Abstract
The cerebellum, traditionally linked to voluntary motor coordination, is now recognized for its role in nonmotor functions, including cognitive and social behaviors. This expanded understanding is vital for identifying neurodevelopmental disorders such as autism spectrum disorder (ASD), where cerebellar abnormalities are common. Recent research has identified specific cerebellar circuits contributing to these diverse functions, revealing interconnected pathways that regulate both motor and social behaviors. The cerebellum communicates extensively with the cerebral cortex, thalamus, and limbic structures through converging and diverging pathways, integrating sensory and motor information to fine-tune outputs and influence higher-order functions. Mouse models have been instrumental in dissecting cerebellar functions, with studies using genetic and neuroanatomical techniques to manipulate specific circuits and observe behavioral outcomes. Disruptions in cerebellar pathways can lead to motor deficits and social impairments, mirroring human neurodevelopmental disorders. This review explores the anatomical and functional organization of cerebellar pathways in mice, their role in behavior, and the implications of cerebellar dysfunction in disorders such as ASD. Understanding these pathways enhances knowledge of cerebellar contributions to behavior and informs therapeutic strategies for cerebellar and neurodevelopmental disorders, emphasizing the integral role of the cerebellum in motor and social functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum, traditionally associated with the coordination of voluntary motor activity, has been increasingly recognized for its role in a range of nonmotor functions, including cognitive and social behaviors[1, 2]. This expanded understanding is particularly relevant in the context of neurodevelopmental disorders such as autism spectrum disorder (ASD), where cerebellar abnormalities are frequently reported [3]. Recent studies have begun to elucidate the specific neural circuits within the cerebellum that contribute to these diverse functions, highlighting the presence of distinct yet interconnected pathways that regulate both motor and social behaviors [4]. Understanding the contribution of the cerebellum to these functions involves dissecting the intricate network of cerebellar connections and their influence on broader neural circuits [5]. The cerebellum communicates extensively with various regions of the brain, including the cerebral cortex, thalamus, and limbic structures, through a series of well-orchestrated pathways [6]. These pathways can be broadly categorized into converging and diverging circuits. Converging pathways integrate sensory and motor information to fine-tune motor outputs, while diverging pathways project to regions involved in higher-order functions, influencing cognition and social interactions [7]. Mouse models have proven invaluable in dissecting the cellular and molecular mechanisms underlying cerebellar function. Through advanced genetic and neuroanatomical techniques, researchers have been able to manipulate specific cerebellar circuits and observe the resulting behavioral outcomes [8, 9]. These studies have provided significant insights into how disruptions in cerebellar pathways can lead to motor deficits and social impairments, mirroring aspects of human neurodevelopmental disorders [10]. In this review, we explore the current understanding of cerebellar pathways that contribute to motor and social behaviors in mice [11]. We will discuss the anatomical and functional organization of these pathways, their role in behavior, and the implications of cerebellar dysfunction in neurodevelopmental disorders [12]. By examining the converging and diverging pathways within the cerebellum, we aim to shed light on the complex neural mechanisms that underlie the cerebellum's multifaceted contributions to both motor and social functions.
Traditional Role of the Cerebellum in Motor Control
Historically, the cerebellum has been predominantly studied in the context of motor control. Classical studies have demonstrated that the cerebellum is essential for the coordination, precision, and timing of new movements, and therapeutic possibilities may be revealed by discovering how the cerebellum is connected to pain. pain is considered more than sensation but also emotion and perception. This complexity is reflected in the network of cortical and subcortical systems that represent and process pain [13]. Lesions in the cerebellum lead to ataxia, characterized by uncoordinated and imprecise movements, underscoring its critical role in motor function [14]. Early cerebellar manipulations significantly impact behavior, as observed in adult mice with specific cerebellar mutations. The link between abnormal cerebellar improvement and autism-like behaviors in more genetically relevant models of early cerebellum involvement in nonmotor functions influences behavior during the preadult stages of cerebellar manipulation during development and can induce autism-like behaviors [15]. This review highlights recent advances and insights into the traditional understanding of cerebellar function in motor activities, integrating findings from the last decade that enhance our comprehension of its mechanisms and influence on motor behavior [16]. Traditionally recognized for its role in motor control, has been the focus of extensive research to elucidate its anatomical structures, functional integration, and contributions to motor learning and coordination [17, 18]. Anatomically, the cerebellum is divided into the vestibulocerebellum, spinocerebellum, and cerebrocerebellum, each contributing uniquely to balance, limb movement, and the planning of voluntary actions [19]. Recent studies have highlighted the cerebellum’s ability to integrate sensory inputs with motor commands, ensuring precise and smooth movements [20]. This integration is crucial for motor learning, where synaptic plasticity within the cerebellar cortex allows for the adaptation and refinement of motor skills through practice [21, 22]. Additionally, the cerebellum’s role in timing and predicting motor actions is essential for executing movements at the correct moment, as supported by evidence from neuroimaging studies [18]. The cerebellum's extensive connectivity with other motor regions, such as the motor cortex and basal ganglia, facilitates the coordination and modulation of motor commands [23]. Clinically, understanding cerebellar function has led to therapeutic strategies for cerebellar disorders, including neurostimulation techniques, intensive rehabilitation programs, and pharmacological interventions aimed at improving motor control [10]. These advances underscore the integral role of the cerebellum in motor function and its potential as a target for therapeutic interventions in motor disorders [24].
Expanding the Role of the Cerebellum to Nonmotor Functions
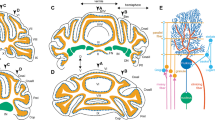
Recent research has expanded the understanding of the cerebellum beyond motor control, implicating it in cognitive processes and social behaviors [25, 26]. Schmahmann’s dysmetria of thought hypothesis proposed that the cerebellum contributes to cognitive functions through its extensive connections with the cerebral cortex [27, 28]. Functional imaging studies in humans have shown cerebellar activation during tasks that require working memory, language processing, and social cognition. The cerebellum is structurally divided into three main parts: the vestibulocerebellum, the spinocerebellum, and the cerebrocerebellum, each contributing differently to motor control [29]. The vestibulocerebellum, including the flocculonodular lobe, is involved in balance and eye movements. The spinocerebellum, comprising the vermis and intermediate zones, regulates body and limb movements [30]. The cerebrocerebellum, which consists of the lateral hemispheres, is associated with planning and initiating voluntary activity [31].
Sensorimotor Integration
Recent studies have emphasized the role of the cerebellum in integrating sensory inputs with motor commands to fine-tune motor actions [32]. Functional imaging and electrophysiological studies have shown that the cerebellum receives proprioceptive feedback from muscles and joints, which it uses to adjust movements dynamically [4, 33]. This study demonstrated how proprioceptive signals are processed in the cerebellum to ensure smooth and coordinated movements [20, 34]. The cerebellum plays a crucial role in sensorimotor integration, seamlessly combining sensory inputs with motor commands to produce coordinated and precise movements [35]. It receives proprioceptive feedback, vestibular information, and visual and auditory inputs, integrating these signals to adjust and refine motor actions in real time [32]. Key mechanisms include intricate neural circuits involving Purkinje cells, synaptic plasticity, and predictive coding, which allow the cerebellum to anticipate and correct movement errors [5]. Advances in functional imaging and electrophysiological studies have deepened our understanding of these processes, highlighting the importance of the cerebellum in maintaining balance and coordination. Clinically, this knowledge informs therapeutic approaches such as neurostimulation and targeted rehabilitation to improve motor performance in individuals with cerebellar disorders.
Motor Learning and Adaptation
The cerebellum plays a crucial role in motor learning by integrating sensory feedback with motor commands to refine and optimize movements through practice [36]. Recent research has highlighted the cerebellum’s involvement in error-based learning, where it detects discrepancies between intended and actual movements and adjusts future actions accordingly [37]. Key mechanisms include synaptic plasticity at parallel fiber-Purkinje cell synapses, where long-term depression (LTD) and long-term potentiation (LTP) enable the adaptation of motor commands [22, 38]. Additionally, the cerebellum acts as an internal clock, ensuring precise timing for coordinated movements, and employs predictive coding to anticipate and correct errors [39, 40]. Advances in neuroimaging and electrophysiological studies have provided deeper insights into these processes, demonstrating the essential function of the cerebellum in motor learning. Clinically, this understanding has informed the development of targeted therapies, such as neurostimulation and specialized rehabilitation programs, aimed at enhancing motor learning and recovery in individuals with cerebellar disorders [23]. The cerebellum is crucial for motor learning, particularly in the adaptation and refinement of motor skills through practice [22]. This function is mediated by long-term synaptic plasticity within the cerebellar cortex, especially in parallel fiber-Purkinje cell synapses [41]. Studies have shown that synaptic modifications in the cerebellum are essential for acquiring new motor skills and adapting to changing environments. Notably, motor learning involves changes in the strength of synaptic connections, which are critical for the accurate execution of learned movements [32].
Timing and Prediction
One of the key contributions of the cerebellum to motor control is the precise timing and prediction of motor actions [42]. The cerebellum is thought to act as a predictive model that anticipates the sensory consequences of motor commands, allowing timely adjustments [43]. Studies using neuroimaging techniques have shown cerebellar activation during tasks that require precise timing and sequencing of movements, such as playing a musical instrument or typing. provided evidence that the cerebellum's predictive capabilities are crucial for coordinating complex motor sequences [44, 45].
Cerebellar pathways and neuroanatomical connections
The cerebellum interacts with various brain regions through a network of converging and diverging pathways. Converging pathways primarily involve the integration of sensory and motor information [19, 46]. These pathways include connections from the spinal cord, vestibular nuclei, and cerebral cortex to the cerebellar cortex and deep cerebellar nuclei [19]. Diverging pathways project from the cerebellum to multiple brain regions, including the prefrontal cortex, which is involved in higher-order cognitive functions, and the limbic system, which regulates emotions and social behaviors (Table 1).
Mouse models for cerebellar research
Mouse models have been instrumental in elucidating the role of specific cerebellar circuits in behavior [52]. Genetic tools, such as Cre–loxP recombination, have allowed for the selective manipulation of cerebellar neurons [53]. Studies using these techniques have revealed that disrupting cerebellar Purkinje cells can lead to deficits in both motor coordination and social behavior, suggesting a shared neural substrate for these functions [54]. For instance, deletion of the Tsc1 (Tuberous Sclerosis Complex 1) gene in Purkinje cells results in autism-like behaviors in mice (Table 2). Tuberous sclerosis complex gene 1 (TSC1) plays a pivotal role in the insulin receptor pathway and is often disrupted in tumors. Encoded via the TSC1 gene, hamartin plays an essential role in regulating human cells. Understanding the role of phosphorylation requires identifying upstream kinases [55]. The TSC is a compelling model for examining how the cerebellum contributes to ASD pathogenesis. TSC patients exhibit cerebellar pathology and a strong association with ASD, while also revealing novel roles for Tsc1 in Purkinje cell function, shedding light on the role of the cerebellum in cognitive disorders such as autism [56]. Sufferers with familial TSC inherit a germline mutation in TSC1 or TSC2, leading to somatic loss and diverse renal epithelial tumors. TSC is an autosomal dominant genetic disease characterized by hamartomatous tumors across organs caused by mutations in TSC1 or TSC2. Chronic kidney disease is a critical risk factor for renal neoplasms, and TSC-associated renal tumors are often compared with sporadic cases [57]. Initially, thought to cause phenotypic variability in TSC, mtDNA variants were strongly correlated across tissues and tumors. Several key genotype–phenotype correlations in TSC have been identified [58, 59]. Considerably, traditional genetic testing fails to reveal mutations in 15% of patients. It is postulated that the majority of these cases are caused by somatic TSC1/TSC2 variants, creating complicated diagnostic challenges [59]. Half of those with TSC1/TSC2 variants have a second hit in affected tissues, such as facial angiofibromas, renal AMLs, and LAM. Missense variants make up 6% of TSC1 pathogenic variants, while small indels make up more than 57% of TSC1 pathogenic variants [60]. Using a 3D CNN model with multicontrast (multicontrast magnetic resonance imaging) MRI, we were able to identify TSC in children early. Fluid-attenuated inversion recovery (FLAIR3), a novel modality, enhances the visibility of TSC lesions and improves classification accuracy. The technique utilizes 3D-EfficientNet and 3D-ResNet as feature extractors. FLAIR3 was previously used most effectively in MS [61]. Genetic disorder (TSC) is a caused by mutations inside the TSC1 and TSC2 genes cell growth and benign tumor formation in multiple organs of affect the brain, skin, and other organs.
Cerebellar involvement in autism spectrum disorder (ASD)
There is substantial evidence linking cerebellar dysfunction to ASD. Neuroimaging studies have identified structural and functional abnormalities in the cerebellum of individuals with ASD [76, 77]. Animal studies have further supported this link, showing that mutations in genes associated with ASD often lead to cerebellar abnormalities [78]. For example, knockout models for genes such as Shank3 and Cntnap2 exhibit both motor and social deficits along with cerebellar anomalies (Table 3).
Specific Pathways Implicated in Motor and Social Behaviors
Research has identified specific cerebellar pathways involved in distinct aspects of motor and social behaviors [17, 25]. For motor control, the spinocerebellar and cerebrocerebellar pathways are crucial because they integrate sensory feedback and fine-tune motor outputs [84]. For social behaviors, cerebellar connections to the prefrontal cortex and limbic system are particularly important [85]. These pathways are thought to modulate social cognition and emotional regulation through their influence on cortical and subcortical circuits (Table 4).
Human and mouse models play a critical role in understanding the genetic and molecular underpinnings of those conditions. These models new therapies effectively, paving the way for targeted treatments (Table 5). Current studies focuses on various interventions, including pharmacological approaches, gene therapy, and behavioral strategies to develop effective treatments that can significantly improve the quality of lifestyles for people with cerebellar dysfunction and ASD, with ongoing studies continually refining these therapeutic approaches.
Clinical Implications
Understanding the role of the cerebellum in motor control has significant clinical implications, particularly for individuals with cerebellar disorders [32]. Conditions such as cerebellar ataxia, dystonia, and tremor are characterized by impaired motor coordination and balance. Recent therapeutic approaches, including cerebellar stimulation and targeted rehabilitation strategies, aim to mitigate these motor deficits by enhancing cerebellar function [91]. Studies have shown that cerebellar transcranial magnetic stimulation (TMS) can improve motor performance in patients with cerebellar damage, highlighting the potential for neuromodulation in treating cerebellar disorders.
Conclusion
The literature underscores the dual role of the cerebellum in motor and social functions, mediated through its converging and diverging pathways. Understanding these pathways in detail not only enhances our knowledge of cerebellar function but also provides insights into the neural mechanisms underlying neurodevelopmental disorders such as ASD. Continued research using advanced genetic and neuroanatomical techniques in mouse models will be crucial in unraveling the complex contributions of cerebellar circuits to behavior.
Availability of data and materials
The data will be made available upon request.
References
Manto M, Adamaszek M, Apps R, Carlson E, Guarque-Chabrera J, Heleven E, Kakei S, Khodakhah K, Kuo S-H, Lin C-YR, Joshua M, Miquel M, Mitoma H, Larry N, Péron JA, Pickford J, Schutter DJLG, Singh MK, Tan T, Tanaka H, Tsai P, Van Overwalle F and Yamashiro K. Consensus Paper: Cerebellum and Reward. The Cerebellum 2024. https://doi.org/10.1007/s12311-024-01702-0
Sendhilnathan N, Bostan AC. Strick PL and Goldberg MEJNC. A cerebro-cerebellar network for learning visuomotor associations. 2024;15:2519.
Kent JSJCOiBS. Transdiagnostic emergence of cerebellar abnormalities: implications for cerebellar contributions to psychopathology. 2024: 57:101354.
Bress KS, Cascio CJJN and Reviews B. Sensorimotor regulation of facial expression–an untouched frontier. 2024:105684.
Payne HL, Raymond JL, Goldman MSJE. Interactions between circuit architecture and plasticity in a closed-loop cerebellar system. 2024;13: e84770.
Wu Z. Spacetime in the brain. Dissertation, Magdeburg, Otto-von-Guericke-Universität Magdeburg, 2024.
Morgunova A. Tiny clues, monumental impact: navigating from microRNAs to brain development in the landscape of adolescent depression risk. 2024.
Doszyn O. Dulski T and Zmorzynska JJFiMN. Diving into the zebrafish brain: exploring neuroscience frontiers with genetic tools, imaging techniques, and behavioral insights. 2024;17:1358844.
Han KA, Yoon T-H, Kim J, Lee J, Lee JY, Jang G, Um JW. Kim JK and Ko JJnc. Specification of neural circuit architecture shaped by context-dependent patterned LAR-RPTP microexons. 2024;15:1624.
Ciricugno A, Oldrati V, Cattaneo Z, Leggio M, Urgesi C and Olivito GJTC. Cerebellar Neurostimulation for Boosting Social and Affective Functions: Implications for the Rehabilitation of Hereditary Ataxia Patients. 2024:1–27.
Wahl L. Serra I and Badura AJCOiBS. Impact of cerebellar-specific genetic and circuit manipulations on the behavioral phenotype and cerebellar physiology in murine autism models. 2024;55: 101330.
Morgado F, Vandewouw MM, Hammill C, Kelley E, Crosbie J, Schachar R, Ayub M, Nicolson R, Georgiades S, Arnold PJTP. Behaviour-correlated profiles of cerebellar-cerebral functional connectivity observed in independent neurodevelopmental disorder cohorts. 2024;14:173.
Li CN, Keay KA, Henderson LA and Mychasiuk RJJoN. Re-examining the Mysterious Role of the Cerebellum in Pain. 2024: 44.
Nudelman CJJFPeLOOotIAoL and Phoniatrics. Sensorimotor Integration in Patients with Voice Disorders: A Scoping Review of Behavioral Research. 2024.
Kakizawa S, Arasaki T, Yoshida A, Sato A, Takino Y, Ishigami A, Akaike T, Yanai S and Endo SJRB. Essential role of ROS–8-Nitro-cGMP signaling in long-term memory of motor learning and cerebellar synaptic plasticity. 2024: 70:103053.
Ocklenburg S and Guo ZVJN. Cross-hemispheric communication: Insights on lateralized brain functions. 2024.
Prati JM, Pontes-Silva A and Gianlorenço ACLJBBR. The cerebellum and its connections to other brain structures involved in motor and non-motor functions: a comprehensive review. 2024:114933.
Langlois ET, Bennequin D and de Marco GJTC. Role of the Cerebellum in the Construction of Functional and Geometrical Spaces. 2024:1–26.
Kim LH, Heck DH and Sillitoe RVJARoN. Cerebellar Functions Beyond Movement and Learning. 2024: 47.
Zobeiri OA, Cullen KEJNC. Cerebellar Purkinje cells in male macaques combine sensory and motor information to predict the sensory consequences of active self-motion. 2024;15:4003.
Gmaz JM, Keller JA. Dudman JT and Gallego JAJCOiN. Integrating across behaviors and timescales to understand the neural control of movement. 2024;85: 102843.
Bruel A, Abadía I, Collin T, Sakr I, Lorach H, Luque NR, Ros E and Ijspeert AJPCB. The spinal cord facilitates cerebellar upper limb motor learning and control; inputs from neuromusculoskeletal simulation. 2024: 20:e1011008.
Torbati AHM, Jami S, Kobravi H, Davoudi N, Gholibeigi MA, Ashkzari AJN and Physiology B. Underlying interactive neural mechanism of motor learning governed by the cerebellum, the basal ganglia, and motor/sensory cortex: a review from theoretical perspective. 2024:1–10.
Chen Y, Xu Z, Liu T, Li D, Tian X, Zheng R, Ma Y, Zheng S, Xing J and Wang WJJoN. Application of deep brain stimulation and transcranial magnetic stimulation in stroke neurorestoration: A review. 2024:100120.
Manto M, Adamaszek M, Apps R, Carlson E, Guarque-Chabrera J, Heleven E, Kakei S, Khodakhah K, Kuo S-H and Lin C-YRJTC. Consensus Paper: Cerebellum and Reward. 2024:1–24.
Faris P, Pischedda D. Palesi F and D’Angelo EJFiCN. New clues for the role of cerebellum in schizophrenia and the associated cognitive impairment. 2024;18:1386583.
Wang Z, Diedrichsen J, Saltoun K, Steele CJ, Arnold-Anteraper SR, Yeo BT, Schmahmann J and Bzdok DJb. Intrinsic structural covariation links cerebellum subregions to the cerebral cortex. 2024:2024.02. 16.580701.
Hodgdon EA, Anderson R, Al Azzawi H, Wilson TW, Calhoun VD, Wang Y-P, Solis I, Greve DN, Stephen JM and Ciesielski KTJDCN. MRI morphometry of the anterior and posterior cerebellar vermis and its relationship to sensorimotor and cognitive functions in children. 2024:101385.
Kebschull JM, Casoni F, Consalez GG, Goldowitz D, Hawkes R, Ruigrok TJ, Schilling K, Wingate R, Wu J and Yeung JJTC. Cerebellum lecture: the cerebellar nuclei—core of the cerebellum. 2024: 23:620–77.
Kaski D, Bamiou DE, Bronstein A and Koohi NJNAQST. Neuro‐Otology: Dizziness, Balance and Hearing. 2024:797–838.
Rios-Zermeno J, Ballesteros-Herrera D, Dominguez-Vizcayno P, Carrillo-Ruiz JD, Moreno-Jimenez SJAN. Dentate nucleus: a review and implications for dentatotomy. 2024;166:1–9.
Martins LA, Schiavo A, Paz LV, Xavier LL, Mestriner RGJP and Behavior. Neural Underpinnings of Fine Motor Skills Under Stress and Anxiety: A Review. 2024:114593.
Parziale A and Marcelli AJAIR. Understanding upper-limb movements via neurocomputational models of the sensorimotor system and neurorobotics: where we stand. 2024: 57:73.
Liu MF, Gaunt RA, Collinger JL, Downey JE, Batista AP, Boninger ML and Weber DJJm. Volitional control of movement interacts with proprioceptive feedback in motor cortex during brain-computer interface control in humans. 2024:2024.02. 26.24303289.
Cienfuegos M, Maycock J, Naceri A, Tobias D, Kõiva R, Schack T and Ritter H. Exploring Motor Skill Acquisition in Bimanual Coordination: Insights from Navigating a Novel Maze Task. 2024.
Vandervert L, Manto M, Adamaszek M, Ferrari C, Ciricugno A and Cattaneo ZJTC. The Evolution of the Optimization of Cognitive and Social Functions in the Cerebellum and Thereby the Rise of Homo sapiens Through Cumulative Culture. 2024:1–12.
Nardi F, Faisal AA and Haar SJb. Motor Learning Mechanisms are not modified by Feedback Manipulations in a Real-World Task. 2024:2024.04. 10.588812.
Kamesh A. Increased striatal glutamate and dopamine transmission in young VPS35 D620N knock-in mouse model of Parkinson’s disease precedes reduced neurotransmission in old age. 2024.
Narayanan S, Varma A and Thirumalai VJSA. Predictive neural computations in the cerebellum contribute to motor planning and faster behavioral responses in larval zebrafish. 2024: 10:eadi6470.
Parras GG, Delgado-García JM, López-Ramos JC. Gruart A and Leal-Campanario RJnSoL. Cerebellar interpositus nucleus exhibits time-dependent errors and predictive responses. 2024;9:12.
Schreurs BG, O’Dell DE, Wang DJB. The Role of Cerebellar Intrinsic Neuronal Excitability. Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning. 2024;13:200.
Berlijn AM, Huvermann DM, Schneider S, Bellebaum C, Timmann D, Minnerop M and Peterburs JJTC. The Role of the Human Cerebellum for Learning from and Processing of External Feedback in Non-Motor Learning: A Systematic Review. 2024:1–20.
Misaghian K. Lugo JE and Faubert JJFian. Immediate fall prevention: the missing key to a comprehensive solution for falling hazard in older adults. 2024;16:1348712.
Flüthmann N. From sports-related to sport-specific movement performances: Approaching a method transfer in neuronal motor behavior. 2024.
Alhwaiti Y, Alrashdi I. Ahmad I and Khan AJCiHB. A computational deep learning approach for establishing long-term declarative episodic memory through one-shot learning. 2024;156: 108213.
van der Heijden MEJTC. Converging and Diverging Cerebellar Pathways for Motor and Social Behaviors in Mice. 2024:1–14.
Washburn S, Oñate M, Yoshida J, Vera J, Bhuvanasundaram R, Khatami L, Nadim F and Khodakhah KJNN. The cerebellum directly modulates the substantia nigra dopaminergic activity. 2024:1–17.
Kasiraman G, Gousebasha YI, Sridaran HJA and Research V. Effect of Vestibular Rehabilitation on Postural Stability in Children with Visual Impairment. 2024;33:85–92.
Bonassi G, Zhao M, Samogin J, Mantini D, Marchese R, Contrino L, Tognetti P, Putzolu M, Botta A and Pelosin EJS. Brain Networks Modulation during Simple and Complex Gait: A “Mobile Brain/Body Imaging” Study. 2024: 24:2875.
Rajamani SK, Iyer RS, Venkatraman AJJoO, Hearing and Medicine B. Comparison of Halmágyi–Curthoys Head Impulse (Thrust) Test with Romberg’s Test in Detection of Vestibular Hypofunctioning in Vertigo Patients. 2024: 5:4.
Silva-Batista C, Lira J, Coelho DB, de Lima-Pardini AC, Nucci MP, Mattos ECT, Magalhaes FH, Barbosa ER, Teixeira LA, Amaro Junior EJBS. Mesencephalic Locomotor Region and Presynaptic Inhibition during Anticipatory Postural Adjustments in People with Parkinson’s Disease. 2024;14:178.
Ji Y, McLean JL and Xu RJNB. Emerging Human Pluripotent Stem Cell-Based Human–Animal Brain Chimeras for Advancing Disease Modeling and Cell Therapy for Neurological Disorders. 2024:1–18.
Kojima L, Seiriki K, Rokujo H, Nakazawa T, Kasai A and Hashimoto HJi. Optimization of AAV vectors for transactivator-regulated enhanced gene expression within targeted neuronal populations. 2024.
Zhou Y, Sanchez VB, Xu P, Roule T, Flores-Mendez M, Ciesielski B, Yoo D, Teshome H, Jimenez T and Liu SJJi. Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency. 2024.
Hsieh T-C, Nicolay BN, Frolov MV and Moon N-SJPG. Tuberous sclerosis complex 1 regulates dE2F1 expression during development and cooperates with RBF1 to control proliferation and survival. 2010: 6:e1001071.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51. https://doi.org/10.1038/nature11310.
Machacek ME, Wu C-L, Cornejo KM. Pathology of hereditary renal cell carcinoma syndromes: Tuberous sclerosis complex (TSC). Semin Diagn Pathol. 2024;41:8–19. https://doi.org/10.1053/j.semdp.2023.09.001.
Giannikou K, Martin KR, Abdel-Azim AG, Pamir KJ, Hougard TR, Bagwe S, Tang Y, MacKeigan JP, Kwiatkowski DJ, Henske EP. Spectrum of germline and somatic mitochondrial DNA variants in Tuberous Sclerosis Complex. Front Genet. 2023;13: 917993.
Man A, Di Scipio M, Grewal S, Suk Y, Trinari E, Ejaz R, Whitney R. The Genetics of Tuberous Sclerosis Complex and Related mTORopathies: Current Understanding and Future Directions. Genes. 2024;15:332.
Ikeda KM, House AA, Connaughton DM, Pautler SE, Siu VM, Jones M-L. Potential Pitfalls in Pre-implantation Genetic Diagnosis in a Patient with Tuberous Sclerosis and Isolated Mosaicism for a TSC2 Variant in Renal Tissue. Molecular Syndromology. 2021;12:154–8.
Gabr RE, Hasan KM, Haque ME, Nelson FM, Wolinsky JS, Narayana PA. Optimal combination of FLAIR and T2-weighted MRI for improved lesion contrast in multiple sclerosis. J Magn Reson Imaging. 2016;44:1293–300.
de Xivry J-JO, Diedrichsen JJCOiBS. Diversity of the nature of input and output signals in the cerebellum suggests a diversity of function. 2024;57: 101386.
Allen JP, Garber KB, Perszyk R, Khayat CT, Kell SA, Kaneko M, Quindipan C, Saitta S, Ladda RL and Hewson SJHMG. Clinical features, functional consequences, and rescue pharmacology of missense GRID1 and GRID2 human variants. 2024: 33:355–73.
Vicente-Acosta A, Herranz-Martin S, Pazos MR, Galan-Cruz J, Amores M, Loria F and Diaz-Nido JJb. Glial cell activation precedes neurodegeneration in the cerebellar cortex of the YG8–800 murine model of Friedreich's ataxia. 2024:2024.05. 17.594658.
Hamel K, Moncada EL, Sheeler C, Rosa J-G, Gilliat S, Zhang Y and Cvetanovic MJNoD. Cerebellar heterogeneity and selective vulnerability in spinocerebellar Ataxia type 1 (SCA1). 2024:106530.
Kumar G, Zhou Z, Wang Z, Kwan KM, Tin C. Ma CHEJCN and Therapeutics. Real-time field-programmable gate array-based closed-loop deep brain stimulation platform targeting cerebellar circuitry rescues motor deficits in a mouse model of cerebellar ataxia. 2024;30: e14638.
Raslan IR, Silva TYT, Kok F, Rodrigues MM, Aragão MM, Pinho RS, França MC Jr. Barsottini OG and Pedroso JLJNG. Clinical and Genetic Characterization of a Cohort of Brazilian Patients With Congenital Ataxia. 2024;10: e200153.
Prytkova I, Liu Y, Fernando M, Gameiro-Ros I, Popova D, Kamarajan C, Xuei X, Chorlian DB, Edenberg HJ and Tischfield JAJJoN. Upregulated GIRK2 counteracts ethanol-induced changes in excitability & respiration in human neurons. 2024.
Mahoney HL, Bloom CA, Justin HS, Capraro BM, Morris C, Gonzalez D, Sandefur E, Faulkner J, Reiss S and Valladares AJFiCN. DISC1 and reelin interact to alter cognition, inhibition, and neurogenesis in a novel mouse model of schizophrenia. 2024: 17:1321632.
Kaiyrzhanov R, Ortigoza‐Escobar JD, Stringer BW, Ganieva M, Gowda VK, Srinivasan VM, Macaya A, Laner A, Onbool E and Al‐Shammari RJMD. Clinical and Molecular Spectrum of Autosomal Recessive CA8‐Related Cerebellar Ataxia. 2024.
Alfayyadh MM, Maksemous N, Sutherland HG, Lea RA, Griffiths LRJG. Unravelling the Genetic Landscape of Hemiplegic Migraine: Exploring Innovative Strategies and Emerging Approaches. 2024;15:443.
Sakai Y, Kassai H, Nakayama H, Fukaya M, Maeda T, Nakao K, Hashimoto K, Sakagami H, Kano M, Aiba A. Hyperactivation of mTORC1 disrupts cellular homeostasis in cerebellar Purkinje cells. Sci Rep. 2019;9:2799.
Abbott PW, Hardie JB, Walsh KP, Nessler AJ, Farley SJ, Freeman JH, Wemmie JA, Wendt L, Kim Y-c and Sowers LP. Knockdown of the Non-canonical Wnt Gene Prickle2 Leads to Cerebellar Purkinje Cell Abnormalities While Cerebellar-Mediated Behaviors Remain Intact. The Cerebellum 2024:1-13.
Dewa K-i, Arimura N, Kakegawa W, Itoh M, Adachi T, Miyashita S, Inoue YU, Hizawa K, Hori K and Honjoya N. Neuronal DSCAM regulates the peri-synaptic localization of GLAST in Bergmann glia for functional synapse formation. Nature communications 2024: 15:458.
Wang Y, Zhai Y and Wang J. Insight into the early pathogenesis and therapeutic strategies of spinocerebellar ataxia type 3/machado–joseph disease from mouse models. Parkinsonism & Related Disorders 2024:106991.
Klaus J. Stoodley CJ and Schutter DJJDCN. Neurodevelopmental trajectories of cerebellar grey matter associated with verbal abilities in males with autism spectrum disorder. 2024;67: 101379.
Vilela J, Rasga C, Santos JX, Martiniano H, Marques AR, Oliveira G and Vicente AMJIJoMS. Bridging Genetic Insights with Neuroimaging in Autism Spectrum Disorder—A Systematic Review. 2024: 25:4938.
Gambini D, Ferrero S, Bulfamante G, Pisani L, Corbo M, Kuhn EJN, Neurobiology A. Cerebellar phenotypes in germline PTEN mutation carriers. 2024;50: e12970.
Kolinko Y, Kralickova M, Cendelin JJTC. Reduction of microvessel number and length in the cerebellum of Purkinje cell degeneration mice. 2024;23:471–8.
Cealie MY, Douglas JC, Swan HK, Vonkaenel ED, McCall MN, Drew PD, Majewska AKJC. Developmental Ethanol Exposure Impacts Purkinje Cells but Not Microglia in the Young Adult Cerebellum. 2024;13:386.
Carrión-Castillo A, Boeckx CJSR. Insights into the genetic architecture of cerebellar lobules derived from the UK Biobank. 2024;14:9488.
Nikova A. Thalamocortical connectivity in schizophrenia: a systematic. 2024.
Gkintoni E, Skokou M, Gourzis PJM. Integrating Clinical Neuropsychology and Psychotic Spectrum Disorders: A Systematic Analysis of Cognitive Dynamics. Interventions, and Underlying Mechanisms. 2024;60:645.
Christova M, Sylwester V, Gallasch E, Fresnoza SJTC. Reduced Cerebellar Brain Inhibition and Vibrotactile Perception in Response to Mechanical Hand Stimulation at Flutter Frequency. 2024;23:67–81.
Terburg D, van Honk J and Schutter DJJC. Doubling Down on Dual Systems: A Cerebellum-Amygdala Route towards Action-and Outcome-Based Social and Affective Behavior. 2024.
Rowe HP, Stipancic KL, Campbell TF, Yunusova Y. Green JRJCL and Phonetics. The association between longitudinal declines in speech sound accuracy and speech intelligibility in speakers with amyotrophic lateral sclerosis. 2024;38:227–48.
De Marchi I, Buffone F, Mauro A, Bruini I, Vismara LJM. Manual Therapy of Dysphagia in a Patient with Amyotrophic Lateral Sclerosis: A Case Report. 2024;60:845.
Passaretti M, Piervincenzi C, Baione V, Pasqua G, Colella D, Pietracupa S, Petsas N, Angelini L, Cannavacciuolo A and Paparella GJTC. The Role of Cerebellum and Basal Ganglia Functional Connectivity in Altered Voluntary Movement Execution in Essential Tremor. 2024:1–22.
Qneibi M, Bdir S, Bdair M, Aldwaik SA, Sandouka D, Heeh M and Idais TIJEJoMC. AMPA receptor neurotransmission and therapeutic applications: A comprehensive review of their multifaceted modulation. 2024:116151.
Upadhyay A, Gradwell MA, Vajtay TJ, Conner J, Sanyal AA, Azadegan C, Patel KR, Thackray JK, Bohic M and Imai FJb. The Dorsal Column Nuclei Scale Mechanical Sensitivity in Naive and Neuropathic Pain States. 2024.
Mushtaq S, Bilal T, Khurshid I, Sheikh WM, Khursheed A, Jan J, Ali SA, Nabi SU, Ganie MA and Muzamil S. Clinical Perspective and Overview of Spinal Cord Disorders and Cerebellar Ataxias. Evidence‐Based Neurological Disorders. Jenny Stanford Publishing; 2024. pp. 229–66.
Cascino G and Monteleone AMJP. Early traumatic experiences and the hypothalamus-pituitary-adrenal axis in people with eating disorders: A narrative review. 2024: 159:106665.
Robayo AMM. Hypotalamus-Pituitary-Adrenal (HPA) Axes and Their Relationship with Stress, Mood, Personality, and Neurocognitive Functioning. The Theory of Mind Under Scrutiny: Psychopathology, Neuroscience, Philosophy of Mind and Artificial Intelligence. Springer; 2024. pp. 341–65.
Fernández de Gamarra-Oca L, Lucas-Jiménez O, Ontañón J, Loureiro-Gonzalez B, Peña J, Ibarretxe-Bilbao N, García-Guerrero M, Ojeda N, Zubiaurre-Elorza LJBS and Function. Amygdala structure and function and its associations with social-emotional outcomes in a low-risk preterm sample. 2024:1–12.
Mu C, Dang X and Luo X-JJNhb. Mendelian randomization analyses reveal causal relationships between brain functional networks and risk of psychiatric disorders. 2024:1–12.
He J, Bore MC, Jiang H, Gan X, Wang J, Li J, Xu X, Wang L, Fu K and Li LJm. Shared Neural Dysregulations Mediate Dysregulated Empathy for Pain across Mental Disorders-a Pre-registered Neuroimaging Meta-Analysis. 2024:2024.02. 07.24302440.
Ibrahim K, Iturmendi-Sabater I, Vasishth M, Barron DS, Guardavaccaro M, Funaro MC, Holmes A, McCarthy G. Eickhoff SB and Sukhodolsky DGJSR. Neural circuit disruptions of eye gaze processing in autism spectrum disorder and schizophrenia: An activation likelihood estimation meta-analysis. 2024;264:298–313.
Licchetta L, Bruschi G, Stipa C, Belotti LMB, Ferri L, Mostacci B, Vignatelli L, Minardi R, Di Vito L, Muccioli L. Tuberous sclerosis complex in adulthood: focus on epilepsy prognosis. Epilepsy Behav. 2024;153: 109688.
de Fátima dos Santos Sampaio M, de Paiva YB, Sampaio TB, Pereira MG and Coimbra NC. Therapeutic applicability of cannabidiol and other phytocannabinoids in epilepsy, multiple sclerosis and Parkinson's disease and in comorbidity with psychiatric disorders. Basic & Clinical Pharmacology & Toxicology 2024: 134:574–601.
Verduzco‐Gutierrez M, Raghavan P, Pruente J, Moon D, List CM, Hornyak JE, Gul F, Deshpande S, Biffl S and Al Lawati Z. AAPM&R consensus guidance on spasticity assessment and management. PM&R 2024.
Golmohammadi M, Mahmoudian M, Hasan EK, Alshahrani SH, Romero-Parra RM, Malviya J, Hjazi A, Najm MA, Almulla AF, Zamanian MY. Neuroprotective effects of riluzole in Alzheimer’s disease: A comprehensive review. Fundam Clin Pharmacol. 2024;38:225–37.
Atella TC, Medina JM, Atella GC, Allodi S and Kluck GE. Neuroprotective Effects of Metformin Through AMPK Activation in a Neurotoxin-Based Model of Cerebellar Ataxia. Molecular Neurobiology 2024:1–15.
Affrald RJ, Narayan S. Exploring the Versatility of 4-Aminopyridine and Its Analogues in Managing Demyelinating Diseases (A Review). Russ J Bioorg Chem. 2024;50:824–54.
Verma M. Plant Metabolites and Vegetables for Brain Disease Prevention and Treatment Madhuri Verma. Plant Metabolites and Vegetables as Nutraceuticals. Apple Academic Press. pp. 275–97.
Ashwlayan VD, Ratnesh RK, Sharma D, Sharma A, Sangal A, Saifi A and Singh J. A Comprehensive Review on Plant-Based Medications and Chemical Approaches for Autism Spectrum Disorders (ASDs) Psychopharmacotherapy. Indian Journal of Microbiology 2024:1–17.
Khot KB, DS S, Gopan G, Deshpande N S, Shastry P, Bandiwadekar A and Jose J. Enhancing selegiline hydrochloride efficacy: Box Behnken-optimized liposomal delivery via intranasal route for Parkinson’s disease intervention. Journal of Liposome Research 2024:1–18.
Boleti APdA, Cardoso PHdO, Frihling BEF, de Moraes LFRN, Nunes EAC, Mukoyama LTH, Nunes EAC, Carvalho CME, Macedo MLR and Migliolo L. Pathophysiology to Risk Factor and Therapeutics to Treatment Strategies on Epilepsy. Brain Sciences 2024: 14:71.
Duncan J, Sander J, Alim‐Marvasti A, Balestrini S, Baxendale S, Bindman D, Chinthapalli K, Chowdhury F, Diehl B and Eriksson S. Epilepsy and Related Disorders. Neurology: A Queen Square Textbook 2024:247–318.
Acknowledgements
The corresponding author, Assistant Prof. Azhagu Madhavan Sivalingam, is thankful to all the authors for their collaborative efforts in writing this paper. This work is supported by the Natural Products & Nanobiotechnology Research Lab, Department of Community Medicine, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, Chennai-600 105, Tamil Nadu, India.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Azhagu Madhavan Sivalingam: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization. Arjun Pandian:- Formal analysis, Methodology, Investigation, Visualization. All the authors gave final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing Interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivalingam, A.M., Pandian, A. Cerebellar Roles in Motor and Social Functions and Implications for ASD. Cerebellum (2024). https://doi.org/10.1007/s12311-024-01720-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-024-01720-y