Abstract
Nuclear medicine physics is a subspecialty of medical physics. Nuclear medicine practice includes diagnostic procedures of imaging and non-imaging and radionuclide therapy protocols primarily for cancer treatment. Nuclear medicine physics is to apply physics principles and technology to support clinical nuclear medicine. Nuclear medicine is a branch of physics that utilizes nuclear technology for the diagnosis and treatment of diseases. It covers radionuclide production, interaction, detection, and imaging. It also involves radiation dosimetry and radionuclide therapy procedures.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
11.1 Introduction
Nuclear medicine physics is a subspecialty of medical physics. Nuclear medicine practice includes diagnostic procedures of imaging and non-imaging and radionuclide therapy protocols primarily for cancer treatment. Nuclear medicine physics is to apply physics principles and technology to support clinical nuclear medicine. Nuclear medicine is a branch of physics that utilizes nuclear technology for the diagnosis and treatment of diseases. It covers radionuclide production, interaction, detection, and imaging. It also involves radiation dosimetry and radionuclide therapy procedures.
Nuclear medicine is very unique, because it helps doctors view how your body is functioning. This type of imaging takes very small amounts of radioactive pharmaceuticals and follows their path and progress through your body.
In nuclear medicine procedures, chemical compounds are labeled with radio isotope to form radiopharmaceuticals. The radiopharmaceutical is administered through intravenous injection for majority of nuclear medicine studies. Some are taken in the form of gas or pill. Following the administration, these radioactive drugs can localize to specific organs or cellular receptors. Isotope attached to the molecule emits gamma ray, i.e., photons. Some of those photons interact with tissue through photoelectric absorption or Compton scattering or pair production depending on their initial energy and interaction probability. Others travel through the tissue and hit the detectors on a gamma camera. The gamma camera detects those photons and forms a distribution of the radioactivity in the patient body to generate an image. The images represent the distribution of the radiopharmaceutical, which may not necessarily be the anatomical structure of organs. This is a key difference between nuclear medicine image and X-ray CT scan. Similar to X-ray CT and other tomographic imaging modalities, nuclear medicine imaging can provide three-dimensional distribution of radiopharmaceuticals using image reconstruction to generate single photon emission computed tomography (SPECT) or positron emission tomography (PET) (Cherry et al. 2012). In recent years, multimodality imaging with combination of two or more imaging modalities has been widely used in clinical radiology, nuclear medicine, and radiation oncology such as SPECT-CT, PET-CT, and PET-MRI.
An essential function of medical physicists is providing technical support on quality assurance for imaging equipment. American College of Radiology (ACR) issues accreditation to major imaging modalities including nuclear medicine cameras: SPECT and PET-CT. Medical physicists play a key role in the ACR accreditation.
A medical physicist is also responsible for safe use of radionuclides in clinical nuclear medicine. The physicist participates in the hospital radiation safety program as divisional radiation safety officer or provides technical support to radiation safety officer and radiation safety committee to maintain regulatory compliance with radiation safety codes. It has been an ongoing effort by medical physicists to work with radiologists to reduce radiation exposure to patients during radiology procedures using X-ray or radiopharmaceuticals.
11.2 Radiation in Nuclear Medicine
Various radiations are used in nuclear medicine. Those radiations are usually obtained from radiation-emitting materials called radioisotopes or radiopharmaceuticals. Radioisotopes could be a naturally existing radioactive material like 92U238 or a stable atom of a material can be converted to a radioisotope by destabilizing it (Cherry et al. 2012; Chandra et al. 2011). The following radiations are usually used in nuclear medicine.
11.2.1 Alpha Radiation
Alpha radiation or α-rays (also called α-particles) are considered to be the helium nuclei. A helium atom consists of two protons and two neutrons inside its nucleus. Two electrons orbit around the nucleus. When these two electrons are knocked out of a helium atom, the remaining part is called an α-ray. The mass of an α-ray is Mα = 6.6447 × 10−27 kg or 4.00153 u. In terms of energy, the rest mass energy of an α-particle is 3727.4012 MeV. An α-ray carries two units of charge number Z and four units of mass number A. Therefore, when an α-ray is emitted from a radioisotope isotope (usually called parent element), the charge number of the radioisotope decreases by 2 and its mass number goes down by 4 units. Charge number of an atom represents the nature of the atom; therefore, after the emission of an α-ray, then new atom or element (usually called daughter element) is a different element based on the elements’ periodic table classification. The following reaction shows the emission of an α-ray from a radioisotope:
where X and Y represent the parent and daughter elements, respectively.
11.2.2 Beta Radiation
Beta radiation or β-rays originate from the nucleus of an unstable atom as a result of
nuclear transitions. β-rays are of two types: β − rays and β + rays.
11.2.2.1 β − Rays
Negative beta radiations or β − rays are considered to be like an electron. The charge, mass, and other properties of a β − ray are exactly the same as an electron. The only difference is in their origin. A β − ray comes out of a nucleus as a result of nuclear transitions, but an electron is obtained from an atom as a result of transitions in atomic orbits. The rest mass of a β − ray is Mβ- = 9.109 × 10−31 kg or 5.4858 × 10−4 u. In terms of energy, the rest mass energy of a β − ray is 0.511 MeV. The charge number of a β − ray is Z = −1 and its mass number is A = 0. Therefore, when a β − ray is emitted from a radioisotope isotope, the charge number of the radioisotope increases by 1 and its mass number does not change at all. Due to the change in charge number, a daughter element obtained in a β − emission is different than its parent element. The following reaction shows the emission of a β − ray from a radioisotope:
11.2.2.2 β + Rays
Positive beta radiations or β + rays are considered to be like a positron. Like the case of a β − ray and an electron, the charge, mass, and other properties of a β + ray are exactly the same as a positron. The only difference is in their origin. A β + ray comes out of a nucleus as a result of nuclear transitions, but a positron is obtained as a result of pair production, where a photon converts into an electron-positron pair in the presence of heavy nucleus. The rest mass of a β + ray is Mβ+ = 9.109 × 10−31 kg or 5.4858 × 10−4 u. In terms of energy, the rest mass energy of a β + ray is 0.511 MeV. The charge number of a β + ray is Z = +1 and its mass number is A = 0. Therefore, when a β + ray is emitted from a radioisotope isotope, the charge number of the radioisotope decreases by 1 and its mass number does not change at all. Due to the change in charge number, a daughter element obtained in a β + emission is different than its parent element. The following reaction shows the emission of a β + ray from a radioisotope:
11.2.3 Gamma Radiation
Gamma radiations or γ-rays are radiation with zero charge number and zero mass number. These radiations also originate inside the nucleus of an unstable element. These are electromagnetic radiations or photons of high energy. They travel with the speed of light and carry no rest mass. The entire mass of a γ-ray is in the form of energy. Since a γ-ray has Z = 0 and A = 0, therefore, when a γ-ray is emitted from a radioisotope isotope, both Z and A do not change at all. As a result, the parent and daughter elements are the same, but the daughter element has less energy and in many cases more stable than the parent element. The following reaction shows the emission of a γ-ray from a radioisotope:
11.2.4 Common Radioisotopes
Radioisotopes are produced by two methods. One method is called accelerator-based production method and the other method is known as nuclear reactor-based radioisotope production method (Chandra et al. 2011). Common accelerators to produce radioactive isotopes are cyclotron and linear accelerator. A cyclotron is pictured in Fig. 11.1.
A list of commonly used radioisotopes along with their uses is given Table 11.1.
11.3 Radioactive Decay Law and Activity
All radio isotopes emitting radiation follow radioactive decay law. Consider N 0 number of nuclei or atoms of parent element are present at certain instant. The material is disintegrating and losing number of nuclei. Assume that dN is the number of nuclei decayed in this time dt and N is the number left behind then mathematically:
or dN/N = − λ dtIntegrating lnN = − λt + C Using the initial condition, at t = 0, N = N 0, we get
The last equation becomes
Simplifying, we get
Equation (11.5) is called radioactive decay law. Radioactive isotopes obey radioactive decay law when emitting radiation. Moreover, λ is called radioactive decay constant. Each radioactive element has its own unique λ.
11.3.1 Activity
Activity of a radioactive element is defined as
It is a measure of how many nuclei or atoms disintegrate in a unit time. The unit of activity is curie (Ci). One Ci is defined as (Cherry et al. 2012; Christian et al. 2011)
A smaller unit of activity is becquerel (Bq):
11.3.2 Half-Life
The time taken by a radioisotope to reduce to half of its initial number of atoms is called its half-life T 1/2. A mathematical expression for half-life is obtained by replacing N = N 0/2 and t = T 1/2. Making these substitutions, we get
This natural half-life of a radioisotope is also called its physical half-life.
11.3.3 Mean Life
When a radioactive element decays, the first atom takes almost no time to decay. On the other hand, some atoms may take hours, days, and years to decay. Therefore, the idea of mean life describes the average time for which an atom survives before it decays. Mathematically, the mean life T is defined as
11.4 Examples
Knowing the activity, mass, number of atoms, volume, and other parameters of a radioisotope is very important before it can be used in nuclear medicine. The following examples give an idea how to work on finding various parameters before a radioisotope is used clinically.
Example 11.1: Calculating Mass
A patient needs iodine (I131) for treatment. What is the mass of iodine in 550 MBq of I131 used as therapy dose? Half-life of I131 is 8.04 days.
Solution
Half-life of I131 = T 1/2 = 8.04 days = 694,000 s
λ = 0.693/T 1/2 = 0.693/694,000
λ = 9.985 × 10−7 s
Activity = A = 550 MBq = 550 × 106 disint./s
Activity = A = λN = > N = A/λ
N = 550 × 106 / 9.985 × 10−7
N = 5.508 × 1014
To calculate the mass, we take help of Avogadro’s number N A = 6.02 × 1023 atoms/mole
1 mole of I131 = 131 g
6.02 × 1023 atoms of I131 has mass = 131 g
Therefore,
The mass of 5.508 × 1014 atoms of I131 = 131 × 5.508 × 1014 /6.02 × 1023
Thus, the mass of 550 MBq I131 = 1.2 × 10−7 g
Example 11.2: Calculating Mass
What is the mass of 1 × 10−3 Ci of mTc99? mTc 99 has a decay constant of
λ = 3.2 × 10−5 s−1.
Solution
λ = 3.2 × 10−5 s−1, mass =?
A = λN = 1 × 10−3 Ci
Since 1 Ci = 3.7 × 1010 disint./s, therefore
A = 1 × 10−3 × 3.7 × 1010
A = 3.7 × 107 disint./s
Using A = λN = > N = A/λ
N = 3.7 × 107/3.2 × 10−5
N = 1.156 × 1012
1 mole of mTc99 = 99 g
6.02 × 1023 atoms of mTc99 has mass = 99 g
Therefore,
The mass of 1.156 × 1012 atoms of mTc99 = 99 × 1.156 × 1012 / 6.02 × 1023
Thus, the mass of 1 × 10−3 Ci mTc99 = 1.9 × 10−11 g
Example 11.3: Calculating Activity
Find the activity of 160 g of Ga67 with half-life of 78.3 h. This source is used to inject in a patient after 2 days. What is its activity at that time?
Solution
Activity = A = λN
To find λ, we have T 1/2 = 78.3 h = 281,880 s.
λ = 0.693/T 1/2 = 0.693 / 281,880 = 2.46 × 10−6/s
To find N, we take help of N A = 6.02 × 1023 atoms per mole
67 g of Ga has number of atoms = N A = 6.02 × 1023
160 g of Ga has number of atoms = N = 160 × 6.02 × 1023/67
which gives N = 1.4376 × 1024
Activity = A = λN = 2.46 × 10−6 × 1.4376 × 1024
A = 3.536 × 1024 disint./s
Since 1 Ci = 3.7 × 1010 disint./s
Therefore, A = 3.536 × 1024 / 3.7 × 1010
or A = 9.55 × 1013 Ci
To calculate its activity after t = 2 days, we have to find N after 2 days. Using radioactive decay law,
N = N 0.e−λt
Using N 0 = 1.4376 × 1024,
t = 2 days = 48 h,
and λ = 0.693/T 1/2 = 0.693/78.3 = 0.00885 h−1, we get
N = 1.4376 × 1024 × e(− 0.00885 × 48)
which gives N = 9.4 × 1023 atoms
Therefore, the activity after 2 days is
A = λN = 0.00885 × 9.4 × 1023 = 8.319 × 1021 disintegrations/h
which gives A = 2.31 × 1018 disintegrations/s
or A = 6.243 × 107 Ci
Example 11.4: Volume of Radioisotope
A radioactive sample of mTc99 with half-life of 6 h was calibrated at 7:00 AM and contained 15 mCi/ml (555 MBq/ml) of radioactivity at that time. If this is the desired amount of radioactivity to be administered to the patient, determine the volume of the preparation which will have to be injected at 10:00 AM?
Solution
Using the radioactive decay law and replacing number of atoms by the volume of the radioactive solution,
N 0 = V 0 = 15 mCi/ml, T 1/2 = 6 h
N = V =? λ = 0.693 / T 1/2 = 0.693 / 6 × 3600
λ = 3.21 × 10−5/s
t = 10:00 AM–7:00 AM = 3 h = 10,800 s.
using N = N 0.e−λt
N 0 = (15 mCi/ml).exp.(− 3.21 × 10−5 × 10,800)
N = 10.5 mCi/ml or 388.5 MBq/ml
Amount desired = 15 mCi
Volume needed = 15 mCi / 10.5 mCi/ml
Volume needed = 1.43 ml
Example 11.5: Disposal Time
A sealed bag of contaminated waste measures 185 MBq. This bag is contaminated by radioactive I125 with half-life of 60 days. The permitted activity of I125 for waste discharge is 45,000 Bq. How long this contaminated bag should be stored before safe discharge?
Solution
T 1/2 = 60 days => λ = 0.693/60 = 0.01155 /day, t =?
N 0 = 185 × 106 Bq, N = 4.5 × 104 Bq
Using N = N 0.e−λt
Simplifying N / N 0 = e−λt
N 0 / N = eλt
ln (N 0 / N) = λt
t = ln (N 0 / N) / λ
t = [ln (185 × 106 / 4.5 × 104)] / 0.01155
t = [ln (4111.11)] / 0.01155
t = 8.32 / 0.01155
t = 720.5 days or about 2 years
11.5 Physical Half-Life, Biological Half-Life, and Effective Half-Life
Physical half-life T p 1/2
The natural half-life of a radioactive isotope (discussed already).
Biological half-life T b 1/2
The time in which half of the radioisotope is excreted by the body through metabolic activities. Bodies’ metabolic activities affect different foods and materials in a different way. Some materials can be excreted by the body with a slower rate and others can be excreted faster.
Effective half-life T e 1/2 :
The time in which a radioisotope reduces to half when both methods of decay (natural decay and biological excretion) act at the same time. Mathematically,
Example 11.6
A radioisotope is taken inside the body of a patient to treat a tumor. The physical half-life of this radioisotope is 6 h. The body excretes the isotope with a half-life of 8 h. Find the effective half-life of this radioisotope.
Solution
-
T p 1/2 = 6 h, T b 1/2 = 8 h, T e 1/2 =?
-
1/T e 1/2 = (1/T p 1/2 + 1/T b 1/2)
-
1/T e 1/2 = 1/6 + 1/8 = 14/24
-
T e 1/2 = 1.7 h
Example 11.7
The physical half-life of a radioactive material is 40 min. With what rate (biological half-life) should the body excrete the materials so that its effective half-life is one fifth of its biological half-life?
Solution
-
T p 1/2 = 40 m, T b 1/2 =?, T e 1/2 = (T b 1/2) / 6
-
Using 1/T e 1/2 = (1/T p 1/2 + 1/T b 1/2)
-
Replacing T e 1/2 by (T b 1/2) / 6
-
6/T b 1/2 = (1/T p 1/2 + 1/T b 1/2)
-
Which gives 6/T b 1/2 - 1/T b 1/2 = 1/T p 1/2
-
5/T b 1/2 = 1 / 40 or T b 1/2 = 200 m or 3 h and 20 m
11.6 Radiation Detectors in Nuclear Medicine
There are many radiation detectors which can be divided into four categories: gas-filled detectors, scintillation detectors, semiconductors, and others. These detectors are designed for detection of different particles at various energy levels (Christian et al. 2011; Buschberg et al. 2011).
Gas-filled detector has an air chamber where with incoming gamma phones deposit energy to generate electrons. Those electrons are collected through electrical field produced by applied voltage. When the voltage increases, the interaction mechanism changes from ionization, proportional, and Geiger-Müller, respectively. Thus ionization chamber and GM counter are manufactured based on their working voltages and are widely used in medical facilities. A dose calibrator is an ionization chamber. It is built as a well counter to increase geometric efficiency. It is common to have the air in the chamber be pressurized to increase detection efficiency. The dose calibrator measures every dose to be administered to patient. Thus it is essential to ensure the quality and reliability of the dose calibrator by performing routine quality assurance (Q/A) procedures which includes daily constancy, quarterly linearity, annual accuracy, and geometry upon installation and major repair. The GM counter is primarily used as a survey meter for low-level contamination of radioactivity and patient measurement. It is required to have the GM detector checked before its use and calibrated annually.
Thyroid uptake probe is a NaI (Tl) crystal in a cylindrical collimation that focuses the radiation detection to thyroid or other organ of interest. The NaI (Tl) detector is able to record low activity admitted to a patient and then distributed in the thyroid. Using the thyroid uptake measurement, the patient thyroid function could be evaluated. Quality assurance of the thyroid uptake probe includes daily constancy, quarterly energy calibration, Chi-square test, minimum detectable activity, and annual evaluation. In modern thyroid uptake system, there is a well counter attached to the same computer as the uptake probe. This well counter is built with a NaI (Tl) crystal which is manufactured as a well in geometry to increase detection efficiency. The well counter is widely used to measure a sample of blood or other body fluid containing radioactive material. It is also used in package receiving, contamination survey, etc. where low-level radioactivity is normally involved. The thyroid uptake system is not intended for high-activity measurement due to the saturation of the NaI (Tl) crystal with a large amount of photons. Quality assurance for the well counter is similar to the uptake probe.
The daily constancy test is to ensure the detectors are functioning consistently every day in clinical service. It measures a radioactive sample, like Cs-137. This sample is used every day in the same condition so that similar reading is expected after decay correction. The numbers from multiple measurements may not be identical because of statistical nature of the radiation.
Scintillation detectors have a scintillation crystal, for example, NaI (Tl), to stop the gamma ray and then convert the gamma ray energy to visible light photons. This is called scintillation process. The visible light photon then interacts with photocathode on surface of a photoelectrical multiplier (PMT) to generate electrons. Those electrons travel through multiple collisions within the PMT to be “multiplied” to form electrical signals. The scintillation crystals are used to build scintillation detectors with high detection efficiency and good energy resolution. It is also used to generate energy spectrum in multichannel analyzer. However, the most important application of the scintillation detection in nuclear medicine is for gamma photon imaging. Another form of the scintillation detection is to use liquid scintillation counter (LSC). The LSC is primarily used in beta detection since the sample has directly contacted with scintillation material to avoid absorption of beta rays by the protection coating of the scintillation crystals.
Semiconductors have the advantage of high efficiency and excellent energy resolution compared with gas-filled and scintillation detectors. However, Ge and Si are to be kept at very low temperature which makes it difficult for general purpose applications. In recent years, some semiconductor materials are introduced in nuclear medicine for detection (gamma probe) and imaging (cardiac scanner).
Film badge is used for monitoring radiation exposure. It is named film badge for its original design of a film as detection medium to record the exposure level. New generation of radiation exposure monitoring device uses optically stimulated luminescence dosimeters (OSL badges) to assess total body, eye (lens), and shallow (skin) exposures, and thermoluminescent dosimeters (TLD or ring badges) are used to monitor hand exposures. The OSL/TLD is assigned to radiation worker for a period of time (monthly or quarterly). Radiation exposure recorded on to the OSL badge is measured by stimulating the detector material and causing it to luminesce in proportion to the exposure (Table 11.2).
The TLD is collected and read by a device that measures ionizing radiation exposure by recording the intensity of visible light emitted from a crystal in the TLD detector when the crystal is heated. The intensity of light emitted is dependent upon the radiation exposure. The TLD is based on a thermoluminescent process which is different from any of the gas-filled, scintillation, or semiconductor detectors.
In nuclear medicine, survey meters, dose calibrator, and uptake probe and well counter are the most important detectors. GM counter is widely used as survey meter in nuclear medicine and other departments in a medical facility. Dose calibrator is a key element of nuclear medicine instrumentation. It is used to measure every dose before administration to patient.
11.7 Gamma Cameras in Nuclear Medicine
In gamma photon (gamma ray and X-ray) detection, scintillation crystals are used for their high detection efficiency and good energy resolution. Among the crystals, NaI(Tl) is by far the most widely used in detectors to record counts of photons similar to GM counter, in spectrometer to record the counts and energy information to generate energy spectrum, and in scanner to register events of photon emission to produce distribution (image) of the photon emission including the counts and the energy spectra. Furthermore, gamma camera can generate images without rectilinear scan but utilizing array of PMTs to record spatial information of gamma emission. The key component in a gamma camera to locate a gamma emission is the collimator (Fig. 11.2) (Bushberg et al. 2011; Chandra et al. 1992).
An Anger camera consists of collimator, scintillation crystal, array of PMTs, electronics, and display device. Gamma photons emitted from radioactive decay of the radioisotope attached to the pharmaceutical compound administered to patient are statistically independent. They are emitted randomly toward to all directions. In order to localize those photons (to define the positions of the emission from the patient body), a collimator is needed to register a photon from an emission site to a picture element of imaging metrics. This is called a pixel. The imaging metrics normally has 64 by 64, 128 by 128, 256 by 256, or 1024 by 1024 pixels in a two-dimensional image (or planar image). The position of the pixel in the metrics corresponds to the emission site of the patient body. The intensity of the pixel reflects the number of photons registered on to that pixel. A photon can only pass through a collimator hole when it is in the right direction; otherwise, it will hit the collimator hole septum and then be absorbed. Therefore the primary function of the collimator is to define the location of emission site. Since the emission site is where the radioisotope decays, and the radioisotope is attached to (labeled) the pharmaceutical, then the image is the distribution of the compound. This way, the gamma camera image provides the biological and physiological distribution of the compound to describe the physiological function. Thus the gamma camera imaging is called function imaging.
There are parallel hole, diverging hole, converging hole, and pine hole collimators in clinical nuclear medicine imaging. The parallel hole collimator is the most widely used in gamma cameras. It is made of lead. The parallel hole collimators are composed of thousands of aligned holes parallel to each other, which are formed by casting hot lead in most of collimators. The collimator conveys only those photons traveling directly along the long axis of each hole. Photons emitted in other directions are absorbed by the septum wall between the holes through photoelectric interaction. Compton scattering and septum penetration can reduce image quality of the gamma camera (Table 11.3).
In summary, gamma camera imaging involves of following:
-
1.
Injection of radio isotope-labeled radiopharmaceutical to patient.
-
2.
After certain period of time for uptake of the radiopharmaceutical, the patient is positioned on the imaging table for scan (planar or SPECT).
-
3.
Gamma rays emitted may have photoelectric interaction which leads to tissue absorption of the gamma photons or may be Compton scattered which lose some of the initial energy and change the direction of traveling. If the gamma ray energy is higher than 1.02 MeV, there may be chance to have pair production. Therefore, photons traveling through patient body may end up with photon beams with an energy distribution and emissions from scattered photons.
-
4.
When the photon arrives at the surface of the collimator, only the gamma rays passing through the holes may deposit their energy to crystal to generate scintillation lights. Many photons are absorbed; some may make their way to the crystal at lower energy since a fraction of the initial energy is carried away during the Compton scattering.
-
5.
When incoming gamma rays interact with scintillation crystal, they may go through photoelectric interaction which lead to full absorption of the gamma photon or may be scattered to different direction at lower energy in Compton scattering. Some scattered photon may be absorbed by photoelectric interaction after being scattered once or more times.
-
6.
Scintillation lights are converted to electrons on the surface of those PMTs, and then the electrons are collected and processed to generate pulse signals. Three components are included in the pulse signals: location, intensity of the event, and energy of the gamma rays.
-
7.
Those signals are further processed (smoothing, enhancing contrast, etc.) in planar imaging.
-
8.
Images are archived, transferred, and displayed for analysis and clinical diagnosis.
11.8 SPECT
A planar image is a 2-D picture of 3-D distribution of radioactivity. Tomographic imaging is to generate a 3-D picture of the 3-D distribution. In order to collect 3-D data, a SPECT (single photon emission computed tomography) camera scans patient from multiple angles (e.g., 180 or 360°) to generate a set of 2-D images (projections). Those 2-D projection images form the basis for reconstruction (Buschberg et al. 2011). Similar to X-ray CT, SPECT utilizes image reconstruction from multiple projection images to provide a set of images (e.g., transverse slices) to represent 3-D distribution. A key difference between the SPECT and the X-ray CT is that the SPECT is emission tomography and the X-ray CT is transmission tomography. This is due to the fact that gamma camera and SPECT scanner detect gamma rays emitted from patient and the X-ray pass through patient body before being detected.
Filtered back projection is an image reconstruction method to mathematically construct a 3-D image based on the multiple projection date from tomographic scan. Iterative reconstruction is developed to improve image quality by including physics characters in the imaging process. It has been used as a major reconstruction method in emission tomography.
Mathematical filters are used in the image reconstruction and processing to remove noise and select useful components in image data by filtering some spatial frequency out. It is an important part of imaging processing to select a filter and set up filter parameters in clinical nuclear medicine imaging.
SPECT imaging can provide quantitative information for data analysis. Those quantitative data can be used for dosimetry and diagnostic evaluation of disease. Therefore a SPECT image provides pictures and quantitative data to describe 3-D distribution of radionuclides in patient body. It is essential to maintain quality of the SPECT and gamma camera images to the level that that current technology can provide. This involves quality product by manufacturer, equipment quality assurance, and right clinical protocol by nuclear medicine service (Fig. 11.3).
The dual-head detectors can be arranged 180° or 90° (for cardiac scan) or other angles for certain imaging protocols. Those configurations are to position the detector heads close to the patient for better special resolution.
There are many factors that may impact performance of a gamma camera: photon attenuation, Compton scattering, and source to detector distance. In addition to those factors, tomographic acquisition, reconstruction, and image processing will change the SPECT images.
Nuclear medicine services have quality control/assurance program for all gamma cameras. These include daily testing, periodic testing, and calibration of the gamma cameras. The following table lists the tests and calibrations as routine QC procedures.
A common QC test is uniformity imaging of a flood source or a point source to simulate a uniform flood emission where uniformity analysis provides four parameters: integral and differential uniformity in central field of view and in useful field of view (Table 11.4) (Figs. 11.4, 11.5, 11.6, and 11.7).
Planar image of Tc-99 m source to measured camera sensitivity. Source activity was 200 uCi in a plastic container. Imaging time: 120 s. Detector 1: 77,000 counts per 200 uCi, Detector 2: 75,000 counts per 200 uCi (Images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
Intrinsic uniformity image of Tl-201 point source on a Siemens eCam Gamma Camera. A point source of Tl-201 was positioned in the center of two detector heads. Since the source to detector distance was too short, the gamma emission to the two heads was not uniform as shown on the left. Images on the right were curvature corrected (Images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
Planar image of a Tc-99 m line source and count profile showing spatial resolution measured by FWHM. The line source was a glass tube filled with radioactivity and then positioned at the center of the two detector heads. Upper part of the display below are images from the two heads, and lower portion show the profile of the activity distribution on images (Images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
Extrinsic resolution image of Co-57 flood source on parallel hole collimator. A Co-57 sheet source was on top of a four-quadrant resolution phantom positioned on top of the parallel hole collimator loaded onto the detector head (images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
American College of Radiology (ACR) implemented an accreditation program for advanced imaging. Since 1987, the ACR has accredited more than 35,000 medical facilities in 10 imaging modalities, which includes CT, MRI, ultrasound, mammography, nuclear medicine, and PET. Medical physicists work together with physicians and technologists to obtain and maintain the accreditation Figs. 11.8 and 11.9.
Intrinsic resolution image of In-111 point source on dual-head camera. A point source of I-111 was at certain distance from the four-quadrant resolution phantom. The collimator was removed from detector head (images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
SPECT uniformity and resolution images of Tc-99 m filled ACR phantom on a dual-head camera. 15 to 20 mCi of Tc-99 m was normally put in to the phantom. Transverse images below were from image reconstruction following tomographic acquisition. Image on the left was a slice in the upper section where there was no inserts but only water. Image on the right was a transverse display of the rods in the phantom (images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
In summary, SPECT imaging involves of following:
(1) to (6) are similar to the planar imager.
(7) Multi-angle acquisitions are needed to provide set of projections.
(8) Image reconstructed of the projection data is to provide tomographic images in SPECT.
(9) Image processing before and/or after reconstruction may be used to improve images for reading.
(10) Images are archived, transferred, and displayed for analysis and clinical diagnosis.
11.9 PET
In gamma camera imaging (planar and SPECT), photons are emitted and then detected as single photons. Therefore, a collimator is needed to define the locations of the events. However, the collimator can only pass a small fraction of photons hit the surface of the camera. It significantly reduces gamma camera detection efficiency. PET (positron emission tomography) detects pairs of gamma rays of 511 keV which are emitted through radioactive decay of positron emitter (positron-emitting radionuclide). The positron then interacts with electron to have a positron-electron annihilation. This annihilation generates a pair of gamma rays of 511 keV, at 180° and emitted the same time (Buschberg et al. 2011; Sorenson et al. 1987). Because of these three features of the gamma rays, they can be imaged without collimation but through coincidence detection (Fig. 11.10).
Positron-electron annihilation and coincidence detection. Two gamma rays are 511 keV, emitted at the same time and in opposite direction. The event is recorded when the two gamma rays are detected by a pair of detectors at 180°; they are within energy window (30%) centered at 511 keV and arrived (being detected) detectors within a timing window (<10 ns)
In the coincidence detection, two 511 keV gamma rays are detected with a pair of detectors at opposite direction (180°). The PET registers an event of positron-electron annihilation only when the two photons are within 511 keV energy window (e.g., 30% peaked at 511 keV) at 180° and hit the detector at the same time. Here, the same time actually means within a timing window of about 10 ns. Comparing this detection with single photon imaging, an event of gamma emission is recorded only when the photons are within energy window (e.g., 20% centered at 140 keV for Tc-99 m) and pass through collimator holes; here the coincidence detection is similar to electronics collimation (Fig. 11.11).
A modern PET camera utilizes multiple rings of thousands of scintillation detectors. The ring structure provides many paired detectors for coincidence detection. The multiple rings cover a “section” rather than a “slice” of the imaging subject to improve efficiency of the PET imaging. A typical whole-body scan can be done with five to seven sections.
Scintillation crystals in PET scanner must be able to stop 511 keV photons. NaI (Tl) crystal is the primary detector in nuclear medicine imaging because of its high scintillation efficiency for gamma ray energy of 140 keV (Tc-99 m). However, the NaI (Tl) is not the choice for PET scanner since its stopping power for high-energy photons is low compared with BGO or LSO.
BGO (bismuth germanate) is the standard crystal for PET before LSO was introduced to improve scintillation speed or to reduce decay time of the crystal. With its elevated stopping power, high scintillation efficiency, good energy resolution, and non-hygroscopes, BGO is an excellent scintillation material and is ideal for PET imaging. LSO crystal is a new-generation scintillator crystal. LSO (lutetium orthosilicate) crystal has the advantages of high light output and density, quick decay time, excellent energy resolution, and low cost. These properties make LSO an ideal candidate for a range of gamma ray detection applications in PET imaging. Similar to the LSO crystal, GSO (gadolinium oxyorthosilicate) and LYSO (cerium-doped lutetium yttrium orthosilicate) are used by different manufacturers of PET scanners (Table 11.5) (Fig. 11.12).
PET image of F-18 in an ACR phantom. Black holes are cold spheres, white holes are hot spheres, and gray region shows background activity in the cylindrical container. Picture on the right describes distribution of the activity in each sphere (Images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
11.10 Multimodality Imaging
The highly sensitive PET scan images the biological and physiological process to find disorders at the molecular level (functional imaging), while the CT scan provides a detailed picture of the body’s internal anatomy (anatomical imaging). The PET-CT scan combines the strengths of these two well-established imaging modalities into a single scan. The CT portion of a PET-CT scanner provides anatomical definition of an internal organ and body attenuation map for attenuation correction. In recent years, MRI has been used to build a PET-MRI imaging unit to take advantage of both well-established imaging modalities.
Similar to PET-CT scanner, there is SPECT-CT imaging system for single photon imaging where the CT portion is to generate attenuation map and to provide anatomical definition of the organ of interest.
Since the imaging modalities (PET and CT or PET and MRI) are available before they are merged into one imaging unit, most multimodality imaging systems use image registration techniques to “combine” images from different modalities to generate a “fussed” image. For example, in PET-CT, the fussed image maintains the functional distribution of radioactive isotope with anatomically defined organ structure by CT scan.
Since the two modalities are calibrated at the factory by manufacturer, then there is no need for users to create calibration factors to register two sets of images. However, if the two sets of images come from different modalities that are independent imaging units, software fusion is needed and the calibration factors may be generated by using face markers.
11.11 PET-CT QC
Similar to SPECT, a routine QC (quality control) program is a key component in a clinical PET-CT facility. ACR established an accreditation program for PET and then PET-CT. Manufacturers of the PET-CT provide users with a guideline for routine QC protocols. Because of variety in PET-CT design, those QC guidelines are specific for each unit. ACR listed general steps in performing the QC procedures, which normally involves imaging a line source of a positron emitter and scanning a phantom of F-18 similar to the SPECT phantom (Figs. 11.3 and 11.14).
PET image of F-18 ACR phantom showing hot (white) cold (black) lesions. Upper curve shows count profile across the phantom and lower count profile measures the size of the lesion and counts (activity) distribution (Images were from Nuclear Medicine Service, Virginia Commonwealth University Hospital System, Richmond, Virginia)
An important part of nuclear medicine practice is radionuclide therapy. The therapy procedures apply radionuclides to treat various diseases including cancer. The radionuclide (e.g., I-131) is administered orally or through intravenous injection (e.g., Sm-153 Quadramet) or by catheter (e.g., Y-90 microspheres) inserted by interventional radiologists. Medical physicists support physicians to handle the radioactive materials and provide dosimetry calculations (Ollinger et al. 1997; Muehllehner et al. 2006).
Questions
-
1.
Why is Tc-99 m chosen as the radioisotope to label majority of the pharmaceuticals in clinical nuclear medicine?
-
2.
What is the primary function of a collimator in a gamma camera? There are three parameters in a parallel hole collimator design: hole length, hole diameter, and septa thickness. Fill in the table for effect of adjusting those parameters (change one of the two parameters while keep the other two) to camera detection efficiency and special resolution.
Parallel hole collimator
Detection efficiency
Spatial resolution
Increase length of the hole
Increase diameter of the hole
Increase septa thickness
Decrease length of the hole
Decrease diameter of the hole
Reduce septa thickness
-
3.
NaI (Tl) crystal is installed on most of gamma cameras. However, it is not the choice of detector for PET. An important feature of BGO or LSO crystal is their high stopping power for 511 keV gamma rays. Why is the BGO or LSO not a good candidate for SPECT cameras?
-
4.
Gamma rays may have photoelectric absorption and/or Compton scattering when traveling through tissue and interacting with scintillation crystal. What is going to happen when a group of photons is attenuated in tissue or when those photons are absorbed by scintillation crystal?
-
5.
In gamma camera imaging, one challenge is limited counts of gamma rays being detected. List possible ways to increase the counts in clinical imaging.
-
6.
Select detectors for the following applications, and justify your selection based on physics and instrumentation:
-
(a)
Contamination survey
-
(b)
Wipe test
-
(c)
Measure doses to be injected to patients
-
(d)
Thyroid uptake
-
(e)
Lymph node localization during breast surgery
-
(f)
Area monitoring
-
(g)
Real time monitoring of personnel exposure
-
(h)
Personal dosimeter (film badge in the past)
-
(a)
-
7.
Select parallel hole collimators (LEGP, MEGP, HEGP, LEHR) or pinhole collimators for the following clinical applications, and justify your selection based on physics of the radionuclides and characteristics of the collimators:
-
(a)
Tc-99 m brain
-
(b)
Tc-99 m whole body
-
(c)
In-111 whole body
-
(d)
I-131 whole body
-
(e)
I-131 thyroid
-
where LEGP stands for low-energy general purpose collimator,
-
MEGP stands for medium-energy general purpose collimator,
-
HEGP stands for high-energy general purpose collimator, and
-
LEHR stands for low-energy high-resolution collimator.
-
(a)
-
8.
The following diagram shows the camera system spatial resolution (top) and system efficiency (bottom) vs. distance between patient and camera head:
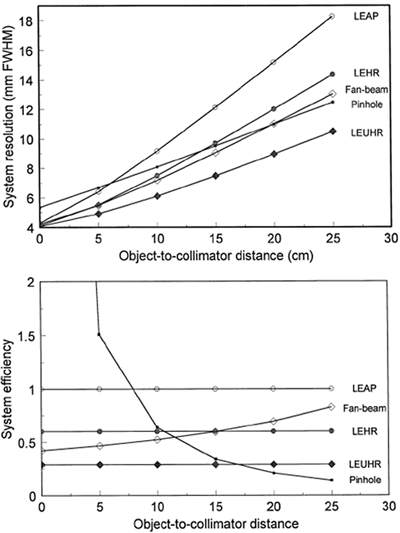
-
(a)
Would you position a patient at 5 cm, 10 cm, or 20 cm from the collimator?
-
(b)
Do you expect the same spatial resolution for organs near body surface as organs deep inside the body?
-
(c)
Does the system spatial resolution change with the distance if parallel hole collimator is loaded?
-
(d)
Does the system detection efficiency change with the distance if the parallel hole collimator is loaded?
-
(a)
-
9.
Major problems in PET imaging include the following:
-
(a)
Attenuation, random coincidence, center of rotation, dead time, and collimator penetration
-
(b)
Uniformity, random coincidence, Compton scattering, and dead time
-
(c)
Spatial resolution, random coincidence, and Compton scattering
-
(d)
Attenuation, random coincidence, Compton scattering, and dead time
-
(e)
None of the above
-
(a)
-
10.
Key features of the 511 keV gamma rays from positron-electron interaction are that:
-
(a)
Each photon has energy of 511 keV, two photons travel in opposite direction (about 180°), and both are emitted at the same time.
-
(b)
Two photons have total energy of 511 keV, two photons travel in opposite direction (about 180°), and both are emitted at the same time.
-
(c)
Each photon has energy of 511 keV, two photons travel in same direction, and both are emitted at the same time.
-
(d)
Each photon has energy of 511 keV, two photons travel in opposite direction (about 180°), and two photons are emitted within 12 ns.
-
(a)
Key
Gas-filled detectors | Detector medium is a chamber filled with air or a specific gas. When radiation hits the chamber, ionization of the molecules in the air occurs. The positive ions will be attracted to the negative side of the detector (the cathode), and the free electrons will travel to the positive side (the anode). These charges are collected by the anode and cathode which then form a current. |
Scintillation crystals | The crystal will generate visible light photons when excited by ionizing radiation. Then the visible light photons are collected and converted to electrons on surface of the photomultiplier tube (PMT). The electrons get multiplied in the PMT and form a pulse as signal. |
Uptake probe | A NaI (Tl) well counter to measure the tissue/organ uptake of radioisotope-labeled compound. |
Survey meters | Radiation detectors used to check out possible contaminations of radioactive materials and exposure from majority of radioactive sources and from X-rays. |
Anger cameras | Gamma camera developed by Hal Anger in 1957. An array of PMTs is behind a scintillation crystal. The crystal is covered with a collimator to locate sites of gamma emissions. |
SPECT | Based on the Anger camera, applied multi-angle acquisition and tomographic image reconstruction to form three-dimensional distribution of the radiopharmaceutical in tissue |
The gamma rays are detected as independent events. | |
PET | Similar to the SPECT but with a key difference in detection method. The gamma rays are detected in a coincidence mode |
Image registration | It is to register multiple image sets into one to take advantage of unique features from each imaging modality. |
Multimodality imaging | More than one imaging modality is utilized to image one subject. |
Radiation dosimetry | Measurement of ionizing radiation to determine energy deposit to the tissue or organ. |
References
Bushberg JT, Anthony Seibert J, Leidholdt EM. Jr, Boone JM (2011) Essential physics of medical imaging, 3rd edn. ISBN-13: 978–0781780575
Chandra R (1992) Introductory physics of nuclear medicine, 4th edn. Lea & Febiger, Philadelphia
Chandra R (2011) Nuclear medicine physics: the basics, 7th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia. ISBN-13: 978–1451109412
Cherry SR, Sorenson JA, Phelps ME (2012) Physics in nuclear medicine, 4th edn. Saunders, Philadelphia. ISBN: 978–1–4160-5198-5
Christian PE, Waterstream-Richand KM, Langan JK (2011) Nuclear medicine and PET/CT - technology and techniques, 7th edn. Mosby-Year Book, Inc. ISBN-13: 978–0323071925
Muehllehner G, Karp JS (2006) Positron emission tomography. Physics in Medicine and Biology 51(13):R117
Ollinger JM, Fessler JA (1997) Positron emission tomography. IEEE Signal Process Mag 14(1):43–55
Sorenson JA, Phelp ME (1987) Physics in nuclear medicine, 2nd edn. Grune and Stratton, New York
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Luo, J., Maqbool, M. (2017). Nuclear Medicine Physics. In: Maqbool, M. (eds) An Introduction to Medical Physics. Biological and Medical Physics, Biomedical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-61540-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-61540-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61538-7
Online ISBN: 978-3-319-61540-0
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)



















