Abstract
The cerebellum controls ongoing motor function and motor learning. Therefore, damage to its circuits causes a number of movement disorders such as ataxia, dystonia, and tremor. Cerebellar connectivity in both normal and abnormal states has been intensely studied. As a result, its anatomy, circuitry, and neuronal firing properties are among the best understood in the brain. This knowledge has directly facilitated efforts to uncover the mechanisms that cause motor dysfunction. Here, we discuss several mouse models of cerebellar disease. We focus on how cerebellar development depends on genes and neural activity to assemble circuits for behavior.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The cerebellum is best known for its crucial role in controlling smooth, purposeful movements. Cerebellar circuits receive motor planning information from the cerebral cortex about the goals and commands of movement in addition to feedback information from the brain stem and spinal cord about the sensory consequences of movement execution. This activity within the cerebellum can be modified through multiple cellular and molecular mechanisms of synaptic plasticity . The resultant output of cerebellar activity influences connected motor systems in the cerebral cortex, brain stem, and spinal cord to allow for calibration of motor programs that can be initiated and executed without immediate sensory feedback. There are currently two general models for how the cerebellum controls motor behavior during both ongoing movement (motor coordination) and repetitions of the same movement (motor learning). One model is that cerebellar computations evaluate the accuracy of actions by comparing predicted outcomes of intended movements to the outcomes of actual movements and then reduce error by providing signals for adaptive corrections [1]. The other model is that the cerebellum participates in the timing of movement rather than error correction [2]. It is also possible that the cerebellum performs both functions. In either case, it is not surprising that physical, pharmacological, and genetic insults to the cerebellar circuit result in movement disorders, and descriptions of motor symptoms after cerebellar damage date back to Flourens [3], Babinski [4,5,6], Holmes [7], and other pioneers in the field [8]. Cerebellar insults typically disrupt the coordination and accuracy of movement, conditions cumulatively referred to as “ataxia” (Greek, loss of order). Numerous distinct motor symptoms can arise from cerebellar damage, including the inability to judge distance or scale during target-oriented movements (“dysmetria,” Greek, abnormal measure), oscillatory shaking of muscles during movement (tremor), diminished reflexive resistance to passive limb displacements (“hypotonia,” Greek, low tone), and impaired production of speech (“dysarthria,” Greek, abnormal articulation). Symptoms arise from the loss or disruption of normal cerebellar functions, and the ultimate motor behavioral consequences may also be due to movement control in a pathological state. Here, we discuss the mechanisms for different manifestations of cerebellar disease from the perspective of insights gained from mouse models, as they are currently one of the most common tools used in the study of cerebellar disorders. In order to understand the behavioral consequences of the diseased cerebellar circuit, we will consider cerebellar structure and development in the context of the functional motor system in vivo.
Structure of the Cerebellum
The cerebellum is interconnected with the rest of the brain by three pairs of large fiber tracts on its ventral surface, the cerebellar peduncles, and located dorsal to the pons and medulla (see chapter “The Embryology and Anatomy of the Cerebellum”). Though it is a predominantly continuous structure, there are three gross anatomical divisions of the cerebellum: a “wormlike” region along the midline called the vermis (Latin, worm), lateral regions that are relatively enlarged in humans called the hemispheres , and an intermediate region called the paravermis. The cerebellum comprises a three-layered cortex surrounding an inner core of white matter and three pairs of cerebellar nuclei. The sheet of cortex folds as cells proliferate during cerebellar development into folia and fissures along the anteroposterior axis, which form a series of lobules that are evolutionarily conserved and reproducible in mammals and birds [9]. Based on the work of Olof Larsell, Roman numerals are used to identify lobules in the vermis (I – X), whereas the hemispheres comprise CrusI, CrusII, lobulus simplex (LS), paramedian lobules (Pml), copula pyramids (Cop), the flocculus (Fl), and the paraflocculus (Pfl). Though lobule form is distinct across the anatomical divisions of the cerebellum, they contain the same repeated circuit and all the major cerebellar cell types [10,11,12] (Fig. 1), with the Purkinje cell at the center of each circuit. Purkinje cell somata form a monolayer, the Purkinje cell layer, across the cerebellar cortex and extend elaborate dendritic arbors into the outermost of the three layers, the molecular layer. Climbing fibers , one of the two major afferent pathways to the cerebellum, originate in the inferior olivary nucleus of the medulla and form excitatory synapses on the smooth shafts of Purkinje cell dendrites in the molecular layer. Mossy fibers, the second major afferent pathway to the cerebellum, terminate on granule cells within the third and innermost layer of cerebellar cortex, the granule cell layer, and originate from over two-dozen brainstem and spinal cord nuclei [14]. These nuclei include the basilar pontine nuclei relaying input from the cerebral cortex, dorsal nucleus of Clarke, vestibular nuclei, cuneate nuclei, and lateral reticular nuclei. Mossy fibers communicate with Purkinje cells indirectly through granule cell axons, known as parallel fibers, which ascend the granule cell and Purkinje cell layers and bifurcate to form excitatory synapses on the spines of Purkinje cell dendrites in the molecular layer. Numerous interneurons are present that influence the activity of local circuits, such as stellate and basket cells in the molecular layer and Golgi and unipolar brush cells in the granule cell layer. Neuromodulatory afferents also terminate in all three layers of the cerebellar cortex and within the cerebellar nuclei to influence local activity [15, 16]. Purkinje cell axons are the sole output of the cerebellar cortex and integrate all cerebellar inputs before projecting to the core of the cerebellum to form inhibitory synapses on their target cerebellar nuclei neurons . The cerebellar nuclei are the final efferent pathway to the rest of the brain and spinal cord; however, a small minority of Purkinje cells project directly to vestibular nuclei [17]. Despite this relatively simple and repeated cytoarchitecture (Fig. 1), a more complex circuit map is revealed by molecular, anatomical, and physiological approaches and by symptoms of disease. Subsets of Purkinje cells are divided into a series of reproducible parasagittal stripes, “zones,” (Fig. 2) that run along the anteroposterior axis and are defined by gene expression patterns [12]. The classical and most thoroughly studied molecular marker of zones is known as zebrinII , which is an antigen on the metabolic enzyme aldolase C [18]. The topographical map of zebrinII expression in mice has been detailed extensively [19,20,21]. However, zebrinII is conserved, and its general pattern of expression is identical across different taxa [22,23,24,25,26,27,28]. ZebrinII-expressing Purkinje cells alternate with zones that do not express the antigen. Together, the two subsets form a striking array of zebrinII-positive and zebrinII-negative stripes that are symmetrically distributed across the midline. More than 40 molecular markers of zones have been identified [29] including excitatory amino acid transporter 4 (EAAT4), phospholipase C beta 3 (PLCβ3), and gamma-aminobutyric acid type B receptor subunit 2 (GABAβR2), which are expressed in zebrinII-positive zones, and phospholipase C beta 4 (PLCβ4), metabotropic glutamate receptor 1 splice variant 1b (mGlurR1b), and neuroplastin, which are expressed in the complementary zebrinII-negative zones. Bands of zones do not run uninterrupted from anterior lobules to posterior lobules, and a unique pattern of zones is observed in four domains of the vermis: anterior = lobules I – V, central = lobules VI – VII, posterior = lobules VIII and dorsal IX, and nodular = lobules ventral IX and X [30] (Fig. 2). These domains are also innervated by functionally distinct mossy fiber afferents; for example, the spinocerebellar tract projects to the anterior and posterior domains, the pontocerebellar tract projects to the central and posterior domains, and the vestibulocerebellar tract projects to the nodular domain [12, 31]. These domains are not equivalent to the traditional functional compartments known as the spinocerebellum (regulation of muscles, tendons, and joints), cerebrocerebellum (planning and initiation of movement), and vestibulocerebellum (body equilibrium and oculomotor function). However, there is clearly some overlap in the functional attributes of each. These divisions are also reflected by the phenotypes of cerebellar disease in naturally occurring mutant mice, which often display differential structural defects along the anteroposterior axis [30]. Furthermore, the axon termination patterns of mossy and climbing fiber afferents within each of these domains exhibit parasagittal zones that have a reproducible anatomical relationship with the zones of their target Purkinje cells [32, 33] or the narrower functional microzones [34]. Climbing fibers originating from a specific subnucleus of the inferior olive typically terminate in one or two of these longitudinal zones [35, 36], and mossy fibers from specific sources branch to terminate in multiple longitudinal zones [37,38,39,40]. Zones are also distinct in their topographically defined Purkinje cell output to specific subnuclei of their three target cerebellar nuclei, fastigial (medial), interposed (middle; = globose and emboliform in primates), and dentate (lateral), each of which, too, has a unique efferent pathway to the rest of the brain and spinal cord [31, 41, 42], including projections back to the inferior olive to form a patterned cortico-nucleo-olivary tripartite loop [43, 44]. Together, units of topographically organized cerebellar afferents, their target Purkinje cell zones, and Purkinje cell efferent projections to the cerebellar nuclei compose cerebellar “modules,” the basic functional circuit of the cerebellum [45]. Retrograde transsynaptic tracing shows that individual muscle groups are linked to specific Purkinje cell zones [46]. Functional mapping of the cerebellar circuit using imaging and electrophysiology also exhibits topography consistent with the zonal plan [47,48,49,50]. Within each zone, receptive fields mapped by recording responses to tactile stimuli reveal a “fractured somatotopy” of spinocerebellar mossy fibers with multiple sensory representations of body parts in mosaic patches [47, 51, 52]. Due to the relatively uniform cytoarchitecture of the cerebellum, it has been thought that these topographical differences in function are caused by differences in afferent and efferent connectivity; however, recent evidence suggests that this is also due to other regional variations such as Purkinje cell morphology, Purkinje cell packing density , granule cell packing density, neuronal soma size, the position of mossy fiber and climbing fiber synapses within their target layers, distribution of interneurons, intrinsic Purkinje cell firing properties, and synaptic plasticity [53]. Distinct computational processes within and between zones can potentially arise from variations in the cytoarchitecture and physiology of local circuits in these functional compartments. This exquisite organization of connections and the precise circuitry they form require carefully executed developmental programs for proper function and behavior [54]. During this complex coordination, there are many opportunities for insults to cause disorders with devastating consequences for motor and perhaps even non-motor behavior.
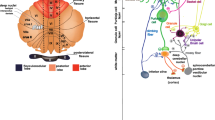
Architecture of the cerebellar circuit . (a) Mouse brain shown from a lateral view with the cerebellum highlighted in color. (b) The basic cerebellar circuit comprises Purkinje cells, granule cells, stellate and basket cell interneurons, and the cerebellar nuclei. Afferent information is delivered to the cerebellum as climbing fibers or mossy fibers. Note that the Purkinje cell is the sole output of cerebellar cortex and the cerebellar nuclei deliver efferent information of the circuit. The + and – signs indicate whether each synapse is excitatory or inhibitory, respectively. For simplicity we have not shown Golgi cells, unipolar brush cells, Lugaro cells, or candelabrum cells. The different types of glia were also omitted. (Modified with permission from Reeber et al. [13])
ZebrinII zones in the mouse cerebellum. (a, b) Wholemount immunohistochemical staining of the mouse cerebellum with zebrinII reveals the intricate patterning of the cerebellar cortex into parasagittal zones. Roman numerals identify the lobules of the vermis. Pfl paraflocculus, Fl flocculus, LS lobulus simplex, Pml paramedian lobule, Cop copula pyramidis. Scale bar = 2 mm. (Modified with permission from Reeber et al. [13])
Development of the Cerebellar Circuit
Due to the cerebellum’s well-understood circuitry and roles in developmental and adult-onset diseases, it is an important model for understanding normal and abnormal brain circuit map formation [54]. Positional cues must be present to set up the patterns of specific lobules on the anteroposterior axis and zones on the mediolateral axis. Studies resolving how genes establish the coordinates of this functional framework have increased our understanding of the impact of complex neurological diseases [12]. The embryonic cerebellum is initially smooth without external morphological landmarks, but fissures that distinguish five cardinal lobes in the vermis begin to form by late embryonic development, embryonic day 17 (E17) in mice. Purkinje cells are derived from the ventricular zone of dorsal rhombomere 1 from E10 to E13 and migrate along radial glia into symmetrical clusters by ~E14. The granule cells are derived between ~E12 and E17 from a germinal zone called the rhombic lip, which produces a specialized transient progenitor layer on the surface of the cerebellum called the external granule cell layer by E16.5 [54]. Cerebellar granule cells are the most numerous cell type in the adult brain. They undergo extensive proliferation and are the main driving force for cerebellar growth and lobule patterning. During postnatal development, the five cardinal lobes expand and fold as they subdivide into the conserved stereotyped lobules, and this process (foliation) is complete by postnatal day 14 (P14) in mice. Genetic cues allowing for the precision and reproducibility of foliation between animals are not fully understood but may involve the “anchoring” of Purkinje cells to the future base of lobules by their projections to the cerebellar nuclei and the proliferation of granule cell precursors mechanically forcing lobule outgrowth [55] under the control of Purkinje cell-derived sonic hedgehog (Shh) signals [56, 57] and the function of Engrailed homeobox genes (En1/2) [58, 59]. The molecular heterogeneity of Purkinje cells may provide a scaffold that guides the patterns of neural circuit formation in the developing cerebellum, which is consistent with evidence that Purkinje cell subsets differentially express intrinsic molecular markers as early as E14 [60,61,62], including cell adhesion and guidance molecules [63, 64]. Purkinje cells are critical not only for shaping morphogenesis but also for guiding topographical map formation. Purkinje cells of similar birthdates may determine the adult patterns of Purkinje cell gene expression and restrict the boundaries of zones as the map forms. This is accomplished during embryogenesis when Purkinje cell subsets migrate and cluster into similar coordinate positions [65]. Afferents arrive in the cerebellum spanning mid-embryonic and postnatal development [66] in positions that later correspond to specific lobules, and Purkinje cell cues are thought to provide the scaffold that guides afferents into longitudinal zones following the initial patterning of Purkinje cell clusters [54]. Retrograde tracing in fixed embryonic rat tissue shows that mossy fibers from the vestibular ganglion arrive in the cerebellum by E13, and those from the vestibular nuclei and spinal cord arrive at E15 [66]. Climbing fibers arrive at ~E17, followed by mossy fibers from the lateral reticular nucleus and pontine nuclei at P0 [66]. In mice, spinocerebellar and vestibular mossy fibers arrive at E13/14 [67], climbing fibers arrive at E14/15 [68], and the remaining mossy fibers arrive during late embryonic and postnatal development [54]. Climbing fiber afferents exhibit rudimentary parasagittal stripes by E15/16 in mice [68], soon after Purkinje cell clusters initially express transient parasagittal molecular markers such as En1/2 [61]. Climbing fiber termination patterns and Purkinje cell zones correspond topographically by E17 [69]. Though mossy fibers synapse on granule cells in the adult cerebellum, they form transient contacts with Purkinje cells during embryonic development that may be critical for the segregation of spinocerebellar afferents into parasagittal zones [32, 70,71,72,73]. Unlike climbing fibers, mossy fibers do not exhibit clear-cut zones until after birth [74]. Purkinje cells are innervated by five to six climbing fibers by P3, and during early postnatal development one of these connections is selectively strengthened while the other synapses are eliminated; by P17 each Purkinje cell is innervated by a single climbing fiber, and each climbing fiber may contact up to ten Purkinje cells [75]. Cerebellar postnatal development also involves changes in the firing properties of both Purkinje cell simple spikes, which are intrinsically generated and modulated by mossy fiber to granule cell inputs via granule cell parallel fiber projections, and Purkinje cell complex spikes, which are generated by climbing fiber afferents [76] (Fig. 3). Both frequency and regularity of Purkinje cell spikes are dynamic as climbing and parallel fiber synapses mature and intrinsic Purkinje cell gene expression changes during development [76]. Neural activity , mediated by spontaneous activity and sensory experience, likely intersects with genetic programs to properly assemble the cerebellum and its circuits [77]. Genetic mouse models demonstrate that if genes regulating organization of the circuit are disrupted, there are severe impacts on map formation and motor function although external morphological defects typically associated with cerebellar disease may be subtle. For example, En1/2 genes are critical for establishing the organization of the cerebellar circuit, and En1/2 mutants exhibit altered formation of lobules and parasagittal Purkinje cell gene expression [59, 78,79,80,81]. Furthermore, adult patterns of mossy fiber afferents in distinct lobules and parasagittal zones are sensitive to En1/2 deletions [72]. Spontaneous mutant mouse models of ataxia identified by their motor phenotypes also demonstrate an active role for Purkinje cells in setting up the topography of cerebellar afferents and the importance of the cerebellar circuit map for motor control. Mossy fiber termination patterns are altered in the staggerer mutant mouse with intrinsically affected Purkinje cells [70]. The dreher mutation causes cell fate changes of cerebellar progenitors, and anteroposterior and parasagittal patterns are distorted but present despite external morphological phenotypes [82]. The cerebellar deficient folia (cdf) mutation causes a selective failure of a zebrinII-positive Purkinje cell cluster to disperse, and adult mutants have abnormal parasagittal zone widths in the anterior vermis [83]. Scrambler mutant mice are able to maintain Purkinje cell zones and topographical circuits despite the abnormal placement of 95 % of Purkinje cells due to severe ectopia [84]. The reeler mutation causes the cerebellum to contain a “single lobule” composed of hypogranular cortex and a central mass of Purkinje cell clusters mixed with cerebellar nuclei, but the spinocerebellar and vestibulocerebellar afferents of reeler mice are able to maintain targeting to specific regions despite the lack of external morphological landmarks [85, 86]. These mouse models of motor dysfunction , which have cerebellar abnormalities due to structural and circuit defects, have been invaluable for furthering our understanding of how circuit maps are generated. Moreover, the use of spontaneous and engineered (knockout and conditional) mice has helped shed light on the mechanisms of complex cerebellar diseases.
Purkinje cells fire simple spikes and complex spikes. (a) Purkinje cell labeled using the classical Golgi-Cox staining method , demonstrating the elaborate morphology and dendritic branching of the Purkinje cell. (b) Extracellular single-unit recording from a Purkinje cell of an adult mouse in vivo. Purkinje cells fire two types of action potentials: high-frequency simple spikes that are driven by intrinsic activity and modulated by mossy fiber-granule cell inputs and low-frequency complex spikes that are triggered by climbing fiber input (astericks). (c) Higher power image of the Purkinje cell recording shown in panel (b) with individual spike waveforms visible. (Modified with permission from Reeber et al. [13])
The Role of Cerebellar Development in Ataxia, a Classical Cerebellar Movement Disorder
As the genes and specific mutations causing human disorders continue to be identified, genetic mouse models of individual diseases have shed light on how the cerebellum is affected at the levels of pathology, physiology, and circuit patterning to cause symptoms with which patients present in the clinic. Ataxia is the most common symptom of cerebellar disease and a common phenotype of the aforementioned mutant mice. Upon neurological examination, patients with ataxia usually exhibit uncoordinated limbs, impaired balance, gait disturbance, and diminished fine motor control [87]. Cerebellar ataxia is the most common form of ataxia, and there currently are over 60 identified forms of inherited cerebellar ataxia [13, 88]. Although ataxia and other cerebellar motor deficits are typically discussed in relation to specific genetic mutations, defects in cerebellar circuitry can also be sporadic or acquired as a result of stroke, tumors, multiple sclerosis, alcoholism, peripheral neuropathy, metabolic disorders, and vitamin deficiencies [89]. The following genetic cerebellar manipulations demonstrate the diversity of paths that can lead to ataxia and related motor deficits. We focus on Purkinje cells due to their crucial role during cerebellar development and their central function in the adult circuit.
SCA1 (Spinocerebellar Ataxia Type 1)
Spinocerebellar ataxia type 1 (SCA1 ) is a dominantly inherited form of ataxia. SCA1 causes progressive loss of motor coordination, impaired balance, and gait disturbance. Other symptoms typically include dysarthria, dysmetria, difficulty swallowing, muscle atrophy, kyphosis, nystagmus, spasticity, and cognitive impairments [90]. SCA1 belongs to a family of neurodegenerative conditions that are caused by abnormal CAG repeat expansions that encode polyglutamine tracts. The mutated gene responsible for SCA1 was cloned and identified as the transcriptional regulator ATAXIN-1 [91]. The polyglutamine ataxin-1 protein product is widely expressed in the brain but in SCA1 becomes toxic primarily to Purkinje cells of the cerebellum [92]. Polyglutamine ataxin-1 remains uniquely soluble in Purkinje cells, allowing it to enter the nucleus and disrupt the function of multiple protein complexes [93]. In humans, the onset of motor deficits most often occurs in the third or fourth decade of life followed by death 10–15 years later; however, the age of onset and survival time depend on the number of repeats in the expanded polyglutamine sequence and can occur as late as the sixth decade of life or as early as the first decade [94]. Neuroimaging of late-stage SCA1 patients reveals gross atrophy of the cerebellum primarily due to the degeneration of Purkinje cells [90, 92, 95]. SCA1 patients also typically exhibit atrophy of the dentate cerebellar nuclei, pons, inferior olive, and other brain stem nuclei as the disease progresses [92]. Thus, degeneration eventually impacts both the cerebellar afferent and the efferent pathways. Postmortem examination of cerebellar tissue from SCA1 patients shows morphological abnormalities of remaining Purkinje cells in addition to Purkinje cell loss [95, 96]. The generation of mutant SCA1 transgenic mice has been critical in furthering our understanding of SCA1 progression [97,97,99]. First, electrophysiological properties of Purkinje cells such as intrinsic firing and the strength of glutamatergic synapses are abnormal preceding both onset of ataxia and Purkinje cell structural alterations in SCA1 mutant mice [100, 101]. Furthermore, specific genes involved in glutamate and calcium signaling are downregulated in Purkinje cells of SCA1 mutants before the morphological changes or behavioral deficits are obvious [102, 103]. Impaired performance on motor tasks in SCA1 mutant mice appears subsequently but before Purkinje cell morphological changes [100], suggesting changes in gene expression and altered circuit activity initiate SCA1 symptoms rather than the degeneration of Purkinje cells. Motor performance continues to decline as the dendritic morphology of Purkinje cells begins to deteriorate, dendritic arborization is reduced, the number of dendritic spines decreases, and the molecular layer shrinks as cells regress [97, 100]. Structural abnormalities become more evident as the proximal Purkinje cell dendrites atrophy and when the Purkinje cell somata begin to exhibit heterotopic positioning in the molecular layer [97, 99, 100]. It is not until the later stages of disease progression that Purkinje cell loss is detected [97, 99, 100]. The ages at which these events occur in SCA1 mutant mice differ between models containing shorter or longer knocked-in CAG repeats, consistent with what is observed in human patients [94]. The longer repeats cause an earlier onset of the disease and more severe symptoms. Despite the earlier onset, analysis of disease progression in juvenile and young adult mutant mice reveals that abnormalities in circuit activity and motor performance precede Purkinje cell degeneration. Progressive impairment of motor function in SCA1 thus reflects not only the degeneration of cells in the cerebellum and associated brain stem nuclei but also the earlier and sustained dysfunction of key neuronal populations that are integrated within the circuit.
SCA6 (Spinocerebellar Ataxia Type 6)
Spinocerebellar ataxia type 6 (SCA6), like SCA1, is a dominantly inherited form of ataxia and a triplet repeat disease. In SCA6, a CAG repeat expansion occurs within the gene CACNA1A, which encodes the pore-forming subunit of voltage-dependent P/Q-type calcium channels [104, 105]. The mutated polyglutamine P/Q-type calcium channels are widely expressed in the brain but become toxic primarily to Purkinje cells [106], where they are highly expressed in the plasma membrane [107]. Age of onset and survival time depend on the number of repeats in the expanded polyglutamine sequence, but SCA6 onset most commonly occurs in the fifth or sixth decade of life followed by death 20–30 years later [94]. SCA6 patients experience slowly progressive ataxia of the limbs and gait in addition to dysarthria and nystagmus [104, 108], and neuroimaging reveals cerebellar atrophy [108]. Neurodegeneration in SCA6 occurs mostly in Purkinje cells, but death of neurons in the dentate cerebellar nuclei and inferior olive is also observed [105, 109, 110]. Postmortem examination of cerebellar tissue from SCA6 patients shows morphological abnormalities of remaining Purkinje cells in addition to the loss of Purkinje cells [106]. In transgenic mouse models of SCA6, the onset of ataxia occurs before morphological changes or loss of Purkinje cells [111]. Electrophysiological examination reveals that Purkinje cells exhibit reduced firing rates and rhythmicity at ages coinciding with the onset of ataxia [112] and at later disease stages [113]. Though the polyglutamine mutation occurs in an ion channel that regulates the firing patterns of Purkinje cells in adult mice [114], SCA6 symptoms do not result from changes in channel current but rather age-dependent gain-of-function effects of aggregated mutant protein on cellular function [113, 115, 116]. Although SCA6 symptoms manifest in midlife, P/Q channels are expressed soon after birth [117] and are involved in synapse elimination of climbing fiber innervation onto Purkinje cells during development [75, 118, 119]. Interestingly, Purkinje cells of SCA6 mutant mice exhibit transiently increased firing rates and rhythmicity as well as abnormal climbing fiber innervation during early postnatal development without causing behavioral abnormalities [120]. These alterations disappear once the mice reach weanling age when the circuit has largely developed [54], and cellular and synaptic function of Purkinje cells return to normal [120]. These transient electrophysiological phenotypes during development are different from those observed in adult SCA6 mice, and they do not appear to impact motor coordination nor represent a mild initial stage of the ultimate phenotype that would progressively worsen. However, compensatory adaptations prior to disease onset have been observed in the Purkinje cells of SCA1 mutant mice [101], and such homeostatic alterations to the cerebellar circuit in response to transient electrophysiological dysfunction have not yet been detected in developing SCA6 mice but may not become pathological until later in life, if they are present [120]. In addition to SCA1 and SCA6, a prolonged period of Purkinje cell dysfunction prior to neuronal loss has emerged as a common feature in other polyglutamine disorders including spinocerebellar ataxia type 3 (SCA3); Purkinje cells in a genetic mouse model of SCA3 exhibit abnormal intrinsic activity and motor symptoms prior to neurodegeneration [121]. These early manifestations of hereditary ataxias could be effective targets for therapy as the circuits could be rescued before the cells die [100, 112, 121].
Car8 wdl (The waddles Spontaneous Mutant Mouse)
The carbonic anhydrase 8 gene (Car8) is abundantly expressed in Purkinje cells [122, 123]. Lower levels of expression can also be seen in the cerebellar nuclei and brainstem due to the termination of Purkinje cell axons in these regions. The CAR8 protein is involved in calcium modulation pathways [124] and is expressed beginning during embryonic development and continuing into adulthood [125, 126]. A spontaneous mutant mouse, waddles (Car8 wdl), contains a deletion within the Car8 gene and exhibits progressive ataxia that is evident by 2 weeks of age in addition to appendicular dystonia and tremor [122]. In humans, mutations in the homologous gene (CA8) also cause ataxia [127]. Unlike in the SCAs, Purkinje cells do not degenerate, and the cerebellum does not show gross anatomical defects [122, 123]. However, adult Car8 wdl mice have microcircuit abnormalities including denser climbing fiber innervation that extends to distal Purkinje cell dendrites and reduced parallel fiber synapse formation on Purkinje cell dendritic spines [128]. The mutation also impairs the topography of cerebellar circuits during development; the segregation of Purkinje cell subsets into distinct parasagittal zones is developmentally delayed in Car8 wdl mice, and the topography of spinocerebellar afferents is abnormal in early postnatal and adult mice [123] (Fig. 4). Furthermore, electrophysiological examination of mutant mice reveals that the developing Purkinje cells exhibit abnormal firing frequency and patterns [123, 128], but Purkinje cells do not degenerate even as ataxia worsens [123]. The ataxia observed in Car8 wdl mice thus may result from both miswiring of the cerebellum’s functional map and aberrant electrophysiological output of adult Purkinje cells. The CAR8 protein is a binding partner for inositol triphosphate receptor type 1 (IP3R1) [122, 124], an intracellular calcium release channel that is mutated in SCA15. Interestingly, IP3R1 is one of the genes downregulated in SCA1 mice preceding the onset of ataxia or morphological changes [102, 103]. Impaired calcium homeostasis in Purkinje cells appears to mediate a central mechanism of pathogenesis common to many types of ataxia that manifest with or without neurodegeneration. However, CAR8 likely has calcium-independent functions as well.
The termination pattern of spinocerebellar mossy fibers is altered in Car8 wdl mice. (a) Schematic of the postnatal day 5 (P5) mouse cerebellum from a lateral view with the cerebellum highlighted in blue and the primary target domains of spinocerebellar mossy fiber projections highlighted in magenta. Roman numerals identify the lobules of the vermis. Note that the anteriormost lobules are also innervated by the spinocerebellar tract and are not visible as they are hidden from view by the colliculi. Cb cerebellum, BS brain stem, Ctx cerebral cortex, IC inferior colliculus, SC superior colliculus. (b) Fluorescent mapping of spinocerebellar mossy fiber terminal fields in lobule III of a Car8 wdl mouse and a control mouse at P5 after injection of WGA-Alexa 555 into the lower thoracic-upper lumbar spinal cord and transport of the tracer up the spinocerebellar tract. Mossy fiber topography is altered in Car8 wdl mice because the sensory pathways are incorrectly targeted and weakly innervate the cerebellum during early postnatal development. Scale bar = 250 μm. Panel (b) was modified with permission from White et al. [123]
L7 Cre ; Vgat flox/flox (Conditional Genetic Silencing of Purkinje Cell Neurotransmission)
Effective cerebellar control of motor behavior depends on the ability of Purkinje cells to integrate incoming sensorimotor inputs and communicate appropriately with their target neurons in the cerebellar nuclei. In the L7 Cre ;Vgat flox/flox mouse , inhibitory synaptic transmission of Purkinje cells is constitutively blocked using conditional genetics [129]. Under control of the cell type-specific promoter L7, Cre recombinase excises the floxed vesicular GABA transporter gene (Vgat) that encodes the transporter for loading neurotransmitter into synaptic vesicles [129]. This eliminates the ability of Purkinje cells, the sole output of the cerebellar cortex, to communicate with the cerebellar nuclei, the final output of the cerebellum and its link to the rest of the motor system. Purkinje cell output to the vestibular nuclei is also silenced by this approach. L7Cre ;Vgat flox/flox mice exhibit motor coordination defects, gait disturbance, and impaired balance. Though the absence of Purkinje cell output does not affect the gross morphology of the cerebellum, segregation of Purkinje cells into zones is disrupted, and the zonal topography of spinocerebellar afferents develops abnormally [129]. Although the basic circuit map is intact, the normally sharp boundaries of zones are compromised [129]. Purkinje cells of L7 Cre ;Vgat flox/flox mice exhibit abnormal electrophysiological activity, but their output is not signaled downstream in this model [129]. However, loss of Purkinje cell signaling causes the cerebellar nuclei to fire abnormally, impacting the ultimate output of the cerebellum. Taken together with other models of cerebellar dysfunction, it is clear that ataxia and other motor deficits can arise due to insults in wiring, firing, or survival of Purkinje cells in a wide range of diseases with diverse causes.
Cerebellar Development and Non-motor Disorders
Over the past 30 years, evidence from functional neuroimaging studies has mounted indicating that the cerebellum is active during non-motor behaviors such as perception, cognition, and emotion [130,131,132]. This idea is supported by evidence of extensive afferents and efferents interconnecting the cerebellum with prefrontal and parietal cortex [41, 133, 134]. Lesioning studies also suggest that cerebellar damage can lead to a variety of non-motor behavioral deficits [132, 135, 136]. However, the extent of the cerebellum’s role in cognitive function remains unclear and is a topic of lively debate [137,138,139,140]. The adult cerebellum appears to be particularly relevant to those non-motor tasks requiring complex spatial and temporal judgments, such as prediction and perceptual sensory discrimination, or in which skilled mental responses are developed using an internal model [134, 141, 162]. It could be that the computational capacities of the cerebellum to discriminate patterns and use these patterns to learn to make context-dependent predictions with respect to motor behavior would be also useful to non-motor areas of the brain [142]. Signals from cerebellar cortex to both motor and non-motor areas of the cerebral cortex synapse in the interposed and dentate cerebellar nuclei and are then relayed through the thalamus [54]. In return, mossy fibers originating in the basal pontine nuclei relay information from cerebral cortex to the cerebellar cortex, with non-motor information likely going to the hemispheres [54]. Together, these cerebro-cerebellar connections form closed loops in which regions of the cerebellar cortex projecting to a given area of the cerebral cortex in turn receive input originating in those same areas of the cerebral cortex [41]. Each of these regions is involved in specific functions, forming a topographical map across the cerebellar cortex, cerebellar nuclei, thalamus, pons, and cerebral cortex [31, 41, 42]. Functional neuroimaging links different cognitive and motor behaviors to activity in specific cerebro-cerebellar closed loops [143], and focal cerebellar damage can cause different motor or non-motor deficits in a location-dependent manner [132, 136]. This anatomical and functional segregation of cerebro-cerebellar connections might respect the modular architecture of the cerebellum [45]. Anatomical and functional abnormalities in the cerebellar circuit have been implicated in several non-motor neurodevelopmental disorders [144] and may play a particularly important role during sensitive periods of development [145]. Clinical studies have also noted increased cognitive deficits in children who suffer cerebellar damage during posterior fossa tumor resection [146]. How the cerebellum interacts with the cerebral cortex during development remains poorly understood. Some non-motor diseases linked to cerebellar development include autism spectrum disorder [145, 147, 148] and dyslexia [149, 150]. The cerebellum could also be involved in schizophrenia [151, 152]. The study of cerebellar non-motor diseases has required both human patients and genetic mouse models. For example, the most consistently affected structure in postmortem examination of tissue from autistic individuals is the cerebellum, including hypoplasia and reduced numbers of Purkinje cells without signs of neurodegeneration [147, 153, 154]. The En2 gene is necessary for establishing the structure and circuit organization of the cerebellum during mouse development [54], and EN2 mutations are linked to autism susceptibility in humans [155,156,157]. Loss-of-function mutations and transgenic misexpression of En2 in mice cause autism-like behaviors [158, 159]. These mice show some morphological abnormalities in the cerebellum that are broadly similar to those reported in humans with autism as well as abnormal foliation and afferent topography [59, 79,80,81]. In addition to cerebellar defects being implicated in non-motor diseases, cerebellar “motor” diseases can also feature non-motor symptoms. For example, human and mouse studies show that SCA1 [99, 160] and human CA8 mutations [127] cause cognitive deficits in addition to ataxia. It could be that the Purkinje cell and its associated microcircuits underlie both motor [129] and non-motor problems [162]. This would suggest that the basic operational properties of a Purkinje cell could be tuned to different behaviors. Future experimental work will reveal whether this is the case.
References
Gilbert PFC, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128(2):309–28.
Llinás RR. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2013;7:96.
Flourens M. Recherches expérimentales sur les propriétés et les fonctions du système nerveux dans les animaux vertébrés. Paris: Crevot; 1824.
Babinski J. De l’asynergie cérébelleuse. Rev Neurol. 1899;6:806–16.
Babinski J. Sur le rôle du cervelet dans les actes volitionnels nécessitant une succession rapide de mouvements (diadococinésie). Rev Neurol. 1902;10:1013–5.
Babinski J. Asynergie et inertie cérébelleuse. Rev Neurol. 1906;14:685–6.
Holmes G. The cerebellum of man. Brain. 1939;62:2–30.
Manto M. The cerebellum, cerebellar disorders, and cerebellar research – two centuries of discoveries. Cerebellum. 2008;7(4):505–16.
Larsell O. In: Jansen J, editor. The comparative anatomy and histology of the cerebellum from monotremes through apes. Minneapolis: University of Minnesota Press; 1970. p. 31–58.
Cajal S. Histologie du Systeme Nerveux de l’Homme et des Vertebres, vol. 2. Madrid: Consejo Superior de Investigaciones Cientificas; 1911.
Altman J, Bayer S. Development of the cerebellar system: in relation to its evolution, structure, and functions. Boca Raton: CRC Press; 1997.
Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–77.
Reeber SL, Otis TS, Sillitoe RV. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83.
Fu Y, Tvrdik P, Makki N, Paxinos G, Watson C. Precerebellar cell groups in the hindbrain of the mouse defined by retrograde tracing and correlated with cumulative Wnt1-cre genetic labeling. Cerebellum. 2011;10(3):570–84.
Schweighofer N, Doya K, Kuroda S. Cerebellar aminergic neuromodulation: towards a functional understanding. Brain Res Rev. 2004;44(2–3):103–16.
Reeber SL, Sillitoe RV. Patterned expression of a cocaine- and amphetamine-regulated transcript peptide reveals complex circuit topography in the rodent cerebellar cortex. J Comp Neurol. 2011;519(9):1781–96.
Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in Purkinje cells. J Neurosci. 2003;23(21):7904–16.
Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291(4):538–52.
Eisenman LM, Hawkes R. Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. J Comp Neurol. 1993;335(4):586–605.
Armstrong C, Hawkes R. Pattern formation in the cerebellar cortex. Biochem Cell Biol. 2000;78(5):551–62.
Sillitoe RV, Hawkes R, Sillitoe RV, Hawkes R. Screen for patterning defects in the mouse cerebellum. J Histochem Cytochem. 2002;50(2):235–44.
Sillitoe RV, Marzban H, Larouche M, Zahedi S, Affanni J, Hawkes R. Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog Brain Res. 2005;148:283–97.
Pakan J, Iwaniuk A, Wylie D, Hawkes R, Marzban H. Purkinje cell compartmentation as revealed by zebrin II expression in the cerebellar cortex of pigeons (Columba livia). J Comp Neurol. 2007;501(4):619–30.
Marzban H, Chung SH, Pezhouh MK, Feirabend H, Watanabe M, Voogd J, et al. Antigenic compartmentation of the cerebellar cortex in the chicken (Gallus domesticus). J Comp Neurol. 2010;518(12):2221–39.
Marzban H, Hawkes R. On the architecture of the posterior zone of the cerebellum. Cerebellum. 2011;10(3):422–34.
Marzban H, Hoy N, Marotte L, Hawkes R. Antigenic compartmentation of the cerebellar cortex in an Australian marsupial, the tammar wallaby Macropus eugenii. Brain Behav Evol. 2012;80(3):196–209.
Marzban H, Hoy N, Buchok M, Catania KC, Hawkes R. Compartmentation of the cerebellar cortex: adaptation to lifestyle in the star-nosed mole Condylura cristata. Cerebellum. 2015;14(2):106–18.
Wylie D, Hoops D, Aspden J, Iwaniuk A. Zebrin II is expressed in sagittal stripes in the cerebellum of dragon lizards (Ctenophorus sp.). Brain Behav Evol. 2017;88(3-4):177–86.
Hawkes R. Purkinje cell stripes and long-term depression at the parallel fiber-Purkinje cell synapse. Front Syst Neurosci. 2014;8:41.
Ozol K, Hayden JM, Oberdick J, Hawkes R. Transverse zones in the vermis of the mouse cerebellum. J Comp Neurol. 1999;412(1):95–111.
Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10(9):670–81.
Ji Z, Hawkes R. Developing mossy fiber terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. J Comp Neurol. 1995;359(2):197–212.
Voogd J, Pardoe J, Ruigrok TJH, Apps R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J Neurosci. 2003;23(11):4645–56.
Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol. 1994;476(2):229–44.
Ekerot CF, Larson B. Branching of olivary axons to innervate pairs of sagittal zones in the cerebellar anterior lobe of the cat. Exp Brain Res. 1982;48(2):185–98.
Apps R, Trott JR, Dietrichs E. A study of branching in the projection from the inferior olive to the x and lateral c1 zones of the cat cerebellum using a combined electrophysiological and retrograde fluorescent double-labelling technique. Exp Brain Res. 1991;87(1):141–52.
Ji Z. Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience. 1994;61(4):935–54.
Serapide MF, Pantó MR, Parenti R, Zappalá A, Cicirata F. Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J Comp Neurol. 2001;430(4):471–84.
Gerrits NM, Voogd J, Nas WSC. Cerebellar and olivary projections of the external and rostral internal cuneate nuclei in the cat. Exp Brain Res. 1985;57(2):239–55.
Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411(1):97–118.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23(23):8432–44.
Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89(1):634–9.
Voogd J, Ruigrok TJH. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol. 2004;33:5–21.
Pijpers A, Voogd J, Ruigrok TJH. Topography of olivo-cortico-nuclear modules in the intermediate cerebellum of the rat. J Comp Neurol. 2005;492(2):193–213.
Ruigrok TJH. Ins and outs of cerebellar modules. Cerebellum. 2011;10(3):464–74.
Ruigrok TJH, Pijpers A, Goedknegt-Sabel E, Coulon P. Multiple cerebellar zones are involved in the control of individual muscles: a retrograde transneuronal tracing study with rabies virus in the rat. Eur J Neurosci. 2008;28(1):181–200.
Chockkan V, Hawkes R. Functional and antigenic maps in the rat cerebellum: zebrin compartmentation and vibrissal receptive fields in lobule IXa. J Comp Neurol. 1994;345(1):33–45.
Ebner TJ, Chen G, Gao W, Reinert K. Optical imaging of cerebellar functional architectures: parallel fiber beams, parasagittal bands and spreading acidification. Prog Brain Res. 2004;148:125–38.
Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8(10):1329–34.
Schonewille M, Luo C, Ruigrok TJ, Voogd J, Schmolesky M, Rutteman M, Freek HE, de Jeu MTG, de Zeeuw CI. Zonal organization of the mouse flocculus: physiology, input, and output. J Comp Neurol. 2006;487:670–82.
Shambes G, Gibson J, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15(2):94–140.
Hallem JS, Thompson JH, Gundappa-Sulur G, Hawkes R, Bjaalie JG, Bower JM. Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIa of the rat. Neuroscience. 1999;93(3):1083–94.
Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16(2):79–93.
White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip Rev Dev Biol. 2013;2(1):149–64.
Sudarov A, Joyner AL. Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2007;2:26.
Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270(2):393–410.
Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133(9):1811–21.
Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991;251(4998):1239–43.
Kuemerle B, Zanjani H, Joyner A, Herrup K. Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development. J Neurosci. 1997;17(20):7881–9.
Oberdick J, Schilling K, Smeyne R, Corbin J, Bocchiaro C, Morgan J. Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron. 1993;10(6):1007–18.
Millen KJ, Hui CC, Joyner AL. A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development. 1995;121(12):3935–45.
Larouche M, Hawkes R. From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum. 2006;5(2):77–88.
Arndt K, Nakagawa S, Takeichi M, Redies C. Cadherin-defined segments and parasagittal cell ribbons in the developing chicken cerebellum. Mol Cell Neurosci. 1998;10(5–6):211–28.
Luo J, Treubert-Zimmermann U, Redies C. Cadherins guide migrating Purkinje cells to specific parasagittal domains during cerebellar development. Mol Cell Neurosci. 2004;25(1):138–52.
Hashimoto M, Mikoshiba K. Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J Neurosci. 2003;23(36):11342–51.
Ashwell K, Zhang L. Ontogeny of afferents to the fetal rat cerebellum. Acta Anat. 1992;145(1):17–23.
Grishkat H, LM E. Development of the spinocerebellar projection in the prenatal mouse. J Comp Neurol. 1995;363(1):93–108.
Paradies MA, Eisenman LM. Evidence of early topographic organization in the embryonic olivocerebellar projection: a model system for the study of pattern formation processes in the central nervous system. Dev Dyn. 1993;197(2):125–45.
Paradies MA, Grishkat H, Smeyne RJ, Oberdick J, Morgan JI, Eisenman LM. Correspondence between L7-lacZ-expressing Purkinje cells and labeled olivocerebellar fibers during late embryogenesis in the mouse. J Comp Neurol. 1996;374(3):451–66.
Arsénio Nunes M, Sotelo C, Wehrlé R. Organization of spinocerebellar projection map in three types of agranular cerebellum: Purkinje cells vs. granule cells as organizer element. J Comp Neurol. 1988;273(1):120–36.
Sotelo C, Wassef M. Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos Trans R Soc Lond Ser B Biol Sci. 1991;331:307–13.
Sillitoe RV, Vogel MW, Joyner AL. Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J Neurosci. 2010;30(30):10015–24.
Sillitoe RV. Mossy fibers terminate directly within Purkinje cell zones during mouse development. Cerebellum. 2016;15(1):14–7.
Arsénio NM, Sotelo C. Development of the spinocerebellar system in the postnatal rat. J Comp Neurol. 1985;237(3):291–306.
Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34(10):1697–710.
Arancillo M, White JJ, Lin T, Stay TL, Sillitoe RV. In vivo analysis of Purkinje cell firing properties during postnatal mouse development. J Neurophysiol. 2015;113(2):578–91.
Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, Hoshino M, Joyner AL, Kano M, Kilpatrick DL, Koibuchi N, Marino S, Martinez S, Millen KJ, Millner TO, Miyata T, Parmigiani E, Schilling K, Sekerkova G, Sillitoe RV, Sotelo C, Uesaka N, Wefers A, Wingate RJT, Hawkes R. Consensus paper: cerebellar development. Cerebellum. 2016;15:789–828.
Sgaier SK, Lao Z, Villanueva MP, Berenshteyn F, Stephen D, Turnbull RK, Joyner AL. Genetic subdivision of the tectum and cerebellum into functionally related regions based on differential sensitivity to engrailed proteins. Development. 2007;134(12):2325–35.
Sillitoe RV, Stephen D, Lao Z, Joyner AL. Engrailed homeobox genes determine the organization of Purkinje cell sagittal stripe gene expression in the adult cerebellum. J Neurosci. 2008;28(47):12150–62.
Cheng Y, Sudarov A, Szulc KU, Sgaier SK, Stephen D, Turnbull DH, Joyner AL. The Engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum. Development. 2010;137(3):519–29.
Millen KJ, Wurst W, Herrup K, Joyner AL. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development. 1994;120(3):695–706.
Sillitoe RV, George-Jones NA, Millen KJ, Hawkes R. Purkinje cell compartmentalization in the cerebellum of the spontaneous mutant mouse dreher. Brain Struct Funct. 2014;219(1):35–47.
Beierbach E, Park C, Ackerman S, Goldowitz D, Hawkes R. Abnormal dispersion of a purkinje cell subset in the mouse mutant cerebellar deficient folia (cdf). J Comp Neurol. 2001;436(1):42–51.
Reeber SL, Loeschel CA, Franklin A, Sillitoe RV. Establishment of topographic circuit zones in the cerebellum of scrambler mutant mice. Front Neural Circ. 2013;7:122.
Vig J, Goldowitz D, Steindler DA, Eisenman LM. Compartmentation of the reeler cerebellum: segregation and overlap of spinocerebellar and secondary vestibulocerebellar fibers and their target cells. Neuroscience. 2005;130(3):735–44.
Goffinet AM, So KF, Yamamoto M, Edwards M, Caviness VS. Architectonic and hodological organization of the cerebellum in reeler mutant mice. Dev Brain Res. 1984;16(2):263–76.
Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, Marien P, Nowak DA, Schmahmann JD, Serrao M, Steiner KM, Strupp M, Tilikete C, Timmann D, van Dun K, Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. 2016;15(3):369–91.
Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9(9):885–94.
Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol. 2010;9(1):94–104.
Zoghbi HY, Orr HT. Spinocerebellar ataxia type 1. Semin Cell Biol. 1995;6(1):29–35.
Orr H, Chung M, Banfi S, Kwiatkowski TJ, Servadio A, Beaudet A, McCall AE, Duvick LA, Ranum LPW, Zoghbi HY. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4(3):221–6.
Gilman S, Sima A, Junck L, Kluin K, Koeppe R, Lohman M, Little R. Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol. 1996;39(2):241–55.
Lim J, Crespo-barreto J, Jafar-nejad P, Bowman AB, Richman R, Hill DE, Orr HT, Zoghbi HY. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452(7188):713–8.
Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–47.
Koeppen A. The Purkinje cell and its afferents in human hereditary ataxia. J Neuropathol Exp Neurol. 1991;50(4):505–14.
Ferrer I, Genís D, Dávalos A, Bernadó L, Sant F, Serrano T. The Purkinje cell in olivopontocerebellar atrophy. A Golgi and immunocytochemical study. Neuropathol Appl Neurobiol. 1994;20(1):38–46.
Clark H, Burright E, Yunis W, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17(19):7385–95.
Burright EN, Brent Clark H, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82(6):937–48.
Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K, Kano M, Atkinson R, Sun Y, Armstrong DL, Sweatt JD, Orr HT, Paylor R, Zoghbi HY. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron. 2002;34(6):905–19.
Hourez R, Servais L, Orduz D, Gall D, Millard I, de Kerchove d’Exaerde A, Cheron G, Orr HT, Pandolfo M, Schiffmann SN. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2011;31(33):11795–807.
Dell’Orco JM, Wasserman AH, Chopra R, Ingram MAC, Hu Y-S, Singh V, Wulff H, Opal P, Shakkottai VG. Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. J Neurosci. 2015;35(32):11292–307.
Serra HG, Byam CE, Lande JD, Tousey SK, Zoghbi HY, Orr HT. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet. 2004;13(20):2535–43.
Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci. 2000;3(2):157–63.
Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansion in the α1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–9.
Ishikawa K, Watanabe M, Shoji S, Tsuji S. Japanese families with autosomal dominant pure cerebellar ataxia map to chromosome 19p13.1-p13.2 and are strongly associated with mild CAG expansions in the spinocerebellar ataxia type 6 gene in chromosome 19p13.1. Am Soc Hum Genet. 1997:336–46.
Yang Q, Hashizume Y, Yoshida M, Wang Y, Goto Y, Mitsuma N, Ishikawa K, Mizusawa H. Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathol. 2000;100(4):371–6.
Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15(10):6403–18.
Schols L, Kruger R, Amoiridis G, Przuntek H, Epplen JT, Riess O. Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. J Neurol Neurosurg Psychiatry. 1998;64(1):67–73.
Tsuchiya K, Oda T, Yoshida M, Sasaki H, Haga C, Okino H, Tominaga I, Matsui K, Akiyama H, Hashizume Y. Degeneration of the inferior olive in spinocerebellar ataxia 6 may depend on disease duration: report of two autopsy cases and statistical analysis of autopsy cases reported to date. Neuropathology. 2005;25(2):125–35.
Stefanescu MR, Dohnalek M, Maderwald S, Thürling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain. 2015;138(Pt 5):1182–97.
Jayabal S, Ljungberg L, Erwes T, Cormier A, Quilez S, El Jaouhari S, Watt AJ. Rapid onset of motor deficits in a mouse model of spinocerebellar ataxia type 6 precedes late cerebellar degeneration. eNeuro. 2015;2(6):ENEURO. 0094–15.2015.
Jayabal S, Chang HHV, Cullen KE, Watt AJ. 4-Aminopyridine reverses ataxia and cerebellar firing deficiency in a mouse model of spinocerebellar ataxia type 6. Sci Rep. 2016;6:29489.
Mark MD, Krause M, Boele HJ, Kruse W, Pollok S, Kuner T, Dalkara D, Koekkoek S, De Zeeuw CI, Herlitze S. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J Neurosci. 2015;35(23):8882–95.
Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24(40):8818–22.
Watase K, Barrett CF, Miyazaki T, Ishiguro T, Ishikawa K, Hu Y, Unno T, Sun Y, Kasai S, Watanabe M, Gomez CM, Mizusawa H, Tsien RW, Zoghbi HY. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci U S A. 2008;105(33):11987–92.
Saegusa H, Wakamori M, Matsuda Y, Wang J, Mori Y, Zong S, Tanabe T. Properties of human Cav2.1 channel with a spinocerebellar ataxia type 6 mutation expressed in Purkinje cells. Mol Cell Neurosci. 2007;34(2):261–70.
Indriati DW, Kamasawa N, Matsui K, Meredith AL, Watanabe M, Shigemoto R. Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. J Neurosci. 2013;33(8):3668–78.
Miyazaki T. P/Q-type Ca2+ channel 1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24(7):1734–43.
Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin H-S, Watanabe M, Sakimura K, Kano M. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2011;108(24):9987–92.
Jayabal S, Ljungberg L, Watt AJ. Transient cerebellar alterations during development prior to obvious motor phenotype in a mouse model of spinocerebellar ataxia type 6. J Physiol. 2016;0:1–18.
Shakkottai VG, do Carmo Costa M, Dell’Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the Polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31(36):13002–14.
Jiao Y, Yan J, Zhao Y, Donahue LR, Beamer WG, Li X, Roe BA, LeDoux MS, Gu W. Carbonic anhydrase-related protein VIII deficiency is associated with a distinctive lifelong gait disorder in waddles mice. Genetics. 2005;171(3):1239–46.
White JJ, Arancillo M, King A, Lin T, Miterko LN, Gebre SA, Sillitoe RV. Pathogenesis of severe ataxia and tremor without the typical signs of neurodegeneration. Neurobiol Dis. 2016;86:86–98.
Hirota J, Ando H, Hamada K, Mikoshiba K. Carbonic anhydrase-related protein is a novel binding protein for inositol 1,4,5-trisphosphate receptor type 1. Biochem J. 2003;372:435–41.
Taniuchi K, Nishimori I, Takeuchi T, Ohtsuki Y, Onishi S. cDNA cloning and developmental expression of murine carbonic anhydrase-related proteins VIII, X, and XI. Mol Brain Res. 2002;109(1–2):207–15.
Kato K. Sequence of a novel carbonic anhydrase-related polypeptide and its exclusive presence in Purkinje cells. FEBS Lett. 1990;271(1–2):137–40.
Türkmen S, Guo G, Garshasbi M, Hoffmann K, Alshalah AJ, Mischung C, Kuss A, Humphrey N, Mundlos S, Robinson PN. CA8 mutations cause a novel syndrome characterized by ataxia and mild mental retardation with predisposition to quadrupedal gait. PLoS Genet. 2009;5(5):1–8.
Hirasawa M, Xu X, Trask RB, Maddatu TP, Johnson BA, Naggert JK, Nishina PM, Ikeda A. Carbonic anhydrase related protein 8 mutation results in aberrant synaptic morphology and excitatory synaptic function in the cerebellum. Mol Cell Neurosci. 2007;35(1):161–70.
White JJ, Arancillo M, Stay TL, George-Jones NA, Levy SL, Heck DH, Sillitoe RV. Cerebellar zonal patterning relies on Purkinje cell neurotransmission. J Neurosci. 2014;34(24):8231–45.
Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47.
Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–60.
Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6:159–62.
Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA. Consensus paper: The Role of the Cerebellum in Perceptual Processes. Cerebellum. 2014:197–220.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34.
Tavano A, Borgatti R. Evidence for a link among cognition, language and emotion in cerebellar malformations. Cortex. 2010;46(7):907–18.
Schmahmann J, Sherman J. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–79.
Glickstein M. What does the cerebellum really do? Curr Biol. 2007;17(19):824–7.
Lemon R, Edgley S. Life without a cerebellum. Brain. 2010;133(3):652–4.
Galliano E, Potters J-W, Elgersma Y, Wisden W, Kushner SA, De Zeeuw CI, Hoebeek FE. Synaptic transmission and plasticity at inputs to murine cerebellar Purkinje cells are largely dispensable for standard nonmotor tasks. J Neurosci. 2013;33(31):12599–618.
Leiner H, Leiner A, Dow R. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100(4):443–54.
Bastian AJ. Moving, sensing and learning with cerebellar damage. Curr Opin Neurobiol. 2011;21(4):596–601.
D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2012;6:116.
Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. Cognitive and motor loops of the human Cerebrocerebellar system. J Cogn Neurosci. 2010;22(11):2663–76.
Stoodley CJ. The cerebellum and neurodevelopmental disorders. Cerebellum. 2016;15(1):34–7.
Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518–32.
Cantelmi D, Schweizer TA, Cusimano MD. Role of the cerebellum in the neurocognitive sequelae of treatment of tumours of the posterior fossa: an update. Lancet Oncol. 2008;9(6):569–76.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus paper: Pathological Role of the Cerebellum in Autism. Cerebellum. 2012;11(3):777–807.
Becker EBE, Stoodley CJ. Autism spectrum disorder and the cerebellum. 1st, vol. 113. Int Rev Neurobiol. Elsevier Inc.; 2013. 1–34 p.
Stoodley CJ, Stein JF. Cerebellar function in developmental dyslexia. Cerebellum. 2013;12(2):267–76.
Nicolson R, Fawcett A, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends Neurosci. 2001;24(9):508–11.
Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–8.
Mothersill O, Knee-Zaska C, Donohoe G. Emotion and theory of mind in schizophrenia – investigating the role of the cerebellum. Cerebellum. 2016;15(3):357–68.
Bauman M, Kemper T. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35(6):866–74.
Kemper T, Bauman M. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11(1):175–87.
Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Assoc homeobox Transcr factor, ENGRAILED 2, 3, with autism Spectr Disord. Mol Psychiatry. 2004;9(5):474–84.
Wang L, Jia M, Yue W, Tang F, Qu M, Ruan Y, Lu T, Zhang H, Yan H, Liu J, Guo Y, Zhang J, Yang X, Zhang D. Association of the ENGRAILED 2 (EN2) gene with autism in Chinese Han population. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147(4):434–8.
Sen B, Surindro Singh A, Sinha S, Chatterjee A, Ahmed S, Ghosh S, Usha R. Family-based studies indicate association of Engrailed 2 gene with autism in an Indian population. Genes Brain Behav. 2010;9(2):248–55.
Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116(1):166–176.
Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, Millonig JH, DiCicco-Bloom E, Crawley JN. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One. 2012;7(7):40–2.
Bürk K, Bösch S, Globas C, Zühlke C, Daum I, Klockgether T, Dichgans J. Executive dysfunction in spinocerebellar ataxia type 1. Eur Neurol. 2001;46(1):43–8.
Tsai PT, Hull C, Chu YX, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488(7413):647-651.
Ito M. Control of mental activities by internal models in the cerebellum. Nature Reviews Neuroscience. 2008;9(4):304–13.
Acknowledgments
This work was supported by funds from Baylor College of Medicine (BCM) and Texas Children’s Hospital. R.V.S. received support from the National Institutes of Neurological Disorders and Stroke (NINDS) R01NS089664. The BCM IDDRC Neuropathology Sub-Core performed the tissue staining (the BCM IDDRC Neurovisualization Core is supported by U54HD083092). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). We thank Amanda M. Brown for suggestions and comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Lackey, E.P., Sillitoe, R.V. (2017). Motor Circuit Abnormalities During Cerebellar Development. In: Marzban, H. (eds) Development of the Cerebellum from Molecular Aspects to Diseases. Contemporary Clinical Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-59749-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-59749-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59748-5
Online ISBN: 978-3-319-59749-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)