Abstract
Therapeutic management of the cancer patients with symptoms of acute coronary syndrome has to be tailored to patient’s comorbidities while balancing potential risks of invasive revascularization. Careful selection of patients with ischemia-inducing stenosis necessitating cardiac catheterization is required to avoid hazardous complications in cancer patients with good prognosis. In general in patients with acute coronary syndrome, an early invasive strategy (coronary angiography and percutaneous coronary intervention or coronary artery bypass graft) is superior to a conservative strategy of optimum medical treatment alone. Intraprocedural tools for lesion assessment (intravascular ultrasonography, optical coherence tomography) allow a better characterization of the luminal processes and assessment of the hemodynamic impact of the lesion. A fractional flow reserve of >0.75 permits postponing stent placement and prompt continuation on anticancer therapy with no increased mortality risk. Special considerations have to be made in respect to primary or acquired thrombocytopenia, the increased propensity to thrombosis associated with cancer as a pro-inflammatory state, and the potential drug interactions. The use of percutaneous coronary angiography with either bare metal stents or drug eluting stents requires combined antiplatelet therapy (aspirin and P2Y12 inhibitors) to prevent early stent thrombosis. Significant collaborative efforts between cardiologists and hematologists/oncologists is of prime importance in order to optimize the care of oncology patients and increase overall survival.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Coronary artery disease (CAD)
- Cancer
- Thrombocytopenia
- Percutaneous coronary intervention (PCI)
- Fractional flow reserve (FFR)
- Intravascular ultrasound (IVUS)
- Optical coherence tomography (OCT)
- Coronary artery bypass graft (CABG)
- Cardiotoxicity
- Takotsubo syndrome
Introduction
Ischemic heart disease in cancer patients can either be longstanding or as consequence of the exposure to “cardiotoxic” therapies (chemotherapy, radiation) along with the hypercoagulable state created by malignancies [1].
Unique issues arise when this patient population develops the need for interventional cardiovascular procedures such as indications, timing of the procedure, additional comorbidities including thrombocytopenia and paraneoplastic disease, vascular access choice, etc. Often, a combined medical and interventional approach is required in order to best balance the risk-benefit profile of these patients [2].
This chapter highlights the treatment approaches to cancer patients with acute myocardial infarction, giving emphasis to percutaneous coronary interventions (PCI) and the importance of intraprocedural tools to improve outcomes in this patient population.
In this chapter we will focus on the following:
-
1.
Management of Myocardial Infarction in Cancer Patients: General Considerations
-
2.
Coronary Interventions
-
Intraprocedural tools for lesion assessment
-
Fractional Flow Reserve (FFR)
-
Intravascular Ultrasound (IVUS)
-
Optical Coherence Tomography (OCT)
-
-
Left main coronary artery disease
-
-
3.
Takotsubo stress cardiomyopathy
-
4.
Special considerations in patients with thrombocytopenia
Management of Myocardial Infarction in Cancer Patients: General Considerations
Therapeutic management of the cancer patients with symptoms of acute myocardial infarction (MI) pose significant challenges. Tailored treatment to patient’s comorbidities and balancing potential risks are recommended. The available treatment options for MI in cancer patients (aspirin, β blockers, statins, percutaneous coronary intervention (PCI) without stenting, PCI with bare metal stent or drug eluting stent, CABG) rely on studies done in general population, as evidence-based treatments lack for this particular group of patients [2]. In one study in which very few patients underwent coronary intervention, medical therapy with aspirin and beta blockers improved survival [3]. In another study, cardiac death at 1-year was found to be similar in cancer patients and non-cancer patients, probably as result of early reperfusion therapy with coronary intervention [4].
Early invasive approach versus conservative management provide conflicting results and controversies still persist in the literature [4]. The traditional approach in cancer patients with symptoms of acute coronary syndrome (ACS) includes an intense medical management and risk stratification using non-invasive means to identify patients who may need coronary angiography [2]. However, reports have shown that higher risk patients appear to benefit from an early invasive strategy [5]. Results of a recently published randomized controlled multicenter trial including only patients aged ≥80 years presenting with non-ST elevation myocardial infarction (NSTEMI) or unstable angina (UA), showed an early invasive strategy (coronary angiography and subsequent treatment with percutaneous coronary intervention, coronary artery bypass graft) being superior to a conservative strategy of optimum medical treatment alone in the reduction myocardial infarction, need for urgent revascularization, stroke, or death [6]. Early angiography (within 24 h) speeds revascularization, prevents occurrence of further complications of ACS, and facilitates early discharge [7].
Current guidelines in general population recommend early invasive strategy for higher risk patients based on TIMI score and/or GRACE (Global Registry of Acute Coronary Events) risk predictor model [8]. Additional recommendations include [8]:
-
Patients with NSTEMI who have refractory angina or hemodynamic or electrical instability.
-
Signs or symptoms of heart failure or new or worsening mitral regurgitation.
-
Sustained ventricular tachycardia or ventricular fibrillation
The initial step embraces risk stratification by MDACC Risk Score. Presence of ≥3 of these factors favors early angiography (ideally within 24 h) with possible revascularization by percutaneous intervention or bypass surgery. If none of the above, patients should begin intensive medical management according to current cardiovascular guidelines. The MD Anderson Cancer Center approach in cancer patients is shown in Fig. 8.1.
Placement of IABP should be considered in patients with cardiogenic shock or with hemodynamic instability until revascularization can be done or in patients with recurrent ischemia despite maximal medical treatment [2]. Recent data from MD Anderson Cancer Center on patients diagnosed with acute myocardial infarction showed that cancer patients are more likely to benefit from aggressive medical therapy, with a significant overall survival improvement with the use of aspirin and beta-blockers [3]. The study also reported that patients with hematological malignancies have worse outcomes than patients with solid tumors [3].
If angina symptoms cannot be controlled with optimal medical therapy and further pain palliation is required, PCI or Coronary artery bypass graft (CABG) should be considered as further options (Figs. 8.2 and 8.3). When invasive revascularization is considered, the choice between PCI and CABG is a matter of debate. The general condition of the patient, stage of malignancy and severity of the cardiac disease are factors that influence the decision. CABG is preferred when patients have a good outcome and a potentially curable malignancy, while PCI is reserved for more aggressive disease [2]. If PCI is the option, balloon angioplasty, stenting with implantation of BMS (bare metal stent) or DES (drug eluting stent) are possibilities available. However, cancer patients with coronary artery disease and BMS placed have an increased risk of stent thrombosis compared to general population [9]. In cancer patients with normal platelet counts and no other contraindications, dual antiplatelet therapy with Aspirin and Clopidogrel is recommended for all patients with acute MI [10]. Early discontinuation of Clopidogrel has been associated with subacute and late stent thrombosis and recurrent myocardial infarction. Cancer patients with bare metal stents (BMS) appear to have a higher risk of stent thrombosis compared to the general population with most events in patients on DAPT. This risk could be enhanced by the pro-inflammatory state in cancer and susceptibility for clotting. Some of the chemotherapeutic drugs are thrombogenic (cisplatinum and thalidomide) or might induce thrombocytopenia causing concerns about the need to use the platelet-suppressing agents [11]. Moreover, re-endothelialization after implantation of stent can take longer in cancer patients under chemotherapeutic regimen. Postponement of any non-cardiac surgery is suggested to be done for 6 weeks up 3 months after implantation of BMS and 6–12 months after DES [2].
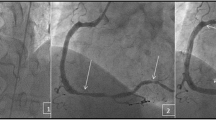
(Panel a–d) Sixty-eight year old man with stage IIIB non-small cell lung cancer on chemo-radiation therapy was admitted with chest pain and elevated troponin levels. (a) A complete total occlusion (CTO) of the Left Circumflex artery; (b) left circumflex artery angioplasty; (c) stent deployment in the left coronary artery; (d) flow restoration of the left coronary artery
(Panel a–c) Sixty-one-year-old male with a past medical history of hypertension, multiple sclerosis, nonsmall-cell lung cancer diagnosed in 2004, status post multiple chemotherapy regimens with variable response, who was started on experimental chemotherapy. (a) A subtotal occlusion of the proximal to mid left descending artery (LAD); (b) LAD with residual >60% stenosis after balloon angioplasty (POBA), stenting was required; (c) flow restoration of the LAD with <10% residual stenosis after post dilation of the stent
Coronary Interventions: Intraprocedural Tools for Lesion Assessment
Fractional Flow Reserve
Interventional treatment of moderate lesions based on angiographic findings alone can be influenced by the patient’s symptoms, and stenosis severity can be overestimated [12]. There is suboptimal overlap between the degree of stenosis and coronary blood flow. Hyperemic flow may be limited in stenosis as low as 45% diameter as other factors, such as residual cross-sectional area, lesion length, and collateral circulation influence resistance and flow, and therefore, symptomatology [13].
Fractional flow reserve (FFR) represents a lesion-specific index defined as the maximum achievable blood flow in the presence of a stenosis divided by maximum flow if there was no obstructive epicardial coronary disease [14] (Fig. 8.4). FFR guided PCI is associated with less coronary events when compared with revascularization driven by angiographic aspect. FAME trial results have shown, in general population, a decreased primary composite end point of death, myocardial infarction, and repeat revascularization at 1 year, by quantifying the hemodynamic significance of the lesion by FFR [15]. The combined rate of death and myocardial infarction was also significantly reduced. Justification of FFR guided-PCI use in ischemic coronary disease has been provided by several studies; a recent meta-analysis by Nascimento et al. [16] looked at a total of 19 studies and highlights the safety in deferral of patients with normal FFR and in those receiving intervention with an abnormal FFR.
FFR measurement in 72-year-old gentleman with a longstanding history of progressive myelodysplasia syndrome and history of non-ST segment elevation myocardial infarction. Echocardiographic findings showed hypokinesis in the LAD territory. Platelet count was 15 × 109/L. Special considerations were taken to decrease bleeding risk such as radial approach and the use of micro puncture needle. Initial angiographic findings revealed a distal LAD lesion corresponding with 80% stenosis. We proceeded to measure FFR, which showed non-significant hemodynamic compromise. The patient was medically managed and was able to resume cancer therapy
Controversy in cancer patients regarding the extent of the benefit from PCI exists. Careful selection of patients with ischemia-inducing stenosis requiring revascularization and balancing risks is required in order to avoid hazardous complications in patients with good prognosis. A fractional flow reserve (FFR) of 0.80 or less in non-cancer patients reflects a hemodynamically significant stenosis with an accuracy of 90% [15].
Previous results from unpublished data on cancer patients who underwent coronary angiography and FFR measurement have shown that deferring cancer patients with FFR > 0.75 allows prompt continuation on anticancer therapy and is not related with increased mortality risk. Recommendation is to measure FFR when possible to assess hemodynamic involvement of the lesion.
Intravascular Ultrasound
Intravascular ultrasound (IVUS) due to its higher special resolution is superior to angiography alone in determining lesion severity and allows for better characterization of luminal processes (Fig. 8.5) [17]. It provides information on pre-intervention related to lesion characteristics, including vulnerable plaques, lesion severity, length, and morphology; on post-intervention optimal stent implantation for stent expansion, extension, and apposition; and on possible complications after stent implantation [18]. Recent published data suggest that IVUS-guided DES implantation decreases the rates of major cardiac events, stent thrombosis and target lesion revascularization when compared to angiography-guided PCI [19]. The use of FFR and IVUS have significantly improved detection of coronary stenosis and are frequently used to assess the severity of left main coronary coronary artery stenosis.
IVUS guided stent placement in a patient with metastatic melanoma. Patient was treated with Carboplatin and Taxol with Avastin for a year and then with GSK-MEK inhibitor. The primary cancer was located in the left neck area, for which he had a surgery and postoperative radiation. Patient had left sided neck discomfort and the troponin level was elevated. Panel a—coronary angiography shows stenosis of the proximal segment of LAD; Panel b—IVUS assessment before stent placement; Panel c—IVUS guided stent placement, Panel d—restoration of blood flow in LAD
Optical Coherence Tomography
Optical coherence tomography (OCT) is a high-resolution imaging modality that uses infrared light emission to provide cross-sectional images of tissue with a resolution of ≤10–20 μm [20]. Due to its high resolution, it enables the differentiation of the various layers of the coronary arterial vessel wall to accurately classify the tissue characteristics and identify morphological features of vulnerable plaque, such as a thin fibrous cap, lipid-rich plaque, and thrombus formation [21]. Ex vivo studies of the coronary arteries have demonstrated the accuracy of optical coherence tomography imaging for definition of plaque characteristics (revealing nearly identical images when compared with the histology) [22] (Fig. 8.6).
(Panel a–d) OCT images for preoperative evaluation in a 60 year old female with breast cancer with multiple PCI and recurrent chest pain. Panel a—Optical coherence tomography (OCT) appearance of lipid pool with overlying thin fibrous cap—the lipid core has a diffuse border and high light attenuation resulting in poor tissue penetration. This is the typical appearance of thin cap fibro-atheroma (TCFA). Panel b—OCT appearance of calcified plaques—calcified regions with a sharp border, low signal, low attenuation, permitting deeper penetration. Panel c—OCT appearance of overlying thrombus on some of the stent struts. Panel d—Optical coherence tomography images of common neointima and neoatherosclerosis. Common neointima is recognized by its high-signal. Common neointima is recognized by its high-signal intensity and homogeneous region inside stent struts. All the images were obtain from the same patient, who developed neoatherosclerosis after stent implantation
OCT has great utility in cancer patients identifying stents with adequate strut apposition and endothelialization [2]. Such findings support a decreased risk of in stent thrombosis and help guide temporary discontinuation of antiplatelet therapy to continue cancer treatment without experiencing adverse cardiovascular effects.
OCT also allows visualization of the key components of the atherosclerotic plaque that appear to confer vulnerability to rupture thickness of the fibrous cap, size of the necrotic core, and the presence of macrophages [23]. Thin fibrous cap cutoff by OCT is <65 μm [24]. Necrotic core (and the broader histopathological category of a lipid pool) is seen as a signal-poor region with poorly defined borders and fast OCT signal drop-of [23, 25]. Macrophage accumulations can sometimes be seen at the border of the fibrous cap and necrotic core, and can appear as punctate signal-rich spots that exceed the background noise of the image [25].
Left Main Coronary Assessment and Therapeutic Strategies
Left main coronary stenosis has become increasingly common in cancer survivors due to mediastinal exposure to radiation therapy [26]. Assessment of the lesion includes coronary angiography in addition to FFR and IVUS to improve the diagnostic accuracy (Fig. 8.7). There is no current data is available for invasive assessment of left main disease in cancer patients. In our experience on patients that underwent coronary angiography we have used a FFR value of >0.80 or absolute cross sectional area by IVUS of >7 mm2 for symptomatic and >6 mm2 for asymptomatic patients as cutoff criteria to defer further revascularization strategies. Interventions were deferred in 50% of patients and cancer care was resumed without interruptions.
IVUS-guided LM stenosis assessment in a 45-year-old gentleman with metastatic melanoma to the lung and liver and recurrent pulmonary edema. At that time, left heart catheterization revealed an ostial left main stenosis. The arrow points to the stenosis (Panel a). Patient was considered high-risk for bypass surgery. Decision was made to proceed with left main stenting with a drug-eluting stent (DES). Left main was stented with a Cypher drug-eluting stent 35 × 8 deployed at 14 atmospheres post-dilatation was performed using a quantum 40 × 18 inflated at 14 atmospheres with flare-up of the ostium (Panel b, c). Intravascular ultrasound post-procedure confirmed good stent apposition (Panel d). Patient was transferred to the ICU with intraaortic balloon pump and Swan Ganz catheter
Stent vs. CABG
Left main stenting has been adopted among clinical practices as a response to previously published studies which reported comparable outcomes when compared to coronary artery bypass graft (CABG) revascularization [27].
Current guidelines of LMCA disease in general population recommend PCI (class IIa recommendation) in patients with ostial or shaft disease, those with low SYNTAX score (<23), or those where PCI can be performed more rapidly and safely than CABG [28].
Takotsubo Stress Cardiomyopathy
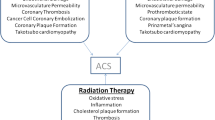
Takotsubo stress cardiomyopathy (TSC) is a syndrome characterized by transient myocardial dysfunction with unknown etiology [29]. It represents a clinical syndrome that mimics acute myocardial infarction, with indistinguishable electrocardiographic findings, as well as non-specific biomarker elevation. Recently studied data at MD Anderson Cancer Center found that almost 10% of patients with cancer who exhibited clinical characteristics of NSTEMI had TSC [30]. Physical and psychological stress have been identified as most common triggers in general population, mostly due to sympathetic activation [31]. Takotsubo syndrome is also considered to be a side effect of chemotherapeutic use for antineoplastic agents such as 5-FU, Sunitinb, Daunorubicin, Cytarabine [32]. However, in cancer patients, surgical procedures account for most of the cases (Fig. 8.8).
Researchers at the Mayo Clinic proposed the following diagnostic criteria for TSC [33]:
-
1.
Transient hypokinesis, akinesis, or dyskinesis in the left ventricular mid segments with or without apical involvement;
-
2.
Regional wall motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger;
-
3.
Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture;
-
4.
New ECG abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and,
-
5.
Absence of pheochromocytoma and myocarditis.
The gold standard for definitive differentiation between AMI and TSC is cardiac catheterization (Fig. 8.9). Identifying TSC in cancer patients is essential since they may represent a subgroup of patients that could promptly resume cancer therapy with complete ejection fraction recovery. Also identifying TSC may allow withholding antiplatelet therapy in patients who are at risk of bleeding and in the absence of confirmed diagnosis of TSC would otherwise may receive antiplatelet therapy. In absence of significant underlying comorbidities the prognosis is good. Cancer therapy should be resumed in 2–4 weeks and for long term treatment β-blockers can be used to reduce the sympathetic heart stimulation. Past experience have shown that 95% of the patients who required further oncologic treatment were able to continue it without recurrent TSC with a mean time of 21 days [34].
Angiographic study in a 70-year-old Caucasian woman with a history of marginal cell lymphoma who had undergone threecycles of Fludarabine with Mitoxantrone and presented with progressive dyspnea on exertion. She was found to have new onset systolic heart failure with regional wall motion abnormalities and elevated cardiac enzymes. (a) Coronary angiography showing clean coronaries without any signs of obstruction and (b) LV gram showing apical balloon. A diagnosis of Takotsubo cardiomyopathy was made and the patient made an unremarkable recovery and was discharged home without any sequelae
Studies on long-term prognosis of TSC have found no difference in terms of survival when compared to AMI [29], suggesting that this clinical syndrome is less benign than previously thought. Physicians should be aware and low threshold for cardiac catheterization should be considered in highly suspicious patients.
Special Considerations in Patients with Thrombocytopenia
Prevalence of thrombocytopenia (TP) varies from 10 to 25% among cancer patients, approximately 10% having platelet counts less than 100 × 109/L [35].Thrombocytopenia may be a feature of the underlying malignancy or may result from the treatment of cancer itself, and increases the risk of bleeding and other cardiac events. Chemotherapy-induced thrombocytopenia triggers spontaneous bleeding (not life threatening or intracranial) in patients with platelet counts less than 10 × 109/L [36]. .Bleeding risk in patients with TP is increased, however, low platelet counts does not protect against thrombotic events (Fig. 8.10).
A platelet count of 40–50 × 10 × 109/L should be sufficient to perform most of the interventional procedures. Safety measures during the procedure include ultrasound guidance, micro puncture needles, and fluoroscopic guidance, may contribute to the best possible outcomes [2]. 30–50 U/kg unfractionated heparin is the initial recommended dose for thrombocytopenic patients undergoing PCI who have platelets <50 × 109/L with ACT monitoring during the procedure and additional heparin administration if ACT < 250 s.
The use of antiplatelet therapy remains controversial; however, experience at MD Anderson Cancer Center in patients with thrombocytopenia and ischemic heart disease showed a significant improvement in survival among the patient cohort when therapy with ASA was added to the treatment regimen, and no bleeding complications were found [37]. This finding implies that regardless of the platelet count, antiplatelet therapy should be considered Administration of Aspirin can be used in patients with platelet counts more than 10,000/mL. Given the high risk of early stent thrombosis, dual antiplatelet therapy with Aspirin or Clopidogrel is recommended when platelet counts are >30–50 × 109/L. Usually, in cancer patients with thrombocytopenia, Clopidogrel can be administered with a 75 mg oral dose daily, after an initial loading dose of 150–300 mg [38].
DAPT may be restricted to 2 weeks following PTCA alone, 4 weeks after bare-metal stent (BMS), and 6 months after second or third generation drug-eluting stents (DES) if platelet counts are more than 50 × 109/L. Consultation with hematology/oncology specialists is recommended for severely thrombocytopenic cancer patients with MI undergoing cardiac catheterization. In MD Anderson Cancer Experience, no patient with thrombocytopenia has received GPIIb/IIIa inhibitors [10]. There is a lack of data on the use of Abciximab, Eptifibatide, Tirofiban in association with medical and invasive treatment in cancer patients with MI, while in general population a low risk of bleeding (<25%) and of thrombocytopenia (<0.5%) has been reported.
Conclusions
In cancer patients with stable coronary disease, symptoms can be managed conservatively, with medical treatment only as with general population. In contrast, in patients with severe three vessel disease involving left anterior descending artery and symptoms of UA/MI there is a critical need for revascularization. Data on the outcomes after performing invasive procedures in cancer patients with concomitant active coronary artery disease are lacking, as major clinical trials have excluded this particular group of patients. Special considerations have to be made in respect to cancer’ comorbidities such as thrombocytopenia, the increased propensity to thrombosis, and the potential drug interactions between drugs commonly used in the management of coronary disease and antineoplastic agents in cancer treatment. In order for patients to receive an appropriate treatment and avoid hazardous consequences of invasive treatment proper selection of patients who will benefit from revascularization has to be made. Detection of angiographically significant coronary disease can be made by using FFR or intravascular ultrasound (IVUS) in order to identify patients in whom interventions can be deferred. If FFR or IVUS are unavailable, optical coherence tomography (OCT) or noninvasively using cardiac PET may be considered. The use of PCI with either bare metal stents or drug eluting stents requires combined antiplatelet therapy (aspirin and P2Y12 inhibitors) to prevent early stent thrombosis. Significant collaborative efforts between cardiologists and hematologists/oncologists is of prime importance in order to optimize the care of oncology patients and increase overall survival.
Abbreviations
- ACS:
-
Acute coronary syndrome
- BMS:
-
Bare metal stents
- CABG:
-
Coronary artery bypass graft surgery
- CAD:
-
Coronary artery disease
- DAPT:
-
Dual antiplatelet therapy (aspirin and a thienopyridine)
- DES:
-
Drug eluting stents
- FFR:
-
Fractional flow reserve
- IVUS:
-
Intravascular ultrasonography
- NSTEMI:
-
Non ST elevation myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- POBA:
-
Plain balloon angioplasty
- UA:
-
Unstable angina
References
Whitlock MC, Yeboah J, Burke GL, Chen H, Klepin HD, Hundley WG. Cancer and its association with the development of coronary artery calcification: an assessment from the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4(11).
Iliescu CA, Grines CL, Herrmann J, et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv. 2016;87(5):E202–23.
Yusuf SW, Daraban N, Abbasi N, Lei X, Durand JB, Daher IN. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35(7):443–50.
Kurisu S, Iwasaki T, Ishibashi K, Mitsuba N, Dohi Y, Kihara Y. Comparison of treatment and outcome of acute myocardial infarction between cancer patients and non-cancer patients. Int J Cardiol. 2013;167(5):2335–7.
Pratap P, Gupta S, Berlowitz M. Routine invasive versus conservative management strategies in acute coronary syndrome: time for a “hybrid” approach. J Cardiovasc Transl Res. 2012;5(1):30–40.
Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387(10023):1057–65.
Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59(9):857–81.
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Gross CM, Posch MG, Geier C, et al. Subacute coronary stent thrombosis in cancer patients. J Am Coll Cardiol. 2008;51(12):1232–3.
Iliescu C, Durand JB, Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex Heart Inst J. 2011;38(3):259–60.
Krone RJ. Managing coronary artery disease in the cancer patient. Prog Cardiovasc Dis. 2010;53(2):149–56.
Takashima H, Waseda K, Gosho M, et al. Severity of morphological lesion complexity affects fractional flow reserve in intermediate coronary stenosis. J Cardiol. 2015;66(3):239–45.
Abbott JD. More than addition the use of fractional flow reserve in serial stenoses. J Am Coll Cardiol Interv. 2012;5(10):1019–20.
Pijls NHJ, Van Gelder B, Van der Voort P, et al. Fractional flow reserve: a useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183–93.
Tonino PAL, De Bruyne B, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New Engl J Med. 2009;360(3):213–24.
Nascimento BR, Belfort AF, Macedo FA, et al. Meta-analysis of deferral versus performance of coronary intervention based on coronary pressure-derived fractional flow reserve. Am J Cardiol. 2015;115(3):385–91.
Cheneau E, Leborgne L, Mintz GS, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108(1):43–7.
Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314(20):2155–63.
Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7(3):233–43.
Khandhar SJ, Yamamoto H, Teuteberg JJ, et al. Optical coherence tomography for characterization of cardiac allograft vasculopathy after heart transplantation (OCTCAV study). J Heart Lung Transplant. 2013;32(6):596–602.
Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551–5.
Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11(7):379–89.
Sinclair H, Bourantas C, Bagnall A, Mintz GS, Kunadian V. OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc Imaging. 2015;8(2):198–209.
Miyamoto Y, Okura H, Kume T, et al. Plaque characteristics of thin-cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovasc Imaging. 2011;4(6):638–46.
Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058–72.
Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract. 2011;2011:317659. doi:10.4061/2011/317659.
Buszman PE, Buszman PP, Banasiewicz-Szkróbka I, et al. Left main stenting in comparison with surgical revascularization: 10-year outcomes of the (Left Main Coronary Artery Stenting) LE MANS Trial. JACC: Cardiovasc Interv. 2016;9(4):318–27.
Dash D, Chen SL. Stenting of left main coronary artery stenosis: data to clinical practice. J Cardiovasc Dis Diagn. 2015;3:222.
Tornvall P, Collste O, Ehrenborg E, Jarnbert-Petterson H. A case-control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67(16):1931–6.
Munoz E, Iliescu G, Vejpongsa P, et al. Takotsubo stress cardiomyopathy: “good news” in cancer patients? J Am Coll Cardiol. 2016;68(10):1143–4.
Akashi YJ, Nef HM, Lyon AR. Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev Cardiol. 2015;12(7):387–97.
Fakhri Y, Dalsgaard M, Nielsen D, Lav Madsen P. 5-Fluorouracil-induced acute reversible heart failure not explained by coronary spasms, myocarditis or takotsubo: lessons from MRI. BMJ Case Rep. 2016;2016.
Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754–62.
Vejpongsa P, Banchs J, Reyes M, Iliescu G, Akinyemi M, Yusuf SW, Iliescu C. Takotsubo cardiomyopathy in cancer patients. Triggers, recovery, and resumption of therapy. J Am Coll Cardiol. 2015;65(10S):A927.
Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19(4):1137–46.
Wang J, Cai X, Cheng X, Song P, Jiang S, Gong J. Acute myocardial infarction caused by tumor-associated thrombotic thrombocytopenic purpura: case report. Med Princ Pract. 2014;23(3):289–91.
Sarkiss MG, Yusuf SW, Warneke CL, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer. 2007;109(3):621–7.
Yusuf SW, Iliescu C, Bathina JD, Daher IN, Durand JB. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Tex Heart Inst J. 2010;37(3):336–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Munoz, E., Giza, D.E., Bellera, R., Iliescu, C. (2018). Acute Coronary Syndrome in Patients with Cancer. In: Yusuf, S., Banchs, J. (eds) Cancer and Cardiovascular Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-62088-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-62088-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62086-2
Online ISBN: 978-3-319-62088-6
eBook Packages: MedicineMedicine (R0)