Abstract
Inherited cerebellar malformations cause lifelong disability and are not well studied in the newborns because there is a lack of appropriate clinical examination tools. Recently, inherited cerebellar malformations have been investigated using emerging advanced neuroimaging technology such as MRI, which revealed many cerebellar developmental disorders. These malformations cause impairments that involve motor and non-motor functions. Cerebellar hypoplasia, Dandy–Walker syndrome, Joubert syndrome, pontocerebellar hypoplasia, and rhombencephalosynapsis are examples of cerebellar malformations. In this chapter we will focus on cerebellar malformations that have been reported using characteristic symptoms and signs. The current approach for evaluation of the affected patients, differential diagnosis, and management will be discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebellar imaging
- Cerebellar disorder
- Cerebellar hypoplasia
- Dandy–Walker syndrome
- Joubert syndrome
- Pontocerebellar hypoplasia
- Rhombencephalosynapsis
Introduction
The cerebellum cytostructure is discussed in chapter “The Embryology and Anatomy of the Cerebellum.” Cerebellar development begins during an early embryonic stage with a complicated developmental process that continues well into the first year after birth in humans. Recent advances in neonatal intensive care, neuroimaging techniques such as positron emission tomography (PET), magnetic resonance imaging (MRI), and functional MRI (fMRI) have improved our ability to understand the structural and functional anomalies that implicate cerebellar involvement in numerous motor and non-motor functions, ranging from motor/sensory integration and working memory to various higher-order cognitive processes [1,2,3,4]. Despite the advanced technology, understanding cerebellar malformations in children requires additional research regarding their prognosis, and they have lifelong consequences. Because of a lack of appropriate treatment, up to 80% of parents choose to terminate pregnancy after a prenatal diagnosis of a cerebellar malformation [1, 5]. The prolonged developmental process in the cerebellum makes it more vulnerable to perturbation caused by genetic factors, environmental insults, or a combination of both that occurs during development. Cerebellar abnormalities range from subtle impairments including cognition to significant structural defects with life-threatening or lifelong disabilities. Before the introduction of MRI, Dandy–Walker variants were a term used to characterize several types of cerebellar malformations.
Cerebellar dysfunction disturbs the regulation of muscle tone, motor control, and coordination of movement, which is called ataxia —a broad term that refers to a disturbance in the smooth performance of the motor activities. The non-motor dysfunction that results from cerebellar manifestations includes cognitive affective syndrome that includes impairment in executive function, spatial cognition, personality changes, and language deficits [6,7,8,9]. Cerebellar structural and functional abnormalities have been reported in psychiatric disorders such as schizophrenia, bipolar disorder, depression, anxiety disorders, attention deficit hyperactivity disorder (ADHD), and autism [10,11,12,13,14,15].
The specific constellation of symptoms is sometimes useful for localizing the cerebellar lesion, but often there is considerable overlap. Because of a complex developmental process during cerebellum formation, clinical classification of cerebellar neurodevelopmental disorder is difficult; however, a classification has been suggested that is based on embryological and genetic considerations [16].
Cerebellar malformations can either be primary or secondary. In the latter group, the cerebellar defects are secondary to a developmental disorder in structures around the cerebellum such as Chiari malformation or vein of Galen malformation. Chiari malformations (Fig. 1) are posterior cranial fossa defects that range from herniation of the cerebellar tonsils through the foramen magnum to complete agenesis of the cerebellum. Chiari malformations are classified into four types (I–IV), with type IV being the most severe malformations [17]. The interruption of the surrounding mesodermal development causes congenital hypoplasia of the posterior cranial fossa, and therefore part of the cerebellum herniates through the foramen magnum. Other conditions sometimes associated with Chiari-type I malformation include hydrocephalus, bone abnormalities such as craniosynostosis (especially lambdoid craniosynostosis), hyperostosis (craniometaphyseal dysplasia), and X-linked vitamin D-resistant rickets, syringomyelia, spinal curvature, tethered spinal cord, and connective tissue disorders such as Ehlers–Danlos syndrome and Marfan syndrome [18,19,20,21]. Because of familial clustering in some cases of Chiari-type I malformation, a genetic susceptibility such as gene mutations in chromosomes 9 and 15 has been suggested [22]. In Chiari malformation type II, both cerebellar vermis and tonsil herniation accompany a lumbar or lumbosacral myelomeningocele [23, 24]. The hypoplasia in Chiari-type IV malformation corresponds to primary cerebellar agenesis [25, 26].
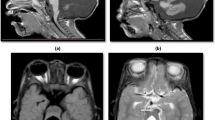
Chiari malformation type I. (a, b) Sagittal and coronal T2-weighted brain images. There is slight inferior herniation of cerebellar tonsils through the foramen magnum that is less than 5 mm, and it shows benign tonsillar ectopia that could be a mild variant of Chiari malformation. (c, d) Axial and sagittal T2-weighted MRI of the brainstem and cervical spinal cord. Note the presence of a large syrinx in association with tonsillar ectopia in Chiari malformation type I
Another example is the vein of Galen malformation that results from the presence of one or more arteriovenous fistulas, which constitute up to 30% of intracranial vascular malformations presenting among pediatric patients [27, 28]. The left-to-right cardiac shunt causes a noncyanotic flow condition with resultant heart failure and accompanying macrocephaly. In patients with vein of Galen malformation, the superior cerebellar arteries also discharge into the vein of Galen [29]. It is reasonable to assume that the dilated vein causes direct compression of cerebrospinal fluid (CSF) flow, increased intracranial pressure, and caudal displacement of the cerebellar tonsils [30], which leads to cerebellar signs and symptoms.
In this chapter, we summarize primary cerebellar malformations and discuss current treatment in these patients. Based on our clinical experience and available knowledge, there is no appropriate treatment and most of the patients will be managed conservatively (see chapter “Clinical Features, Assessment, and Management of Patients with Developmental and Other Cerebellar Disorders”). Treatment depends on the specific symptoms and requires a team of specialists including neonatologists, pediatricians, neurologists, and therapists. It is important to refer families of affected children for genetic counseling. In this chapter, we include selected primary cerebellar malformations such as the cerebellar hypoplasia, Dandy–Walker malformation (DWM), Joubert syndrome (JS), and the related diseases pontocerebellar hypoplasia (PCH), rhombencephalosynapsis (RES), lissencephaly, cerebellar dysplasia, and dysplastic cerebellar gangliocytoma or Lhermitte–Duclos disease (LDD).
Cerebellar Hypoplasia
The cerebellar primordium emerges at approximately 28 days postfertilization in humans (embryonic day 7–8 in the mouse) as a neuroepithelial swelling of the rostral lip of the fourth ventricle, which is part of the alar plate of the metencephalon (rhombomere-1) [31,32,33]. Therefore, any developmental dysregulation that targets the rhombomere-1 causes failure to specify the anterior hindbrain and results in cerebellar aplasia/hypoplasia because of defects in dorsal patterning mechanisms [34,35,36]. Cerebellar hypoplasia is a heterogeneous group of disorders that was first reported by Crouzon in 1929.
The causes of the cerebellar hypoplasia are broad and include chromosomal aberrations (such as trisomy 9, 13, and 18), metabolic disorders [37], teratogens (drugs and infections; see chapter “Teratogenic Influences on Cerebellar Development”), or isolated genetic cerebellar hypoplasia (such as reelin receptor, very low density lipoprotein (VLDL) [38], dyskerin pseudouridine synthase 1 (DKC1) [39], oligophrenin 1 (OPHN1) [40], pancreas-specific transcription factor 1a (PTF1A) [41], and carbohydrate-deficient glycoprotein syndrome types I and II (CDG1 and 2) [42, 43]). Similar to most developmental anomalies, cerebellar hypoplasia may be associated with other brain malformations and there may be multi-organ involvement. Patel (2002) suggested a classification in which cerebellar hypoplasia is divided into focal hypoplasia (e.g., isolated vermis or hemisphere hypoplasia) and generalized hypoplasia with and without an enlarged fourth ventricle [44, 45].
Clinically, ataxia and poor motor learning are the most common presentations and are nonprogressive compared with atrophic cerebellar disorders [46]. In infancy, hypotonia and global developmental delay are present earlier, and other signs include ocular motor disorders, dysarthria, intention tremor, and microcephaly. Behavioral abnormalities, intellectual disability, and speech and language disorders can vary from mild to severe impairment [26].
Management
It is important to consider that ataxia or other neurological signs in cerebellar hypoplastic patients usually do not worsen over time compared with atrophic cerebellar disorder. There is no standard course of treatment. The principal treatment is supportive including physical therapy, occupational therapy, speech therap y, psychiatric/behavioral medications, and special education.
Dandy–Walker Malformation
The fundamental structure that is affected in DWM is the cerebellum [47,48,49]. DWM is a genetic disorder, with the most common and severe type being the Dandy–Walker syndrome malformation [47]. The zinc finger protein of the cerebellum 1 (ZIC1) and zinc finger protein of the cerebellum 4 (ZIC4) genes on 3q24 [50] and FOXCI are candidate genes, and DWM results when they are deleted [51]. It is suggested that Zic1 and Zic4 are required for the full responsiveness of granule cell precursors (GCPs) to sonic hedgehog (SHH), but the role of the Foxc1 is not understood [36].
DWM is characterized by partial or complete agenesis of the vermis, upward rotation of the vermis, and an enlarged posterior cranial fossa [1, 36, 52, 53]. It is also characterized by cystic dilation of the fourth ventricle into the posterior cranial fossa. Enlargement of the posterior cranial fossa causes an abnormally high tentorium above the internal occipital protuberance and transverse occipital sulcus (location of transverse sinus) and also a variable degree of hydrocephalus [1, 54]. During cerebellar development, the right and left cerebellar primordia are fused at the midline. Any misregulation in this developmental process leads to a lack of cerebellar fusion at the midline. The lack of midline fusion causes the extension of membranous area/roof plate anteriorly, resulting in a large fourth ventricle. Cerebrospinal fluid pulsations cause roof plate expansion posteriorly within the posterior fossa , forming a large posterior cyst that represents the fourth ventricle [55].
Clinically, DWM can be defined via the characteristic triad consisting of the following: (1) complete or partial agenesis of the vermis, (2) cystic dilatation of the fourth ventricle, and (3) an enlarged posterior cranial fossa with upward displacement of the transverse sinuses [56, 57]. If hydrocephalous is present, it suggests a common developmental disorder in which multiple brain regions are affected [58].
The signs and symptoms associated with DWM are broad. DWM patients often have hypotonia, delayed motor development, ataxia, lack of coordination, jerky movements of the eyes, and progressive enlargement of the skull. Some patients may have normal cognition, whereas others have mild to severe mental retardation, even when hydrocephalus is effectively treated. The enlarged head circumference, which may bulge at the back of the skull, can increase pressure on the brain stem and nerves and can cause difficulties in controlling face and neck and abnormal breathing patterns. Sagittal and axial MRI (Fig. 2) can distinguish DWM from other cerebellar malformations. In DWM, it is important to consider mega cisterna magna, retro-cerebellar cysts, and Blake’s pouch cyst [55, 59]. It should be noted that in addition to the lack of the middle part of the cerebellum, the midline structures in the forebrain such as the corpus callosum may be absent. Systemic malformations associated with DWM may include cardiac anomalies, urogenital anomalies, and other abnormalities may occur collectively in about half of the patients [60,61,62,63].
Dandy–Walker malformation . (a) Sagittal T2-weighted brain image. There is hypoplasia of the inferior vermis. A connection between the cisterna magna and the fourth ventricle is seen. (b) Axial T1-weighted brain image. This image shows isolated inferior vermian hypoplasia and cerebellar hemispheres that appear normal. This is referred to as part of the Dandy–Walker variant
Management
If there is hydrocephalous, treatment could include shunting and cerebrospinal fluid (CSF) drainage from the lateral ventricles and/or posterior fossa cyst, which is currently considered the surgical treatment of choice [49]. Treatment also consists of physiotherapy, occupational therapy, speech therapy, and specialized education. Although diagnosis of DWS during intrauterine development is difficult, if an ultrasound suggests DWS , then amniocentesis should be performed to aid in the diagnosis [64]. It is important that the families of affected children be referred for genetic counseling.
Joubert Syndrome and Related Disorders
JS was first identified by Marie Joubert in Montreal, Canada [65]. JS is a group of autosomal recessive conditions that are characterized by developmental anomalies, which are caused by defects in the structure or function of the primary cilium [66, 67]. JS consists of midbrain-hindbrain malformation, which causes a developmental delay, motor disability, hypotonia, ataxia, abnormal eye movements, and neonatal breathing abnormalities. One of the gold standards for JS diagnosis is the molar tooth sign (MTS) observed on a plain MRI (Fig. 3). When other organs, such as the retina, kidneys, and liver, are involved, it is called JS and related disorders (JSRDs) , and these patients also have the MTS [66]. JSRDs are the most common inherited congenital cerebellar malformation.
Joubert syndrome and related disorders. (a) Coronal T2 FLAIR brain image. The cerebellar vermis is aplastic and superior cerebellar peduncles are elongated. (b) Axial T2 FLAIR brain image. This image shows a deep interpeduncular fossa, elongated superior cerebellar peduncles with cerebellar vermis hypoplasia, which are characteristic of the molar tooth sign in Joubert syndrome
Ciliopathy is a fundamental mechanism in JSRD. The primary cilia are important in neuronal development and function as cellular antenna that are found in nearly all cell types. The function of cilia in cells includes protein trafficking, photoreception, embryonic axis patterning, and cell cycle regulation. Therefore, dysfunction of this microtubule-based extension of cellular membranes can affect a single tissue or manifest as having multi-organ involvement, which is called ciliopathy [68]. Within the developing cerebellum, primary cilia have been shown to be essential for reception of the cell signaling ligand sonic hedgehog , which in turn is essential for proliferation of cerebellar neurons such as granule cells [69, 70].
The causative gene of many ciliopathies in individuals with JSRD has defined a new class of neurological diseases [68]. To date, over 16 causative genes have been associated with JSRD and all encode proteins in the primary cilium or its apparatus [66]. For example, mutations in genes such as AHII, INPP5E, CC2D2A, and ARL13B cause JS with MTS and retinal blindness [71]. However, mutations in TMEM216 and RPGRIPIL genes lead to MTS and renal involvement. In more severe cases, mutations in the CEP290 gene causes MTS together with retinal and renal involvement, while mutations in TMEM67 are the most common cause of MTS with liver involvement [72].
Clinically, JSRD patients have hypotonia, ataxia, dysregulated breathing rhythms (that result from dysfunction of the respiratory centers in the brainstem or cerebellum [73]), abnormal eye and tongue movements, and subsequent mental retardation. As mentioned above, the key MRI finding in JSRD is MTS, which is the result of cerebellar vermis hypoplasia, and is accompanied by thick superior cerebellar peduncles with deep interpeduncular fossa. Because ciliopathy interrupts a broad range of developmental process, a defect could be seen in other organs such as kidney, retina, and liver, and there were also facial abnormalities (cleft lip or palate, tongue abnormalities) and polydactyly (extra fingers and toes) [74,75,76].
In mild JSRD , ataxic movement lessens with age and the ability to walk is delayed to age 4–5 years. Some neonates have died as a result of apnea , and therefore it is important to monitor neonates with JSRD during the first year of life. These patients should be periodically examined for any non-neurological signs and symptoms.
Management
The treatment is symptomatic and supportive such as physical therapy, occupational therapy, and speech therapy. Infants with abnormal breathing patterns should be monitored closely for apnea, and this may be required during the first year of life. In this case, caffeine may be helpful to promote respiratory drive. Because of the heterogeneity of these conditions, genetic testing will show specific gene mutations , which can help predict the range of organ involvement such as the retina, kidney, and liver [52].
Pontocerebellar Hypoplasia Type
PCH is a group of autosomal recessive neurodevelopmental and neurodegenerative disorders with hypoplasia of the cerebellum and ventral pons, followed by atrophy. It is also characterized by variable cerebral involvement such as microcephaly, seizures, and a severe delay in cognitive and motor development, which in many cases is fatal early in life [77,78,79].
Ten different subtypes have been reported based on clinical and genetic features (i.e., PCH1-10) [80], and they are summarized in Table 1. Mutations in the following genes cause PCH because of molecular malfunctions that are important for normal development of the neurons and nonneuronal cells. Mutations in the VRK1 gene on chromosome 14q32.2 cause PCH IA (or spinal muscular atrophy with pontocerebellar hypoplasia; SMA-PCH) , in which there is spinal cord anterior horn cell degeneration [81, 82]. Mutations in the EXOSC3 gene on chromosome 9p13.2 lead to PCH1B [83]. Mutations in three genes , TSEN2, TSEN34, and TSEN54, encoding three of four subunits of the tRNA splicing endonuclease complex have been found to underlie PCH2, PCH4, and PCH5 [77]. PCH2 is characterized by cerebellar hypoplasia in which the hemispheres are more severely affected than the vermis, and in contrast to PCH I, there is no anterior horn cell degeneration in the spinal cord. These patients have other signs and symptoms such as progressive cerebral atrophy, microcephaly, dyskinesia, and seizures [77, 78]. Gene mutations affect PCH1-10, as follows: mutations in TSEN54 on chromosome 17q25.1 cause PCH2A; mutations inTSEN2 on chromosome 3p25.2 cause PCH2B; mutations in TSEN34 on chromosome 19q13.42 cause PCH2C; mutations in SEPSECS on chromosome 4p15.2 cause PCH2D (as known as progressive cerebello-cerebral atrophy; PCCA ); mutations in VPS53 on chromosome 17p13.3 cause PCH2E; and mutations in TSEN15 on chromosome 1q25 cause PCH2F [84,85,86]. PCH3 is caused by a mutation in the PCLO gene on chromosome 7q21 [87], and PCH4 is caused by a mutation in the TSEN54 gene on chromosome 17q25.1 [84]. A mutation in the TSEN54 gene on chromosome 17q25 causes PCH5, and a mutation in the gene encoding mitochondrial arginyl-tRNA synthetase (RARS2) on chromosome 6q15 causes PCH6 [88]. The gene involved in PCH7 is unknown [89, 90]. PCH8 is caused by a mutation in the CHMP1A gene on chromosome 16q24 [91]. A mutation in the AMPD2 gene on chromosome 1p13 causes PCH9 [92] and a mutation in CLP1 on chromosome 11p12 causes PCH10 [93]. Finally, loss of function of SLC25A46 causes lethal congenital pontocerebellar hypoplasia [94].
Clinically, PCH patients have hypotonia and difficulty with coordination of sucking and swallowing and problems handling their oral and respiratory secretions [95]. There are no criteria to distinguish precisely between the different subtypes based on clinical signs and symptoms, and therefore genetic testing is important. The cerebellum and pontine hypoplasia can be revealed by MRI in which the cerebellar hemispheres may be more severely affected than the midline vermis (Fig. 4).
Pontocerebellar hypoplasia . (a) Sagittal T2-weighted brain images. Sagittal image: the pons is very small but has a relative sparring bulging in its superior part. Vermis hypoplasia predominates at the inferior site. (b) Coronal T2-weighted brain images. Cerebellar hemispheric hypoplasia with vermis relatively spared leading to classic dragonfly image
Management
Treatment is symptomatic and requires the teamwork of health-care professionals. Patients with PCH need a gastrostomy tube and airway control, and they may not survive beyond 1 year of age. There is no known cure for PCH . It is important to refer families of affected children for genetic counseling.
Rhombencephalosynapsis
RES is a neurodevelopmental malformation that is characterized by midline fusion of the two cerebellar hemispheres, which is caused by failure of the midline structure development in the rhombencephalon. It is suggested that disruption of dorsoventral patterning of the rhombencephalon may cause RES [96]. RES is rare condition with unknown etiology, and the most specific and key MRI finding is agenesis or hypogenesis of the vermis, in which the cerebellar vermis is completely or partially absent with a fused cerebellar hemisphere and midline dentate nucleus [96]. RES may be associated with other cerebellar abnormalities, such as Purkinje cell heterotopias [97]. RES can be seen as an isolated cerebellar disorder or together with other developmental malformations in the nervous system or other organs. Although RES is seen most frequently in isolated form, it is a highly consistent finding in Gomez–Lopez–Hernandez syndrome (GLHS) . GLHS is also known as cerebellotrigeminal-dermal dysplasia (a neurocutaneous disorder). Isolated RES malformation should be distinguished from cerebellotrigeminal-dermal dysplasia, which presents with parietal/temporal alopecia (lack of hair), trigeminal anesthesia (loss of sensation in the face), midface hypoplasia with towering skull shape, corneal opacities, mental retardation, and short stature. RES is also associated with midline brain structural defects including absent olfactory bulbs, dysgenesis of the corpus callosum, absent septum pellucidum, and in rare patients, atypical forms of holoprosencephaly [96]. RES has also been reported in vertebral anomalies, anal atresia, cardiovascular anomalies, trachea–esophageal fistula, renal anomalies, limb defects (VACTERL) association, and hydrocephalus [26, 97,98,99,100].
Ishak et al. [96] proposed four groups based on the severity of cerebellar vermis defect: (1) mild, in which the nodulus, anterior, and posterior vermis are partially absent; (2) moderate, where there is a lack of posterior vermis with some anterior vermis but the nodulus is present; (3) severe, which is a lack of posterior and anterior vermis with the nodulus partially absent; and (4) complete, where there is a lack of the entire vermis [96]. They also divided RES-affected patients into four clinical categories using the following criteria: (1) RES in patients with GLHS, (2) RES plus at least one of the VACTERL features without scalp alopecia, (3) RES plus a focal or diffuse forebrain midline fusion defect without alopecia, and (4) RES in patients with malformations that do not fit into the categories 1–3 (with abnormal head shape, midface hypoplasia, low-set and/or posteriorly rotated ears, telecanthus, and/or hypertelorism).
Clinically, signs and symptoms in patients with the isolated form of RES are variable including developmental delay, in which motor learning and skills develop between 3 and 6 years of age, hypotonia, and ataxia [101].
Management
Treatment for RES infants is generally supportive and includes physical therapy and occupational therapy. If hydrocephalus is present in patients with RES and it is symptomatic, this can be an indication for surgical intervention with a ventriculostomy or ventricular shunt. It is important to refer families of affected children for genetic counseling.
Lissencephaly and Cerebellar Dysplasia
Lissencephaly with cerebellar hypoplasia is a neurodevelopmental malformation in which cellular migration is severely impaired. The cerebellum in patients with lissencephaly is underdeveloped with prominent vermis hypoplasia or aplasia [102,103,104,105]. Mutations in the gene encoding reelin (RELN), which is mapped on chromosome 7q22, cause lissencephaly with severe abnormalities of the cerebellum, hippocampus, and brainstem. Reelin is a large extracellular matrix-associated protein [106] that is involved in migration of neurons through binding to its receptors (very low density lipoprotein receptor [VLDLR]), the apolipoprotein E receptor 2 (ApoER2; [107,108,109]), and also α3β1 integrin and protocadherins [110]. In a mouse model of lissencephaly, mutations in RELN and DAB1 prominently cause neuronal migration defects in the brain with accompanying cerebellar hypoplasia, and there is also abnormal circuitry development [111, 112]. Mutations in reelin show abnormal developmental disorders outside the brain as well such as neuromuscular connectivity and congenital lymphedema [104]. It is also reported that mutations in α-dystroglycan may result in lissencephaly and central nervous system developmental malformations [113].
Clinically, the important approach is magnetic resonance imaging (MRI) from the cerebellum, which shows severe vermis and cerebellar hypoplasia and cerebellar peduncle malformation.
Management
Treatment of patients who have lissencephaly with cerebellar hypoplasia is supportive care and treatment of symptoms. In case of difficulties with feeding, a gastrostomy tube may be considered. If seizures are present, anti-seizure medications are administered, and in the case of hydrocephalus, shunting is performed. It is important to refer families of affected children for genetic counseling.
Dysplastic Cerebellar Gangliocytoma or Lhermitte–Duclos Disease
The first case of the LDD was reported by Lhermitte and Duclos in 1920 as a cerebellar ganglion cell tumor or dysplastic cerebellar gangliocytoma [114, 115]. LDD is a rare developmental disorder of the cerebellum and features both malformation and benign neoplasm. Most patients with LDD appear to have mutations in the phosphatase and tensin homologue (PTEN) gene [115,116,117]. Most frequently, LDD occurs in young adults in the third and fourth decades of life [118, 119]. Because LDD presents in previously healthy children with features of a unilateral cerebellar mass, the main considerations are the posterior fossa tumor and secondary hydrocephalus. LDD is not diagnosed as medulloblastoma in most patients because of differences in the age group, medical history, and unique imaging features. Neuroimaging with MRI is sufficient and important in the diagnostic process. Long-standing unilateral space-occupying skull lesions in the posterior fossa leads to thinning of the skull in the occipital region [120, 121]. Histopathological findings show dysplastic gangliocytoma of the cerebellum in front of a hamartoma lesion with widening of the molecular layer occupied by abnormal ganglion cells, absence of the Purkinje cell layer, and hypertrophy of the granular layer [122].
Clinically, patients with LDD present with headache, nausea, cerebellar signs, hydrocephalus, and increased intracranial pressure. Patients may have symptoms for many years, such as cranial nerve palsies and cerebellar symptoms, because of the slowly progressive nature of this disease [120]. LDD patients may show mental retardation . LLD is commonly associated with other congenital malformations such as familial hamartoma-neoplasia syndrome and Cowden’s disease (CD) , an inherited cancer/hamartoma syndrome involving the breast, thyroid gland, and other organs [123].
Management
Decompressive surgery for symptomatic patients is the accepted choice of treatment. The risk of performing surgery is the lack of clear tumor margins . Symptomatic and supportive treatments such as physical therapy and occupational therapy should be offered.
Summary
In this chapter, we summarized cerebellar malformations and current treatment. Treatment is in response to symptoms and requires a team of specialists, health-care professionals, and genetic counselors. Based on available knowledge and our experience, there is no curative treatment and most of the patients are managed using conservative approaches.
References
Bolduc ME, Limperopoulos C. Neurodevelopmental outcomes in children with cerebellar malformations: a systematic review. Dev Med Child Neurol. 2009;51(4):256–67.
Allen G, et al. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275(5308):1940–3.
Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–61.
Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44(2):113–28.
Hutchinson S, et al. Cerebellar volume of musicians. Cereb Cortex. 2003;13(9):943–9.
Bhatia MS, Saha R, Gautam P. Cerebellar cognitive affective syndrome: a case report. Prim Care Companion CNS Disord. 2016;18(2). doi:10.4088/PCC.15l01851
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79.
Chang C, Siao SW. Cerebellar cognitive affective syndrome: attention deficit-hyperactivity disorder episode of adolescent with cerebellar atrophy in a psychiatric ward. Kaohsiung J Med Sci. 2016;32(1):52–4.
Marien P, et al. Developmental coordination disorder: disruption of the cerebello-cerebral network evidenced by SPECT. Cerebellum. 2010;9(3):405–10.
Marko MK, et al. Behavioural and neural basis of anomalous motor learning in children with autism. Brain. 2015;138(Pt 3):784–97.
Salman MS, Tsai P. The role of the pediatric cerebellum in motor functions, cognition, and behavior: a clinical perspective. Neuroimaging Clin N Am. 2016;26(3):317–29.
Mothersill O, Knee-Zaska C, Donohoe G. Emotion and theory of mind in schizophrenia-investigating the role of the cerebellum. Cerebellum. 2016;15(3):357–68.
Minichino A, et al. The role of cerebellum in unipolar and bipolar depression: a review of the main neurobiological findings. Riv Psichiatr. 2014;49(3):124–31.
Schutter DJ. A cerebellar framework for predictive coding and homeostatic regulation in depressive disorder. Cerebellum. 2016;15(1):30–3.
Phillips JR, et al. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66.
Barkovich AJ, Millen KJ, Dobyns WB. A developmental and genetic classification for midbrain-hindbrain malformations. Brain. 2009;132(Pt 12):3199–230.
Abd-El-Barr MM, Strong CI, Groff MW. Chiari malformations: diagnosis, treatments and failures. J Neurosurg Sci. 2014;58(4):215–21.
Tubbs RS, et al. The pediatric Chiari I malformation: a review. Childs Nerv Syst. 2007;23(11):1239–50.
Marin-Padilla M, Marin-Padilla TM. Morphogenesis of experimentally induced Arnold-Chiari malformation. J Neurol Sci. 1981;50(1):29–55.
Wang J, et al. Acquired Chiari malformation and syringomyelia secondary to space-occupying lesions: a systematic review. World Neurosurg. 2016.
Fisahn C, et al. The Chiari 3.5 malformation: a review of the only reported case. Childs Nerv Syst. 2016;32(12):2317–9.
Boyles AL, et al. Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet A. 2006;140(24):2776–85.
Victorio MC, Khoury CK. Headache and Chiari I malformation in children and adolescents. Semin Pediatr Neurol. 2016;23(1):35–9.
Ejarque I, et al. Arnold-Chiari malformation in Noonan syndrome and other syndromes of the RAS/MAPK pathway. Rev Neurol. 2015;60(9):408–12.
Yu F, et al. A new case of complete primary cerebellar agenesis: clinical and imaging findings in a living patient. Brain. 2015;138(Pt 6):e353.
Poretti A, Boltshauser E, Doherty D. Cerebellar hypoplasia: differential diagnosis and diagnostic approach. Am J Med Genet C: Semin Med Genet. 2014;166C(2):211–26.
Wilkins RH. Natural history of intracranial vascular malformations: a review. Neurosurgery. 1985;16(3):421–30.
Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology. 1989;31(2):109–28.
Rao VR, Mathuriya SN. Pediatric aneurysms and vein of Galen malformations. J Pediatr Neurosci. 2011;6(Suppl 1):S109–17.
Jones BV, et al. Vein of Galen aneurysmal malformation: diagnosis and treatment of 13 children with extended clinical follow-up. AJNR Am J Neuroradiol. 2002;23(10):1717–24.
Marzban H, et al. Cellular commitment in the developing cerebellum. Front Cell Neurosci. 2014;8:450.
Millet S, et al. The caudal limit of Otx2 gene expression as a marker of the midbrain/hindbrain boundary: a study using in situ hybridisation and chick/quail homotopic grafts. Development. 1996;122(12):3785–97.
Millen KJ, et al. Neurogenetics of the cerebellar system. J Child Neurol. 1999;14(9):574–81; discussion 581–2.
Eddison M, et al. Segmental identity and cerebellar granule cell induction in rhombomere 1. BMC Biol. 2004;2:14.
Chizhikov VV, et al. The roof plate regulates cerebellar cell-type specification and proliferation. Development. 2006;133(15):2793–804.
Basson MA, Wingate RJ. Congenital hypoplasia of the cerebellum: developmental causes and behavioral consequences. Front Neuroanat. 2013;7:29.
Vermeer S, et al. Cerebellar ataxia and congenital disorder of glycosylation Ia (CDG-Ia) with normal routine CDG screening. J Neurol. 2007;254(10):1356–8.
Turkmen S, et al. Cerebellar hypoplasia, with quadrupedal locomotion, caused by mutations in the very low-density lipoprotein receptor gene. Eur J Hum Genet. 2008;16(9):1070–4.
Pearson T, et al. An intronic mutation in DKC1 in an infant with Hoyeraal-Hreidarsson syndrome. Am J Med Genet A. 2008;146A(16):2159–61.
des Portes V, et al. Specific clinical and brain MRI features in mentally retarded patients with mutations in the Oligophrenin-1 gene. Am J Med Genet A. 2004;124A(4):364–71.
Sellick GS, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36(12):1301–5.
Jaeken J, Matthijs G. Congenital disorders of glycosylation: a rapidly expanding disease family. Annu Rev Genomics Hum Genet. 2007;8:261–78.
Tentler D, et al. Deletion including the oligophrenin-1 gene associated with enlarged cerebral ventricles, cerebellar hypoplasia, seizures and ataxia. Eur J Hum Genet. 1999;7(5):541–8.
Patel S, Barkovich AJ. Analysis and classification of cerebellar malformations. AJNR Am J Neuroradiol. 2002;23(7):1074–87.
Massoud M, et al. Prenatal unilateral cerebellar hypoplasia in a series of 26 cases: significance and implications for prenatal diagnosis. Ultrasound Obstet Gynecol. 2014;44(4):447–54.
Wichman A, Frank LM, Kelly TE. Autosomal recessive congenital cerebellar hypoplasia. Clin Genet. 1985;27(4):373–82.
Osenbach RK, Menezes AH. Diagnosis and management of the Dandy-Walker malformation: 30 years of experience. Pediatr Neurosurg. 1992;18(4):179–89.
Cueva-Nunez JE, et al. Dandy-Walker variant: case report. Rev Chil Pediatr. 2016;87(5):406–10.
Klein JL, et al. Clinical and neuroimaging features as diagnostic guides in neonatal neurology diseases with cerebellar involvement. Cerebellum Ataxias. 2016;3:1.
Grinberg I, et al. Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation. Nat Genet. 2004;36(10):1053–5.
Aldinger KA, et al. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009;41(9):1037–42.
Parisi MA, Dobyns WB. Human malformations of the midbrain and hindbrain: review and proposed classification scheme. Mol Genet Metab. 2003;80(1–2):36–53.
Kim JH, et al. Impulsive behavior and recurrent major depression associated with Dandy-Walker variant. Psychiatry Investig. 2013;10(3):303–5.
Abdel Razek AA, Castillo M. Magnetic resonance imaging of malformations of midbrain-hindbrain. J Comput Assist Tomogr. 2016;40(1):14–25.
Cotes C, et al. Congenital basis of posterior fossa anomalies. Neuroradiol J. 2015;28(3):238–53.
D’Agostino AN, Kernohan JW, Brown JR. The Dandy-Walker syndrome. J Neuropathol Exp Neurol. 1963;22:450–70.
Hart MN, Malamud N, Ellis WG. The Dandy-Walker syndrome. A clinicopathological study based on 28 cases. Neurology. 1972;22(8):771–80.
Spennato P, et al. Hydrocephalus in Dandy-Walker malformation. Childs Nerv Syst. 2011;27(10):1665–81.
Nelson MD Jr, Maher K, Gilles FH. A different approach to cysts of the posterior fossa. Pediatr Radiol. 2004;34(9):720–32.
Tonni G, et al. Complete trisomy 9 with unusual phenotypic associations: Dandy-Walker malformation, cleft lip and cleft palate, cardiovascular abnormalities. Taiwan J Obstet Gynecol. 2014;53(4):592–7.
Zaki MS, et al. Dandy-Walker malformation, genitourinary abnormalities, and intellectual disability in two families. Am J Med Genet A. 2015;167A(11):2503–7.
Klein O, et al. Dandy-Walker malformation: prenatal diagnosis and prognosis. Childs Nerv Syst. 2003;19(7–8):484–9.
Sasaki-Adams D, et al. The Dandy-Walker variant: a case series of 24 pediatric patients and evaluation of associated anomalies, incidence of hydrocephalus, and developmental outcomes. J Neurosurg Pediatr. 2008;2(3):194–9.
Guibaud L, et al. Prenatal diagnosis of 'isolated' Dandy-Walker malformation: imaging findings and prenatal counselling. Prenat Diagn. 2012;32(2):185–93.
Joubert M, et al. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19(9):813–25.
Valente EM, Dallapiccola B, Bertini E. Joubert syndrome and related disorders. Handb Clin Neurol. 2013;113:1879–88.
Usta M, et al. Joubert syndrome and related disorders: a rare cause of intrahepatic portal hypertension in childhood. Eur Rev Med Pharmacol Sci. 2015;19(12):2297–300.
Sattar S, Gleeson JG. The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev Med Child Neurol. 2011;53(9):793–8.
Chizhikov VV, et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27(36):9780–9.
Spassky N, et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317(1):246–59.
Bachmann-Gagescu R, et al. The ciliopathy protein CC2D2A associates with NINL and functions in RAB8-MICAL3-regulated vesicle trafficking. PLoS Genet. 2015;11(10):e1005575.
Brancati F, et al. MKS3/TMEM67 mutations are a major cause of COACH syndrome, a Joubert syndrome related disorder with liver involvement. Hum Mutat. 2009;30(2):E432–42.
Kamdar BB, et al. Self-reported sleep and breathing disturbances in Joubert syndrome. Pediatr Neurol. 2011;45(6):395–9.
Brancati F, Dallapiccola B, Valente EM. Joubert syndrome and related disorders. Orphanet J Rare Dis. 2010;5:20.
Nag C, et al. Joubert syndrome: the molar tooth sign of the mid-brain. Ann Med Health Sci Res. 2013;3(2):291–4.
Lopez Ruiz P, et al. Uncrossed epileptic seizures in Joubert syndrome. BMJ Case Rep. 2015; 2015.
Bierhals T, et al. Pontocerebellar hypoplasia type 2 and TSEN2: review of the literature and two novel mutations. Eur J Med Genet. 2013;56(6):325–30.
Sanchez-Albisua I, et al. Natural course of pontocerebellar hypoplasia type 2A. Orphanet J Rare Dis. 2014;9:70.
Millen KJ, Gleeson JG. Cerebellar development and disease. Curr Opin Neurobiol. 2008;18(1):12–9.
Eggens VR, et al. EXOSC3 mutations in pontocerebellar hypoplasia type 1: novel mutations and genotype-phenotype correlations. Orphanet J Rare Dis. 2014;9:23.
Rudnik-Schoneborn S, et al. Extended phenotype of pontocerebellar hypoplasia with infantile spinal muscular atrophy. Am J Med Genet A. 2003;117A(1):10–7.
Renbaum P, et al. Spinal muscular atrophy with pontocerebellar hypoplasia is caused by a mutation in the VRK1 gene. Am J Hum Genet. 2009;85(2):281–9.
Wan J, et al. Mutations in the RNA exosome component gene EXOSC3 cause pontocerebellar hypoplasia and spinal motor neuron degeneration. Nat Genet. 2012;44(6):704–8.
Budde BS, et al. tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat Genet. 2008;40(9):1113–8.
Samanta D, Willis E. Intractable epileptic spasms in a patient with pontocerebellar hypoplasia: severe phenotype of type 2 or another subtype? Ann Indian Acad Neurol. 2016;19(3):385–7.
Feinstein M, et al. VPS53 mutations cause progressive cerebello-cerebral atrophy type 2 (PCCA2). J Med Genet. 2014;51(5):303–8.
Rajab A, et al. A novel form of pontocerebellar hypoplasia maps to chromosome 7q11-21. Neurology. 2003;60(10):1664–7.
Edvardson S, et al. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am J Hum Genet. 2007;81(4):857–62.
Anderson C, et al. Early pontocerebellar hypoplasia with vanishing testes: a new syndrome? Am J Med Genet A. 2011;155A(4):667–72.
Namavar Y, et al. Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet J Rare Dis. 2011;6:50.
Mochida GH, et al. CHMP1A encodes an essential regulator of BMI1-INK4A in cerebellar development. Nat Genet. 2012;44(11):1260–4.
Akizu N, et al. AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell. 2013;154(3):505–17.
Karaca E, et al. Human CLP1 mutations alter tRNA biogenesis, affecting both peripheral and central nervous system function. Cell. 2014;157(3):636–50.
Wan J, et al. Loss of function of SLC25A46 causes lethal congenital pontocerebellar hypoplasia. Brain. 2016;139:2877–90.
Christiansen S, Roos LK, Miranda MJ. Pontocerebellar hypoplasia is a rare cause of floppy infant syndrome. Ugeskr Laeger. 2015;177(40):V05150380.
Ishak GE, et al. Rhombencephalosynapsis: a hindbrain malformation associated with incomplete separation of midbrain and forebrain, hydrocephalus and a broad spectrum of severity. Brain. 2012;135(Pt 5):1370–86.
Pasquier L, et al. Rhombencephalosynapsis and related anomalies: a neuropathological study of 40 fetal cases. Acta Neuropathol. 2009;117(2):185–200.
Sukhudyan B, et al. Gomez-Lopez-Hernandez syndrome: reappraisal of the diagnostic criteria. Eur J Pediatr. 2010;169(12):1523–8.
Gomez MR. Cerebellotrigeminal and focal dermal dysplasia: a newly recognized neurocutaneous syndrome. Brain Dev. 1979;1(4):253–6.
Lopez-Hernandez A. Craniosynostosis, ataxia, trigeminal anaesthesia and parietal alopecia with pons-vermis fusion anomaly (atresia of the fourth ventricle). Report of two cases. Neuropediatrics. 1982;13(2):99–102.
Kruer MC, et al. Truncal ataxia, hypotonia, and motor delay with isolated rhombencephalosynapsis. Pediatr Neurol. 2009;41(3):229–31.
Ross ME, Swanson K, Dobyns WB. Lissencephaly with cerebellar hypoplasia (LCH): a heterogeneous group of cortical malformations. Neuropediatrics. 2001;32(5):256–63.
al Shahwan SA, Bruyn GW, al Deeb SM. Non-progressive familial congenital cerebellar hypoplasia. J Neurol Sci. 1995;128(1):71–7.
Hong SE, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26(1):93–6.
Kroon AA, et al. Lissencephaly with extreme cerebral and cerebellar hypoplasia. A magnetic resonance imaging study. Neuropediatrics. 1996;27(5):273–6.
D’Arcangelo G, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–23.
D’Arcangelo G, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24(2):471–9.
Hiesberger T, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24(2):481–9.
Trommsdorff M, et al. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97(6):689–701.
Senzaki K, Ogawa M, Yagi T. Proteins of the CNR family are multiple receptors for Reelin. Cell. 1999;99(6):635–47.
Caviness VS Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326.
Lambert de Rouvroit C, Goffinet AM. The reeler mouse as a model of brain development. Adv Anat Embryol Cell Biol. 1998;150:1–106.
Yis U. Lissencephaly with brainstem and cerebellar hypoplasia and congenital cataracts. J Child Neurol. 2015;30(5):625–6.
Klisch J, et al. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. AJNR Am J Neuroradiol. 2001;22(5):824–30.
Shinagare AB, Patil NK, Sorte SZ. Case 144: dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease). Radiology. 2009;251(1):298–303.
Zhou XP, et al. Germline inactivation of PTEN and dysregulation of the phosphoinositol-3-kinase/Akt pathway cause human Lhermitte-Duclos disease in adults. Am J Hum Genet. 2003;73(5):1191–8.
Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–8.
Roessmann U, Wongmongkolrit T. Dysplastic gangliocytoma of cerebellum in a newborn. Case report. J Neurosurg. 1984;60(4):845–7.
Vieco PT, et al. Dysplastic gangliocytoma (Lhermitte-Duclos disease): CT and MR imaging. Pediatr Radiol. 1992;22(5):366–9.
Milbouw G, et al. Clinical and radiological aspects of dysplastic gangliocytoma (Lhermitte-Duclos disease): a report of two cases with review of the literature. Neurosurgery. 1988;22(1 Pt 1):124–8.
Ashley DG, et al. Lhermitte-Duclos disease: CT and MR findings. J Comput Assist Tomogr. 1990;14(6):984–7.
Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): a malformation, hamartoma or neoplasm? Acta Neurol Scand. 2002;105(3):137–45.
Padberg GW, et al. Lhermitte-Duclos disease and Cowden disease: a single phakomatosis. Ann Neurol. 1991;29(5):517–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Marzban, A., Vafaee-shahi, M., Azarkhish, K. (2017). Clinical Aspects of the Inherited Cerebellar Malformations. In: Marzban, H. (eds) Development of the Cerebellum from Molecular Aspects to Diseases. Contemporary Clinical Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-59749-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-59749-2_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59748-5
Online ISBN: 978-3-319-59749-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)