Abstract
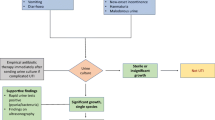
Urinary tract infection (UTI) is the most common bacterial infection. It accounts for 8.6 million visits in the ambulatory care setting in the United States. Women are at higher risk for UTI, with a self-reported annual incidence of 12%, and by the age of 32, half of them have suffered at least one UTI. Moreover UTI recurrence is high. Luckily acute pyelonephritis is much less common than cystitis, with a peak annual incidence of 25 cases per 10,000 women 15–34 years of age [1]. Infection is initiated when potential pathogens migrate from the bowel lumen to urethra and ascend to the kidneys. Accurate differentiation between uncomplicated UTI (episodes of acute cystitis and pyelonephritis in healthy premenopausal, non-pregnant woman, with no history suggesting abnormalities of urinary tract) and complicated UTI (all the other cases) is mandatory. Imaging helps in disease detection, definition its extent, reveal predisposing conditions and complications. Ultrasound is iexpensive, immediate, painless, widely available and radiation-free, so it should represents he first line imaging modality in the majority of patients. However uncomplicated UTI usually do not require imaging for diagnosis and treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Urinary tract infection (UTI) is the most common bacterial infection. It accounts for 8.6 million visits in the ambulatory care setting in the United States [1]. Women are at higher risk for UTI, with a self-reported annual incidence of 12%, and by the age of 32, half of them have suffered at least one UTI [1]. Moreover UTI recurrence is high. Most UTI infections are acute cystitis that regress rapidly with proper antibiotic therapy. Luckily acute pyelonephritis is much less common than cystitis, with a peak annual incidence of 25 cases per 10,000 women 15–34 years of age [1]. Incidence of UTI in men younger than 60 years of age is rare, but after 85, incidence duplicates but still remains half that of women. UTI is the most common cause of bacteraemia in older men [2]. UTI is really common also in children. Infection is initiated when potential pathogens migrate from the bowel lumen to urethra and ascend to the kidneys. Risk factors are different for women (sexual intercourse, use of spermicides, previous urinary tract infection and a new sex partner) and men (benign prostatic hyperplasia and increased ratio of catheterization). It is mandatory to differentiate uncomplicated UTI (episodes of acute cystitis and pyelonephritis in healthy premenopausal, non-pregnant woman, with no history suggesting abnormalities of urinary tract) and complicated UTI (all the other cases). Usually imaging is not required for diagnosis and treatment of uncomplicated UTI in adult patients. Moreover patients with conditions predisposing to infections (immunocompromised or diabetic patients) may benefit from early imaging. Imaging is used to detect disease and define its nature and extent and reveal predisposing conditions and complications.
2 Imaging Modalities
Several imaging modalities have been used to image UTIs in the past decades. Even though we will later discuss each technique, ultrasound (US) is the modality of choice for initial evaluation in children. Children should be scanned both in supine and prone position. Computed tomography (CT) and magnetic resonance (MR) are inacceptable for routine use. Even though the usage of CT is common in adult medicine, we highly recommend US as a first-line imaging modality also in adults since it is inexpensive, immediate, painless, widely available and radiation-free. CT and MR should be used as a second-line imaging modality in complicated patients.
2.1 Plain Film
Abdominal radiography is a rapid and relatively inexpensive examination, so it was used as a part of the initial examination study in patients with suspected pyelonephritis. However abdominal radiography had low sensitivity and specificity. Moreover it became the initial step of every excretory urographic study, but the successive use of CT overcame that of plain films in almost all institutions. The CT scout radiography allows the detection of urinary calcifications and gas but has several drawbacks including the unreliable differentiation of abdominal gas from the presence of gas in the UT and the difficult detection of small urinary calcification superimposed on normally calcified structures such as vertebral transverse process [3].
2.2 Intravenous Urography
Intravenous urography (IVU) had a well-established role in the evaluation of the UT; in fact it allows the visualization of the kidneys, ureters and bladder after the intravenous injection of contrast medium. Kidneys contrast medium removal (filtration) from the blood stream and excretion in the UT enable the opacification of the structures mentioned above and to image them thanks to several plain films taken at predefined image intervals as the contrast flows through the different portions of the UT. The possible imaging findings in positive IVU were a diffuse oedema and an enlargement of the affected kidneys, a delayed and attenuated nephrogram, a retarded filtration and excretion of the contrast medium which determine an effacement or a delayed filling of the renal collecting system with decreased opacity and a dilatation of the renal collecting system [4]. Nowadays IVU has increasingly been replaced by computed tomography urography (CTU) even though there is a paucity of comparative studies between the two techniques [5]. Referring physician is pleased by the large amount of data CTU provides not only on UT but also on other abdominal organs and structures, and patients prefer CTU due to the absence of preparatory bowel cleansing. Two major drawbacks are costs and radiation dose: CTU undoubtedly costs more and exposes patients to a higher dose (10–35 mSv) compared to IVU (5 mSv), but CTU data should be critically reviewed on the basis of the number of phased used, newest low kilovolt (kV) and milliampere second (mAs) protocol and automatic tube-current modulation. In conclusion literature suggests that a dose reduction is feasible technically, but exam protocols should be tailored on patient’s characteristics and clinical problems [5].
2.3 Ultrasonography
US is an inexpensive, repeatable, widely available, radiation-free imaging technique, based on properties of acoustic physic. Its widespread use makes it essential in the initial evaluation of the kidneys. Moreover, in urological pathologies, US frequently confirm clinical suspicion or even lead to final diagnosis. In US high-frequency ultrasound waves are generated within the probe, where an electric field is applied to an array of piezoelectric crystals causing them to vibrate, thus generating ultrasound waves. After being generated, waves are sent in the tissues where they interact with multiple human body surfaces being attenuated, reflected and/or refracted. The retuned echoes (to the probe) are reconverted to electrical impulses and generate images. Given the assumptions of a constant waves’ speed within human tissues (1540 m/s) and constant attenuation and waves’ straight path, different echo times are used to characterize (define the echogenicity) and localize different tissue types; the same assumptions lead also to artefact formation. Ring-down artefact (typical of air presence) occurs when US beam encounters fluid, trapped within a tetrahedron of air bubbles. It determines a repeated vibration of the air-fliud system creating a continuous echo transmitted back to the probe. A bright reflector with a continuous bright line extending posteriorly is the way this artefact is displayed [6]. Shadowing artefact is characterized by a signal void behind a high attenuating zone. In fact when the ultrasound beam encounters an area with an extremely higher attenuation, compared to the neighbouring tissues, most of the echoes are reflected, so the echoes returning from structures beyond are highly attenuated as well. Bones and calcified structures such as nephrolithiasis typically present shadowing [6].
US-Doppler imaging utilizes Doppler effect (calculating frequency shift) to imagine moving structures, usually blood, within a region of interest. Structures can be imaged, quantitatively and qualitatively, as moving towards the probe or away from it. US-Doppler imaging is helpful in vessel imaging as well as in the definition of the vascularization of a specific tissue area. Anyhow even US-Doppler imaging has its own artefact. In twinkling artefact, which is typically associated with rough hyperechoic, irregular surfaces, an alternating scintillation of colours on US-Doppler imaging is displayed beneath the hyperechoic structure. It is commonly referred to a form of intrinsic noise related to the multiple internal reflections of the incident ultrasound beam on multiple cracks, which broaden the spectrum. This phenomenon may aid in detection of renal calculi.
A proper and detailed US examination of kidneys starts with patient in supine position. At first renal length (longitudinal axis) and cortical thickness should be analysed. Even though normal right kidney mean longitudinal diameter is 10.74 ± 1.35 cm, and left kidney mean longitudinal diameter is 11.10 ± 1.15 cm, length should be related to patient’s height and phenotype. Normal renal anatomy is arranged in an outer echogenic cortex, echo-poor pyramids and the sinus. Ribs sometimes obscure some portion of kidneys. In such cases the sonographer should find a proper acoustic window locating the probe between ribs or asking the patient to breathe deeply in order to rise them up.
The recent widespread use of contrast-enhanced ultrasonography (CEUS) raised concern to an otherwise old technique; in fact at first it was described in 1968. CEUS is based on microbubble-based contrast agents (3–10 μm) composed of a shell of biocompatible materials (lipids and proteins) filled with gas (air, perfluorocarbon or sulphur hexafluoride), each of the components determine specific physical and mechanical properties. Particularly the biocompatible shell affects their capability to oscillate. In CEUS the US beam interacts with microbubbles in several different ways: at high acoustic power, microbubbles are destructed, while at low acoustic power, they produce a sound with the same frequency (f0) of the insonating beam (Fig. 7.1). At their resonant frequency, higher than the latter one, a non-linear vibration is produced, so harmonic (2f0, 3f0, etc.) and subharmonic (f0/2, f0/3, etc.) peaks are generated after the first resonance frequency peak. The mechanism underlying US contrast medium is completely different from other types of contrast media; in fact microbubbles are confined within vascular, so they do not pass in the interstice.
At low acoustic power (10-20 Kilo Pascal) microbubbles mantains the same radius after compression and relaxation so produce a sound with the same frequency of the insonating bram (higher portion of the image). At higher iacoustic power (40-50 Kilo Pascal) microbubbles interact with the US beam expanding and contracting in a non-linear mode (lower portion of the image). Both of these characteristics has been used to obtain CEUS images
Sonoelastography (SE) is an ultrasound technique to image the relative elastic/mechanical properties of soft tissue. Tissue could be imaged using strain elastography or shear-wave elastography. In strain elastography tissue is manually compressed with the probe. Speckle tracking is used to image speckle displacement, which is correlated to tissue displacement. Harder materials are deformed less, so speckle are more stable, contrary softer materials are easier to compress and speckle are displaced more. Shear-wave elastography uses US beam both to compress tissues at a specific depth and to image the propagation of shear-wave displacement in the tissue. The US equipment produce a push pulse which compress parenchyma generating low-frequency waves (low than 1000 Hz) radiating in a plane perpendicular to the image plane. Shear waves are then imaged using real-time Doppler or time of flight techniques. A specific vendor has also developed an innovative way to image larger tissue portion. Multiple push pulse are sent at different depth creating a conical wavefront that is used to image mechanical properties of larger sampled areas. However each technique has its own advantages and drawbacks. Strain elastography is simpler and cheaper, but tissue compression is subjective and deeper organs are difficult to evaluate. Moreover strain elastography is nonquantitative. It may only express the ratio of stiffness/elasticity of the pathological portion to the normal one. Shear-wave elastography does not necessitate compression, and it is more quantitative; anyhow quantitative results of different vendors are not comparable, and renal parenchymal complexity modifies shear-wave propagation. Kidneys have a very complex architecture with a multitude of tubules parallel to the papilla axis. Shear-wave velocity reduces encountering tubules and vascular structures thus altering elasticity values.
3 Acute Infection
3.1 Pyelonephritis
Pyelonephritis is defined as an infection of the renal parenchyma, calyces and renal pelvis and represents a potentially organ- and/or life-threatening infection. Usually, renal infection is caused by direct bacterial infection throughout two different routes: ascending the UT (most common) and via the bloodstream (less common). Therefore in ascending pyelonephritis, the medulla is firstly involved; contrary haematogenous spread is first seen as cortical, even though, after 48, this distinction is not feasible. Moreover in haematogenous spread, both kidneys are typically involved. Common pathogens in ascending forms are Escherichia coli and Enterococcus faecalis, two bowel organisms that frequently infect UT. Streptococci and staphylococci are the most involved organisms in haematogenous diffusion [7]. In younger patients pyelonephritis is more frequent in women [7]. Common symptoms are fever, chills, leukocytosis, unilateral or bilateral flank pain, dysuria and urinary frequency and urgency. Infrequently gastrointestinal symptoms may be seen such as abdominal pain, nausea, vomiting and diarrhoea. Elevated C-reactive protein, elevated erythrocyte sedimentation rate and leukocytosis with a neutrophilic shift are common findings at blood tests. Other typical findings are pyuria, granular or leukocyte casts, bacteriuria and positive urinary cultures. Sometimes blood cultures and urine cultures share the same bacteria. If untreated pyelonephritis often leads to renal scaring, so timely diagnosis and management is such important on patients’ outcomes. At gross pathology involved kidneys are generally enlarged and display whitish patchy areas of varying size alternating to spared parenchyma. Thin yellow streaks crossing through the medulla representing collecting ducts filled with pus may also be seen [7]. On sectioning, diffuse involvement of parenchymal surface by microabscesses may be present [7]. In cases associated with urinary obstruction, renal pelvis and calices may be dilated. Sometimes, severe infection may lead to papillary necrosis. At microscopy evaluation acute bacterial pyelonephritis is characterized by prominent neutrophilic inflammation of the renal tubules, typically sparring glomeruli and vessels [7]. There is an associated destruction of tubular basement membranes, resulting in inflammation spilling into the renal interstitium. Parenchymal involvement is often patchy, with alternating areas of intense inflammation and relatively normal-appearing zones [7].
Abdominal radiograph is a rapid, inexpensive examination that was routinely obtained as the first component of IVU. However the widespread use of CT has overtaken that of radiography. Moreover the information derived from abdominal radiograph were scarce: urinary tract gas and calcifications. IVU helped in delineating the anatomy of the UT and pelvicalyceal system. Renal enlargement, striated or delayed nephrogram, delayed calyceal appearance and dilatation or effacement of the collecting system were typical findings of acute renal infection. However only a paucity of patients had abnormal IVU findings and there was a low parenchymal detail; therefore other imaging techniques are preferred. Even though US is negative in a vast majority with suspected pyelonephritis, it is frequently used as a first-line diagnostic tool. Interstitial nephritis is not visible on routine greyscale images. Positive US findings are renal enlargement, loss of renal sinus fat due to oedema, congenital anomalies, hydronephrosis and modifications in renal echogenicity and corticomedullary differentiation system [3, 8]. The latter finding could represent haemorrhage (hyperechoic), oedema (hypoechoic) or abscess formation (Fig. 7.2). Colour Doppler improves sensitivity to parenchymal abnormality as most pyelonephritic lesions are ischaemic, and power Doppler helps in evaluating of hypoperfused areas. Several pitfalls are associated with US kidney evaluation such as differentiations of calcification from intraparenchymal or collecting system gas (manifested, respectively, as “clean” shadowing and “dirty” shadowing with echoes and reverberations) and identification of perinephric extension of infection system. The urinary bladder should always be imaged in a US evaluation for suspected pyelonephritis. Newer applications such as tissue harmonic and US contrast agents may enhance sensitivity. With tissue harmonic imaging, pyelonephritic lesions were commonly seen as focal or segmental, patchy, hypoechoic areas extending from the medulla to the renal capsule [3]. CT is the modality of choice in acute bacterial pyelonephritis. In fact it is superior to US providing detailed anatomic and physiologic information allowing the definition of both extrarenal and intrarenal pathologic conditions. However CEUS is an alternative that has been proven to be equally accurate in the detection of acute pyelonephritis [9] even though there is a paucity of literature and EFSUMB 2008 guidelines do not include complicated pyelonephritis as an indication for CEUS [10]. Parenchymal enhancement after microbubble injection is evaluated continuously. Anyhow two major phases could be considered: a cortical phase, in which there is a pronounced enhancement of the cortex (15–30 s after contrast injection), and a parenchymal phase, in which both the cortex and medullae enhance (25 s–4 min after contrast injection). Parenchymal phase may also be divided into early parenchymal phase (25 s–1 min) and late parenchymal phase (after 1 min) [11]. Typical CEUS features of pyelonephritis are a cortical or corticomedullary focal wedge-shaped or round lesion, less enhancing compared to the surrounding parenchyma [11] (Fig. 7.2), but CEUS may also demonstrate normal parenchymal enhancement. However microbubbles are not nephrotoxic so it is safe to use them, even in patients with marginal renal function as a first-line exam. US elastography has not been used yet to differentiate areas of nephritis from surrounding healthy parenchyma. However infection and inflammation determine tissue oedema and urine flow blockage due to tubular obstruction, so renal stiffness increases (Fig. 7.2). Moreover abscess formation leads to colliquation, so during early stages, when abscess cavity is not still formed, parenchyma may show reduced tissue stiffness values compared to nephritis areas. Even though its clinical utility in acute pyelonephritis is debated, alteration at renal cortex scintigraphy may be seen and may last for at least 3 months after infections. Early scintigraphic findings usually do not predict the late outcome [12]. That is why renal cortex scintigraphy is advocate in the detection of post-pyelonephritis renal scarring, even though there is no consensus between authors on the correct timing. Moreover renal cortical scintigraphy using 99mTc-DMSA has proved to be more sensitive than US and IVU and as accurate as CT and MRI in the diagnosis of acute pyelonephritis [13].
US findings in a female patient with fever, chills, and right flank pain in who right kidney pyelonephritis was suspected. A paracoronal US scan (a) showing an enlarged hyperechoic area (arrow) of parenchyma in which there is loss of corticomedullary differentiation. The corresponding color-Doppler findings (b) are an ischaemic area (arrow). CEUS (c) and SE (d) enhance diagnostic confidence confirming that the area has no enhancement (arrow in c) after microbubbles injection and is stiffer compared to the adjacent renal structures (white box in d)
3.2 Pyonephrosis and Hydronephrosis
As detailed previously, hydronephrosis, a distention and dilatation of renal pelvis and calyces, caused by an obstruction of the free urine flow, is a predisposing factor for UTI and permanent renal dysfunction. It may be unilateral or bilateral. The distention of both the ureter and the renal pelvis and calices is defined hydroureteronephrosis. Pyonephrosis represents an infected, obstructed and frequently enlarged, collecting system [3]. Symptoms are non-specific and similar to those of other UTI and include fever, chills and flank pain. It should be suspected in patients with known UT obstruction associated with flank pain and fever. Early diagnosis is mandatory because direct, immediate intervention is crucial. When pyonephrosis is left untreated, renal function deteriorates rapidly and permanently [3]. Moreover patients frequently develop septic shock. Pyonephrosis may be caused by a broad spectrum of pathologic conditions. Pathogens reach the collecting system through ascending infection or haematogenous spread. There are wide predisposing risk factors such as obstruction due to calculi, ureteropelvic junction obstruction, tumours, complications from pyelonephritis or strictures and immunosuppression (diabetes, steroids, acquired immunodeficiency syndrome).
In the past IVU was an important clinical tool in pyonephrosis in the detection of obstructive uropathy. US is valuable in the identification of pelvicalyceal system dilatation, echogenic debris, fluid-fluid levels within the collecting system and occasionally gas. The presence of debris is the most reliable sign of pyonephrosis. Furthermore US can detect calculi at the vesicoureteric junction, but the detection of ureteric calculi is more challenging [3, 8]. It is thought that the evaluation of vascular resistive indices ameliorates sensitivity, but there has been paucity of studies. Drainage of the infected collecting system can be accomplished under CT, US or fluoroscopic guidance, placing a percutaneous nephrostomy. Drainage decompresses the collecting system, allowing better renal plasma flow and delivery of antibiotics to both parenchyma and urine.
3.3 Emphysematous Pyelonephritis
Emphysematous pyelonephritis (EPN) refers to a unilateral, fulminant, necrotizing, gas forming, infection of the renal parenchyma and the perinephric tissues [14]. Renal emphysema and pneumonephritis are other terms that have been used to describe this condition; contrary emphysematous pyelitis is the presence of gas in the renal pelvis alone, without parenchymal involvement. EPN is a rare and often life-threatening condition that usually occurs in uncontrolled diabetic patients, more frequently in women [8]. Mortality is high, and without early therapeutic intervention, the condition generalizes to fulminant sepsis, and urgent nephrectomy is mandatory. Escherichia coli, Enterobacter, Klebsiella pneumoniae and Proteus mirabilis account for most of the reported cases, with Escherichia coli accounting for 60% [14]. Severely damaged, ischaemic kidneys in uncontrolled diabetic patients are the substrate on which pathogenic bacteria form gas causing mixed acid fermentation in a hyperglycaemic environment. The subsequent evolution is tissue destruction, purulent infection and inhibition of removal of locally produced gas [14].
In about 70% of patients, an abnormal collection of gas, either mottled gas within the renal fossa or crescentic collection within the Gerota fascia, could be detected in up to 85% of patients [3, 8]. US demonstrates an enlarged kidney with hyperechoic nondependent foci within the renal parenchyma or the collecting system that present distal shadowing reverberation. This characteristic feature helps in the distinction from renal calculi. Nevertheless US ability to correctly characterize EPN is low due to the presence of adjacent bowel gas or calculi that may confuse the interpretation.
3.4 Emphysematous Pyelitis
In emphysematous pyelitis (EP), the presence of gas is limited to the renal excretory system. It is a very rare and benign condition with a low overall mortality rate as compared to EPN. Thus, it is mandatory to distinguish between these two entities because of the prognostic differences and different clinical management. As in EPN, also in EP, gas production in the excretory system is secondary to acute bacterial infection [10]. EP is frequently associated with diabetes mellitus and obstruction of the UT. The diagnosis is frequently delayed due to the non-specificity of symptoms, similar to the clinical presentation of uncomplicated acute pyelonephritis [10].
Abdominal radiography demonstrates gas outlining the pelvicalyceal system and ureters. However, abdominal radiography sensitivity is low (33%), due to difficulty in differentiating renal gas from air in overlying loops of bowel [10]. At US high-amplitude nondependent flat echoes are typically within the renal sinus or calices but could be mistaken with calculi or the surrounding intra bowel air.
3.5 Renal Abscess
Renal abscess may result from an untreated or inadequately treated acute pyelonephritis that progress to tissue necrosis and liquefaction or from haematogenous spread of bacteria from a primary extrarenal focus of infection. Renal abscess has to be suspected when appropriate pyelonephritis therapy does not lead to clinical response [3]. Moreover immunocompromised, diabetic patients and those with UT obstruction are at greater risk. Gram-negative pathogens are more frequently encountered in ascending infections; conversely gram-positive organisms, such as Staphylococcus aureus, are associated with haematogenous spreading [8]. Severe renal parenchymal involvement and corticomedullary abscess could reach the renal capsule and perforate it, forming a perinephric abscess that is contained by Gerota fascia. If the UT is unobstructed, renal abscesses often drain spontaneously into the calyces and ureter [8]. It has to be kept in mind that pancreatitis, diverticulitis and Crohn’s disease may also reach the perirenal space and produce similar appearances.
At US abscess appears as hypoechoic mass with borders that become more defined as the abscess encapsulates (Fig. 7.3). Sometimes in the cavity internal echoes, representing debris, and hyperechogenic foci with acoustic shadowing, suggestive of air, may also be seen. Debris are movable with patient decubitus. Loculation and septations are other possible imaging findings. Typically there is no internal flow on colour Doppler images [3, 8]. At CEUS abscesses present as rounded or geographical areas lacking of enhancement throughout the whole exam. Some present with rim enhancement or enhancing thick septa [11]. Moreover, US- and CT-guided percutaneous placement of drains helps most of the patients to improve clinical status and is sometimes a definitive procedure [8].
US (a, b, c) and CT (d) findings in a young female patient, with known right kidney pyelonephritis, irresponsive to antibiotic treatment. A para-axial US scan demonstrating a bulky hypoechoic mass (arrow) arising from the right renal parenchyma protruding within the fat tissue of perirenal space. A para-axial (b) and a paracoronal (c) color-Doppler evaluation, showing the lack of flow signal in the mass (arrow). Axial contrast enhanced CT scan at nephroparenchymal phase confirms (arrow) the presence of an abscess, revealing a hypodense area (*) delimited by a hyperdense rim, and the involvement of the surrounding fat tissue
4 Chronic Infection
4.1 Chronic Pyelonephritis
Chronic pyelonephritis (CP) is a somewhat controversial disease that is debated on whether it reflects a long-standing infection, arises from multiple recurrent infections or represents the residuum of an old, inactive disease. In the past it was diagnosed when significant lymphocytic inflammation of the renal interstitium was noted. However, kidneys’ scarring from non-infectious conditions is also accompanied by prominent, non-specific chronic inflammation [7]. Moreover, chronic inflammation in areas of tubulointerstitial scarring cannot fully support CP diagnosis [7]. CP is usually associated with multiple urinary infections, vesicoureteral reflux or a history of past or present urinary. However it can also occur in other clinical setting, such as calculi, urinary diversion and neurogenic bladder [8]. Scarring could determine renal failure, hypertension and complications during pregnancy [8]. At gross examination of the kidney, the capsular surface demonstrates broad “U-shaped” scars. A cortical and medullary thinning underlies these scarred areas [7]. Renal pelvis is normally dilated, blunted and deformed. At microscopic findings mononuclear inflammation involves the tubulointerstitial compartment of both the cortex and medulla, with severe tubular atrophy.
IVU was once considered the imaging modality of choice; however US and CT are more sensitive in the detection of CP. There are several typical imaging findings: renal scarring, atrophy and cortical thinning, hypertrophy of sparred renal parenchyma, thickening and dilatation of calyceal system and renal asymmetry. On US scars are linear hyperechoic areas perpendicular to kidney’s surface [8]. Focal areas of fibrosis and cortical thinning may also be recognized as well as an increased echogenicity of renal pelvis due to an increased renal sinus fat [8]. Moreover a dilatation of calices and the entire collecting system may be seen due to parenchymal fibrosis [8].
4.2 Xanthogranulomatous Pyelonephritis
Xanthogranulomatous pyelonephritis (XGP) is a destructive, rare, chronic inflammatory process of the kidneys that was initially described by Schlagenhaufer in 1916. Middle-aged females (45–55 years) are more frequently affected than males in a ratio of 2:1 [15]. Even though almost all patients are symptomatic, symptoms are non-specific such as pain, fever, weight loss, lower urinary symptoms and malaise [3, 15], so XGP is commonly misdiagnosed because it mimics other pathologic conditions. Flank pain and haematuria may help in narrowing the differential diagnosis and direct imaging evaluation. The aetiology of XGP is not fully understood, but it is thought to derive from an atypical, incomplete immune response in association to a long-term UT obstruction and infection. Although calculi are not a prerequisite for the diagnosis of XGP, they are present in a wide percentage of cases; moreover staghorn type of calculi is frequent. The two most common pathogens associated with XGP are Escherichia coli and Proteus mirabilis. Three forms have been recognized: diffuse, segmental and focal. The long-lasting granulomatous immune response to recurrent infections led to a replacement of renal parenchyma with lipid-laden (foamy) macrophages [3, 15]. Loss of renal function is attributable to the severe renal inflammation rather than to UT obstruction [3]. At gross pathology affected kidneys are usually enlarged with single or multiple yellow to orange nodules, abscess, cortical scarring and atrophy and involvement of perinephric fat [15]. As detailed before in the microscopic evaluation, the inflammatory infiltrate mainly composed of xanthomatous cells, which have a foamy cytoplasm with an abundant clear to vacuolated cytoplasm and are consistent with histiocytes, is mixed with fibrosis and cholesterol clefts. The parenchyma nearby is characterized by calyceal mucosa ulceration, necrotic debris and tubular atrophy.
A large staghorn is commonly depicted in most, but not all, cases at abdominal radiographs even though it is a non-specific sign (Fig. 7.4). Renal contour enlargement and loss of the ipsilateral psoas margin are additional radiographic findings [3]. IVU demonstrates a markedly decreased renal function with an extremely retarded or absent excretion of contrast medium even at delayed imaging. US findings in diffuse XGP demonstrate an enlarged kidney, with a loss of the typical architecture and a large amorphous central echogenicity that corresponds to the staghorn calculus which generally is associated with acoustic shadowing [3, 15]. Contrary there are no specific US imaging features in focal XGP; in fact it is impossible to differentiate it from a renal abscess.
Plain film (a), US (b) and CT (c) of a young man with fever and abdominal pain; he suffered of severe perinatal brain hypoxia. Large bilateral calculi (arrows) were identified at abdominal X-ray. The one on the right side had staghorn conformation. Abdominal US (b) examination performed to rule out presence of XGP confirmed the presence of a large, hyperechoic, staghorn calculus within the right collecting system, which produced acoustic shadowing underneath. A subsequent CT scan was acquired (c) which ruled out the presence of XGP, demonstrating acute cholecystitis (not shown), but better imaged the staghorn calculus
4.3 Tubercular Infection
The genitourinary system involvement in patients with extrapulmonary tuberculosis is well known and accounts for 15–20% of patients. The correlation with pulmonary tuberculosis is debated in literature: some claim a relation between the two with a delay between pulmonary and genitourinary disease that varies from 5 to 40 years [8, 16], while other suggests that less than 50% of patients with urinary tuberculosis actually have abnormal results from chest radiography [3]. Symptoms are non-specific and range from fever, weight loss and fatigue, which are less common to dysuria, increased frequency of micturition and microscopic or macroscopic haematuria associated with back, abdominal or flank pain. Also, purified protein derivative skin testing is inconclusive in 20% of patients, and cultures of urine from affected patients may be distorted or confounded by the simultaneous presence of more common urinary pathogens yielding to a difficult diagnosis.
Renal involvement is related to haematogenous spread of mycobacterium tuberculosis, and even though dissemination is possible bilaterally, clinical involvement is usually prominent on one side. The high oxygen tension and blood flow in glomeruli and peritubular capillary beds determine an excellent environment for bacteria development and proliferation [16]. Initially small cortical granulomas, nearby glomeruli, form when host immunity prevails and disease lasts dormant for decades [3, 8, 16]. The further compromission of immune system leads granulomas to enlarge and coalesce. With ruptured capillary bacteria gain access to proximal tubules and loops of Henle creating caseating granuloma and papillary necrosis [3, 16]. Further extension in the collecting system often occurs, which generates fibrosis [3]. Moreover, if left untreated, subsequent evolution of the disease determines a loss of renal function and may spread to retroperitoneal organs including the colon [3]. In fact, the involvement of the ureter and bladder is secondary to renal involvement. Granuloma formation within the transitional epithelium could lead to fibrosis, thus to ureteral strictures and ureteral shortening and calcification [8]. It is important to specify that there is no specific imaging clue for the diagnosis of urinary tuberculosis since similar imaging findings could be caused by several other pathogens; that is the reason why it is defined as the “great imitator”. But nevertheless the presence of several abnormalities at the same time allows the correct diagnosis [4].
Several different types of calcifications may be seen at abdominal radiographs including amorphous, speckled or curvilinear patterns, “putty kidney” (calcified thick material filling a dilated collecting system) and calcium in parenchymal mass and lobar calcifications [8]. Calcifications are present in 24–44% of patients with UT tuberculosis [16]. Common findings on IVU include focal scars, dystrophic parenchymal calcifications, cavitary lesions and infundibular stenosis which lead to focal or generalized hydronephrosis [8]. Ureteral ulceration, wall thickening and focal dilatation determine a sawtooth- or corkscrew-like aspect of the ureter; the subsequent progressive fibrosis determines a straighter and more fixed appearance of the ureter, an aspect that is normally defined as “pipestem ureter” [8]. Even though US has limited use in the definition of urinary tuberculosis, two patterns have been described: an infiltrating one with higher echogenicity related to the presence of calcifications and an hydronephrotic or pyonephrotic one with dilated calices and a renal pelvis with reduced dimensions.
4.4 Human Immunodeficiency Virus Relate Infections and Nephropathy
Despite the prevention programme and their impact in controlling human immunodeficiency virus (HIV) spreading in population of some countries, the HIV-acquired immunodeficiency syndrome (AIDS) epidemic continues to grow, and by 2005 there were more than 40 million people infected worldwide [17]. Even though respiratory, neurologic and gastrointestinal involvement and imaging characteristics have been widely described, there is a lower definition of HIV renal and UT involvement and its imaging aspects. There are several different causes attributed to renal impairment in HIV patients: HIV acquired nephropathy, opportunistic infections, drug-related renal disease (especially in patients treated with HAART antiviral therapy), neoplasia and vascular causes [17]. The disease-related reduction in T-helper lymphocytes cells and the subsequent immunosuppression make AIDS patients highly susceptible of opportunistic fungal infections, such as Pneumocystis jirovecii, although P. jirovecii most frequently infects lung, haematogenous and lymphatic spread occur in 1% of patients [17]. Histological evaluation of kidneys infected with P. jirovecii demonstrated multiple calcific nodules particularly in renal cortex, representing areas of pathogen infiltration and consequent destruction of renal tubules [17].
Focal areas of increased echogenicity have been described in the renal cortex and medulla [18] but also in the liver, spleen, pancreas and adrenal gland. At CT evaluation calcification was described in the renal cortex [17]. However all these findings were non-specific for the diagnosis of P. jirovecii infection and in fact were subsequently described also in Mycobacterium avium infection and Cytomegalovirus.
Other fungal infections associated with AIDS are Candida albicans and Aspergillus infection. They can manifest as the presence of several focal microabscess in the kidney parenchyma and hydronephrosis. At histologic analysis, kidneys contain a combination of fungal spores, hyphae and pseudohyphae [17].
HIV-acquired nephropathy is a relatively new disease (the first published description was in 1984) recognized as a complication of HIV infections. HIV viruses directly infect renal epithelial cells and led to direct expression of HIV genes within those cells. It is characterized by progressive renal failure often associated with proteinuria and bland urinary sediment and has a mortality rate of 100% within 6 months from the onset of uraemia [8, 19]. HIV-related nephropathy is more frequent in patients with a CD4 cell count <200 cells/mm3. On gross specimen evaluation at autopsy, the kidneys were pale, swollen and enlarged due to the presence of several tubular microcyst distending the parenchyma [19]. Even though imaging could help in the diagnostic workup of patients, an in vivo diagnosis is only achieved with renal biopsy. At microscopic evaluation in the acute setting a severe form of collapsing focal segmental glomerulosclerosis is seen. As the disease evolves, glomeruli evolve in a tight solidified ball crowded by overlying enlarged, vacuolated visceral epithelial cells [19]. In addition to glomeruli modifications also, tubular involvement is present with atrophy, interstitial fibrosis, oedema, inflammation and widespread tubular degenerative and regenerative changes which determine distended tubules containing loose proteinaceous casts to form tubular microcysts [19].
At US kidneys appear as normal size or enlarged. The initial enlargement is on axial dimension with kidneys losing their normal aspect and gaining a bulbous shape. A high echogenic parenchyma is the most characteristic feature, with a loss of differentiation between fat renal sinus and renal cortex which have to be related with parenchymal disease [17]. Moreover the reduction of renal sinus dimensions due to renal oedema has been described in up to 49% of patients. Also a thickening of pelvicalyceal system has also been described both at US and CT [17].
4.5 Malakoplakia
Malakoplakia is a rare granulomatous condition first reported by Michaelis and Gutmann in 1902, characterized histopathologically by von Hansemann histiocytes and Michaelis-Gutmann bodies. Von Hansemann cells are large ovoid eosinophilic macrophages with intracytoplasmic bodies, the Michaelis-Gutmann bodies [20]. The aetiology is unknown; it is believed to be associated with defective bacterial digestion due to impaired macrophage function [20]. It can present in several different ways; moreover since it can occur in almost any part of the body, symptoms depend on the organ involved, thus presenting a huge diagnostic challenge. Anyhow malakoplakia is most commonly found in the genitourinary tract [20]. The incidence rate is three to four times higher in middle-aged female who develop malakoplakia than their male counterparts. The most frequently recovered organism in urine culture and upon flexible cystoscopy is Escherichia coli [16]. Moreover malakoplakia is more frequent in immunosuppressed patients. It usually affects the bladder, prostate, ureters and kidneys. Advanced disease may lead to renal failure. Treatment of malakoplakia is not well established, usually medical with surgical intervention sometimes advocated.
Since it manifests as a mucosal mass of the bladder and ureters, renal findings are those of a lower urinary obstruction. When kidneys are directly involved, an infiltrative multifocal process is typically observed [16]. Kidney lesions range from a few millimetres up to 3–4 cm and tend to coalesce. At US lesions are poorly marginated, hypoechoic lesions in a markedly enlarged, deformed, renal parenchyma. There is scarce literature evidence in the usage of nuclear medicine; however an intense accumulation of fluorine 18 fluorodeoxyglucose was noted at positron emission tomography.
4.6 Fungal Infection
If yeast-like organisms, which are Candida species for the most of times, are discovered in the urine, it is important to decide whether or not this is the clue that outlines an infection or if it represents a colonization or a contamination. A new sample of urine helps in discriminating contamination from colonization and infection. Whether patients are symptomatic or not, candiduria in critically ill patients is very serious and should be considered as a potential marker of invasive candidiasis [13]. Systemic candidiasis is a well-known cause of morbidity and mortality in critically ill, hospitalized and immunocompromised patients such as patients with AIDS and hematologic malignancies or patients treated with immunosuppressive drugs after an organ transplant. In patients at risk, a prophylactic antifungal therapy is administered in order to reduce the prevalence of Candida infection [21]. Candida invades the intestinal mucosa and infects the liver through portal circulation. Acute infection is accompanied by generic symptoms such as non-specific gastrointestinal complaints, fever and hepatosplenomegaly. After this initial phase normally, there are three different scenarios: patients die of overwhelming infection despite therapy, patients respond favourably to therapy or patients recover after candidiasis has become widespread and the resultant response to infection causes organ failure and death [21].
There is a paucity of descriptions on the aspects of US findings in renal candidiasis. The US manifestations are divided in two categories, which may coexist or may be consecutive to one another: parenchymal involvement and creation of a mycetoma (also known as fungus ball) in the collecting system [22]. In parenchymal involvement normally papillae become hyperechoic, a finding that is challenging to differentiate from nephrocalcinosis and transient hyperechogenicity of the pyramids in neonates. Moreover papillae may slough and a fungus ball, which appears as a non-shadowing hyperechoic focus, may develop in the collecting system [22]. IVU can reveal hydronephrosis, a focal mass in the collecting system or a non-functioning kidney.
4.7 Parasitic Infection
Several parasites (Plasmodium malariae and falciparum, Leishmania donovani, Trypanosoma brucei, Filarioidea, etc.) may cause renal damage and are associated with glomerular lesions [23]. Schistosomiasis or bilharzia is a tropical disease due to Schistosoma worms. The transmission involves the water where the larvae seek the skin of a suitable definitive hosts and migrate in the blood via the lungs to the liver and here transform into worms that gradually reach their perivesicular (Schistosoma haematobium) or mesenteric (other species) destination [24]. Females then produce hundreds to thousands of eggs that reach bladder or intestinal lumen and from there are eliminated in the environment. This complex life cycle determines a granulomatous inflammation, ulceration and pseudopolyposis of the bladder and ureteral walls [24]. Common signs are haematuria, dysuria, pollakiuria and proteinuria. Chronic lesions of the bladder and terminal part of ureters evolve to fibrosis or calcifications, subsequently resulting in hydroureter and hydronephrosis. Surprisingly renal function in early stages of the disease is preserved; contrary chronic compression is associated with parenchymal damage and renal failure [24].
Due to the specific involvement of the bladder, imaging findings mirror the pathologic course. Acute phase is characterized by nodular bladder wall thickening at IVU or CTU. End-stage schistosomiasis leads to bladder wall thickening, contraction and calcification; the latter is easily seen at plain radiograph. Calcifications are typically linear or curvilinear. Ureter involvement is rare and typically limited to the lower third. Findings in the affected part are the same described for the bladder walls. Fibrosis of the lower third ureters produces a partial urine obstruction. Upper parts of ureters initially compensate by dilatation hypertrophy that generates enough pressure to overcome distal obstruction. IVU and CTU may demonstrate ureteral calcifications and stenosis, ureterectasis and obstructive uropathy.
Hydatid disease is a zoonosis caused by Echinococcus granulosus’ larvae. It is endemic in many regions of the world including the Mediterranean, Africa, South America, Australia, Middle East and New Zealand [25]. Sheep, cattle and camels are the common intermediate hosts for this worm. The worm’s eggs are passed in stool and are transmitted to humans by dogs [23]. Larvae escape from eggs and penetrate the human body throughout the intestinal mucosa. From there they spread in the portal circulation. Even though the majority of larvae are filtered by the liver and the lungs, some of them reach the general circulation and involve other organs, such as the kidneys [23]. The undestroyed larvae form cysts. The wall of hydatid cyst is formed by three layers: the outermost (pericystic) is produced by modified host cell that forms in the presence of the parasite; the middle is a thin, easily ruptured, acellular, membrane that allows the passage of nutrients; and the inner is where the laminated membrane and scolices are produced [25]. Cyst fluid is a clear transudate that is antigenic if released into the host’s body as a consequence of cyst rupture causing severe reactions and anaphylaxis. Of all human organs, renal involvement is rare (2–3% of cases) and patients are often asymptomatic for several years. Most common symptoms are flank mass, pain and dysuria [25]. Renal hydatid cysts are more frequently unilateral, solitary and localized in the cortex of the superior or inferior pole. A severe, but uncommon (18%), complication is the rupture of the cysts in the collecting system with resultant renal colic and hydatiduria [25].
Abdominal radiography images a soft tissue mass that corresponds to the cyst. In a minority of cases, curvilinear or ring-shaped calcifications may be seen. IVU demonstrates infundibular and calyceal distortion [25]. US appearance of renal hydatid cysts may vary between a unilocular simple renal cyst and multiseptated daughter cysts. Three signs raise suspicion on a cyst: a thick, bilayered wall, the “falling snowflake” which is the presence of multiple echogenic foci produced by hydatid sand that modify their locations as the patients change position and a “floating membrane” that represents the detachment of the endocyst from the pericyst [25].
References
Hooton T (2012) Uncomplicated urinary tract infection. N Engl J Med 366:1028–1037
Solomon CG, Schaeffer AJ, Nicolle LE (2016) Urinary tract infections in older men. N Engl J Med 374(6):562–571. https://doi.org/10.1056/NEJMcp1503950
Craig WD, Wagner BJ, Travis MD (2008) From the archives of the AFIP: pyelonephritis: radiologic-pathologic review. Radiographics 28:255–276
Quaia E (2014) In: Quaia E (ed) Radiological imaging of he kidney, 2nd edn. Springer, Heidelberg
Stacul F, Rossi A, Cova MA (2008) CT urography: the end of IVU? Radiol Med 113(5):658–669
Feldman MK (2009) US artifacts 1. Radiographics 29(4):1179–1189
Hou J, Herlitz LC (2014) Renal infections. Surg Pathol Clin 7(3):389–408
Browne RFJ, Zwirewich C, Torreggiani WC (2004) Imaging of urinary tract infection in the adult. Eur Radiol Suppl 14(3):168–183
Mitterberger M, Pinggera GM, Colleselli D et al (2008) Acute pyelonephritis: comparison of diagnosis with computed tomography and contrast-enhanced ultrasonography. BJU Int 101(3):341–344
Roy C, Pfleger DD, Tuchmann CM, Lang HH, Saussine CC, Jacqmin D (2001) Emphysematous pyelitis: findings in five patients. Radiology 218:647–650
Fontanilla T, Minaya J, Corteás C et al (2012) Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging 37(4):639–646
Rossleigh MA (2001) Renal cortical scintigraphy and diuresis renography in infants and children. J Nucl Med 42(1):91–95
Kauffman CA, Fisher JF, Sobel JD, Newman CA (2011) Candida urinary tract infections - diagnosis. Clin Infect Dis 52(SUPPL. 6):S452–S456
Laway BA, Bhat MA, Bashir MI, Ganie MA, Mir SA, Daga RA (2012) Conservative management of emphysematous pyelonephritis. Indian J Endocrinol Metab 16(2):303–305
Li L, Parwani AV (2011) Xanthogranulomatous pyelonephritis. Arch Pathol Lab Med 135(5):671–674
Wong A, Dhingra S, Surabhi VR (2012) AIRP best cases in radiologic- pathologic correlation genitourinary tuberculosis. Radiographics 32(3):839–844
Symeonidou C, Standish R, Sahdev A, Katz RD, Morlese J, Malhotra A (2008) Imaging and Histo- pathologic features of HIV-related renal disease. Radiographics 28(5):1339–1354
Kay CJ (1992) Renal diseases in patients with AIDS: sonographic findings. AJR Am J Roentgenol 159(3):551–554
Wyatt CM, Klotman PE, D’Agati VD (2009) HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin. Nephrol. 28(6):513–522
Dong H, Dawes S, Philip J, Chaudhri S, Subramonian K (2015) Malakoplakia of the urogenital tract. Urol Case Reports 3(1):6–8
Moore NJE, Leef JL, Pang Y (2003) Systemic candidiasis. Radiographics 23(5):1287–1290
Daneman A, Navarro OM, Somers GR, Mohanta A, Jarrín JR, Traubici J (2010) Renal pyramids: focused sonography of normal and pathologic processes. Radiographics 30(5):1287–1307
Van Velthuysen MLF, Florquin S (2000) Glomerulopathy associated with parasitic infections. Clin Microbiol Rev 13(1):55–66
Gryseels B, Polman K, Clerinx JKL (2006) Human schistosomiasis. Lancet 368(9541):1106–1118
Ishimitsu DN, Saouaf R, Kallman C, Balzer BL (2010) Best cases from the AFIP: renal hydatid disease. Radiographics 30(2):334–337
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Quaia, E., Gennari, A.G., Cova, M.A. (2018). Ultrasound of Upper Urinary Tract Infections. In: Tonolini, M. (eds) Imaging and Intervention in Urinary Tract Infections and Urosepsis. Springer, Cham. https://doi.org/10.1007/978-3-319-68276-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-68276-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68275-4
Online ISBN: 978-3-319-68276-1
eBook Packages: MedicineMedicine (R0)