Abstract
Sludges, dusts, residues and other wastes originating from ferrous and non-ferrous metallic industries pose a serious environmental threat, if not disposed properly. Disposal of these wastes is expensive and remediation is a necessary step to be implemented to control the adverse environmental effects if disposal is done improperly. Since the past couple of decades, the world’s high-grade metal reserves have been depleted considerably, but the demand for metals in day-to-day life in this electronic era is growing rapidly. The depletion of high-grade ores urges the mineral industry to look for alternative resources for metal extraction. Sludges, dusts, and other wastes generated by the metallurgical industries are interesting options as they still contain significant amounts of valuable base and heavy metals, sometimes even precious metals like gold and silver and also rare earth elements, depending on the nature of the mining site and composition of the primary ores used. This chapter overviews various hydrometallurgical and bio-hydrometallurgical leaching processes for the extraction of metals from these wastes. Different strategies of metal recovery such as solvent-extraction, electrowinning, bio/chemical sorption and bio/chemical precipitation from the wastes generated by various ferrous and non-ferrous metallic industries are overviewed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Owing to the enormous increase in the usage of metals in day-to-day life in the form of electronics, households, ornaments and accessories, the demand for metals is also increasing enormously (Anjum et al. 2012; Gahan et al. 2012). Metals are usually produced from mined mineral ores by ferrous and non-ferrous metallurgical industries. These metallurgical industries are not only producing metals, but also generating bulk quantities of wastes, which are either stored in reservoirs or disposed of in the environment. There are a lot of environmental issues associated with this practice (Lottermoser 2010). This review discusses in detail these different types of wastes, their composition and the environmental considerations. Due to the rapid industrialisation and the demand for metals, there is also a huge depletion of high-grade primary metal resources, which urges the metal producing industries to look for secondary alternative sources for metal extraction (Anjum et al. 2012). Metal bearing wastes from different industries can be such alternative resources for the recovery of metals, as some of the wastes still contain significant levels of valuable metals. In addition, also the adverse effects of the metals on the environment can also thus be reduced. The importance of waste utilisation and recycling has widely increased nowadays in view of the sustainable resource supply, circular economy, waste management and environmental protection. Secondary resources utilisation refers to the usage of waste as the feedstock for the manufacturing of products. This strategy helps the society in two ways: (i) the generation of waste is greatly reduced; consequently its disposal into the environment will also be reduced and (ii) it enables sustainable resource management as well as yielding economic benefits (Rao 2011).
In this chapter, up-to-date available methodologies for the extraction and recovery of base and heavy metals from different metallurgical wastes are discussed. Metal bearing wastes such as dusts and sludges from steel making industries and smelting processes and sludges and leach residues from metallurgical industries will be given a special focus, and their potential to be used as a secondary source for metal extraction will be highlighted. Their nature, elemental and mineralogical composition and various hydrometallurgical (chemical and biological) processes used for metal leaching and recovery will be overviewed.
6.1.1 Solid Wastes as Secondary Resources
There are a lot of studies on the effective utilisation of low grade ores to extract valuable metals in an economic as well as environment friendly manner (Anjum et al. 2012). Different approaches have been proposed for the extraction of heavy metals from industrial wastes (solid wastes and slurry wastes), such as metal rich wastewaters, fly ashes, spent liquors, spent catalysts, spent batteries, slags, shales and sludges, some of which have been patented (Brombacher et al. 1997). Jha et al. (2001) studied the proposed processes to recover zinc from various industrial wastes. Techniques for the utilisation of slags (Shen and Forssberg 2003) and sludges from the steel industries were reviewed by Das et al. (2006). Cui and Zhang (2008) overviewed the different pyrometallurgical and hydrometallurgical processes for the extraction of precious metals from electronic wastes. Lee and Pandey (2012) discussed the available methods for the extraction of various metals (Cu, Zn and Ni) from different industrial wastes by microbially assisted leaching processes. Erust et al. (2013) reviewed the possible applications of biohydrometallurgy to recover metals from spent batteries and spent catalysts. They overviewed biological approaches for the utilisation of secondary resources to supply some of the critical materials, e.g. platinum group elements and rare earths. Kaksonen et al. (2014) reported the ability of microbes to process and recover gold. Johnson (2014) discussed biomining and the possible biotechnological applications to extract metals from ores and waste materials.
6.1.2 Metallurgical Sludges, Dusts and Residues as Secondary Resources
Chemical and mineralogical characteristics and toxicity levels of metallurgical wastes are listed in Table 6.1. Table 6.1 clearly shows the high metal content (above sub-economic) of these waste materials. The toxicity characteristic leaching procedure (TCLP) values shown in Table 6.1 also suggest that at least one of the metal values fails to comply with environmental regulations, making them as ‘hazardous’ and preventing them from being disposed in the environment (Laforest and Duchesne 2006; Erdem and Özverdi 2011; Li et al. 2013; Tutor et al. 2013). In a few instances, Portland cement, ferrous sulphate or glass cullets are mixed with these metallurgical wastes to solidify them and make them more stable (Pereira et al. 2001; Salihoglu and Pinarli 2008; Bulut et al. 2009). In any case, the valuable metals harboured by these solid wastes are wasted. The toxicity levels of these metallurgical wastes form the basic necessity of finding a solution to treat or to reuse them in order to reduce their environmental impacts. Moreover, the mineralogical characteristics indicate the potential of these metallurgical solid wastes to be a secondary resource for metal recovery.
6.2 Wastes from the Metal Producing Industry
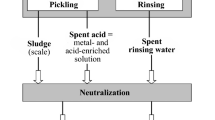
Natural ores consist of the desired metal present in high concentrations in combination with inherent waste compounds, i.e. metals or elements not important for the production process and usually present in lower concentrations. For example, nearly 50% of a zinc concentrate consists of unwanted elements like sulphur, iron, lead, titanium, silicon, copper, calcium, manganese, cadmium, magnesium, arsenic and mercury (Reuter et al. 1998). The metal of interest can be found in its oxidic or sulphidic form, as primary or secondary metallic phases or any other form in the natural ore. Many metallurgical processes, starting from open pit mining to final purification, have to be done to separate the pure metal from the ore. Usually some kind of waste is generated at each step of the metallurgical process; thus the metallurgical industries not only produce metals, but also deposit a huge load of waste materials in the environment (Leonard 1978; Chandrappa and Das 2012). The wastes generated by the metallurgical industries are huge and they are mostly disposed of in the environment (Fig. 6.1). Certain wastes not only contain unwanted elements, but have also considerable quantities of metals, mostly as oxides or sulphides.
Schematic product and waste streams from mining to metal refining (Adapted from Lottermoser 2010). Note the generation of wastes at each and every step of mining and metallurgical processes
Metallurgical industries produce solid, liquid and gaseous wastes. These can be classified as (i) mining wastes, (ii) processing wastes and (iii) metallurgical wastes (Lottermoser 2010). Mining wastes are produced during the initial stages of mining operations like ‘open pit’ or ‘underground’ mining. These operations usually produce waste rocks, overburden, spoil and atmospheric emissions. These mining wastes contain very low levels of or even no metals. Processing wastes are wastes generated by physical ore processing processes applied prior to the extraction of metals, like washing, magnetic separation, gravity separation, crushing, milling, size reduction and floatation (Leonard 1978; Lottermoser 2010). Wastewater streams resulting from the washing and also the mine tailings are categorised as processing wastes. Some of the mine tailings contain significant concentrations of metals and are prone to bioweathering and leaching. Most of them are used for backfilling working sites or reclamation and reconstruction of mining areas, as they do not contain economic levels of metals (Wong 1986).
Metallurgical wastes are mostly residues or muds which are produced at the final stage of the extractive metallurgy and cannot be treated commercially. Extractive metallurgy can be hydrometallurgy, pyrometallurgy or electrometallurgy. Hydrometallurgy involves solvents for the metal extraction, whereas pyrometallurgy involves heat and electrometallurgy involves electric current. These processes separate the metals from their processed ores and also generate vast amounts of metallurgical wastes, like gaseous emissions, dust, slags, sludges, muds, spent ore and residues (Fig. 6.2).
Simplified flow charts of (a) pyrometallurgical and (b) hydrometallurgical operations, in which ore is treated to yield metals accompanied by the generation of wastes (Redrawn from Lottermoser 2010)
Metallurgical wastes contain considerable concentrations of metals, depending on the mineralogy of the ore and geography of the ore mining site. Disposal or storage of these wastes needs to be done carefully because of the adverse environmental impacts such as release of heavy metals by weathering (Gieré et al. 2003; Kierczak et al. 2009), contamination of water bodies (Johnson 2009), metal incorporation into the food chain (Kachur et al. 2003), formation of efflorescences i.e. metal hydrosulphates as a result of evaporation (Keith et al. 2001; Sánchez-España et al. 2005; Romero et al. 2006) or creation of acidic environments (Hammarstrom et al. 2005).
6.2.1 Dusts
Flue dusts are fine, metal containing dust particles collected at the gas exhaust of smelters or any other furnace during metallurgical processing. Copper and zinc producing industries are the major sources of waste dust generation. According to Davenport et al. (2002), dusts emanating from copper smelters consist of 20–40 mass percent of Cu and can be either recycled with concentrates or can be treated by hydrometallurgy for further metal recovery. Massinaie et al. (2006) reported that these wastes originating from copper industries are mostly rich in chalcocite (Cu2S), chalcopyrite (CuFeS2), bornite (Cu5FeS4) and covellite (CuS).
Similarly, metallic dusts are generated during the steel making processes in e.g. electric arc furnace (EAF) smelters. These EAF dusts are rich in zinc and iron oxides and are generated during the heating and cooling of the smelting processes and collected at the gas cleaning system of scraps (Jha et al. 2001). Electric arc furnaces dusts from steel industries typically contain 19.4% Zn, 24.6% Fe, 4.5% Pb, 0.42% Cu, 0.1% Cd, 2.2% Mn, 1.2% Mg, 0.4% Ca, 0.3% Cr, 1.4% Si and 6.8% Cl (Caravaca et al. 1994).
Blast furnace (BF) dusts are similar to EAF, generated during the wet cleaning of the gases in blast furnace mediated steel production. Upon emission, dusts agglomerate after long-term exposure to the earth’s atmosphere because of its inherent moisture content. Elemental analysis revealed that these BF dusts mostly contain iron and carbon in high concentrations. The typical composition of BF dusts is carbon (~30%), Fe2O3 (~51%), SiO2 (~7%), Al2O3 (~3%) and other metals such as Zn, Pb and Mn (Zeydabadi et al. 1997; Das et al. 2002).
6.2.2 Sludges
Sludges are co-products generated during various stages in ferrous and non-ferrous industries. They can be blast furnace sludges (BFS), electric arc furnace sludges (EAFS), converter sludges, basic oxygen furnaces sludges (BOFS) from steel-making industries, sludges from plating industries and also sludges from metal-producing industries. Steel-making industries generate significant quantities of sludge (2–4 tonnes of wastes per tonne of steel (Das et al. 2006)), which consists of approximately 2.5% of Zn and 61% of Fe (Trung et al. 2011). Mansfeldt and Dohrmann (2004) studied the mineralogical and chemical composition of the pig-iron-making sludges and found that, apart from the iron mineral phases magnetite (Fe3O4, 3%), hematite (Fe2O3, 4%) and wuestite (FeO, 2%), they also contain primary and secondary phases of the metals Zn (3%), Pb (1%), Cd (0.01%), and As (0.1). The sludges from the metallurgical industries are also polymetallic, containing significant mass concentrations of Fe 44%, S 28%, As 0.38%, and Zn 0.13% (Hita et al. 2006, 2008). The mineralogical and elemental composition of the metallurgical sludge depends on the nature of the ores.
6.2.3 Residues
Residues can be mainly classified as leach residues and purification residues, based on their generation during the operational processes. Purification residues are produced during the separation of the pure zinc metal from its impurities (for e.g. copper and cobalt), while leach residues are derived during the filtration of the purified acid (mostly sulphuric acid) leached products prior to electrolysis. Recovery of metals from plant residues, like zinc plant residues (ZPR), has gained importance in recent years. Copper and cobalt are often found in the residues generated at the end of zinc-production processes.
There are only few investigations on the extraction of these metals from ZPR. Min et al. (2013) investigated the chemical and mineralogical composition of the leaching residues generated during zinc and lead hydrometallurgical operations. They found that ZPR consist of (mass fractions) 5.35% Zn, 4.66% Pb, 0.24% Cu, 0.15% Cd, 0.25% As and 13.54% Fe. Usually the presence of zinc ferrites, which is a spinel (ZnFe2O4) resulting from the desulphurisation of iron containing sphalerite ores in the final leach residues, makes the extraction of metals tedious because of its very stable and insoluble nature.
6.3 Leaching
Leaching is the key unit operation in metallurgical processes. It is the dissolution of metals from their natural ores into a liquid medium. Leaching processes are classified based on the method used for the leaching of metals, i.e. hydrometallurgy (chemicals) or bio-hydrometallurgy (microbial mediated leaching). Different leaching processes and the leaching of metals from various metal bearing solid wastes are discussed in detail below.
6.3.1 Hydrometallurgical Processes
Hydrometallurgy is the extraction of metals from resources with the help of aqueous chemicals. Hydrometallurgical processes have a few advantages over pyrometallurgy, as they are more eco-friendly and economic for low-grade metal reserves. A general process flow diagram of hydrometallurgy is illustrated in Fig. 6.3. Hydrometallurgy is a general term which refers to a range of processes, including chemical leaching or mediated by oxidising agents, higher oxygen partial pressure or microbial activity (National Research Council 2002).
Basic unit processes in hydrometallurgy (Redrawn from Gupta 2006)
Hydrometallurgical processes consist of different steps: (i) leaching of metals from the source and dissolution into the leachate, (ii) separation of the metal-loaded leachate from the residues, (iii) recovery of the metals from the leach solution and (iv) regeneration and reuse of the leachate (Ghosh and Ray 1991). Leaching processes can be done in situ (heaps or dumps) or ex situ (reactors or vessels). There are various parameters which affect the leaching behaviour of metals from their parent material: (i) pH, (ii) temperature, (iii) concentration of the leaching agent(s), (iv) solid-to-liquid phase ratio and (v) particle size of the parent material. The efficiency of hydrometallurgical processes is increased by using improved leaching conditions coupled to high-pressure leaching and ultra-fine grinding (Malhotra et al. 2009). Selective leaching of metals can also be achieved by adjusting the pH or working at elevated temperatures and pressures (Trefry and Metz 1984; National Research Council 2002; Havlik et al. 2004).
6.3.2 Biohydrometallurgical Processes
Biohydrometallurgy is a recent advancement in the mining industry where micro-organisms are used to enhance the leaching of metals and biotechnological processes are used for the recovery of the dissolved metals. Biohydrometallurgy is the conversion of insoluble metals in ores (or other sources like metallurgical wastes) to the soluble form with the help of micro-organisms. Microbial extraction and recovery of metals like Cu has received considerable attention in the past three decades owing to its relative simplicity, eco-friendly operation and low capital requirement when compared to those of the conventional chemical/heat treatment processes (Olson et al. 2003; Watling 2006; Johnson 2013). Commercial applications of bioleaching were also reported in many instances (Brierley and Brierley 1999, 2001; Brierley 2008; Neale et al. 2011; Gahan et al. 2012). A simplified bioheap leaching process and commercial bioleaching plants are given in Fig. 6.4.
Bioleaching process and commercial bioheap leaching plants: (a) schematic representation of the bioheap leaching process, (b) bioleaching plant in Zijinshan copper mine, China (Renman et al. 2006), (c) bioheap leaching plant in Talvivara mining company, Finland (Riekkola-Vanhanen 2010) and (d) bioheap leaching plant in Kasese mine, Uganda (Gahan et al. 2012)
Knowledge and understanding the metal – microbe interactions and the mechanisms of bioleaching are much needed for the effective recovery of metals from metallurgical wastes.
6.3.2.1 Microbe – Metal Interactions
Bacteria and fungi are able to extract metals from metal contaminated soils and metal wastes. These micro-organisms use one of the following processes (Fig. 6.5): (i) non-specific interaction of metal ions with cationic binding sites present outside the cell wall, (ii) specific interactions at the periplasmic sites of the cell wall, (iii) metallo-chemical complex (chemicals secreted by the microbes in the surrounding medium and the metals form a complex) uptake by the cells, (iv) bioaccumulation, (v) metal precipitation by the microbial metabolites or (vi) metal volatilisation (Upadhyay 2002).
Microbe-metal interactions that can be engineered to develop enhanced bioleaching processes (Reproduced from Upadhyay 2002)
6.3.2.2 Bioleaching
Microbes leach metals via various processes (Fig. 6.6): (i) acidolysis, (ii) redoxolysis, (iii) complexolysis and (iv) bioaccumulation (Schinner and Burgstaller 1989; Bosshard et al. 1996; Brandl 2001; Wu and Ting 2006). Recently, reductive dissolution of oxidised Ni-laterites ores was also reported (Johnson et al. 2013).
Mechanisms of bioleaching of metals from primary and secondary resources (Adapted from Uroz et al. 2009)
The most commonly used genera to catalyse the bio-oxidation of sulphides and liberate the desired metals into the liquid phase are chemo-litho-autotrophic bacteria oxidising iron, e.g. Leptospirillum spp. (Sand et al. 1992; Falco et al. 2003; Sethurajan et al. 2012), Ferroplasma spp. (Edwards et al. 2000; Golyshina et al. 2000) and Ferrimicrobium spp. or sulphur, e.g. Acidithiobacillus spp. (Kelly and Wood 2000; Falco et al. 2003), Thiomonas spp. (Han et al. 2013) and Sulfolobus spp. (Norris et al. 2000). These microbes obtain energy by oxidising ferrous into ferric ion and elemental sulphur to sulphuric acid (Rawlings 2005), thereby leaching reduced sulphide minerals. The bacteria thus enable oxidative dissolution and acidolysis by producing the ferric ions and sulphuric acid.
The mechanisms by which Acidithiobacillus ferrooxidans (A. ferrooxidans) leaches out the metal constituents are (Crundwell 2003): (i) direct bioleaching (bacteria adhere on the surface of the ores and oxidise the reduced sulphides) and (ii) indirect bioleaching (bacteria oxidise the ferrous to ferric ion, thereby contributing to the leaching of minerals). This indirect bioleaching by ferric ion can be subdivided into two phenomena: the produced ferric ions are released either into the bulk solution or inside the layer between bacteria and exopolymeric material affecting the mineral surface and thus leach out minerals.
The generalised reactions (R1 and R2) for the bio-oxidation of mineral sulphides leading to (precious) metal leaching are:
where M is a bivalent metal.
Various heterotrophic bacteria, e.g. Pseudomonas spp. (Müller et al. 1995; Lingling et al. 2012; Pradhan and Kumar 2012) and Bacillus spp. (Farbiszewska-Kiczma et al. 2004) as well as fungi, e.g. Aspergillus spp. (Mulligan et al. 1999, 2004; Rao et al. 2002), Penicillium spp. (Acharya et al. 2002; Amiri et al. 2011; Ilyas et al. 2013) and Ganoderma spp. (Nouren et al. 2011) have also been investigated for their ability to bioleach metals. In few instances, gold bioleaching by cyanide producing bacteria were also reported (Chi et al. 2011; Işıldar et al. 2016). Mixed cultures of two or more bacteria or indigenous enrichments of microbes from metal contaminated sites were studied for metal solubilisation from the ores and achieve higher efficiencies than pure cultures (Sandstrom and Petersson 1997; Fu et al. 2008; Plumb et al. 2008).
Fungal bioleaching mechanisms mainly follow acidolysis, i.e. solubilisation of the metals by the acidic dissolution (protonation of oxygen atom) from the parent material (Burgstaller and Schinner 1993). These fungi produce organic acids like citric, oxalic, malic or gluconic acid (Mulligan et al. 2004; Johnson 2006). Aspergillus spp. are the most-studied fungi for the bioleaching processes because of their capacity to produce higher levels of organic acids. Acharya et al. (2002) and Sukla and Panchanadikar (1993) studied Penicillium sp. for the bioleaching of valuable metals from low-grade ores. Sukla et al. (1995) investigated the bioleaching of Sukinda lateritic Ni ore using the fungus Penicillium spp. and reported that, under optimum conditions of pulp density, dextrose concentration and ore size fraction, a maximum of 90% Co, 40% Mn and 12% Ni could be leached.
6.3.3 (Bio)Hydrometallurgical Treatment of Wastes from Metal Industries
6.3.3.1 Dusts
Dusts from the metallurgical industries contain significant amounts of metals. Various researchers used chemical and microbial mediated leaching procedures for the release of heavy metals from these wastes. Different (bio)hydrometallurgical approaches to processing these dusts from the metal industry for the leaching of metals were developed (Table 6.2). Cole et al. (1987) and Gabler and Jones (1988) studied the possibilities of re-using Zn from Brass smelter flue dust and secondary copper converter dust by sulphuric acid and ammonium carbonate, respectively. The recovered Zn was suitable for electrogalvanising and the ZnO could be re-fed to the furnace. Vítková et al. (2011) investigated the effect of pH on the leachability of metals from Cu smelter dusts and found that an acidic pH (pH 3) favoured the maximum leaching of the metals. As these dusts from the copper industries mainly consist of reduced mineral phases of metals, bioleaching is considered as an eco-friendly approach (Rossi 1990; Schnell Henry 1997; Oliazadeh et al. 2006). Acidithiobacillus spp. and Leptospirillum spp. are the genera widely used for the biological leaching of metals from metallurgical dusts. More than 70% of Zn was extracted by A. ferrooxidans from industrial Fe-Mn alloy dust (Solisio et al. 2002). Mixed populations of iron-oxidising and sulphur-oxidising bacteria were proposed to be more efficient than solely the pure cultures. Bakhtiari et al. (2008a, b, 2010) investigated the leaching efficiency of mixed cultures of A. ferrooxidans, Acidithiobacillus thiooxidans (A. thiooxidans) and Leptospirillum ferrooxidans (L. ferrooxidans) in different bioreactor configurations like continuous stirred tank reactors (CSTR) and air-lift bioreactors from different metal-bearing dust samples. They reported that a maximum of 90% of Cu was leached within 2 days at lower solid to liquid phase ratios (2.7%) in air-lift bioreactors configurations.
The bioleaching efficiency of copper from smelter dusts (combined with flotation concentrate) was higher in the stirred tank reactors than in airlift bioreactors (Vakylabad et al. 2012) and thermophilic lithotrophs were slightly better bioleaching bacteria than mesophilic lithotrophs, although the impact of temperature was not very high as observed in the case of primary (chalcopyrite) ores (Vakylabad 2011; Vakylabad et al. 2012).
As with Cu dusts, there are numerous hydrometallurgical processes developed for the utilisation of EAF dusts. Conventionally, these dusts are treated by sulphuric acid (Duyvesteyn et al. 1979; Pearson 1981; Duyvesteyn and Jha 1986; Cruells et al. 1992). The efficiency of acidic leaching is greatly affected by the iron/zinc ratio and the presence of halogens, as these will interfere during the electrolysis (Havlik et al. 2004, 2006). Alkaline leaching is an alternative strategy for overcoming these problems. Xia and Picklesi (2000) proposed microwave assisted caustic leaching for the recovery of zinc from EAF dust and were able to extract more than 90% of Zn at 8 M NaOH at 117 °C. Dutra et al. (2006) demonstrated that 6 M NaOH at 90 °C recovered 74% of Zn from EAF dusts within 4 hours.
6.3.3.2 Sludges
Different hydrometallurgical approaches to processing metallurgical sludges for the effective and economic extraction of metals have been developed (Table 6.3). The use of hydrometallurgical operations for the effective extraction of Zn and Pb from BFS was reported by Van Herck and Vandecasteele (2000), who focused on the effect of the pH and redox potential. Silva et al. (2005) investigated various factors (pulp density, stirring, concentration of leachant and particle size) affecting the leaching of metals from galvanic sludges and stated that 1 M of H2SO4 can leach 88.6% Cu, 98.0% Ni and 99.2% Zn at room temperature in 24 h. Trung et al. (2011) reported that at high temperature (80 °C), approximately 70% of Zn can be leached within 15 minutes by using 1 M H2SO4. Vereš et al. (2012) investigated the extraction of Zn from blast furnace sludge by microwave-assisted procedures. Cantarino et al. (2012) reported the selective leaching of zinc from basic oxygen furnace sludge with a three step leaching procedure (5 M NaOH) coupled to a thermal treatment, and extracted 95% of Zn.
Bioleaching of a steel-plant sludge using A. ferrooxidans was studied by Bayat et al. (2009). A. ferrooxidans extracted comparatively less metals (35% of Zn and 37% of Fe), which might be due to the oxidised mineral phases present in the metallurgical sludge (Fe3O4 and Fe2O3). Metallurgical sludges containing sulphidic minerals can be treated by microbial processing by oxidative dissolution, as acidic leaching involves concentrated acids and high temperatures and is thus expensive. Hita et al. (2008) reported the possibility of bacterial leaching (A. ferrooxidans, Acidithiobacillus caldus and Sulfolobus metallicus) of Fe, Zn and As from pyritic metallurgical sludge.
6.3.3.3 Residues
Hydrometallurgical extraction of zinc, especially from sulphidic ores, results in the generation of leach and purification residues. Different pyrometallurgical and hydrometallurgical leaching processes have been developed for the extraction of metals from these metallurgical residues (Table 6.4). Ngenda et al. (2009) proposed a new thermal treatment coupled to a highly concentrated acid leaching for the extraction of Zn from the zinc plant residues (ZPR) obtained from the Kolwezi Zinc Plant (Republic of Congo). Safarzadeh et al. (2009) extracted almost 99% of Zn from the zinc residues by 1.7 M sulphuric acid. Lu et al. (2014) demonstrated that 99% of Zn, Cd and Co can be extracted from purification residues by 48 g L−1 sulphuric acid. Sethurajan et al. (2017a) reported that hot (80 °C) sulphuric acid (1.5 M) can leach more than 70–90% of the Zn from Zn-hydrometallurgical residues.
The metal release kinetics follow the shrinking core model for the extraction of metals from ZPR (Safarzadeh et al. 2009, 2011; Sethurajan et al. 2017a). Safarzadeh et al. (2009) proposed that the addition of sulphuric acid, particle size, temperature and phenol dosage play an important role in the reductive leaching of manganese and cobalt. In some cases, e.g. residue from a Zn metallurgical plant in the Çinko-Kurşun metal industry (Turkey), the residues obtained during the hydrometallurgical extraction of Zn with a concentrated sulphuric acid solution contained higher levels of Pb and Cd. Turan et al. (2004) and Yan et al. (2014) studied metallic residues from a zinc - lead plant and proposed a combination of pyrometallurgical (roasting) and hydrometallurgical (sulphuric acid, water and NaCl) processes for the extraction of Zn and Pb from these residues. Rusan et al. (2008) reported a similar hydrometallurgical extraction process for Zn and brine leaching for Pb extraction from Zn plant residues.
6.4 Recovery of Metals from Metallurgical Wastes Leachates
After the leaching of the metals from the metallurgical wastes with acids or micro-organisms, the metals are distributed in the leach solution, simply referred to as leachate. Recovery of pure metals from the leachates is extremely important as it is the final and critical stage. Many strategies have been developed for and applied to the extraction of metals from the leachates, with solvent extraction, precipitation, electrowinning and (bio)sorption as the most commonly applied methodologies. Table 6.5 overviews the established metal-recovery strategies. Each of these methods has advantages and disadvantages, so that sometimes a combination of two or three recovery techniques is required to achieve higher metal recovery efficiencies. However, the recovery of metals from metallurgical waste leachates is only in its infancy and requires lots of research and development at laboratory and pilot scale for the development of further commercial applications.
6.4.1 Metal Recovery by Precipitation
Precipitation is a conventional methodology developed for the removal and recovery of metals from metal bearing solutions. Precipitation of metals from metal contaminated aqueous solutions, like acid mine drainage, industrial wastewaters or leaching solutions, can be achieved by the formation of their respective (i) sulphide, (ii) hydroxide, and sometimes (iii) carbonate salts (Manahan 1990). A few metals like arsenic can also be co-precipitated during flocculation with the salts of iron and aluminium. Recovery efficiencies of the metals by precipitation depend highly on the metal concentrations in the solution and also on the system pH. The major disadvantages of precipitation processes are the high requirements of chemicals to adjust the pH and the generation of a not well settling and dewaterable sludge containing toxic compounds (Ahalya et al. 2003).
Metal precipitation occurs when the concentration of ions in solution exceeds the solubility product (Wang et al. 2005), and can be induced by changes in the ionic equilibrium of the system with the addition of the reaction products (either metal or sulphide/hydroxide). Precipitation of metals consists of various stages: (i) nucleation, (ii) growth of nucleus, and (iii) aggregation or crystallisation (Fig. 6.7) (Benning and Waychunas 2008). In some cases, certain chemicals can induce the precipitation (precipitating agents) and subsequent crystal formation (crystallisation nuclei) (Wang et al. 2005).
Various stages in the recovery of metals by precipitation (Benning and Waychunas 2008)
6.4.1.1 Hydroxide Precipitation
Sodium hydroxide (NaOH) and lime or hydrated lime (Ca(OH)2) are the commonly applied chemicals for the precipitation of metal hydroxides. A generalised equation for the metal hydroxide precipitation can be written as:
where M is a divalent metal ion.
Major disadvantages of this process are non selective recovery of metals and the solubility of the precipitated metal hydroxides: when the pH is not optimal, a soluble metal complex (M(OH)+) will be formed when altering the pH.
6.4.1.2 Carbonate Precipitation
Carbonate precipitation is used to precipitate metals as metal carbonates, in which straight precipitation by chemicals such as calcium carbonate is used or the conversion of hydroxides to carbonates is applied (Wang et al. 2005). Carbonate precipitation can also be applied in combination with hydroxide precipitation.
Varga and Török (2013) studied the precipitation of zinc from EAF dusts ammoniacal leach liquors by using CO2 (Table 6.5). The tested EAF dusts contained franklinite (49.5%), magnetite (0.5%) and zincite (29%) as the major mineral phases and these dusts were leached by ammonia and ammonium carbonate to dissolve zinc. These leachates and model synthetic solutions were studied for the precipitation of zinc carbonate by using gaseous CO2. Fifty-eight percent of Zn was precipitated from the leach liquors at 5 L min−1, 700 rpm and 10 °C. The recovery of Zn from synthetic solutions was comparatively poorer (37%) and it was proposed that co-precipitation of Fe and Pb from the leachates might help in enhancing the recovery of Zn from the solutions.
6.4.1.3 Chemical Sulphidic Precipitation
Ferrous sulphide (FeS), calcium sulphide (CaS), sodium sulphide (Na2S), sodium hydrosulphide (NaHS), ammonium sulphide ((NH4)2S) and hydrogen sulphide (H2S) are the major chemicals used for metal sulphide precipitation (MSP). MSP has various advantages over the other precipitation methods, including the fact that metal sulphide precipitates are less soluble, reaction rates are faster, settling properties are better and sulphide precipitates can be combined with ores in metallurgical processes (Lewis 2010). In addition, selective metal recovery by sulfide precipitation is possible with pH or sulfide adjustment (Sampaio et al. 2009, 2010) and can achieve extremely low (ppb range) residual metal concentrations (Kim and Amodeo 1983). The solubility products of different metal sulphides were studied by Sampaio et al. (2009), who found the log KSP values for Cu (I), Cu (II) and Zn (II) to be 48.0, 35.1, and 23.8 respectively. The operational pH (Fig. 6.8) plays an important role in the precipitation of metal sulphides, as various metal sulphides can solubilise as a function of pH (Lewis 2010). The sulphide concentration is another key factor in MSP (Villa-Gomez et al. 2012); if it is exceeded or depleted either, sulphides or metals will remain in the leachate solution (Veeken et al. 2003).
pH dependence of metal sulphide and metal hydroxide solubilities (Resimulated from Lewis 2010 with Visual MINTEQ)
Metal recovery by MSP has various barriers to cross such as (i) the formation of poly-sulphides owing to the poor mixing of supplied sulphides, which results in excessive consumption of sulphide and low metal recovery, (ii) supersaturation conditions in the solution induced by the low solubility of metal sulphides, which results in the formation of fine particles with poor solid-liquid separation (Lewis and Van Hille 2006) and (iii) formation of impurities like thenardite (Na2SO4) limited the selective metal recovery using chemical (Na2S) sulphide precipitation (Sethurajan et al. 2017a). Lewis and Van Hille (2006) proposed that a gaseous hydrogen sulphide source could decrease the level of supersaturation and thus control the formation of fine particles.
Youcai and Stanforth (2001) investigated the sulphide precipitation of EAF dusts alkaline leachates (Table 6.5), which contained 14.4 g L−1 Zn, 2.98 g L−1 Pb, 1 g L−1 Al, and 0.05 g L−1 Fe. These metals were very stable and did not precipitate, even after several months. They proposed that sodium sulphide was a better precipitant than phosphates, sulphates and carbonates. They were able to selectively precipitate lead with a molar ratio of 1.5–1.7 and then the zinc precipitated in the lead free solution. Lenz and Martins (2007) studied the selective chemical precipitation of Pb and Zn (Table 6.5) from EAF dust alkaline leachates. EAF dusts were leached after a series of steps including hydrolysis and alkaline leaching (NaOH). The final leachates contained various metals and the concentrations were 50 g L−1 zinc, 2 g L−1 lead, 1 g L−1 Al and 0.05 g L−1 Fe. Sodium sulphide with a 2.0 (w/w) and 3.0 (w/w) weight ratio could achieve almost complete precipitation of Pb in the leachates and later Zn was selectively precipitated by sodium sulphide (in the Pb free solution). Sethurajan et al. (2017a) demonstrated selective sphalerite (ZnS) precipitation from real Zn-leach residue leachates by chemical sulphide precipitation.
6.4.1.4 Biogenic Sulphidic Precipitation
Sulphate reducing bacteria (SRB) are prokaryotes which utilise sulphate and other oxidised sulphur compounds as their terminal electron acceptor (Jorgensen 1982). These anaerobic bacteria have not only assimilatory sulphate reduction (which synthesise sulphur compounds by reducing sulphates), but also dissimilatory sulphate reduction in which the sulphates are reduced to sulphides in the absence of molecular oxygen (Barton and Hamilton 2007). Most of the metal wastes (solid wastes or wastewaters) contain significant amount of sulphates.
SRBs can use simple organic compounds as electron donors and sulphate as the terminal electron acceptor, and produce sulphide which can be used for MSP. The following reactions illustrate the process:
(M2+ − Metal cation)
Metal sulphide precipitation by SRB occurs in two stages: (1) biological hydrogen sulphide production by SRB and (2) metal sulphide precipitation by the biologically produced H2S. Biological MSP has been reported for the successful recovery of pure metals from various sources like laterite pressure leaching solution (Zhang and Cheng 2007), bioleaching solution from nickel pyrite ore (Cao et al. 2009), industrial wastewater (Kosińska and Miśkiewicz 2012) and Zn leach residue bioleachate (Sethurajan et al. 2016b). The effect of the sulphide concentration and other macro-nutrients on biological MSP (Villa-Gomez et al. 2011, 2012) and the morphological characteristics of the metal sulfides were reported using inversed fluidised bed reactors (Villa-Gomez et al. 2014). The biological MSP technology has been applied at full scale to treat wastewaters containing low metal concentrations (μg – 0.1 g L−1), but not yet to treat metal bearing solid waste leachates (metal concentrations >1 g L−1) at full scale.
6.4.2 Solvent Extraction
Solvent extraction, also referred to as liquid-liquid extraction, requires two liquid phases that are immiscible with each other. The distribution of the solute between the phases greatly depends on the interaction of the solute with the aqueous and organic phases (Choppin and Morgenstern 2000). Solvent extraction has been commercially applied to RLE (Roasting-Leach-Electrowinning technology) liquors. Solvent extraction and electrowinning are often integrated in commercial hydrometallurgical plants to improve the metal recovery efficiency. Prominent developments in the leaching and recovery of metals through solvent extraction and electrowinning were overviewed by Domic (2007). A simplified flow sheet of the unit operations applied in the metallurgical industry (Fig. 6.9a) and a commercial solvent extraction plant are depicted in Fig. 6.9.
Solvent extraction includes three steps to achieve the recovery of pure metals: extraction, stripping and reduction (Fig. 6.10). The major merits of the solvent extraction procedure are: (i) low energy consumption and (ii) regeneration of the solvent.
Flow sheet of the recovery of metals by solvent extraction (Redrawn from Wilson et al. 2014)
Solvent extraction has been applied to many waste materials like galvanic sludge (Silva et al. 2005), industrial effluents (Mansur 2011) and fly ashes (Karlfeldt et al. 2012) for the extraction of Zn, Cu, In and even for rare earths (Xie et al. 2014). Martín et al. (2003) investigated the extraction of copper from converter flue dust by the combination of acid leaching and solvent extraction procedures (Table 6.5). The dust sample’s mineralogical characterisation reveals that it contains 30 wt % of metallic copper (cuprite (Cu2O), chalcocite (Cu1.96S) and 4.5 wt% of Fe (maghemite (γ-Fe2O3). Traces of As (0.18 wt %) and Mo (0.09 wt %) were also identified. Sulphuric acid was used as the leachant and a maximum of 2500 ppm of Cu was leached at 25 °C with 50 g L−1 of sulphuric acid. LIX 860 or MOC-55TD was used to recover the Cu from the acidic leachate. These solvents successfully extracted the maximum of metals at the aqueous/organic phase ratio 4.7 at pH 0.5 (Martin et al. Martín et al. 2003).
Vahidi et al. (2009) studied the recovery of zinc by solvent extraction from the roast leach residues by using di-2-ethylhexyl phosphoric acid (D2EHPA) (Table 6.5). They were able to extract all the zinc from the leach solution with 20% w/w D2EHPA in the kerosene organic phase (ratio 1:1) at pH 2.5. They found that the addition of tri-butyl phosphate (TBP) (5%) or Na2SO4 (0.2 M) enhanced the zinc recovery to the maximum. Interestingly, they found that none of the parameters aqueous organic phase ratio, TBP or Na2SO4 concentration had a significant effect on the zinc recovery above pH 2.5 and thus the pH plays a key role in the extraction of Zn by D2EHPA. Similarly, Koleini et al. (2010) recovered 90% of indium from zinc plant residues using the D2EHPA solvent-extraction method.
6.4.3 Electrowinning
Electrowinning is applied to recover metals from aqueous solutions. Commercial implementations of electrowinning in combination with solvent extraction are often exploited by the industries. Figure 6.11 shows a commercial electrowinning facility operated at Baghdad (Arizona, USA).
(a) Solution-extraction and electrowinning plant and (b) Direct copper electrowinning facility at Bagdad (Arizona, USA) (Marsden 2006)
The design of electrowinning processes consists of a chamber, a cathode (negatively charged electrode), an anode (positively charged electrode) and also an electrolyte solution. The mechanism of electrowinning is simple: applying an electric current to the electrolytic solution (eluate), the dissolved positively charged metal ions migrate to and deposit on the negatively charged cathode through the electrons passage to the anode. Unlike the other recovery methods, separation of elemental metal ions is the major advantage of this process. Other highlights of electrowinning processes are no sludge production, no hazardous chemical usage and low capital costs (Kondos et al. 1991). Though electrowinning is a promising recovery technology, recovery of pure metals from multi-metallic solutions is tedious as non-target metals can greatly influence the metal recovery, e.g. copper influences gold extraction (Steyn and Sandenbergh 2004) and lead affects the recovery of zinc (Youcai and Stanforth 2001).
The electrowinning technology was successfully applied to recover metals from leachates of industrial wastes such as electronic scraps and fly ashes (Jha et al. 2001; Vegliò et al. 2003; Cui and Zhang 2008). Electrowinning extraction is more cost effective (especially for the recovery of Zn) in alkaline solutions than in acidic solutions because of their high – energy requirements (St-Pierre and Piron 1986, 1990). Youcai and Stanforth (2000) worked on the separation of pure Zn from an alkaline medium leached EAF dust solution containing Zn 45.60 g L−1, Pb 3.60 g L−1, Fe 0.06 g L−1, Al 1.14 g L−1, Cu 0.06 g L−1 and Cd 0.04 g L−1 (Table 6.5). The presence of lead in the solution might considerably affect the electrowinning process and Pb was thus pre-removed by sodium sulphide precipitation. The lead depleted solution was used for the electrowinning process to separate pure zinc. 2.4–2.7 kWh electricity was applied to recover 1 kg of pure zinc from the Pb-deprived solution. Mukongo et al. (2009) and Tsakiridis et al. (2010) obtained similar results by applying electrowinning to furnace flue dusts (Table 6.5). They were able to electrolyse more than 90% of Zn from the dust samples at the expense of 3.5 kWh/kg energy.
6.4.4 (Bio)Sorption
Sorption is a widely used and relatively cost-effective metal-recovery technology applied to heavy-metal-containing aqueous solutions. Ion-exchange and expansion properties are important in the selection of suitable sorbent materials. The mechanism of sorption involves three important phases (Das 2010): (i) solid phase (which denotes the sorbent used), (ii) liquid phase (the leachate is usually used as the solvent) and (iii) dissolved phase (refers to the dissolved metal ions). Apart from low cost, sorption has other advantages like low sludge production and multiple use of the sorbent by regeneration of the sorbent. The major limitation of this technique is the early saturation of the (biomass) sorbent (Alluri et al. 2007).
Clay minerals, biological materials, carbon nanotubes, activated carbon, metal oxides and zeolites have been used as sorbents for heavy metals (Zhao et al. 2011). Biological agents such as bacteria, yeasts, fungi and plant materials can also be used in sorption and the process is termed as biosorption. Micro-organisms accumulate metals in the cell wall based on the cell’s metabolism and the properties of the cell wall (Fig. 6.12) (Ahalya et al. 2003). In addition, plant tissues are able to accumulate metals, which take up the metals either by active (at the expense of energy) or passive (electrostatic attachment to the cell wall) processes (Fig. 6.12).
Petrisor et al. (2002) reported the biosorption from Romanian mine tailings. Creamer et al. (2006) and Macaskie et al. (2007) demonstrated the use of bacteria (Desulfovibrio desulfuricans and Klebsiella pneumonia, respectively) to recover precious metals like gold, silver and palladium from electronic scrap leachates. Zinc removal from leachates of solid industrial waste using hazelnut shell was reported by Turan et al. (2011). Jalili Seh-Bardan et al. (2013) investigated the biosorption of metals such as Zn, Pb, Fe, As and Mn using Aspergillus fumigates from gold mine tailing leachates. More rigorous lab scale studies are needed to scale up the biosorption of metals from leachates at large scale levels.
6.5 Conclusions
Huge loads of different metal bearing wastes are produced by different ferrous and non-ferrous metallurgical operations. These metallurgical dusts, sludges, residues and other solid wastes contain high metal concentrations. The two important environmental issues, i.e. growing demand of metals and environmental impacts caused by metallurgical wastes, can be addressed by extraction and recovery of the heavy metals from these wastes. There are different leaching procedures suggested by various authors for distinctly different metal wastes. A variety of metal-recovery strategies have been developed for the successful recovery of metals from the metal containing leachates. Mineralogical phase composition (oxidised or reduced) and metal content play an important role in the selection of suitable leaching and recovery processes. The combination of the knowledge on the mineralogical composition of the waste with the various leaching and metal recovery processes will help to use these metallurgical wastes as potential secondary sources of metals.
References
Acharya C, Kar RN, Sukla LB (2002) Bioleaching of low grade manganese ore with Penicillium citrinum. Eur J Miner Process Environ Prot 2(3):197–204
Ahalya N, Ramachandra TV, Kanamadi RD (2003) Biosorption of heavy metals. Res J Chem Environ 7(4):71–79
Alizadeh R, Rashchi F, Vahidi E (2011) Recovery of zinc from leach residues with minimum iron dissolution using oxidative leaching. Waste Manag Res 29:165–171. doi:10.1177/0734242X10372661
Alluri HK, Ronda SR, Settalluri VS, Bondili JS, Suryanarayana V, Venkateshwar P (2007) Biosorption: an eco-friendly alternative for heavy metal removal. Afr J Biotechnol 6(25):2924–2931. doi:10.4314/ajb.v6i25.58244
Amiri F, Mousavi SM, Yaghmaei S (2011) Enhancement of bioleaching of a spent Ni/Mo hydroprocessing catalyst by Penicillium simplicissimum. Sep Purif Technol 80(3):566–576. doi:10.1016/j.seppur.2011.06.012
Anjum F, Shahid M, Akcil A (2012) Biohydrometallurgy techniques of low grade ores: a review on black shale. Hydrometallurgy 117:1–12. doi:10.1016/j.hydromet.2012.01.007
Asadi-Zeydabadi B, Mowla D, Shariat MH, Fathi Kalajahi J (1997) Zinc recovery from blast furnace flue dust. Hydrometallurgy 47(1):113–125. doi:10.1016/S0304-386X(97)00039-X
Bakhtiari F, Zivdar M, Atashi H, Seyed-Bagheri SA (2008a) Bioleaching of copper from smelter dust in a series of airlift bioreactors. Hydrometallurgy 90(1):40–45. doi:10.1016/j.hydromet.2007.09.010
Bakhtiari F, Atashi H, Zivdar M, Seyed-Bagheri SA (2008b) Continuous copper recovery from a smelter's dust in stirred tank reactors. Int J Miner Process 86(1–4):50–57
Bakhtiari F, Zivdar M, Atashi H, Seyed-Bagheri S (2010) Continuous bioleaching of copper from copper smelters dust with a mixed culture of mesophilic bacteria. J Chem Soc Pak 32:215–222
Barton LL, Hamilton WA (2007) Sulphate-reducing bacteria: environmental and engineered systems. Cambridge University Press, Cambridge
Bayat O, Sever E, Bayat B, Arslan V, Poole C (2009) Bioleaching of zinc and iron from steel plant waste using Acidithiobacillus Ferrooxidans. Appl Biochem Biotechnol 152(1):117–126, https://doi.org/10.1007/s12010-008-8257-5
Benning LG, Waychunas GA (2008) Nucleation, growth, and aggregation of mineral phases: mechanisms and kinetic controls. In: Kinetics of water-rock interaction. Springer, New York, pp 259–333. doi:10.1007/978-0-387-73563-4_7
Bosshard PP, Bachofen R, Brandl H (1996) Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ Sci Technol 30:3066–3070. doi:10.1021/es960151v
Brandl H (2001) Microbial leaching of metals. In: Rehm HJ, Reed G (eds) Biotechnology. Wiley-VHC, Weinheim, pp 191–224. doi:10.1002/9783527620999.ch8k
Brierley JA (2008) A perspective on developments in biohydrometallurgy. Hydrometallurgy 94(1):2–7. doi:10.1016/j.hydromet.2008.05.014
Brierley JA, Brierley CL (1999) Present and future commercial applications of biohydrometallurgy. Process Metallurgy 9:81–89. doi:10.1016/S1572-4409(99)80007-8
Brierley JA, Brierley CL (2001) Present and future commercial applications of biohydrometallurgy. Hydrometallurgy 59(2):233–239. doi:10.1016/S0304-386X(00)00162-6
Brombacher C, Bachofen R, Brandl H (1997) Biohydrometallurgical processing of solids: a patent review. Appl Microbiol Biotechnol 48(5):77–587. doi:10.1007/s002530051099
Burgstaller W, Schinner F (1993) Mini review: leaching of metals with fungi. Biotechnology 27:91–116. doi:10.1016/0168-1656(93)90101-R
Bulut U, Ozverdi A, Erdem M (2009) Leaching behavior of pollutants in ferrochrome arc furnace dust and its stabilization/solidification using ferrous sulphate and Portland cement. J Hazard Mater 162(2-3):893–898
Cantarino MV, de Carvalho-Filho C, Borges-Mansur M (2012) Selective removal of zinc from basic oxygen furnace sludges. Hydrometallurgy 111:124–128. doi:10.1016/j.hydromet.2011.11.004
Cao J, Zhang G, Mao Z, Fang Z, Yang C (2009) Precipitation of valuable metals from bioleaching solution by biogenic sulphides. Miner Eng 22:289–295. doi:10.1016/j.mineng.2008.08.006
Caravaca C, Cobo A, Alguacil FJ (1994) Considerations about the recycling of EAF flue dusts as source for the recovery of valuable metals by hydrometallurgical processes. Resour Conserv Recycl 10:35–41. doi:10.1016/0921-3449(94)90036-1
Chandrappa R, Das DB (2012) Wastes from industrial and commercial activities. In: Solid waste management. Springer, Berlin/Heidelberg, pp 217–247. doi:10.1007/978-3-642-28681-0_9
Chi TD, Lee JC, Pandey BD, Yoo K, Jeong J (2011) Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner Eng 30; 24(11): 1219–1222. doi:10.1016/j.mineng.2011.05.009
Choppin GR, Morgenstern A (2000) Thermodynamics of solvent extraction. Solv Extr Ion Exc 18(6):1029–1049. doi:10.1080/07366290008934721
Cole ER, Dattilo M, O'Keefe TJ (1987) Nonferrous waste as a source of zinc for electrogalvanizing. US Department of the Interior, Bureau of Mines
Creamer NJ, Baxter-Plant VS, Henderson J, Potter M, Macaskie LE (2006) Palladium and gold removal and recovery from precious metal solutions and electronic scrap leachates by Desulfovibrio desulfuricans. Biotechnol Lett 28(18):1475–1484. doi:10.1007/s10529-006-9120-9
Cruells M, Roca A, Núnẽz C (1992) Electric arc furnace flue dusts: characterization and leaching with sulphuric acid. Hydrometallurgy 31(3):213–231. doi:10.1016/0304-386X(92)90119-K
Crundwell FK (2003) How do bacteria interact with minerals? Hydrometallurgy 71:75–81. doi:10.1016/S0304-386X(03)00175-0
Cui JR, Zhang LF (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158:228–256. doi:10.1016/j.jhazmat.2008.02.001
Das N (2010) Recovery of precious metals through biosorption-a review. Hydrometallurgy 103(1):180–189. doi:10.1016/j.hydromet.2010.03.016
Das B, Prakash S, Reddy PSR, Biswal SK, Mohapatra BK, Misra VN (2002) Effective utilization of blast furnace flue dust of integrated steel plants. Eur J Miner Process Environ Protect 2(2):61–68
Das B, Prakash S, Reddy PSR, Misra VN (2006) An overview of utilization of slag and sludge from steel industries. Resour Conserv Recycl 50(1):40–57. doi:10.1016/j.resconrec.2006.05.008
Davenport WGL, King M, Schlesinger M, Biswas AK (2002) Extractive metallurgy of copper. Elsevier, Kent
Domic EM (2007) A review of the development and current status of copper bioleaching operations in Chile: 25 years of successful commercial implementation. In: Biomining. Springer, Berlin/Heidelberg, pp 81–95. doi:10.1007/978-3-540-34911-2_4
Dutra AJB, Paiva PRP, Tavares LM (2006) Alkaline leaching of zinc from electric arc furnace steel dust. Miner Eng 19(5):478–485. doi:10.1016/j.mineng.2005.08.013
Duvesteyn WPC, Wicker GR, Doane RE (1979) An omnivorous process for laterite deposits, chapter 28. In: Evans DJI, Shoemaker RS, Veltman H (eds) International laterite Symposium. Society of Mining Engineers of AIME, New York, pp 553–570
Duyvesteyn W, Jha MC (1986) Mixed Lixiviant for separate recovery of zinc and lead from iron- containing materials. US Patent No 4:614, 543
Edwards KJ, Bond PL, Gihring TM, Banfield JF (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796–1799. doi:10.1126/science.287.5459.1796
Erdem M, Özverdi A (2011) Environmental risk assessment and stabilization/solidification of zinc extraction residue: II. Stabilization/solidification. Hydrometallurgy 105(3):270–276. doi:10.1016/j.hydromet.2009.10.011
Erust C, Akcil A, Gahan CS, Tuncuk A, Deveci H (2013) Biohydrometallurgy of secondary metal resources: a potential alternative approach for metal recovery. J Chem Technol Biotechnol 88(12):2115–2132. doi:10.1002/jctb.4164
España JS, Pamo EL, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20:1320–1356. doi:10.1016/j.apgeochem.2005.01.011
Falco L, Pogliani C, Curutchet G, Donati E (2003) A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 71(1):31–36. doi:10.1016/S0304-386X(03)00170-1
Farbiszewska-Kiczma J, Farbiszewska T, Bąk M (2004) Bioleaching of metals from polish black shale in neutral medium. Physicochem Probl Miner Process 38:273–280
Fu B, Zhou H, Zhang R, Qiu G (2008) Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp. and Leptospirillum ferriphilum. Int Biodeter Biodegr 62:109–115. doi:10.1016/j.ibiod.2007.06.018
Gabler RC, Jones JR (1988) Metal recovery from secondary copper converter dust by ammoniacal carbonate leaching. US Department of the Interior, Bureau of Mines
Gahan CS, Srichandan H, Kim DJ, Akcil A (2012) Biohydrometallurgy and biomineral processing technology: a review on its past, present and future. Res J Recent Sci 1(10):85–99
Ghosh A, Ray HS (1991) Principles of extractive metallurgy. New Age International, New Delh
Gieré R, Sidenko NV, Lazareva EV (2003) The role of secondary minerals in controlling the migration of arsenic and metals from high sulphide wastes (Berikul gold mine, Siberia). Appl Geochem 18:1347–1359. doi:10.1016/S0883-2927(03)00055-6
Golyshina OV, Pivovarova TA, Karavaiko GI, Kondratéva TF, Moore ER, Abraham WR, Lünsdorf H, Timmis KN, Yakimov MM, Golyshin PN (2000) Ferroplasma acidiphilum gen. nov, sp. nov, an acidophilic, autotrophic, ferrous-iron-oxidizing, cell wall- lacking, mesophilic member of the Ferroplasmaceae fam. nov, comprising a distinct lineage of the archaea. Int J Syst Evol Microbiol 50(3):997–1006. doi:10.1099/00207713-50-3-997
Gupta CK (2006) Chemical metallurgy: principles and practice. Wiley, Weinheim
Hammarstrom JM, Seal RR II, Meier AL, Kornfeld JM (2005) Secondary sulphate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments. Chem Geol 215:407–431. doi:10.1016/j.chemgeo.2004.06.053
Han Y, Ma X, Zhao W, Chang Y, Zhang X, Wang X, Huang Z (2013) Sulphur-oxidizing bacteria dominate the microbial diversity shift during the pyrite and low-grade pyrolusite bioleaching process. J Biosci Bioeng 116(4):465–471. doi:10.1016/j.jbiosc.2013.04.012
Havlik T, Friedrich B, Stopic S (2004) Pressure leaching of EAF dust with sulphuric acid. Erzmetall 57(2):83–90
Havlík T, Bernardes AM, Schneider IAH, Miškufová A (2006) Hydrometallurgical processing of carbon steel EAF dust. J Hazard Mater 135(1):311–318. doi:10.1016/j.jhazmat.2005.11.067
Henry A (1997) Schnell, Bioleaching of Copper, Biomining, Biotechnology Intelligence Unit, pp 21–43
Hita R, Torrent J, Bigham JM (2006) Experimental oxidative dissolution of sphalerite in the Aznalcóllar sludge and other pyrite matrices. J Environ Qual 35:1032–1039. doi:10.2134/jeq2005.0371
Hita R, Wang H, Bigham JM, Torrent J, Tuovinen OH (2008) Bioleaching of a pyritic sludge from the Aznalcóllar (Spain) mine spillage at ambient and elevated temperatures. Hydrometallurgy 93(1):76–79. doi:10.1016/j.hydromet.2008.03.004
Ilyas S, Chi RA, Lee JC (2013) Fungal bioleaching of metals from mine tailing. Miner Process Extr M 34(3):185–194. doi:10.1080/08827508.2011.623751
Işıldar A, van de Vossenberg J, Rene ER, van Hullebusch ED, Lens PNL (2016) Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag 57:149–157. doi:10.1016/j.wasman.2015.11.033
Jalili Seh-Bardan B, Othman R, Abd-Wahid S, Sadegh-Zadeh F, Husin A (2013) Biosorption of heavy metals in leachate derived from gold mine tailings using Aspergillus fumigatus. CLEAN Soil Air Water 41(4):356–364. doi:10.1002/clen.201200140
Jha MK, Kumar V, Singh RJ (2001) Review of hydrometallurgical recovery of zinc from industrial wastes. Resour Conserv Recycl 33:1–22. doi:10.1016/S0921-3449(00)00095-1
Johnson DB (2006) Biohydrometallurgy and the environment: intimate and important interplay. Hydrometallurgy 83:153–166. doi:10.1016/j.hydromet.2006.03.051
Johnson DB (2009) Extremophiles: acid environments. In: Schaechter M (ed) Encyclopedia of microbiology. Elsevier, Oxford, pp 107–126
Johnson DB (2013) Development and application of biotechnologies in the metal mining industry. Environ Sci Pollut Res Int 20(11):7768–7776. doi:10.1007/s11356-013-1482-7
Johnson DB (2014) Biomining - biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol 30:24–31. doi:10.1016/j.copbio.2014.04.008
Johnson DB, Grail BM, Hallberg KB (2013) A new direction for biomining: extraction of metals by reductive dissolution of oxidized ores. Fortschr Mineral 3(1):49–58. doi:10.3390/min3010049
Jorgensen BB (1982) Ecology of the bacteria of the sulphur cycle with special reference to anoxic-oxic interface environments. Philos Trans R Soc Lond B 298:543–561. doi:10.1098/rstb.1982.0096
Ju S, Zhang Y, Zhang Y, Xue P, Wang Y (2011) Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J Hazard Mater 192(2):554–558. doi:10.1016/j.jhazmat.2011.05.049
Kachur AN, Arzhanova VS, Yelpatyevsky PV, von Braun MC, von Lindern IH (2003) Environmental conditions in the Rudnaya River watershed – a compilation of Soviet and post- Soviet era sampling around a lead smelter in the Russian Far East. Sci Total Environ 303:171–185. doi:10.1016/S0048-9697(02)00351-0
Kaksonen AH, Mudunuru BM, Hackl R (2014) The role of microorganisms in gold processing and recovery – a review. Hydrometallurgy 142:70–83. doi:10.1016/j.hydromet.2013.11.008
Karlfeldt FK, Ekberg C, Skarnemark G, Steenari B-M (2012) Initial studies on the recovery of Cu from MSWI fly ash leachates using solvent extraction. Waste Manag Res 30:1072–1080. doi:10.1177/0734242X12441385
Ke Y, Chai LY, Min XB, Tang CJ, Chen J, Wang Y, Liang YJ (2014) Sulphidation of heavy-metal-containing neutralization sludge using zinc leaching residue as the sulphur source for metal recovery and stabilization. Miner Eng 61:105–112. doi:10.1016/j.mineng.2014.03.022
Keith DC, Runnells DD, Esposito KJ, Chermak JA, Levy DB, Hannula SR, Watts M, Hall L (2001) Geochemical models of the impact of acidic groundwater and evaporative sulphate salts on Boulder Creek at Iron Mountain, California. Appl Geochem 16:947–961. doi:10.1016/S0883-2927(00)00080-9
Kelebek S, Yörük S, Davis B (2004) Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner Eng 17(2):285–291. doi:10.1016/j.mineng.2003.10.030
Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov, Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int J Syst Evol Microbiol 50:511–516. doi:10.1099/00207713-50-2-511
Kierczak J, Néel C, Puziewicz J, Bril H (2009) The mineralogy and weathering of slag produced by the smelting of lateritic Ni ores, Szklary, southwestern Poland. Can Mineral 47:557–572. doi:10.3749/canmin.47.3.557
Kim BM, Amodeo PA (1983) Calcium sulfide process for treatment of metal containing wastes. Environ Prog 2(3):175–180. doi:10.1002/ep.670020309
Koleini SM, Mehrpouya H, Saberyan K, Abdolahi M (2010) Extraction of indium from zinc plant residues. Miner Eng 23(1):51–53. doi:10.1016/j.mineng.2009.09.007
Kondos PD, Mackinnon DJ, Mccready RGL, Riveros PA, Skaff M (1991) Study on metals recovery/recycling from acid mine drainage phase ia: literature survey, 1–47. http://mend-nedem.org/wp-content/uploads/(2013)/01/3.21.1a.pdf
Kosińska K, Miśkiewicz T (2012) Precipitation of heavy metals from industrial wastewater by Desulfovibrio desulfuricans. Environ Prot Eng 38(2):51–60. doi:10.5277/epe120205
Laforest G, Duchesne J (2006) Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J Hazard Mater 135(1):156–164. doi:10.1016/j.jhazmat.2005.11.037
Lee JC, Pandey BD (2012) Bio-processing of solid wastes and secondary resources for metal extraction – a review. Waste Manag 32:3–18. doi:10.1016/j.wasman.2011.08.010
Lenz DM, Martins FB (2007) Lead and zinc selective precipitation from leach electric arc furnace dust solutions. Revista Matéria 12(3):503–509. http://dx.doi.org/10.1590/S1517-70762007000300011
Leonard RP (1978) Hazardous solid waste from metallurgical industries. Environ Health Perspect 27:251–260
Lewis AE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234. doi:10.1016/j.hydromet.2010.06.010
Lewis A, van Hille R (2006) An exploration into the sulphide precipitation method and its effect on metal sulphide removal. Hydrometallurgy 81(3):197–204. doi:10.1016/j.hydromet.2005.12.009
Li M, Peng B, Chai LY, Peng N, Xie XD, Yan H (2013) Technological mineralogy and environmental activity of zinc leaching residue from zinc hydrometallurgical process. T Nonferr Metal Soc 23(5):1480–1488. doi:10.1016/S1003-6326(13)62620-5
Lingling L, Lu Z, Weiwei J (2012) Screening and identification of Pseudomonas aeruginosa LLJQ-1 and its role in bioleaching of low-grade collophanite. In: World Automation Congress (WAC), 1–6. IEEE
Lottermoser BG (2010) Mine wastes: characterization, treatment, and environmental impacts. Springer, Berlin
Lu D, Jin Z, Shi L, Tu G, Xie F, Asselin E (2014) A novel separation process for detoxifying cadmium-containing residues from zinc purification plants. Miner Eng 64:1–6. doi:10.1016/j.mineng.2014.03.026
Macaskie LE, Creamer NJ, Essa AMM, Brown NL (2007) A new approach for the recovery of precious metals from solution and from leachates derived from electronic scrap. Biotechnol Bioeng 96(4):631–639. doi:10.1002/bit.21108
Malhotra D, Taylor P, Spiller E, LeVier M (2009) Recent advances in mineral processing plant design. SME, Colorado
Manahan SE (1990) Hazardous waste chemistry, toxicology, and treatment. CRC Press, Boca Raton
Mansfeldt T, Dohrmann R (2004) Chemical and mineralogical characterization of blast-furnace sludge from an abandoned landfill. Environ Sci Technol 38(22):5977–5984. doi:10.1021/es040002
Mansur MB (2011) Solvent extraction for metal and water recovery from industrial wastes and effluents. R Esc Minas 64(1):51–55. http://dx.doi.org/10.1590/S0370-44672011000100006
Marsden JO (2006) Recent developments in copper hydrometallurgy. In: Sohn international symposium; advanced processing of metals and materials, Volume 1: Thermo and physicochemical principles: non-ferrous high-temperature processing, Vol 1, pp 833–834
Martín IM, López-Delgado A, López FA, Coedo AG, Dorado MT, Alguacil FJ (2003) Treatment of copper converter flue dust for the separation of metallic/non-metallic copper by hydrometallurgical processing. J Chem Eng Japan 36(12):1498–1502. doi:10.1252/jcej.36.1498
Massianaie M, Oliazadeh M, Bagheri AS (2006) Biological copper extraction from melting furnace dust of Sarcheshmeh copper mine. Int J Miner Process 81:58–62. doi:10.1016/j.minpro.2006.06.005
Min XB, Xie XD, Chai LY, Liang YJ, Li M, Ke Y (2013) Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. T Nonferr Metal Soc 23(1):208–218. doi:10.1016/S1003-6326(13)62448-6
Mukongo T, Maweja K, Mutombo I, Tshilombo K (2009) Zinc recovery from the water-jacket furnace flue dusts by leaching and electrowinning in a SEC-CCS cell. Hydrometallurgy 97(1):53–60. doi:10.1016/j.hydromet.2009.01.001
Müller B, Burgstaller W, Strasser H, Zanella A, Schinner F (1995) Leaching of zinc from an industrial filter dust with Penicillium, Pseudomonas and Corynebacterium: Citric acid is the leaching agent rather than amino acids. J Ind Microbiol 14(3–4):208–212. doi:10.1007/BF01569929
Mulligan CN, Galvez-Cloutier R, Renaud N (1999) Biological leaching of copper mine residues by Aspergillus niger. Process Metallurgy 9:453–461. doi:10.1016/S1572-4409(99)80046-7
Mulligan CN, Kamali M, Gibbs BF (2004) Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J Hazard Mater 110(1):77–84. doi:10.1016/j.jhazmat.2004.02.040
National Research Council (US) (2002) Committee on technologies for the mining industries. In: Evolutionary and revolutionary technologies for mining. National Academy Press, Washington, DC
Neale JW, Gericke M, Ramcharan K (2011) The application of bioleaching to base metal sulphides in southern Africa: prospects and opportunities. In: The Southern African Institute of Mining and Metallurgy 6th Southern African Base Metals Conference, pp 367–388. doi: 10.13140/2.1.2461.6323
Ngenda RB, Segers L, Kongolo PK (2009) Base metals recovery from zinc hydrometallurgical plant residues by digestion method. Hydrometallurgy Conference, The Southern African Institute of Mining and Metallurgy, pp 17–29
Norris PR, Burton NP, Foulis NAM (2000) Acidophiles in bioreactor mineral processing. Extremophiles 4:71–76. doi:10.1007/s007920050139
Nouren S, Bhatti HN, Ilyas S (2011) Bioleaching of copper, aluminium, magnesium and manganese from brown shale by Ganoderma lucidum. Afr J Biotechnol 10:10664–10673. doi:10.5897/AJB11.1380
Oliazadeh M, Massianaie M, Bagheri AS, Shahverdi AR (2006) Recovery of copper from melting furnaces dust by microorganisms. Miner Eng 19:209–210. doi:10.1016/j.mineng.2005.09.004
Olson GJ, Brierley JA, Brierley CL (2003) Bioleaching review (Part B): processing in bioleaching: applications of microbial processes by the minerals industries. Appl Microbiol Biotechnol 63:249–257. doi:10.1007/s00253-003-1404-6
Pearson D (1981) Recovery of zinc from metallurgical dusts and fumes, process and fundamental considerations of selected hydrometallurgical systems. In: Kuhn MC (ed) Chapter 14. Society of Mining Engineers of AIME, New York, pp 153–168
Pereira CF, Rodrıguez-Piñero M, Vale J (2001) Solidification/stabilization of electric arc furnace dust using coal fly ash: analysis of the stabilization process. J Hazard Mater 82(2):183–195. doi:10.1016/S0304-3894(00)00359-9
Petrisor IG, Komnitsas K, Lazar I, Voicu A, Dobrota S, Stefanescu M (2002) Biosorption of heavy metals from leachates generated at mine waste disposal sites. Eur J Miner Process Environ Prot 2(3):158–167
Plumb JJ, Muddle R, Franzmann PD (2008) Effect of pH on rates of iron and sulphur oxidation by bioleaching organisms. Miner Eng 21:76–82. doi:10.1016/j.mineng.2007.08.018
Pradhan JK, Kumar S (2012) Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag Res 30(11):1151–1159. doi:10.1177/0734242X12437565
Qiang L, Pinto IS, Youcai Z (2014) Sequential stepwise recovery of selected metals from flue dusts of secondary copper smelting. J Clean Prod 84:663–670. doi:10.1016/j.jclepro.2014.03.085
Raghavan R, Mohanan PK, Patnaik SC (1998) Innovative processing technique to produce zinc concentrate from zinc production residue with simultaneous recovery of lead and silver. Hydrometallurgy 48(2):225–237. doi:10.1016/S0304-386X(97)00082-0
Rao SR (2011) Resource recovery and recycling from metallurgical wastes. In: Waste management series. Elsevier Science, The Netherlands
Rao DV, Shivannavar CT, Gaddad SM (2002) Bioleaching of copper from chalcopyrite ore by fungi. Indian J Exp Biol 40(3):319–324
Rawlings DE (2005) Characteristics and adaptability of iron- and sulphur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb Cell Factories 4:1–15. doi:10.1186/1475-2859-4-13
Renman R, Jiankang W, Jinghe C (2006) Bacterial heap-leaching: practice in Zijinshan copper mine. Hydrometallurgy 83(1):77–82. doi:10.1016/j.hydromet.2006.03.048
Reuter M, Lans S, Booster J (1998) Zinc. Course notes for Zinc College (1999) Report Department of Raw Materials Technology, Delft University of Technology, The Netherlands, pp 1–50
Riekkola-Vanhanen M (2010) Talvivaara Sotkamo mine - bioleaching of a polymetallic nickel ore in subarctic climate. Nova Biotechnol 10(1):7–13
Romero A, González I, Galán E (2006) The role of efflorescent sulphates in the storage of trace elements in stream waters polluted by acid mine-drainage: the case of Peña Del Hierro, Southwestern Spain. Can Mineral 44:1431–1446. doi:10.2113/gscanmin.44.6.1431
Rossi G (1990) Biohydrometallurgy. McGraw-Hill, Hamburg
Ruşen A, Sunkar AS, Topkaya YA (2008) Zinc and lead extraction from Çinkur leach residues by using hydrometallurgical method. Hydrometallurgy 93(1):45–50. doi:10.1016/j.hydromet.2008.02.018
Safarzadeh MS, Moradkhani D, Ojaghi-Ilkhchi M (2009) Kinetics of sulphuric acid leaching of cadmium from Cd-Ni zinc plant residues. J Hazard Mater 163(2):880–890. doi:10.1016/j.jhazmat.2008.07.082
Safarzadeh MS, Dhawan N, Birinci MD, Moradhani D (2011) Reductive leaching of cobalt from zinc plant purification residues. Hydrometallurgy 106:51–57. doi:10.1016/j.hydromet.2010.11.017
Salihoglu G, Pinarli V (2008) Steel foundry electric arc furnace dust management: stabilization by using lime and Portland cement. J Hazard Mater 153(3):1110–1116. doi:10.1016/j.jhazmat.2007.09.066
Sampaio RMM, Timmers RA, Xu Y, Keesman KJ, Lens PNL (2009) Selective precipitation of Cu from Zn in a pS controlled continuously stirred tank reactor. J Hazard Mater 165(1):256–265. doi:10.1016/j.jhazmat.2008.09.117
Sampaio RMM, Timmers RA, Kocks N, André V, Duarte MT, van Hullebusch ED, Lens PNL (2010) Zn-Ni sulphide selective precipitation: the role of supersaturation. Sep Purif Technol 74(1):108–118. doi:10.1016/j.seppur.2010.05.013
Sánchez-España J, López-Pamo E, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20(7):1320–1356. doi:10.1016/j.apgeochem.2005.01.011
Sand W, Rohde K, Sobotke B, Zenneck C (1992) Evaluation of Leptospirillum ferrooxidans for leaching. Appl Environ Microbiol 58(1):85–92
Sandstrom A, Petersson S (1997) Bioleaching of a complex sulphide ore with moderate thermophilic and extreme thermophilic microorganisms. Hydrometallurgy 46:181–190. doi:10.1016/S0304-386X(97)00012-1
Schinner F, Burgstaller W (1989) Extraction of zinc from industrial waste by Penicillium sp. Appl Environ Microbiol 55:1153–1156
Sethurajan M, Aruliah R, Karthikeyan OP, Balasubramanian R (2012) Bioleaching of copper from black shale ore using mesophilic mixed populations in an air up-lift bioreactor. Environ Eng Manag J 11(10):1839–1848
Sethurajan M, Huguenot D, Horn HA, Figueiredo LAF, Lens PNL, van Hullebusch ED (2016a) Fractionation and leachability of heavy metals from aged and recent Zn-metallurgical leach residues from the Três Maris Zinc plant (MG, Brazil). Environ Sci Pollut R 23(8):7504–7516. doi:10.1007/s11356-015-6014-1
Sethurajan M, Huguenot D, Horn HA, Figueiredo LAF, Lens PNL, van Hullebusch ED (2016b) Selective recovery of copper from hazardous zinc hydrometallurgical purification residues. J Environ Manag 177:26–35. doi:10.1016/j.jenvman.2016.03.041
Sethurajan M, Huguenot D, Jain R, Horn HA, Figueiredo LAF, Lens PNL, van Hullebusch ED (2017a) Leaching and selective zinc recovery from acidic leachates of zinc metallurgical leach residues. J Hazard Mater 324:71–82. doi:10.1016/j.jhazmat.2016.01.028
Sethurajan M, Lens PNL, Rene ER, van de Vossenberg J, Huguenot D, Horn HA, Figueiredo LAF, van Hullebusch ED (2017b) Bioleaching and selective biorecovery of zinc from zinc metallurgical leach residues from the Três Marias zinc plant (Minas Gerais, Brazil). J Chem Technol Biotechnol 92(3):512–521. doi:10.1002/jctb.5026
Shen H, Forssberg E (2003) An overview of recovery of metals from slags. Waste Manag 23(10):933–949. doi:10.1016/S0956-053X(02)00164-2
Silva JE, Paiva AP, Soares D, Labrincha A, Castro F (2005) Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J Hazard Mater 120:113–118. doi:10.1016/j.jhazmat.2004.12.008
Sole KC, Feather AM, Cole PM (2005) Solvent extraction in southern Africa: an update of some recent hydrometallurgical developments. Hydrometallurgy 78(1):52–78. doi:10.1016/j.hydromet.2004.11.012
Solisio C, Lodi A, Veglio F (2002) Bioleaching of zinc and aluminium from industrial waste sludges by means of Thiobacillus ferrooxidans. Waste Manag 22:667–675. doi:10.1016/S0956-053X(01)00052-6
Steyn J, Sandenbergh RF (2004) A study of the influence of copper on the gold electrowinning process. J S Afr I Min Metall 104(3):177–182
St-Pierre J, Piron DL (1986) Electrowinning of zinc from alkaline solutions. J Appl Electrochem 16(3):447–456. doi:10.1007/BF01008856
St-Pierre J, Piron DL (1990) Electrowinning of zinc from alkaline solutions at high current densities. J Appl Electrochem 20(1):163–165. doi:10.1007/BF01012487
Sukla LB, Panchanadikar V (1993) Bioleaching of lateritic nickel ore using a heterotrophic micro-organism. Hydrometallurgy 32(3):373–379. doi:10.1016/0304-386X(93)90048-I
Sukla LB, Kar RN, Panchanadikar VV, Choudhury S, Mishra RK (1995) Bioleaching of lateritic nickel ore using Penicillium sp. Trans Indian Inst Met (India) 48(2):103–106
Terézia V, Tamási T (2013) Experimental investigation of zinc precipitation from eaf dust leaching solutions. Mater Sci Eng 38(1):61–71
Török T (2013) Experimental investigation of zinc precipitation from EAF dust leaching solutions. Mater Sci Eng 38(1):61–71
Trefry JH, Metz S (1984) Selective leaching of trace metals from sediments as a function of pH. Anal Chem 56(4):745–749. doi:10.1021/ac00268a034
Trung ZH, Kukurugya F, Takacova Z, Orac D, Laubertova M, Miskufova A, Havlik T (2011) Acidic leaching both of zinc and iron from basic oxygen furnace sludge. J Hazard Mater 192(3):1100–1107. doi:10.1016/j.jhazmat.2011.06.016
Tsakiridis PE, Oustadakis P, Katsiapi A, Agatzini-Leonardou S (2010) Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part II: Downstream processing and zinc recovery by electrowinning. J Hazard Mater 179(1):8–14. doi:10.1016/j.jhazmat.2010.01.059
Turan MD, Altundoğan HS, Tümen F (2004) Recovery of zinc and lead from zinc plant residue. Hydrometallurgy 75(1–4):169–176. doi:10.1016/j.hydromet.2004.07.008
Turan NG, Mesci B, Ozgonenel O (n.d.2011) Artificial neural network (ANN) approach for modelling Zn (II) adsorption from leachate using a new biosorbent. Chem Eng J 173(1):98–105. doi: 10.1016/j.cej.2011.07.042
Tutor FV, Murciego AM, Valle PA, Ayuso EÁ, Pascual EP, Sánchez AG (2013) Weathering products of arsenopyrite and pyrite from El Bollo mine wastes (Salamanca, Spain) and their environmental hazards. Revista De La Sociedad Española De Mineralogía:111–112
Upadhyay SN (2002) Mineral biotechnology: potential and problems. In: Sukla LB, Misra VN (eds) Proceedings of the national seminar on mineral biotechnology. Allied Publishers Pvt Ltd, India, pp 16–31
Uroz S, Calvaruso C, Turpault MP, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Biotechnol 17(8):378–387. doi:10.1016/j.tim.2009.05.004
Vahidi E, Rashchi F, Moradkhani D (2009) Recovery of zinc from an industrial zinc leach residue by solvent extraction using D2EHPA. Miner Eng 22(2):204–206. doi:10.1016/j.mineng.2008.05.002
Vakylabad AB (2011) A comparison of bioleaching ability of mesophilic and moderately thermophilic culture on copper bioleaching from flotation concentrate and smelter dust. Int J Miner Process 101(1-4):94–99
Vakylabad AB, Schaffie M, Ranjbar M, Manafi Z, Darezereshki E (2012) Bio-processing of copper from combined smelter dust and flotation concentrate: a comparative study on the stirred tank and airlift reactors. J Hazard Mater 241–242:197–206. doi:10.1016/j.jhazmat.2012.09.031
Van Herck P, Vandecasteele C (2000) Zinc and lead removal from blast furnace sludge with a hydrometallurgical process. Environ Sci Technol 34(17):3802–3808. doi:10.1021/es991033l
Veeken AHM, Akoto L, Hulshoff-Pol LW, Weijma J (2003) Control of the sulfide (S2−) concentration for optimal zinc removal by sulfide precipitation in a continuously stirred tank reactor. Water Res 37(15):3709–3717. doi:10.1016/S0043-1354(03)00262-8
Veglio F, Quaresima R, Fornari P (2003) Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning. Waste Manag 23(3):245–252. doi:10.1016/S0956-053X(02)00157-5
Vereš J, Lovás M, Jakabský Š, Šepelák V, Hredzák S (2012) Characterization of blast furnace sludge and removal of zinc by microwave assisted extraction. Hydrometallurgy 129:67–73. doi:10.1016/j.hydromet.2012.09.008
Vestola EA, Kusenaho MA, Narhi HM, Touvinen OH, Puhakka JA, Plumb JJ, Kaksonen AH (2010) Acid bioleaching of solid waste materials from copper, steel and recycling industries. Hydrometallurgy 103:74–79. doi:10.1016/j.hydromet.2010.02.017
Villa-Gomez DK, Ababneh H, Papirio S, Rousseau DPL, Lens PNL (2011) Effect of sulphide concentration on the location of the metal precipitates in inversed fluidized bed reactors. J Hazard Mater 192:200–207. doi:10.1016/j.jhazmat.2011.05.002
Villa-Gomez DK, Papirio S, van Hullebusch ED, Farges F, Nikitenko S, Kramer H, Lens PNL (2012) Influence of sulphide concentration and macronutrients on the characteristics of metal precipitates relevant to metal recovery in bioreactors. Bioresour Technol 110:26–34. doi:10.1016/j.biortech.2012.01.041
Villa-Gomez DK, van Hullebusch ED, Maestro R, Farges F, Nikitenko S, Kramer H, Lens PNL (2014) Morphology, mineralogy, and solid-liquid phase separation characteristics of Cu and Zn precipitates produced with biogenic sulphide. Environ Sci Technol 48(1):664–673. doi:10.1021/es402795x
Vítková M, Ettler V, Hyks J, Astrup T, Kříbek B (2011) Leaching of metals from copper smelter flue dust (Mufulira, Zambian copper belt). Appl Geochem 26:S263–S266. doi:10.1016/j.apgeochem.2011.03.120
Wang LK, Hung YT, Shammas NK (2005) Physicochemical treatment processes, Col 3. Springer, Humana Press, Totowa
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides - a review. Hydrometallurgy 84:81–108. doi:10.1016/j.hydromet.2006.05.001
Wilson AM, Bailey PJ, Tasker PA, Turkington JR, Grant RA, Love JB (2014) Solvent extraction: the coordination chemistry behind extractive metallurgy. Chem Soc Rev 43(1):123–134. doi:10.1039/c3cs60275c
Wong MH (1986) Reclamation of wastes contaminated by copper, lead, and zinc. Environ Manag 10(6):707–713. doi:10.1007/BF01867725
Wu HY, Ting YP (2006) Metal extraction from municipal solid waste (MSW) incinerator fly ash-Chemical leaching and fungal bioleaching. Enz Microbial Technol 38:839–847. doi:10.1016/j.enzmictec.2005.08.012
Xia DK, Picklesi CA (2000) Microwave caustic leaching of electric arc furnace dust. Miner Eng 13(1):79–94. doi:10.1016/S0892-6875(99)00151-X
Xie F, Zhang TA, Dreisinger D, Doyle F (2014) A critical review on solvent extraction of rare earths from aqueous solutions. Miner Eng 56:10–28. doi:10.1016/j.mineng.2013.10.021
Yan H, Chai LY, Peng B, Li M, Peng N, Hou DK (2014) A novel method to recover zinc and iron from zinc leaching residue. Miner Eng 55:103–110. doi:10.1016/j.mineng.2013.09.015
Youcai Z, Stanforth R (2000) Integrated hydrometallurgical process for production of zinc from electric arc furnace dust in alkaline medium. J Hazard Mater 80(1):223–240. doi:10.1016/S0304-3894(00)00305-8
Youcai Z, Stanforth R (2001) Selective separation of lead from alkaline zinc solution by sulphide precipitation. Sep Sci Technol 36(11):2561–2570. doi:10.1081/SS-100106110
Zhang WS, Cheng CY (2007) Manganese metallurgy review. Part II: Manganese separation and recovery from solution. Hydrometallurgy 89(3–4):160–177. doi:10.1016/j.hydromet.2007.08.009
Zhao G, Wu X, Tan X, Wang X (2011) Sorption of heavy metal ions from aqueous solutions: a review. Open Colloid Sci J 4:19–31. doi:10.2174/1876530001104010019
Acknowledgements
The authors thank the financial support provided by the Erasmus Mundus Joint Doctorate programme (EMJD) in Environmental Technologies for Contaminated Solids, Soils and Sediments (ETeCoS3, FPA n°2010–0009) and the International Research Staff Exchange Scheme (IRSES) project Mining wastes bio/weathering, pollution control and monitoring (MinPollControl) (Project reference number 247594).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Sethurajan, M., Lens, P.N.L., Horn, H.A., Figueiredo, L.H.A., van Hullebusch, E.D. (2017). Leaching and Recovery of Metals. In: Rene, E., Sahinkaya, E., Lewis, A., Lens, P. (eds) Sustainable Heavy Metal Remediation. Environmental Chemistry for a Sustainable World. Springer, Cham. https://doi.org/10.1007/978-3-319-61146-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-61146-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61145-7
Online ISBN: 978-3-319-61146-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)