbon isotope composition in leaf bulk organic matter (OM, circles) or water soluble fraction (WSOM, triangles) and that of leaf-respired CO2 (δ13CR). Dashed lines indicates no respiratory fractionation (i.e. ΔR = 0). Negative ΔR values correspond to a 13C-enrichment and positive ΔR values to a 13C-depletion in respired CO2 compared to plant material (shown by dark and white arrows, respectively). The solid line corresponds to the 1:1 relationship and the dotted line to the linear regression (y = 3.86 + 1.10x, r 2 = 0.85)
Highly 13C-enriched leaf respired CO2 has been reported for many C3 species (fractionation of up to −13‰) after 4–5 min in the dark measured rapidly with TDLS (Barbour et al. 2007) or by rapid in-tube incubation (Priault et al. 2009; Werner et al. 2009; Wegener et al. 2010; Lehmann et al. 2015). Similarly, strikingly high δ13C values of up to −4‰ (i.e. heavier than atmospheric CO2 ) are observed in respired CO2 in C4 maize leaves even after about 20 min in darkness, thereafter declining relatively slowly to reach stable values (around −14‰) only after 1h30 in darkness, together with a decrease of leaf malate content (Ghashghaie et al. 2016). Recently, Lehmann et al. (2015) found that the δ13C of leaf respired CO2 (potato plants), for both LEDR and steady-state conditions, was better correlated with that of malate than with soluble sugars, suggesting that variations in malate consumption (remobilized or synthetized de novo) could strongly influence the isotope composition in respired CO2 throughout the night.
Further work is thus needed to clarify the metabolic adjustments occurring upon darkening. It is possible that pathways other than malate decarboxylation are involved, considering the strong effect of non-photorespiratory conditions just prior to switching to darkness. It cannot be excluded that the early phase of LEDR is influenced by the consumption of metabolic pools linked to photorespiration that built up in the light. In this regard, it is worth noting that serine has been shown to be strongly 13C-enriched in Pelargonium leaves (Tcherkez et al. 2011b). However, photorespiration can also significantly impact the redox status of the cell, and thus malate pools in different cell compartments . In fact the subcellular origin of the malate pool involved in LEDR is uncertain. In spinach, the drop in total malate content during the first 15 min after darkening cannot be accounted for by the decrease measured in both the vacuole and the chloroplasts, while the cytosolic pool remained rather constant (Gerhardt et al. 1987), suggesting that inter-organelle regulations are involved.
3.3.4 What About Leaf Respiratory Fractionation in the Light?
Experimental assessment of respiratory fractionation in leaves in the light is a difficult task since (i) CO2 photosynthetic fixation largely dominates the net flux, and (ii) CO2 efflux is a mixture of both photorespiratory (i.e. glycine decarboxylation ) and respiratory processes per se. Indirect estimations of each component are possible by fitting observed net photosynthetic fractionation values to predicted values using an extended model for Δ (Lanigan et al. 2008; Tcherkez et al. 2010). Using such an approach, it has been calculated that respiratory CO2 efflux from illuminated Pelargonium leaves is 13C-depleted as compared to organic matter by 0–10‰, consistent with previous theoretical argument (Tcherkez et al. 2004, 2011b).
In the light, contrasted fractionating processes can be suspected to occur. Both glycolysis and mitochondrial TCA decarboxylating enzymes (PDH , IDH, and 2OGDH) are thought to be strongly down-regulated, so that day respiratory flux is probably dominated by a combination of chloroplastic PDH activity, and non-cyclic TCA pathway decarboxylations, with a potentially large involvement of cytosolic bypasses (Sweetlove et al. 2010; Tcherkez et al. 2012). PDH in the chloroplast is fueled by triose-phosphates , which are slightly 13C-depleted (Gleixner et al. 1998), and will probably further fractionate against 13C since pyruvate is likely to be diverted for a range of biosynthetic processes in the light (e.g. pyruvate derived amino acids and their derivatives). Similarly, a large part of the 2OG pool can be used to form Glu to sustain nitrate assimilation, so that the isotope effect of 2OGDH (probably close to 1.020) can be expressed, although the flux it carries might be rather small. On the other hand, labeling experiments suggest that a substantial proportion of respired CO2 in the light comes from stored carbon pools (40–86%, Pärnik and Keerberg 2006; Tcherkez et al. 2010, 2011b), which could be organic acids accumulated in the night (e.g. citrate or malate , see above). Depending on their degree of enrichment, their contribution can partly compensate for the depleting effects of decarboxylases. Still, one might expect organic acids accumulated at night to be less 13C-enriched than those accumulated in the light, since PEPC activity likely refixes an important proportion of 13C-depleted respired CO2 in the dark.
3.3.5 The Role of the PPP as Illustrated in Roots
Heterotrophic organs of plants entirely rely on the constant export of photosynthetic products from autotrophic organs (leaves) through the phloem . Respiration is their main source of energy to sustain cellular functioning and biosynthesis (except in conditions that favor fermentation), so that it exhibits much less diurnal variability than in leaves. In roots, where it has been the most extensively studied, the isotopic composition of respired CO2 is also less variable than in leaves, and on average, slightly 13C-depleted compared to WSOM (Fig. 3.4).
Labeling experiments on French bean have shown that the organization of the respiratory metabolic network in roots shares some similarities with that of darkened leaves described above (Bathellier et al. 2009). The TCA pathway functions as a cycle, and the commitment of mitochondrial acetyl-CoA to CS is probably relatively high. In fact, fatty acid synthesis also occurs in plastids in roots (at low rates), which possess their own PDH , supplied directly from cytosolic pyruvate (Fisher and Weber 2002). A substantial part of TCA intermediates are also abstracted from the cycle mostly to form glutamate and aspartate for nitrogen assimilation . This feature would be further amplified in conditions where organic acid exudation is high. Accordingly, an important flux through PEPC has been found to occur, compensating for the abstraction of TCA intermediates. Following the arguments exposed for darkened leaves, root respiration would thus be expected not to be associated with a large fractionation, potentially slightly negative (i.e. in favor of 13C) because of both the trapping of positions C-1/C-6 of glucose in abstracted TCA intermediates, and the activity of plastidial PDH . Yet, these effects are probably counterbalanced by the isotope effect of 2OGDH (which can be expressed due to a branching point at 2OG , used for both Glu and succinate synthesis).
That said, a significant proportion of respired CO2 in roots is believed to come from the activity of the oxidative pentose phosphate pathway , PPP (20–25%; Dieuaide-Noubhani et al. 1995; Bathellier et al. 2009). Oxidative PPP activity is important in providing NADPH for nitrate reduction and fatty acid synthesis (Bowsher et al. 2007). During the oxidative phase of PPP, 6-phosphogluconate dehydrogenase (6PGDH ) decarboxylates 6-phophogluconate to form ribulose-5-phosphate, releasing the C-1 atom of glucose as CO2. 6PGDH has been shown to exhibit a kinetic fractionation against 13C of around 9.6‰, while being also capable of reversible catalysis thereby giving an equilibrium fractionation in favor of 13C of about 4‰ (Rendina et al. 1984). The classical textbook view is that the reactions of the oxidative phase of the PPP are mostly irreversible in vivo (Tobin and Bowsher 2005). However, a recent estimate of the standard Gibbs free energy of reaction (ΔGr 0) for the decarboxylation of 6-phophogluconate (using the component contribution method; Noor et al. 2013) predicts a rather high in vivo value of around +8 kJ/mol (Tepper et al. 2013). It is likely then that 6PGDH must exhibit quite a substantial degree of reversibility in vivo, which will vary depending on the relative concentration of 6-phospogluconate to ribose-5-phosphate. The effective isotope effect at this step can thus be expected to be rather small. However, the first enzyme of the PPP , glucose-6-phosphate dehydrogenase (G6PDH ) fractionates by 16.5‰ against 13C in the C-1 atom position of glucose (Hermes and Cleland 1984), which is subsequently decarboxylated by 6PGDH . G6PDH thus tends to deplete in 13C the CO2 released by the PPP. Still, it must be pointed out that in a specific case where oxidative PPP would be predominantly active in plastids, the commitment of Glc-6-phosphate to chloroplastic G6PDH can be high (provided little starch synthesis occurs), so that the isotope effect is not expressed. Therefore, if 6PGDH operates close to equilibrium, the oxidative PPP would then contribute 13C-enriched CO2 (up to 4‰ compared to Glc C-1) to the respiratory efflux.
Taken all together, the isotopic contributions of PDH , TCA and oxidative PPP in roots fit reasonably well the range of observed respiratory fractionations (−4‰ to 4‰; Fig. 3.4). We nevertheless recognize that occurrence of more negative respiratory fractionations (below −4‰), if they were to be confirmed, cannot easily be accounted for without the consumption of 13C-enriched substrates. Since organic acids accumulated in leaves in the light are the only obvious 13C-enriched pool, it would imply transport processes. Such processes are possible (e.g. Peuke et al. 1996), but further work would be required to establish their implication in respiration of heterotrophic organs. The existence of other 13C-enriched pools in the roots is still possible, but remains to be demonstrated. Notably, in respiring heterotrophic organs, the isotopic composition of CO2/HCO3 − in cells should be much less influenced by atmospheric CO2 than in illuminated leaves with open stomata, especially in underground organs such as roots, as many respiring organisms contribute to the overall CO2 pool in the soil. Thus in these tissues there is less room for carboxylases like PEPC to produce strongly 13C-enriched metabolites, although this will be dependent on their specific conductance to CO2 and respiration rates.
3.4 Variations in the Substrate Mixture Sustaining Respiration
The above discussion was focused on conditions where respiration oxidizes carbohydrates , so that the RQ is close to 1. Although this is certainly the most common situation, there are both environmental and developmental circumstances that lead plant respiration to consume alternative substrates to sustain ATP production in mitochondria. This has been clearly illustrated in French bean leaves under prolonged darkness (up to 2 weeks; Tcherkez et al. 2003). As the carbohydrate pool collapses, lipids and proteins are remobilized (providing acetyl-CoA , TCA intermediates, and/or pyruvate) and maintain mitochondrial activity. This switch in respiratory substrates is accompanied by a marked decrease of the RQ, which correlates with a 13C-depletion in respired CO2, reaching values consistent with the isotopic composition of lipids and proteins. A switch similar to that artificially induced by prolonged darkness can be expected during plant senescence. Also, as discussed earlier, respiration decreases throughout the night (together with the carbohydrate pool), suggesting that the contribution of the constant turn-over of proteins and membranes probably increases, thus depleting respired CO2 in 13C (Fig. 3.5).
Switches between substrates can also be expected across ontogenetic developmental stages, especially when seeds contain non-starchy reserve types. In peanut seeds (which contain about 50% lipids), lipid remobilization starts progressively during the early stages of germination. Consistently, the isotopic composition of respired CO2 in the emerging radicle and leaves becomes progressively 13C-depleted as lipid contribution increases in the respiratory substrate mix (Ghashghaie et al. 2015), while it remains rather constant in the case of the starch -containing French bean seeds (Bathellier et al. 2008). However, the depletion in respired CO2 is not accompanied by a change in respiratory apparent fractionation, because lipids reserves are converted to carbohydrates through the glyoxylate cycle and gluconeogenesis before being exported to developing organs. In oil-containing seeds, the glyoxylate cycle converts two acetyl-CoA molecules, resulting from fatty acid breakdown, to succinate (Fig. 3.1). Succinate leaving the glycoxylate cycle transports the relatively 13C-depleted carbon stored in lipids to the mitochondrion where (after being converted to malate and oxaloacetate ) it can be either directly used by the TCA pathway, exported to the cytosol (malate-oxaloacetate shuttle ), or used to fuel gluconeogenesis . Despite the likely contribution of 13C-depleted lipids as substrates, peanut cotyledons exhibited a negative respiratory fractionation (i.e. in favor of 13C) during the heterotrophic phase of germination, suggesting either that inverse fractionations are associated with the metabolism of lipid remobilization , or that their respiration is also fuelled by stored carbohydrates (see Ghashghaie et al. 2015 for a more detailed discussion).
It is worth noting that the time course of the respiratory fractionation in growing organs during ontogeny is so that the isotope composition in respired CO2 eventually reaches the typical values observed in mature plants after the onset of autotrophy, in both C3 and C4 plants (see Bathellier et al. 2008 and Ghashghaie et al. 2015 for C3 legumes, and Ghashghaie et al. 2016 for C4 maize), and this effect cannot be accounted for by a simple switch in respiratory substrates (i.e. from remobilized reserves to photosynthetic assimilates). Rather, it must reflect flux changes in primary carbon metabolism that occur when leaves become photosynthetically active.
4 Conclusions
The recently renewed interest in studying the isotope signature of respired CO2 arises from the non-invasive nature of isotopic measurements of CO2. In fact, it allows monitoring metabolic activities in systems which cannot be replicated and destructively sampled, including the entire biosphere (Ciais et al. 1995; Fung et al. 1997; Kaplan et al. 2002) or entire ecosystems (Yakir and Wang 1996; Buchmann et al. 1998; Ogée et al. 2003; Knohl et al. 2005; Tu and Dawson 2005; Bowling et al. 2008; Wehr and Saleska 2015). At the single plant level between-species differences in ΔR might be related to the growth rate (Ocheltree and Marshall 2004) and the production of secondary aromatic metabolites (Werner et al. 2007) suggesting other potential applications for in vivo diagnosis. A better knowledge of fractionation steps and respiratory metabolism will be instrumental to better understand the origin of the δ13C value in respired CO2 and its physiological significance.
Data accumulated in the past 15 years on the isotope composition of CO2 respired by plants clearly demonstrate that a respiratory fractionation does occur, but it is strikingly variable. Throughout this chapter, we have provided hypotheses to explain the origin of the isotope composition in respired CO2 but refinements are to be expected, after having integrated more knowledge on respiratory metabolic fluxes and metabolic compartmentalization within cells. Difficulties have to be anticipated to fully explain the very wide range of isotopic variation that is observed, highlighting the complexity and plasticity of the underlying metabolic network. Particularly striking is the fact that the respiratory fractionation (in autotrophic organs) is more often found to be in favor of 13C, while most decarboxylating enzymes discriminate against 13C, and most metabolites analyzed so far are 13C-depleted as compared to carbohydrates . This simple observation leverages two comments. First, energetic imperatives of respiratory metabolism might require a rather linear organization of catabolism, i.e., with limited metabolic branching, so that enzyme kinetic isotope effects are not expressed in vivo to a significant extent. Second, isotopically heavier yet unknown metabolites may exist, or there is a systematic 13C-enrichment in C atom positions that are decarboxylated. In any case, a more thorough investigation of the intramolecular isotopic distribution in different classes of metabolites is needed in order to move one step further in the understanding of respiratory fractionation in plants.
References
Abelson PH, Hoering TC (1961) Carbon isotope fractionation of amino acids by photosynthetic organisms. Proc Natl Acad Sci U S A 47:623–632
Angert A, Luz B (2001) Fractionation of oxygen isotopes by root respiration: implications for the isotopic composition of atmospheric O2. Geochim Cosmochim Acta 65:1695–1701
Atkin OK, Evans JR, Siebke K (1998) Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust J Plant Physiol 25:437–443
Atkin OK, Scheurwater I, Pons TL (2007) Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol 174:367–380
Azcon-Bieto J, Osmond CB (1983) Relationship between photosynthesis and respiration – the effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol 71:574–581
Badeck FW, Tcherkez G, Nogués S, Piel C, Ghashghaie J (2005) Post-photosynthetic fractionation of stable carbon isotopes between plant organs – a widespread phenomenon. Rapid Comm Mass Spec 19(11):1381–1391
Barbour MM, McDowell NG, Tcherkez G, Bickford CP, Hanson DT (2007) A New measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ 30(4):469–482
Bathellier C, Badeck FW, Couzi P, Harscoët S, Mauve C, Ghashghaie J (2008) Divergence in δ13C of dark respired CO2 and bulk organic matter occurs during the transition between heterotrophy and autotrophy in Phaseolus vulgaris L. plants. New Phytol 177:406–418
Bathellier C, Tcherkez G, Bligny R, Gout E, Cornic G, Ghashghaie J (2009) Metabolic origin of the δ13C of respired CO2 in roots of Phaseolus Vulgaris. New Phytol 181(2):387–399
Bender M, Sowers T, Labeyrie L (1994) The Dole effect and its variations during the last 130,000 years as measured in the Vostok Ice Core. Glob Biogeochem Cy 8:363–376
Bloemen J, Bauweraerts I, De Vos F, Vanhove C, Vandenberghe S, Boeckx P, Steppe K (2015) Fate of xylem-transported 11C- and 13C-labeled CO2 in leaves of poplar. Physiol Plant 153:555–564
Bowling DR, Pataki DE, Randerson JT (2008) Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol 178(1):24–40
Bowsher CG, Lacey AE, Hanke GT, Clarkson DT, Saker LR, Strulen I, Emes MJ (2007) The effect of Glc6P uptake and its subsequent oxidation within pea root plastids on nitrite reduction and glutamate synthesis. J Exp Bot 58:1109–1118
Brugnoli E, Farquhar GD (2000) Photosynthetic fractionation of carbon isotopes. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism. Kluwer Academic Publishers, Dordrecht, pp 399–434
Buchmann N, Brooks RJ, Flanagan LB, Ehleringer JR (1998) Carbon isotope discrimination of terrestrial ecosystems. In: Griffiths H (ed) Stable isotopes: integration of biological, ecological and geochemical processes. Bios Scientific Publishers, Oxford, pp 203–221
Butzenlechner M, Thimet S, Kempe K, Kexel H, Schmidt HL (1996) Inter- and intramolecular isotopic correlations in some cyanogenic glycosides and glucosinolates and their practical importance. Phytochemistry 43:585–592
Byrd GT, Sage RF, Brown HR (1992) A comparison of dark respiration between C3 and C4 plants. Plant Physiol 100:191–198
Carbone MS, Czimczik CI, McDuffee KE, Trumbore SE (2007) Allocation and residence time of photosynthetic products in a boreal forest using a low-level 14C pulse-chase labeling technique. Glob Chang Biol 13:466–477
Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A et al (2009) Viewpoint: why are non-photosynthetic tissues generally C-13 enriched compared with leaves in C-3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36(3):199–213
Cheng W, Sims DA, Luo Y, Coleman JS, Johnson DW (2000) Photosynthesis, respiration, and net primary production of sunflower stands in ambient and elevated atmospheric CO2 concentrations: an invariant NPP: GPP ratio? Glob Chang Biol 6:931–941
Cheung CYM, Poolman MG, Fell DA, Ratcliffe RG, Sweetlove LJ (2014) A diel flux balance model captures interactions between light and dark metabolism during day-night cycles in C3 and crassulacean acid metabolism leaves. Plant Physiol 165:917–929
Cheung CYM, Ratcliffe RG, Sweetlove LJ (2015) A method of accounting for enzyme costs in flux balance analysis reveals alternative pathways and metabolite stores in an illuminated Arabidopsis leaf. Plant Physiol 169:1671–1682
Chia DW, Yoder TJ, Reiter WD, Gibson SI (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidospis and other plant species. Planta 211:743–751
Ciais P, Tans PP, White JWC, Trolier M, Francey RJ, Berry JA et al (1995) Partitioning of ocean and land uptake of CO2 as inferred by δ13C measurements from the NOAA climate monitoring and diagnostics laboratory global air sampling network. J Geophys Res 100:5051–5070
Clark RB, Wallace A, Mueller RT (1961) Dark CO2 fixation in avocado roots, leaves and fruit. J Am Soc Sci 78:161–168
DeLucia E, Drake JE, Thomas RB, Gonzalez-Meler M (2007) Forest carbon use efficiency: is respiration a constant fraction of gross primary production? Glob Chang Biol 13:1157–1167
Dieuaide-Noubhani M, Raffard G, Canioni P, Pradet A, Raymond P (1995) Quantification of compartmented metabolic fluxes in maize root-tips using isotope distribution from C-13-labeled or C-14-labeled glucose. J Biol Chem 270(22):13147–13159
Duranceau M, Ghashghaie J, Badeck F, Deléens E, Cornic G (1999) δ13C of CO2 respired in the dark in relation to δ13C of leaf carbohydrates in Phaseolus vulgaris L. under progressive drought. Plant Cell Environ 22:515–523
Farquhar GD (1983) On the nature of carbon isotope discrimination in C-4 Species. Aust J Plant Physiol 10(2):205–226
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Fisher K, Weber A (2002) Transport of carbon in non-green plastids. Trends Plant Sci 7(8):345–351
Fung I, Field CB, Berry JA, Thompson MV, Randerson JT, Malmström CM et al (1997) Carbon 13 exchanges between the atmosphere and biosphere. Glob Biogeochem Cy 11:507–533
Gauthier P, Bligny R, Gout G, Mahé A, Nogués S, Hodges M, Tcherkez G (2010) In Folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current CO2 assimilation in illuminated leaves of Brassica napus. New Phytol 185:988–999
Gerhardt R, Stitt M, Heldt HW (1987) Subcellular metabolites levels in spinach leaves. Regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiol 83(2):399–407
Gessler A, Tcherkez G, Peuke A, Ghashghaie J, Farquhar G (2008) Diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant Cell Environ 31:941–953
Gessler A, Tcherkez G, Karyanto O, Keitel C, Ferrio JP, Ghashghaie J, Kreuzwieser J, Farquhar GD (2009) On the metabolic origin of the carbon isotope composition of CO2 evolved from darkened light-acclimated leaves in Ricinus Communis. New Phytol 181(2):374–386
Ghashghaie J, Badeck FW (2014) Opposite carbon isotope fractionation during dark respiration in roots vs leaves – a review. New Phytol 201:751–769
Ghashghaie J, Duranceau M, Badeck F, Cornic G, Adeline MT, Deléens E (2001) δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ 24:505–515
Ghashghaie J, Badeck FW, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem Rev 2:145–161
Ghashghaie J, Badeck FW, Girardin C, Sketriené D, Lamothe-Sibold M, Werner RA (2015) Changes in δ13C of dark respired CO2 and organic matter of different organs during early ontogeny in peanut plants. Isot Environ Healt S 51:93–108
Ghashghaie J, Badeck FW, Girardin C, Huignard C, Aydinlis Z, Fonteny C et al (2016) Changes and their possible causes in δ13C of dark-respired CO2 and bulk tissue of different organs of maize plants during early ontogeny. J Exp Bot 67(9):2603–2615
Gilbert A, Silvestre V, Robins RJ, Tcherkez G, Remaud GS (2011) A 13C NMR spectrometric method for the determination of intramolecular δ13C values in fructose from plant sucrose samples. New Phytol 191:579–588
Gilbert A, Robins R, Remaud G, Tcherkez G (2012) Intramolecular 13C-pattern in hexoses from autotrophic and heterotrophic C3 plant tissues: causes and consequences. Proc Natl Acad Sci U S A 109:18204–18209
Gleixner G, Schmidt HL (1997) Carbon isotope effects on the fructose-1,6-bisphosphate aldolase reaction, origin for a non-statistical 13C distribution in carbohydrates. J Biochem Chem 272(9):5382–5387
Gleixner G, Scrimgeour C, Schmidt HL, Viola R (1998) Stable isotope distribution in the major metabolites of source and sink organs of Solanum tuberosum L.: a powerful tool in the study of metabolic partitioning in intact plants. Planta 207:241–245
Goulden ML, McMillan AMS, Winston GC, Rocha AV, Manies KL, Harden JW, Bond-Lamberty BP (2011) Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob Chang Biol 17:855–871
Gout E, Bligny R, Pascal N, Douce R (1993) 13C nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem 268:3986–3992
Guy RD, Berry JA, Fogel ML, Hoering TC (1989) Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177:483–491
Guy RD, Fogel ML, Berry JA (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol 101:37–47
Hayes J (2001) Fractionation of the isotopes of carbon and hydrogen in biosynthetic processes. Rev Mineral Geochem 43:225–277
Heichel GH (1971) Response of respiration of tobacco leaves in light and darkness and the CO2 compensation concentration to prior illumination and oxygen. Plant Physiol 48:178–182
Hermes JD, Cleland WW (1984) Evidence from multiple isotope effect determinations for coupled hydrogen motion and tunneling in the reaction catalyzed by glucose-6-phosphate dehydrogenase. Biochemistry 23:6257–6262
Igamberdiev AU, Zhou GQ, Malmberg G, Gardestrom P (1997) Respiration of barley protoplasts before and after illumination. Physiol Plant 99:15–22
Kaplan JO, Prentice IC, Buchmann N (2002) The stable carbon isotope composition of the terrestrial biosphere: modeling at scales from the leaf to the globe. Glob Biogeochem Cy 16. https://doi.org/10.1029/2001GB001403
Kendall C, McDonnell JJ (1998) Isotope tracers in catchment hydrology. Elsevier Science, Amsterdam
Knohl A, Werner RA, Brand WA, Buchmann N (2005) Short-term variations in delta C-13 of ecosystem respiration reveals link between assimilation and respiration in a deciduous forest. Oecologia 142:70–82
Kunitake G, Stitt C, Saltman P (1959) Dark fixation of CO2 by tobacco leaves. Plant Physiol 34:123–127
Lanigan GJ, Betson N, Griffiths H, Seibt U (2008) Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiol 148:2013–2020
Lee CP, Millar AH (2016) The plant mitochondrial transportome: balancing metabolic demands with energetic constraints. Trends Plant Sci 21(8):662–678
Lehmann MM, Rinne KT, Blessing C, Siegwolf RTW, Buchmann N, Werner RA (2015) Malate as a key carbon source of leaf dark-respired CO2 across different environmental conditions in potato plants. J Exp Bot 66(19):5769–5781
Lehmann MM, Wegener F, Werner RA, Werner C (2016) Diel variations in carbon isotopic composition and concentration of organic acids and their impact on plant dark respiration in different species. Plant Biol 18(5):776–784
McDonald AE, Sieger SM, Vanlerberghe GC (2002) Methods and approaches to study plant mitochondrial alternative oxidase. Physiol Plant 116(2):135–143
McNevin DB, Badger MR, Kane HJ, Farquhar GD (2006) Measurement of (carbon) kinetic isotope effect by Rayleigh fractionation using membrane inlet mass spectrometry for CO2-consuming reactions. Funct Plant Biol 33:1115–1128
Meija J, Coplen TB, Berglund M, Brand WA, De Bièvre P, Gröning M et al (2016) Isotopic compositions of the elements 2013 (IUPAC Technical Report). Pure Appl Chem 88:293–306
Melzer E, O’Leary MH (1987) Anaplerotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol 84:58–60
Melzer E, Schmidt HL (1987) Carbon isotope effects on the pyruvate dehydrogenase reaction and their importance for relative carbon-13 depletion in lipids. J Biol Chem 262(17):8159–8164
Monson KD, Hayes JM (1980) Biosynthetic control of the natural abundance of carbon 13 at specific positions within fatty acids in Escherichia coli. J Biol Chem 255:11435–11441
Monson KD, Hayes JM (1982) Carbon isotope fractionation in the biosynthesis of bacterial fatty acids. Ozonolysis of unsaturated fatyy acids as a mean of determining the intramolecular distribution of carbon isotopes. Geochem Cosmochim Acta 46:139–149
Nalborczyk E (1978) Dark carboxylation and its possible effect on the values of δ13C in C3 plants. Acta Physiol Plant 1(1):53–58
Noguchi K, Terashima I (1997) Different regulation of leaf respiration between Spinacia oleracea, a sun species, and Alocasia odora, a shade species. Physiol Plant 101:1–7
Noguchi K, Go CS, Miyazawa SI, Terashima I, Ueda S, Yoshinari T (2001) Costs of protein turn-over and carbohydrate export in leaves of sun and shade species. Aust J Plant Physiol 28:37–47
Noor E, Haraldsdottir HS, Milo R, Fleming RMT (2013) Consistent estimation of Gibbs energy using component contributions. PLoS Comput Biol 9(7). https://doi.org/10.1371/journal.pcbi.1003098
Ocheltree TW, Marshall JD (2004) Apparent respiratory discrimination is correlated with growth rate in the shoot apex of sunflower (Helianthus annuus). J Exp Bot 55:2599–2605
Ogée J, Peylin P, Ciais P, Bariac T, Brunet Y, Berbigier P et al (2003) Partitioning net ecosystem carbon exchange into net assimilation and respiration using 13CO2 measurements: a cost-effective sampling strategy. Glob Biogeochem Cy 17. https://doi.org/10.1029/2002GB001995
Oliver DJ, Nikolau BJ, Wurtele ES (2009) Acetyl-CoA – Life at the metabolic nexus. Plant Sci 176(5):597–601
Pärnik TR, Keerberg OT (2006) Advanced radiogasometric method for the determination of the rates of photorespiratory and respiratory decarboxylation of primary and stored photosynthate under steady state photosynthesis. Physiol Plant 129:34–44
Peuke AD, Glaab J, Kaiser WM, Jeschke WD (1996) The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. IV. Flow and metabolism of inorganic nitrogen and malate depending on nitrogen nutrition and salt treatment. J Exp Bot 47(296):377–385
Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorm T, Tschoep H et al (2010) Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J 62:785–795
Priault P, Wegener F, Werner C (2009) Pronounced differences in diurnal variation of carbon isotope composition of leaf respired CO2 among functional groups. New Phytol 181(2):400–412
Reddy MM, Vani T, Raghavendra AS (1991) Light-enhanced dark respiration in mesophyll protoplasts from leaves of pea. Plant Physiol 96:1368–1371
Rendina AR, Hermes JD, Cleland WW (1984) Use of multiple isotope effects to study the mechanism of 6-phosphogluconate dehydrogenase. Biochemistry 23:6257–6262
Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN (1995) Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol 109:829–837
Roeske CA, O’Leary MH (1984) Carbon isotope effects on the enzyme catalyzed carboxylation of ribulose-1,5-bisphosphate. Biochemistry 23:6275–6284
Romek KM, Nun P, Remaud G, Sylvestre V, Sotoing Taïwe G, Lecerf-Schmidt F et al (2015) A retro-biosynthetic approach to the prediction of biosynthetic pathways from position-specific isotope analysis as shown for tramadol. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1506011112
Romek KM, Remaud G, Sylvestre V, Paneth P, Robins R (2016) Non-statistical 13C fractionation distinguishes co-incident and divergent steps in the biosynthesis of the alkaloids nicotine and tropine. J Biol Chem. https://doi.org/10.1074/jbc.M116.734087
Rossmann A, Butzenlechner M, Schmidt HL (1991) Evidence for a non-statistical carbon isotope distribution in natural glucose. Plant Physiol 96(2):609–614
Schmid KM, Ohlrogge JB (2002) Lipid metabolism in plants. In: Vance DE, Vance JE (eds) Biochemistry of lipids, lipoproteins and membranes. Elsevier, Amsterdam, pp 93–126
Schmidt HL, Gleixner G (1998) Carbon isotope effects on key reactions in plant metabolism and 13C-patterns in natural compounds. In: Griffiths H (ed) Stable Isotopes: Integration of biological, ecological and geochemical processes. Bios scientific publishers, Oxford, pp 13–25
Schnyder H, Schaufele R, Lotscher M, Gebbing T (2003) Disentangling CO2 fluxes: direct measurements of mesocosm-scale natural abundance (CO2)-C-13/(CO2)-C-12 gas exchange, C-13 discrimination, and labeling of CO2 exchange flux components in controlled environments. Plant Cell Environ 26:1863–1874
Sweetlove LJ, Beard KFM, Nunes-Nesi A, Fernie AR, Ratcliffe RG (2010) Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci 15:462–470
Tcherkez G (2010) Do Metabolic fluxes matter for interpreting isotopic respiratory signals? New Phytol 186(3):567–568
Tcherkez G, Nogués S, Bleton J, Cornic G, Badeck F, Ghashghaie J (2003) Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiol 131(1):237–244
Tcherkez G, Farquhar GD, Badeck FW, Ghashghaie J (2004) Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Funct Plant Biol 31:857–877
Tcherkez G, Mahé A, Gauthier P, Mauve C, Gout E, Bligny R, Cornic G, Hodges M (2009) In Folio respiratory fluxomics revealed by 13C isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic “cycle” in illuminated leaves. Plant Physiol 103:1147–1154
Tcherkez G, Schäufele R, Nogués S, Piel C, Boom A, Lanigan G et al (2010) On the 13C/12C isotopic signal of day and night respiration at the mesocosm level. Plant Cell Environ 33:900–913
Tcherkez G, Mahé A, Hodges M (2011a) 13C/12C fractionations in plant primary metabolism. Trends Plant Sci 16(9):499–506
Tcherkez G, Mauve C, Lamothe M, Le Bras C, Grapin A (2011b) The 13C/12C isotopic signal of day-respired CO2 in variegated leaves of Pelargonium hortorum. Plant Cell Environ 34:270–283
Tcherkez G, Boex-Fontvieille E, Mahé A, Hodges M (2012) Respiratory carbon fluxes in leaves. Curr Opin Plant Biol 15:308–314
Tcherkez G, Bathellier C, Stuart-Williams H, Whitney S, Gout E, Bligny R, Badger M, Farquhar GD (2013) D2O solvent isotope effects suggest uniform energy barriers in Ribulose-1,5-bisphosphate carboxylase/oxygenase catalysis. Biochemistry 52:869–877
Tepper N, Noor E, Amador-Noguez D, Haraldsdottir HS, Milo R, Rabinowitz J, Liebermeister W, Schlomi T (2013) Steady-state metabolite concentrations reflect a balance between maximizing enzyme efficiency and minimizing total metabolite load. PLoS One 8(9). https://doi.org/10.1371/journal.pone.0075370
Thornley JHM (2011) Plant growth and respiration re-visited: maintenance respiration defined – it is an emergent property of, not a separate process within, the system – and why the respiration: photosynthesis ratio is conservative. Ann Bot 108(7):1365–1380
Tobin AK, Bowsher CG (2005) Nitrogen and carbon metabolism in plastids: evolution, integration, and coordination with reactions in the cytosol. Adv Bot Res 42:113–165
Tovar-Mendez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. FEBS J 270:1043–1049
Tu K, Dawson T (2005) Partitioning ecosystem respiration using stable carbon isotope analyses of CO2. In: Flanagan LB, Ehleringer JR, Pataki DE (eds) Stable isotopes and biosphere-atmosphere interactions: processes and biological controls. Elsevier, Amsterdam, pp 125–153
Wegener F, Beyschlag W, Werner C (2010) The magnitude of diurnal variation in carbon isotopic composition of leaf dark respired CO2 correlates with the difference between δ13C of leaf and root material. Funct Plant Biol 37:849–858
Wehr R, Saleska SR (2015) An improved isotopic method for partitioning net ecosystem-atmosphere CO2 exchange. Agric For Meteorol 214:515–531
Werner C, Gessler A (2011) Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: a review of dynamics and mechanisms. Biogeosciences 8:2437–2459
Werner C, Hasenbein N, Maia R, Beyschlag W, Maguas C (2007) Evaluating high time-resolved changes in carbon isotope ratio of respired CO2 by a rapid in-tube incubation technique. Rapid Commun Mass Spec 21:1352–1360
Werner C, Wegener F, Unger S, Nogues S, Priault P (2009) Short-term dynamics of isotopic composition of leaf-respired CO2 upon darkening: measurements and implications. Rapid Commun Mass Spec 23(16):2428–2438
Werner RA, Buchmann N, Siegwolf RTW, Kornexl BE, Gessler A (2011) Metabolic fluxes, carbon isotope fractionation and respiration – lessons to be learned from plant biochemistry. New Phytol 191:10–15
Yakir D, Wang XF (1996) Fluxes of CO2 and water between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 380:515–517
Acknowledgements
We thank the many colleagues who shared their original data with us and contributed with fruitful discussions to the development of our concepts and approaches to use stable carbon isotopes for the study of respiration.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bathellier, C., Badeck, FW., Ghashghaie, J. (2017). Carbon Isotope Fractionation in Plant Respiration. In: Tcherkez, G., Ghashghaie, J. (eds) Plant Respiration: Metabolic Fluxes and Carbon Balance. Advances in Photosynthesis and Respiration, vol 43. Springer, Cham. https://doi.org/10.1007/978-3-319-68703-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-68703-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68701-8
Online ISBN: 978-3-319-68703-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)