Abstract
Purpose of Review
Coronary artery disease in patients with active cancer presents particular challenges for clinicians, as optimum management is required in order to treat the underlying malignancy and to reduce morbidity and mortality associated with cardiovascular diseases. Special considerations must be made in respect to either primary or secondary thrombocytopenia, the presence of coagulopathies and the propensity of bleeding, vascular access complications, and increased risk of stent thrombosis.
Recent Findings
In presence of acute coronary symptoms, the cardio-oncology team has to make a complex decision between conservative medical management or early angiography (within 24 h) and revascularization. There is a lack of reliable data on the outcomes of patients with active cancer who undergo invasive procedures for the diagnostic and treatment of coronary artery disease.
Summary
Cardiac catheterization recommendations in cancer patients are being currently elaborated by cardio-oncologists in order to improve the overall survival in cancer patients with coronary artery disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer and cardiovascular disease are two dominating causes of death worldwide, being responsible for more than 70% of disease-related mortality [1]. The 5-year relative survival in cancer patients has been estimated to be greater than 80%, meaning that there is a significant risk of dying from cardiovascular diseases rather than of progression of the underlying malignancy [2]. Simultaneous occurrence of coronary artery disease (CAD) and cancer is increasingly frequent and is the result of extending cancer therapies to more elderly individuals with preexisting CAD or due to the side effects of cancer therapy. Cancer survivors and patients undergoing active oncological therapies are at risk of developing CAD and require prompt intervention for risk factor modification, early disease identification, and therapeutic intervention to improve prognosis. In order to identify the patients vulnerable to the cardiac effects of oncologic treatments and to address the problems that occur with concurrent heart disease and cancer, continuous collaborative efforts are being made between cardiologists and oncologists to recognize worse oncological outcomes in the setting of heart disease and risk factors. However, the cardiovascular outcomes in cancer patients are difficult to assess due to ongoing cycles of chemotherapy, surgical procedures, or radiation therapy. Regardless if the patients have CAD prior to cancer treatment or as a consequence of it, there are particular challenges when considering invasive evaluation and treatment in cancer patients with coronary disease. The unique issues that arise in case of an acute coronary syndrome (ACS) in cancer patients are related to technical difficulties of vascular access and to the comorbidities associated with cancer such as thrombocytopenia, coagulopathies, or paraneoplastic disease.
The intent of the present review is to address these unique issues related to the interventional approaches used by operators with increased experience in cardiac catheterization of cancer patients.
The Pathophysiology of Coronary Artery Disease in Cancer Patients
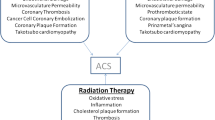
The link between acute coronary events and cancer can be the result of preexisting cardiovascular risk factors, an increased state of inflammation and hemostatic activation triggered by the underlying malignancy, and the toxicity associated with the oncological treatment. Traditional cardiovascular risk factors like increasing age, cigarette smoking, obesity, hypertension, hyperlipidemia, and physical inactivity increase the risk of CAD among cancer survivors or patients undergoing active cancer therapy [3•]. These common risk factors along with cardiac comorbidity increase the short-term and long-term cardiac mortality [4•]. Therefore, a careful and detailed past cardiac personal and family history is required to identify any underlying cardiac disease and its possible association with cancer’s pathogenesis and/or treatment. The development of CAD is strongly associated with smoking-related cancers such as lung or breast cancer, or radiation-treated cancers (i.e., Hodgkin’s lymphoma and non-Hodgkin’s lymphoma) [5,6,7]. Cancer treatment such as radiotherapy, chemotherapy, and hormonal cancer therapies increase CAD risk by direct and indirect cardiotoxic effects, such as reduced physical activity or/and metabolic changes with secondary dyslipidemia [8••].
The presence and severity of CAD is the result of an intricate pathological process that employs the interplay between lipid metabolism, inflammation, and thrombosis [9]. Elevated low-density lipoprotein cholesterol particles stimulate the inflammation process with attraction of the macrophages and smooth muscle cells and formation of plaques. These lipid-rich plaques narrow the vessel lumen, limit the flow, and produce the clinical syndrome of stable angina [10]. Plaques with a thin fibrous cap and large thrombogenic cores (thin-cap fibroatheromas) are considered high risk because are more susceptible to rupture and atherothrombosis, therefore are also called “vulnerable plaques” [11]. To offer a better structural characterization of high-risk plaques, new imaging strategies like CT coronary angiography have emerged and focus on early prediction of atherosclerotic plaque complications, in an attempt to prevent ACS during cancer therapy. The ST segment elevation myocardial infarction (STEMI) is triggered by a more stable occlusive thrombus, fibrin rich and occurs in predictable “hot spots” within the proximal third of coronary arteries [12]. In cancer patients, additional factors such as surgical intervention, chemotherapy drugs, and vascular damage induced by irradiation promote a pro-inflammatory state contributing to plaque formation process. There are multiple suggested mechanisms responsible for the cancer-associated thrombosis: the increased release of pro-inflammatory cytokines (e.g., TNF-α, IL-1) that promote endothelial damage, increased microvasculature permeability and leakage of pro-coagulating factors (platelet activating factors, tissue factor) in extravascular space, thrombin generation by tumor cells [13, 14]. Chemotherapy was also shown to be involved in arterial thromboembolism by altering the hemostatic properties of the endothelium [15]. Several antineoplastic agents have been reported to cause peripheral and coronary vascular injury, leading to myocardial infarction [16]. The effects of these therapies depend on the type and intensity of the therapeutic regimen, the characteristics of the underlying malignancy, and the overall health status of the patient undergoing oncological treatment. The impact is either immediate, manifested as spasms of the blood vessels or dysrhythmia, or later on as atherosclerosis, hypertension, heart failure, which can emerge years after treatment is completed. CAD is considered one of the long-term toxicity or late effects that can manifest months to years after successful treating cancer [17, 18]. The incidence of ischemia varies widely with the class of chemotherapeutics and the intensity/dose of the treatment. The most notorious groups of antineoplastic drugs involved are antimetabolites (Capecitabine, Flourouracil), antimicrotubule agents (Paclitaxel, Docetaxel), monoclonal antibody-based tyrosine kinase inhibitors (Bevacizumab), small molecule tyrosine kinase inhibitors (Erlotinib, Sorafenib), and platinum-containing anti-cancer drugs (Cisplatin) [8••] (Table 1). The exact mechanisms of cardiotoxicity induced by these compounds are fully elucidated only for a small part of these drug classes. Cardiac changes are seen either after the use of one class of drugs or combination of them.
One of the proposed mechanisms of induced vascular toxicity in cancer patients triggered by chemotherapy is abnormal vasoreactivity due to endothelial damage and alterations in the control of vascular smooth muscle tone (Fig. 1). 5-FU, Capecitabine, Docetaxel, and Paclitaxel are known agents that promote myocardial infarction by vasospasm in patients with or without preexisting cardiovascular risks [8••, 19, 20] (Fig. 1). Although the myocardial ischemia is reversible after treatment discontinuation, lethal outcomes have been reported. The use of Sorafenib has also been associated with coronary vasospasm in multiple vessels and poorer cardiovascular outcomes when compared with Sunitib [21]. Microvascular impairment along with vaso-functional imbalance is another mechanism proposed for Sunitinib, a member of the tyrosine kinase inhibitors group [19]. Cardiac ischemia can be triggered by chemotherapeutic agents that cause acute coronary thrombosis, like Bevacizumab, as a result of endothelial dysfunction, inflammation, platelet activation, and vascular remodeling [22]. The use of Cisplatin increases by a 1.5- to 7-fold the long-term risk of CAD and myocardial infarction, and when administered together with Bleomycin and Vinblastine can aggravate the endothelial dysfunction in multiple vascular territories [23]. Nilotinib and Ponatinib contribute to progression of atherosclerosis; Nilotinib has a preferential effect on peripheral arterial circulation, while Ponatinib is associated with a higher incidence of vascular adverse events (6.2%) including (in descending order of frequency) cardiovascular, peripheral vascular, cerebrovascular, and venous thrombotic events [24].
Coronary angiography showing vascular spasm induced by chemotherapy in a cancer patient with no significant obstructive coronary artery disease (panels 1, 2—vasospasm identified in two different cardiac catheterization before nitroglycerin administration, white arrows indicate different areas of vasospasm in the RCA; panel 3—white arrows indicate resolution of vasospasm after nitroglycerin administration)
Other chemotherapeutic regimens like androgen deprivation therapy with gonadotropin-releasing hormone (GnRH) agonists or aromatase inhibitors (anastrazole, letrozole, exemestane) were found to increase the overall cardiovascular risk by decreasing insulin sensitivity, by altering the lipid profile and by interfering with the cardio-protective characteristics of the testosterone [25, 26].
An increased incidence of atherosclerosis and CAD was associated with anterior chest or mediastinal radiation for the treatment of lung, breast, esophageal cancer, or malignant lymphomas [20]. Left anterior descending artery lesions were reported after anterior chest radiotherapy, along with fibrosis and significant stenosis of mitral and aortic valve [27]. Despite growing recognition of the importance of radiotherapy in cancer treatment, with more than 50% of the patients receiving radiation, significant mortality and morbidity due to cardiac adverse effects have been linked to radiation therapy [28]. The impact of radiation on the cardiovascular system depends on the radiation dose (>30 Gy) and the risk factors associated (young age, anterior exposure without shielding, preexisting heart disease) [29]. The rates of major coronary events were shown to increase linearly with the mean dose to the heart by 7.4% per gray (95% confidence interval, 2.9 to 14.5; P < 0.001). This increased risk starts within the first 5 years after radiotherapy and continues into the third decade after radiotherapy [30]. The adverse effects of receiving radiotherapy can be seen in a time range of weeks up to 25 years, with an increased risk of fatal myocardial infarction in first 5 to 10 years after initial therapy [20]. In experimental models, it was shown that an accelerated form of atherosclerosis with cholesterol plaques formation, thrombosis, and fibrosis of all three layers of vascular wall can occur after a few days after radiation exposure [31]. Distribution of CAD depends on the location of radiotherapy, with classic ostial location; however, lesions in mid and distal left anterior descending artery and distal diagonal branch can also be seen in patients who have received breast or chest radiotherapy [32]. There has been significant modification of the radiotherapy techniques to lower radiation doses to the heart, but CAD remains one of the most important manifestation of radiation-related cardiotoxicity. Although the medical management of these patients by now was the same as in all patients with CAD, revascularization (percutaneous or surgical)-related difficulties were encountered due to mediastinal scarring, irradiated coronary vessels, and internal mammary with higher risk of restenosis.
The increase risk of death from CAD was found to be raised up to 2-fold in patients diagnosed with Hodgkin’s disease or breast cancer and treated with radiation therapy [33]. Among the patients with breast cancer, those who received left-sided radiotherapy had a 1.56-fold (95% CI 1.27–1.90) higher risk of dying when compared to those receiving right-sided radiotherapy.
Diagnostic and Treatment of Coronary Artery Disease in Patients with Active Cancer
Compared to general population, the development of CAD in cancer patients is a unique challenge for the cardio-oncology team. The presence of malignancy can limit the use of diagnostic procedures due to increased frailty of the patients undergoing aggressive chemotherapeutic or radiation therapy, due to time constraints imposed by urgent surgical interventions or scheduled administration of chemo/radiotherapy, due to severe pancytopenic status of the patients and increased risk of developing serious infectious complications after invasive procedures. Current CAD guidelines cannot be extrapolated to cancer patients as there is a lack of reliable data on the management of cancer patients with a history of coronary artery disease. There is almost no data available from randomized clinical trials (type A data—good scientific evidence which suggests that the benefits of the clinical service substantially outweigh the potential risks; clinicians should discuss the service with eligible patients), therefore the majority level of evidence is C (at least fair scientific evidence suggests that there are benefits provided by the clinical service, but the balance between benefits and risks is too close for making general recommendations; clinicians need not to offer it unless there are individual considerations) [18, 34]. Strenuous efforts are being made by cardiologists, oncologists, surgical teams, and radiation oncology services to avoid unnecessary procedures that can interfere with cancer care and can modify the overall outcomes. The patient is continuously involved in the decision process and in composing an individualized cardiac therapy.
The initial presentation of ACS in cancer patients may be silent and can go unrecognized due to advanced age of the patients, comorbidities such as diabetes, or simply because symptoms are masked by the use of analgesics and narcotics. Patients with symptoms suggestive of ACS should have an initial diagnosis based on history, risk factors, ECG findings, cardiac biomarkers, and other laboratory data such as coagulation tests. First determination of cardiac biomarkers should be upon presentation, followed by repeated determination at every 6 or 8 h if elevated till trending downward begins [18]. Continuous ECG monitoring should be performed in parallel with measurement of cardiac biomarkers and the differential diagnosis should include other chest pain causes associated with ECG changes such as pericarditis and Tako-tsubo syndrome. Tako-tsubo syndrome, also known as stress cardiomyopathy (SC), is a clinical entity defined as a reversible and transitory ventricular dysfunction with clinical and electrocardiographic presentation similar to myocardial infarction [35]. Recent studies have identified a high prevalence of Tako-tsubo syndrome among cancer patients [36]. The pathogenic mechanisms involved are heterogeneous and multifactorial, and by now there is no clear explanation for this acquired cardiomyopathy. Emotional stress in patients confronting with a difficult cancer diagnosis, microvascular vasospasm induced by catecholamines, inadequate increase in cardiac sympathetic nervous activity, modification of cardiac adreno-receptors sensitivity by the underlying malignancy, and estrogen reduction have been all proposed as possible mechanisms [37]. Tako-tsubo syndrome has been also considered to be a side effect of chemotherapeutic use for antineoplastic agents such as 5-FU, Sunitinb, and Cytarabine [38,39,40]. Patients present with chest pain, shortness of breath, hypotension, and electrocardiographic changes that mimic ST elevation in myocardial infarction or non ST segment elevation coronary syndrome [35, 41]. In absence of significant underlying comorbidities, the prognosis is good. Cancer therapy should be resumed in 2 to 4 weeks, and for long-term treatment, β-blockers can be used to reduce the sympathetic heart stimulation. Therefore, cardiologists and oncologists should be aware of this syndrome in case of a cancer patient who develops acute cardiomyopathy and should order an ECG and rapid echocardiographic assessment.
Risk assessment and management of stable angina in cancer patients follows ACC/AHA guidelines regarding control of symptoms, prevention of atherosclerosis progression, and development of acute coronary syndrome [41]. Stress testing can be used to confirm the presence of coronary artery disease and to predict survival, if patients can tolerate and urgent revascularization is not otherwise indicated.
The first step in treating active cancer patients with symptoms of ACS is intense medical management with bed rest, administration of oxygen, opiate analgesics to relive pain, and anti-ischemic, antiplatelet/antithrombotic drugs. In patients who have no angina relief despite optimal medical therapy or in patients with high TIMI score, an invasive evaluation and treatment should be considered, along with dual antiplatelet therapy (DAPT) (ASA 81 mg and P2Y12 inhibitors) and adjusted doses of heparins for platelets <50,000/mL (Fig. 2). In a recent study published by Yusuf et al., it was shown that the lack of an appropriate medical intervention for myocardial infarction associated with cancer’s comorbidities leads to a high rate of mortality, with a 1-year survival of only 26% [42]. Therefore, invasive cardiac assessment in cancer patients is important for the evaluation and management of concomitant heart disease. Cancer patients with STEMI have significant higher mortality and morbidity rates when compared with STEMI in patients without cancer [43].
Other important recommendations for cancer patients with concomitant CAD are an aggressive medical treatment, especially with statins, and physical activity to stabilize the underlying coronary disease. The interaction between statins and cancer therapy is a matter of debate; several proposed theories suggest that statins can potentiate the antineoplastic agents and can reduce multidrug resistance [44]. However, more data on cancer patients on statins is required at this point.
Special Considerations for Cancer Patients in Interventional Cardiology
One of the initial concerns of cardiac catheterization in cancer patients is thrombocytopenia [45]. The majority of patients diagnosed with hematologic malignancies (acute leukemia, lymphoma, and multiple myeloma), as well as patients with solid tumor cancers (breast cancer, ovarian, germ cell) have thrombocytopenia either as a manifestation of their primary disease or as a consequence of the chemotherapy [46]. Prophylactic platelet transfusion in cancer patients undergoing cardiac catheterization is recommended depending on the platelet count. Transfusions are not recommended if platelet counts are greater than 10,000/mL, with the exception of necrotic tumors or in patients receiving therapy for bladder, gynecologic, colorectal tumors or melanoma who should be transfused if platelets drop under 20,000/mL—or if patients have fever, hyperleukocytosis, and coagulation abnormalities [18]. No minimum platelet level was set as an absolute contraindication to perform coronary angiogram. Most invasive procedure can be performed with comfort if no coagulation abnormalities are associated and the platelet count is around 40,000 to 50,000/mL. Percutaneous coronary intervention (PCI) can be performed with minimal bleeding risk after micropuncture access and careful hemostasis, in patients with platelet counts more than 30,000/mL [47]. Coronary artery bypass grafting (CABG) has to be reserved as an option for patients with more than 50,000/mL platelets. Aspirin can be administered to all patients with platelets greater than 10,000/mL according to SCAI guidelines without worsening the outcomes [18]. In contrast, the administration of P2Y12 agents (Clopidogrel, Ticagrelor) was reserved only for patients with more than 30,000/mL. The standard dose of heparin needs to be adjusted according with the platelet count: if platelet count is less than 50,000/mL, the initial dose of heparin is 30–50 U/kg. Duration of dual platelet therapy (DAPT) can be minimized according with the type of procedure performed and type of stent implanted: 2 weeks after balloon angioplasty, 4 weeks after bare metal stent (BMS), and 6 months after second or third generation drug-eluting stents (DES) are being placed. If bleeding occurs during or after cardiac catheterization, the patient should receive therapeutic platelet transfusions [45].
Another important aspect when considering cardiac catheterization in cancer patients is related to vascular access and potential bleeding complications at the access site. The hypercoagulable state in cancer accompanied by the specific effects of the cancer treatment on hematopoietic cells is associated with a high risk of bleeding [48]. Optimal vascular access assessment needs to be performed before deciding between femoral or radial access site [49]. Femoral access site is preferred in patients with multiple previous arterial lines, patients that underwent total mastectomy or with abnormal Allen’s test. Possible complications are retroperitoneal hemorrhage (RPH), pseudoaneurysm, arterio-venous fistula, excessive bleeding, especially in thrombocytopenic patients, local infections, and delayed epithelization after using vascular closure devices. In comparison, radial access site has a lower bleeding risk and a greater patient comfort and is preferred if patients are candidates for both access types [50]. In patients with thrombocytopenia, radial access is preferred.
The development of effective coronary artery stents has changed the way coronary artery disease is today managed. However, one of their limits is their thrombogenic status, associated with the pro-thrombotic and pro-inflammatory state in cancer [51]. If PCI is indicated, both BMS and newer-generation of DES can be used, with a preference for DES, which have lower rates of stent thrombosis, therefore a reduced length of DAPT therapy is required [52]. Given their high risk of stent thrombosis, bifurcating lesions and overlapping stents should also be avoided. Moreover, coronary angiography can overestimate the significance of ostial or side-branch lesions. Complementary procedures such as the fractional flow reserve (FFR)-guided approach (Fig. 3), intravascular ultrasound (IVUS), and optical coherence tomography (OCT) (Fig. 4) can lead to a better appreciation of coronary stenosis and the need of stent placement. A FFR of 0.80 or less indicates a hemodynamically significant stenosis with an accuracy of greater than 90% in general population [53]. FFR can determine the functional importance of the stenotic lesion and can lead to fewer interventions (PCI and CABG). It should be performed before non-urgent PCI to justify the revascularization option. However, postponing stenting in cancer patients with FFR >0.75 has not been associated with increased mortality within 1 year of procedure and cancer care. In the FAME II study, De Bruyne et al. evaluated the clinical outcomes of patients with CAD who received medical therapy alone with those who underwent PCI and received optimal medical therapy [53]. Patients who underwent PCI and optimal medical therapy had better prognosis, lower rates of urgent revascularization when compared to those who received optimal medical therapy alone. Nascimento et al. in a meta-analysis looking at a total of 19 studies underline the safety in deferral of patients with normal FFR and in those receiving intervention with an abnormal FFR [54].
OCT images showing non-occlusive ulcerative plaques. Panel A, OCT appearance of lipid pool with overlying thin fibrous cap—the lipid core has a diffuse border and high light attenuation resulting in poor tissue penetration. This is the typical appearance of thin-cap fibroatheroma (TCFA). Panel B, OCT appearance of calcified plaques—calcified regions with a sharp border, low signal, low attenuation, permitting deeper penetration
IVUS or OCT should be used to check for apposition, adequate expansion, and edge dissection, as it was shown that incomplete stent coverage, apposition, or intra-stent restenosis are common in cancer patients with CAD. IVUS has been used for a better characterization of the luminal processes, early detection of complications after procedures or a suboptimal stent expansion, malposition, incomplete lesion expansion, and residual plaque [55]. In a recent study, Jang et al. suggested that IVUS-guided DES implantation in non-cancer patient resulted in significant lower rates of major cardiac events, stent thrombosis, and target lesion revascularization [56]. OCT allows better plaque characterization due to cross-sectional view of the tissues with high resolution (≤10–20 μm) and differentiation of the various layers of the coronary arterial vessel wall. OCT is useful in detecting lipid-rich plaques and thin-cap fibroatheromas and also in periprocedural or postprocedural stent analysis [57]. IVUS and OCT procedures are especially important in cancer patients needing temporary discontinuation of platelet therapy. During PCI procedure, activated clotting time should be monitored to be greater than 250 s. Patients with severe thrombocytopenia (less than 50,000/mL) can achieve a therapeutic activated clotting time with lower doses of unfractionated heparin (30–50 U/kg).
A regimen of DAPT with Aspirin and P2Y12 inhibitors should be used in cancer patients with acute coronary syndrome and normal platelet counts. However, on dual antiplatelet therapy, most cancer patients have an increased risk of stent thrombosis when compared with patients without cancer [58]. In patients receiving chemotherapy, there is a delayed re-endothelization of the stent [59]. Moreover, some of the antineoplastic agents, such as Cisplatinum, Thalidomide, require concomitant administration of antithrombotic drugs [60, 61].
CABG is recommended when patients have a good outcome and a potentially curable malignancy, while PCI is reserved for more aggressive and metastatic disease (expected survival <1 year) [27]. Patients who received a recent cancer diagnosis (less than 6 months) and who required PCI for ACS had a threefold higher cardiac mortality when compared to those with a prior cancer diagnosis and a control group (adjusted HR 3.3, CI 1.5–7) [62]. If a non-cardiac surgery is required and the patient has indication for CABG, then both surgeries can be done simultaneously or in a two-stage procedure to reduce hospitalizations and costs, complications, and delay in treating the underlying malignancy. Another important advantage when opting for CABG is that prolonged antiplatelet therapy is not required, therefore bleeding complications are limited [27]. Patients with gastrointestinal malignancies have an increased risk of bleeding and increased rate of cardiac complications after PCI [63]. In these cases, treatment with balloon angioplasty and delayed stenting is a valid option to consider.
Conclusions
In cancer patients with symptoms of ACS, the need for revascularization is important to be assessed before receiving cancer therapy and more elaborate and expensive treatment plans, like solid organ or hematopoietic transplant. In patients with stable coronary disease, symptoms can be managed by conservative medical treatment only. In contrast, in patients with severe three-vessel disease involving left anterior descending artery, there is an urgent need for revascularization by PCI or surgery. Special considerations have to be made in regards to the additional comorbidities in cancer patients such as thrombocytopenia, increased propensity to thrombosis, and the potential interactions between drugs commonly used in the management of coronary disease and antineoplastic agents in cancer treatment. The use of PCI with either bare metal stents or drug-eluting stents requires combined antiplatelet therapy (Aspirin and P2Y12 inhibitors) to prevent early stent thrombosis. To avoid any delay in cancer treatment and perioperative cardiac complications, a complete and careful assessment of the need of invasive cardiac procedures has to be made. There is not enough data on the patient outcomes after performing invasive procedures for patients with concomitant active cancer and coronary artery disease. Therefore, significant collaborative efforts between cardiologists and hematologists/oncologists are of prime importance in order to optimize the care of oncology patients and increase overall survival.
Abbreviations
- CAD:
-
Coronary artery disease
- ACS:
-
Acute coronary syndrome
- BMS:
-
Bare metal stents
- DES:
-
Drug-eluting stents
- CABG:
-
Coronary artery bypass graft surgery
- PCI:
-
Percutaneous coronary intervention
- DAPT:
-
Dual antiplatelet therapy (aspirin and a thienopyridine)
- STEMI:
-
ST segment elevation myocardial infarction
- VEGF:
-
Vascular endothelial growth factor
- FDA:
-
Food and Drug Administration
- 5-FU:
-
5-Fluorouracil
- IVUS:
-
Intravascular ultrasonography
- OCT:
-
Optical coherence tomography
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41.
Hess CN, Roe MT, Clare RM, Chiswell K, Kelly J, Tcheng JE, et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. Journal of the American Heart Association. 2015;4(7).
• Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Survivorship: Res Pract. 2013;7(2):253–61. This is one of the early articles published on CVD risk factors in cancers survivors, which might be overlooked and may compromise long-term health and well-being.
• Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32(7):900–7. This provides evidence that smoking, obesity, poor diet, and inactivity can affect oncological outcomes and lead to increased cancer mortality is cancer survivors.
Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99(3):206–14.
Moser EC, Noordijk EM, van Leeuwen FE, le Cessie S, Baars JW, Thomas J, et al. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood. 2006;107(7):2912–9.
Jagsi R, Griffith KA, Koelling T, Roberts R, Pierce LJ. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer. 2007;109(4):650–7.
•• Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47. This paper provides new insights on the cardiotoxicity of anticancer agents that can lead to significant complications that can affect patients treated for various malignancies. In this article, commonly used chemotherapy agents, including several recently approved medications, for their propensity to cause cardiotoxicity are reviewed.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74.
Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–32.
Herrmann J, Lerman A. An update on cardio-oncology. Trends Cardiovasc Med. 2014;24(7):285–95.
Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004;110(3):278–84.
Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiol Res Pract. 2011;2011:394740.
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076–81.
Wilhelm M. Risk of venous thromboembolism in patients with cancer treated with cisplatin: a systematic review and meta-analysis. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2013;189(8):704–5.
Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol. 2015;65(25):2739–46.
Elder ME, Maclaren NK. Identification of profound peripheral T lymphocyte immunodeficiencies in the spontaneously diabetic BB rat. J Immunol. 1983;130(4):1723–31.
Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions. 2016;87(5):E202–23.
Sen F, Yildiz I, Basaran M, Ekenel M, Oz F, Kilic L, et al. Impaired coronary flow reserve in metastatic cancer patients treated with sunitinib. Journal of BUON: Official Journal of the Balkan Union of Oncology. 2013;18(3):775–81.
Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76(3):656–65.
Naib T, Steingart RM, Chen CL. Sorafenib-associated multivessel coronary artery vasospasm. Herz. 2011;36(4):348–51.
Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49(3):287–97.
Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J. 1988;9(5):552–6.
Tefferi A, Letendre L. Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Hematol. 2011;86(7):610–1.
Ziehr DR, Chen MH, Zhang D, Braccioforte MH, Moran BJ, Mahal BA, et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2015;116(3):358–65.
Nanda A, Chen MH, Braccioforte MH, Moran BJ, D’Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866–73.
Krone RJ. Managing coronary artery disease in the cancer patient. Prog Cardiovasc Dis. 2010;53(2):149–56.
Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract. 2011;2011:317659.
Bahl A, Ghoshal S, Sharma SC. Increased risk of ischemic stroke in young nasopharyngeal carcinoma patients. In regard to Lee et al. (Int J Radiat Oncol Biol Phys 2011;81:e833-e838). Int J Radiat Oncol Biol Phys. 2012;82(4):1321. author reply
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98.
Lee MS, Finch W, Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112(10):1688–96.
Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 2007;69(5):1484–95.
Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57(4):445–52.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–10.
Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz. 2010;35(4):240–3.
Burgdorf C, Kurowski V, Bonnemeier H, Schunkert H, Radke PW. Long-term prognosis of the transient left ventricular dysfunction syndrome (Tako-Tsubo cardiomyopathy): focus on malignancies. Eur J Heart Fail. 2008;10(10):1015–9.
Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602–9.
Basselin C, Fontanges T, Descotes J, Chevalier P, Bui-Xuan B, Feinard G, et al. 5-fluorouracil-induced Tako-Tsubo-like syndrome. Pharmacotherapy. 2011;31(2):226.
Baumann S, Huseynov A, Goranova D, Faust M, Behnes M, Nolte F, et al. Takotsubo cardiomyopathy after systemic consolidation therapy with high-dose intravenous cytarabine in a patient with acute myeloid leukemia. Oncol Res Treat. 2014;37(9):487–90.
Numico G, Sicuro M, Silvestris N, Mozzicafreddo A, Trogu A, Malossi A, et al. Takotsubo syndrome in a patient treated with sunitinib for renal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(24):e218–20.
Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59(9):857–81.
Yusuf SW, Daraban N, Abbasi N, Lei X, Durand JB, Daher IN. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35(7):443–50.
Kurisu S, Iwasaki T, Ishibashi K, Mitsuba N, Dohi Y, Kihara Y. Comparison of treatment and outcome of acute myocardial infarction between cancer patients and non-cancer patients. Int J Cardiol. 2013;167(5):2335–7.
Gonyeau MJ, Yuen DW. A clinical review of statins and cancer: helpful or harmful? Pharmacotherapy. 2010;30(2):177–94.
Yusuf SW, Iliescu C, Bathina JD, Daher IN, Durand JB. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Tex Heart Inst J. 2010;37(3):336–40.
Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(4):1137–46.
Iliescu C, Durand JB, Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex Heart Inst J. 2011;38(3):259–60.
Luzzatto G, Schafer AI. The prethrombotic state in cancer. Semin Oncol. 1990;17(2):147–59.
Nathan S, Rao SV. Radial versus femoral access for percutaneous coronary intervention: implications for vascular complications and bleeding. Curr Cardiol Rep. 2012;14(4):502–9.
Lo TS, Ratib K, Chong AY, Bhatia G, Gunning M, Nolan J. Impact of access site selection and operator expertise on radiation exposure; a controlled prospective study. Am Heart J. 2012;164(4):455–61.
Lee JM, Yoon CH. Acute coronary stent thrombosis in cancer patients: a case series report. Korean Circ J. 2012;42(7):487–91.
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001.
Nascimento BR, Belfort AF, Macedo FA, Sant’Anna FM, Pereira GT, Costa MA, et al. Meta-analysis of deferral versus performance of coronary intervention based on coronary pressure-derived fractional flow reserve. Am J Cardiol. 2015;115(3):385–91.
Mintz GS, Pichard AD, Kovach JA, Kent KM, Satler LF, Javier SP, et al. Impact of preintervention intravascular ultrasound imaging on transcatheter treatment strategies in coronary artery disease. Am J Cardiol. 1994;73(7):423–30.
Jang JS, Song YJ, Kang W, Jin HY, Seo JS, Yang TH, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7(3):233–43.
Staico R, Costa MA, Chamie D, Bezerra H, Armaganijan LV, Costa RA, et al. Very long-term follow-up of strut apposition and tissue coverage with Biolimus A9 stents analyzed by optical coherence tomography. Int J Cardiovasc Imaging. 2013;29(5):977–88.
Gross CM, Posch MG, Geier C, Olthoff H, Kramer J, Dechend R, et al. Subacute coronary stent thrombosis in cancer patients. J Am Coll Cardiol. 2008;51(12):1232–3.
Mauri L, Yeh RW, Kereiakes DJ. Duration of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2015;372(14):1373–4.
Salman MC, Ayhan A. Use of anti-thrombotic agents during chemotherapy for epithelial ovarian cancer. Med Hypotheses. 2006;66(6):1179–81.
Zhang S, Yang J, Jin X, Zhang S. Myocardial infarction, symptomatic third degree atrioventricular block and pulmonary embolism caused by thalidomide: a case report. BMC Cardiovasc Disord. 2015;15:173.
Velders MA, Boden H, Hofma SH, Osanto S, van der Hoeven BL, Heestermans AA, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112(12):1867–72.
Shivaraju A, Patel V, Fonarow GC, Xie H, Shroff AR, Vidovich MI. Temporal trends in gastrointestinal bleeding associated with percutaneous coronary intervention: analysis of the 1998-2006 Nationwide Inpatient Sample (NIS) database. Am Heart J. 2011;162(6):1062–8. e5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dana Elena Giza, Kostas Marmagkiolis, Elie Mouhayar, Jean-Bernard Durand, and Cezar Iliescu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
About this article
Cite this article
Giza, D.E., Marmagkiolis, K., Mouhayar, E. et al. Management of CAD in Patients with Active Cancer: the Interventional Cardiologists’ Perspective. Curr Cardiol Rep 19, 56 (2017). https://doi.org/10.1007/s11886-017-0862-x
Published:
DOI: https://doi.org/10.1007/s11886-017-0862-x