Abstract
Cancer is a complex, often aggressive disease. As such, cancer treatment requires a diverse approach that often includes surgery, chemotherapy, radiotherapy, targeted therapy, or immunotherapy. Despite the potency of these treatments, cancer cells adapt to escape killing and survive either in their original microenvironmental niche, or as disseminated cancer cells in distant organs. Depending on tumour type and treatment modality, tumours display a variety of growth patterns, from rapid proliferation and invasion to a more controlled dormant phenotype. This dormant phenotype is characterized clinically as the asymptomatic period post therapy before relapse, and biologically by an enrichment in cancer cells that are not dividing but survive in a quiescent state, arrested in G0-G1 phase of cell cycle. Dormancy is a tumour intrinsic characteristic that corresponds to the equilibrium phase of the immune-editing hypothesis, in which tumour cells neither proliferate nor are eliminated by the immune response. In this chapter we provide an overview of anti-tumour immunity and ways in which the immune response may shape tumour dormancy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cancer immunoediting
- Immunosurveillance
- Immunotherapy
- Microenvironment
- Cancer-immune system interactions
- Immune evasion
- Tumour dormancy
- Therapy induced dormancy
- Immune checkpoint
- Checkpoint blockade
Anti-cancer Immunity: An Overview
The immune system is an intricate and organized system of cells and organs that functions to protect the body from pathogens. Healthy immunity is achieved when cells of both the innate and adaptive arms of the immune system are able to prevent disease while avoiding destruction of host tissue, or auto-immunity. This “tolerance” of self is essential in a properly functioning immune system, yet it also poses a significant challenge to mounting an immune response against cancer, which arises from self-tissues.
Despite sharing many characteristics of normal tissue, tumour cells do express and produce antigens that are recognized as foreign by the immune response. In the 1950s, Burnet and Thomas were the first to propose that the immune system is able to detect and prevent the growth of tumours; this was the cancer immunosurveillance hypothesis [1]. It took almost 50 years and the development of highly sophisticated transgenic mouse models, where select components of the immune response could be manipulated, to prove that both innate and adaptive immunity are essential to prevent a variety of tumour types. In the early 2000s, the cancer immunosurveillance hypothesis was refined and concept of cancer immunoediting emerged. This process includes three distinct phases; i) elimination, in which cancer cells are recognized and destroyed by immune cells, ii) equilibrium, in which cancer cells survive and may be recognized by the immune response but are not eliminated by them, and iii) escape, in which the immune response is no longer able to prevent cancer cell proliferation or metastasis [2], (also depicted in Fig. 1). In the equilibrium phase, a tumour microenvironment (TME) consisting of tumour cells, immune and non-immune stromal cells, and their secreted products, is established that plays a large role in dictating whether tumours will eventually escape the immune response.
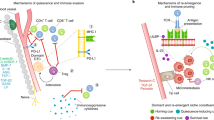
(a) Tumour burden and volume decreases following adjuvant or neoadjuvant therapy prior to tumour recurrence, a period signified as tumour dormancy. (b) Cancer stem cell like interactions with immune system. The three stages of cancer immunoediting involved in growth of clinical tumours describe the intricate relationship between a tumour mass and its infiltrating immune cells. The three phases of editing consist of eradication, equilibrium, and escape. Eradication: Highly immunogenic tumour cells are eradicated by an armamentaria of immune cells. Equilibrium: Moderately immunogenic tumour cells are partially eradicated by immune cells and some remain dormant. Evasion: Poorly immunogenic tumour cells evade immunosurveillance and invade their microenvironment
Many components of the immune system contribute to an effective anti-cancer immune response, however CD8+ cytotoxic T cells have emerged as a major driver of tumour rejection, through the direct killing of tumour cells. Induction of an effective CD8+ T cell response is a multistep process that requires coordinated interactions between numerous cell types [3, 4]. This process begins with the expression of tumour antigens that can be taken up by antigen presenting cells (APC) such as dendritic cells (DCs) and presented in the context of major histocompatibility complex (MHC). These APCs then migrate to draining lymph nodes and present the antigen to a T cell that expresses a T cell receptor (TCR) specific for that antigen-MHC complex. Effective T cell priming and activation depends on the presentation of antigen with concomitant co-stimulatory and cytokine signals, and leads to the proliferation and clonal expansion of tumour-antigen specific effector T cells. Activated T cells then travel via the bloodstream and infiltrate vascularized tumours where they recognize and kill tumour cells.
Each of these steps is carefully controlled by multiple mechanisms of immune-regulation [1, 2, 5,6,7,8,9], many of which may be co-opted by tumours enabling immune escape. Escape from equilibrium depends on both tumour intrinsic mechanisms of immune evasion and mechanisms of immunological tolerance [10, 11]. For example, tumours secrete multiple factors that have pleiotropic suppressive effects on immune cells in the TME. While cytokines and growth factors like IL-1β, GM-CSF, and VEGF have been implicated in driving the expansion of myeloid derived suppressor cells (MDSCs) within the TME that promote tumour growth [12], others like TGF-β [13] and IDO [14] secreted by DCs play important roles in the conversion of effector CD4+ T cells towards a T regulatory (Treg) cell phenotype. The accumulation of MDSCs and Treg cells within the TME is a poor prognostic indicator across multiple cancer types [15,16,17,18].
Tumour intrinsic mechanisms of immune escape also include the expression of surface molecules that interact directly with infiltrating immune cells, thereby preventing their activation or anti-tumour effector functions. The most well studied are Ig family molecules such as programmed death ligand-1 (PD-L1), which acts as an inhibitory signal when bound to its receptor, programmed cell death 1 (PD-1), expressed on activated T cells, natural killer (NK) cells, B cells and some myeloid subsets. Overall, immune escape occurs as a result of induction of potent immunosuppressive mechanisms, or through immune editing, in which the immune system kills immunogenic tumour clones effectively selecting for cancer cells that are non-immunogenic and fall “under the radar” of immune surveillance.
The clinical significance of the tumour immunosurveillance is highlighted by the increased incidence of cancer in patients undergoing immunosuppressive therapy [19, 20]. Furthermore, the effective use of immunotherapies targeting inhibitory receptors, so called checkpoint molecules, that limit T cell effector activity, have now re-established the capacity of the immune system to effectively eradicate tumours. The use of checkpoint inhibitors has led to dramatic and long-lasting clinical responses in a subset of patients with a variety of cancers, including metastatic melanoma and bladder cancer [21]. Indeed, anti-cytotoxic T lymphocyte associated protein 4 (CTLA-4) monoclonal antibodies (mAb) (ipilimumab), and anti-PD-1 mAb (pembrolizumab and nivolumab) have been approved by the FDA for use in metastatic melanoma, while the anti-PD-L1 mAb (atezolizumab) has been approved for use in metastatic bladder cancer, and numerous clinical trials are currently ongoing [11, 21]. This, together with numerous studies identifying positive associations between tumour immune infiltrates with better prognosis, highlight the importance of the immune system in regulating cancer progression [22, 23].
Dormant Tumour-Immune System Interactions
Tumour dormancy can exist as either a state in which rates of cell proliferation match those of cell death, or when tumour cells themselves are in a state of quiescence [10]. Dormant tumour cells are by default in a state of equilibrium with the immune response. In the context of the immune-editing hypothesis, tumour cells exiting dormancy will therefore be either eliminated by, or escape anti-tumour immunity. The length of the dormancy equilibrium period, signified with minimal residual diseased-state, depends on the patient and cancer type [24, 25]. Prostate [26], breast [27], melanoma [28], and non-hodgkin’s lymphoma [29] patients show relatively longer disease free periods post therapy prior to recurrence compared to higher mortality cancers of pancreas [30], brain [31], lung [32] and esophagus [33]. Importantly, although dormant tumours are in equilibrium with immune responses and tumour cells exiting dormancy must evade or trigger immune responses, the variability in dormancy periods across cancers cannot be explained by one “immune-phenotype”. Indeed, how dormant tumour cells specifically interact with immune cells at this stage remains unclear.
The value of immunity directed against cancer stem cells (CSCs) however, is an area of rapidly expanding research that may provide insight as to how dormant cells, which share many features of CSCs in terms of their microenvironmental niche and survival mechanisms [34, 35], induce or prevent immune responses. CSCs across multiple tumour types alter cell surface molecules known to inhibit both innate and adaptive anti-tumour immunity, including the anti-phagocytosis receptor CD47 [36], MHC I [37], MHC II [38], and PD-L1 [39,40,41,42]. In certain CSC types, tumour neoantigens are also expressed at lower levels compared to non-CSCs, and induce expansion of Treg cells [43]. CSCs in renal cell carcinoma have also been shown to prevent the differentiation of mature DCs [44].
Despite these immune evasion strategies, CSCs express multiple tumour associated antigens, which have been exploited as efficacious vaccine strategies in models of ovarian [45], metastatic melanoma [46, 47] and pancreatic [48] cancers. The latter study was recently expanded to a phase I clinical trial (NCI-2010-01868 and NCI-2013-02238) exploring safety and tolerability for a pancreatic cancer CSC vaccine [49]. These studies show selective depletion of CSCs in tumours after pulsing DCs with CSC-derived material, indicating that a specific T cell response can be generated against CSCs in vivo and is efficacious in reducing tumour burden. In addition to cytotoxic T cells, NK cells have also been shown to have preferential killing ability towards CSCs, which upregulate the NK cell recognition ligands MICA/B as well as the death receptors FAS and DR5 [50].
Immunotherapy for Dormant Tumours
While it remains unclear whether dormant tumour cells may share similar immune-modulatory properties as CSCs, if they do, these reports suggest that common immunotherapeutic strategies may target dormant tumour cells [51,52,53]. Certainly, reports of high expression of PD-L1 on CSCs [54] suggests that these cells could be targets of monoclonal antibody immunotherapies directed against the PD-1/PD-L1 checkpoint pathway, such as nivolumab, pembrolizumab and atezolizumab [55,56,57]. Across many solid tumour types, defining checkpoint molecule expression and immune cells in the tumour and circulation predict response to immunotherapy and/or correlate with prognosis. Multiple studies have shown greater objective responses to immunotherapies where targets, such as PD-L1, are present on tumour [58,59,60,61,62] cells. However, this is not an absolute requirement for response, and mounting evidence indicates the importance of tumour infiltrating lymphocytes (TIL) and circulating immune cell correlates in disease progression. For example, expression of PD-L1/PD-1 by circulating innate immune and T cells is a prognostic indicator for glioblastoma, pancreatic, hepatocellular and lung cancer [5, 6, 8, 9] as well as responses to checkpoint blockade with Ipilimumab [7]. Furthermore, in a study that looked at seven different tumour types, PD-L1+ TILs were strongly associated with response to anti-PD-L1 therapy [63]. Importantly however, these studies have all been conducted using sections from primary, or relapsed metastatic tumours, which cannot be defined as dormant tumours. It thus remains highly unclear whether in a dormant setting, the presence of checkpoint molecules on tumour or immune cells are similarly prognostic.
By definition, dormant tumour cells are in equilibrium with the immune response; therefore a rationally designed immunotherapeutic strategy against dormant tumours must either initiate their exit from dormancy or specifically target the unique elements of dormant tumours. Classical interventions like chemotherapy or radiation may provide the initial trigger causing tumour cells to exit the dormant phase, after which an immune response can be mounted. For example, dendritic cells increase tumour antigen presentation at low chemotherapeutic doses [64] and the abscopal effect that is observed after radiotherapy to localized tumours has been attributed to immune-mediated clearance of distant metastases [65, 66]. Chemotherapy can also have direct effects on immune cells; immunogenic drugs, such as oxaliplatin combined with cyclophosphamide, increase sensitivity of tumours to checkpoint blockade therapy [67]. Similarly, epigenetic targeting therapies are associated with upregulation of immune checkpoints. In leukemia [68, 69] and NSCLC [70], treatment with the DNA hypomethylating agent Azacitidine increases PD-1 or PD-L1 promoter demethylation and their expression. Importantly, the exit from dormancy initiated by chemo or radiotherapy is most likely associated with the release of neo-antigens and other damage-associated molecules from the tumour that trigger immune responses [71]. The importance of increasing immunogenicity of tumours is underscored by the widespread efforts to design anti-cancer vaccines [72,73,74]. These may be especially relevant in the context of more dormant tumours such as Prostate, for which the first and only cancer vaccine has been approved [75, 76].
Thus, combining immunotherapies with therapies such as chemotherapies, radiation or epigenetic therapies, that alter the neo-antigen repertoire or checkpoint expression pattern of dormant tumour cells, is a potentially promising treatment strategy.
Ultimately, anticancer immunity is a prerequisite for the successful outcome of conventional cancer therapies [65, 66, 77,78,79]. While the immune response against tumour associated antigens can be elicited by either the innate or adaptive immune systems [78, 80], the goal of active immunotherapy is to achieve anti-tumour immunity. Therefore, apart from designing comprehensive studies related to phenotyping and genotyping of dormant tumours, it is important to consider therapies or combinatorial therapies that are designed for the specific dormant cancer phenotype.
References
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3(11):991–998
Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13(2):95–109
Kim JM, Chen DS (2016) Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 27(8):1492–1504
Chen Daniel S, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39(1):1–10
Basso D, Fogar P, Falconi M, Fadi E, Sperti C, Frasson C et al (2013) Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One 8(1):e54824. PubMed PMID: PMC3554636
Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT (2013) Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res 19(12):3165–3175
Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H et al (2014) Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 9(2):e87705. PubMed PMID: PMC3912016
Waki K, Yamada T, Yoshiyama K, Terazaki Y, Sakamoto S, Matsueda S et al (2014) PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci 105(10):1229–1235. PubMed PMID: PMC4462362
Zeng Z, Shi F, Zhou L, Zhang M-N, Chen Y, Chang X-J et al (2011) Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One 6(9):e23621. PubMed PMID: PMC3164659
Romero I, Garrido F, Garcia-Lora AM (2014) Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res 74(23):6750–6757
Pitt Jonathan M, Vétizou M, Daillère R, Roberti María P, Yamazaki T, Routy B et al (2016) Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44(6):1255–1269
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I (2004) High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 64(17):6337–6343
Chen W, Jin W, Hardegen N, Lei K-J, Li L, Marinos N et al (2003) Conversion of peripheral CD4(+)CD25(−) naive T cells to CD4(+)CD25(+) regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198(12):1875–1886. PubMed PMID: PMC2194145
Mellor AL, Munn DH (2004) Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4(10):762–774
Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ (2013) The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138(2):105–115. PubMed PMID: PMC3575763
Napolitano M, D’Alterio C, Cardone E, Trotta AM, Pecori B, Rega D et al (2015) Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 6(10):8261–8270. PubMed PMID: PMC4480750
Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V et al (2012) Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol 189(12):5602–5611
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J et al (2013) Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One 8(2):e57114. PubMed PMID: PMC3577767
Engels EA, Pfeiffer RM, Fraumeni JF et al (2011) Spectrum of cancer risk among us solid organ transplant recipients. JAMA 306(17):1891–1901
Dahlke E, Murray CA, Kitchen J, Chan A-W (2014) Systematic review of melanoma incidence and prognosis in solid organ transplant recipients. Transpl Res 3(1):10
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56
Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306
Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L (2015) Natural and therapy-induced immunosurveillance in breast cancer. Nat Med 21(10):1128–1138
Quesnel B (2013) Tumor dormancy: long-term survival in a hostile environment. In: Enderling H, Almog N, Hlatky L (eds) Systems biology of tumor dormancy. Springer, New York, pp 181–200
Sosa MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14(9):611–622
Ruppender NS, Morrissey C, Lange PH, Vessella RL (2013) Dormancy in solid tumors: implications for prostate cancer. Cancer Metastasis Rev 32(3–4):501–509. doi:10.1007/s10555-013-9422-z. PubMed PMID: PMC3796576
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN (2007) Overview of resistance to systemic therapy in patients with breast cancer. In: Yu D, Hung M-C (eds) Breast cancer chemosensitivity. Springer, New York, pp 1–22
Faries MB, Steen S, Ye X, Sim M, Morton DL (2013) Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg 217(1):27–34. PubMed PMID: PMC3731060
Davis TA, Maloney DG, Czerwinski DK, Liles T-M, Levy R (1998) Anti-idiotype antibodies can induce long-term complete remissions in non-Hodgkin’s lymphoma without eradicating the malignant clone. Blood 92(4):1184–1190
Beger HG, Rau B, Gansauge F, Leder G, Schwarz M, Poch B (2008) Pancreatic cancer—low survival rates. Dtsch Ärztebl Int 105(14):255–262. PubMed PMID: PMC2696777
Walid MS (2008) Prognostic factors for long-term survival after glioblastoma. Perm J 12(4):45–48. PubMed PMID: PMC3037140
Yang P (2009) Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol (Clifton, NJ) 471:469–486. PubMed PMID: PMC2941142
D’Amico TA (2007) Outcomes after surgery for esophageal cancer. Gastrointest Cancer Res 1(5):188–196. PubMed PMID: PMC2632530
Kleffel S, Schatton T (2013) Tumor dormancy and cancer stem cells: two sides of the same coin? In: Enderling H, Almog N, Hlatky L (eds) Systems biology of tumor dormancy. Springer, New York, pp 145–179
Plaks V, Kong N, Werb Z (2015) The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16(3):225–238
Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D et al (2015) HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 112(45):E6215–E6E23. PubMed PMID: PMC4653179
Lee Y, Sunwoo J (2014) PD-L1 is preferentially expressed on CD44+ tumor-initiating cells in head and neck squamous cell carcinoma. J Immunother Cancer 2(Suppl 3):P270. PubMed PMID: PMC4292581
Jinushi M (2014) Role of cancer stem cell-associated inflammation in creating pro-inflammatory tumorigenic microenvironments. Oncoimmunology 3:e28862. PubMed PMID: PMC4091611
Bishop JL, Davies A, Ketola K, Zoubeidi A (2015) Regulation of tumor cell plasticity by the androgen receptor in prostate cancer. Endocr Relat Cancer 22(3):R165–R182
Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN et al (2015) PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget 6(1):234–242. PubMed PMID: PMC4381591
Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY et al (2016) CD44+ cells in head and neck squamous cell carcinoma suppress T-cell–mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res 22(14):3571–3581
Zhi Y, Mou Z, Chen J, He Y, Dong H, Fu X et al (2015) B7H1 expression and epithelial-to-mesenchymal transition phenotypes on colorectal cancer stem-like cells. PLoS One 10(8):e0135528. PubMed PMID: PMC4540313
Schatton T, Schütte U, Frank NY, Zhan Q, Hoerning A, Robles SC et al (2010) Modulation of t cell activation by malignant melanoma initiating cells. Cancer Res 70(2):697–708. PubMed PMID: PMC2883769
Grange C, Tapparo M, Tritta S, Deregibus MC, Battaglia A, Gontero P et al (2015) Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer 15:1009. PubMed PMID: PMC4690241
Wu D, Wang J, Cai Y, Ren M, Zhang Y, Shi F et al (2015) Effect of targeted ovarian cancer immunotherapy using ovarian cancer stem cell vaccine. J Ovarian Res 8:68. PubMed PMID: PMC4620009
Dashti A, Ebrahimi M, Hadjati J, Memarnejadian A, Moazzeni SM (2016) Dendritic cell based immunotherapy using tumor stem cells mediates potent antitumor immune responses. Cancer Lett 374(1):175–185
Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE et al (2016) Therapeutic efficacy of cancer stem cell vaccines in the adjuvant setting. Cancer Res 76(16):4661–4672
Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J et al (2009) Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 27(8):1734–1740
Lin M, Yuan Y-Y, Liu S-P, Shi J-J, Long X-A, Niu L-Z et al (2015) Prospective study of the safety and efficacy of a pancreatic cancer stem cell vaccine. J Cancer Res Clin Oncol 141(10):1827–1833
Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC et al (2015) NK cells preferentially target tumor cells with a cancer stem cell phenotype. J Immunol 195(8):4010–4019. PubMed PMID: PMC4781667
Canter RJ, Grossenbacher SK, Ames E, Murphy WJ (2016) Immune targeting of cancer stem cells in gastrointestinal oncology. J Gastrointest Oncol 7(Suppl 1):S1–S10. PubMed PMID: PMC4783622
Codony-Servat J, Rosell R (2015) Cancer stem cells and immunoresistance: clinical implications and solutions. Transl Lung Cancer Res 4(6):689–703. PubMed PMID: PMC4700228
Maccalli C, Volontè A, Cimminiello C, Parmiani G (2014) Immunology of cancer stem cells in solid tumours. A review. Eur J Cancer 50(3):649–655
Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N (2016) Immune responses to human cancer stem-like cells/cancer-initiating cells. Cancer Sci 107(1):12–17. PubMed PMID: PMC4724814
Jazirehi AR, Lim A, Dinh T (2016) PD-1 inhibition and treatment of advanced melanoma-role of pembrolizumab. Am J Cancer Res 6(10):2117–2128. PubMed PMID: PMC5088280
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A et al (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387(10031):1909–1920
Sundar R, Cho B-C, Brahmer JR, Soo RA (2015) Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 7(2):85–96. PubMed PMID: PMC4346216
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH et al (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28(19):3167–3175. PubMed PMID: PMC4834717
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH et al (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20(19):5064–5074. PubMed PMID: PMC4185001
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. PubMed PMID: PMC3544539
Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD et al (2013) Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 31(34):4311–4318. PubMed PMID: PMC3837092
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133. PubMed PMID: 23724867
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567
Shurin GV, Tourkova IL, Kaneno R, Shurin MR (2009) Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol 183(1):137–144. PubMed PMID: PMC4005417
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K et al (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8(+) T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 104(9):3360–3365. PubMed PMID: PMC1805580
Radvanyi L (2013) Immunotherapy exposes cancer stem cell resistance and a new synthetic lethality. Mol Therapy 21(8):1472–1474. PubMed PMID: PMC3740219
Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F et al (2016) Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44(2):343–354
Ørskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M et al (2015) Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: a rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 6(11):9612–9626
Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR et al (2014) Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 28(6):1280–1288
Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Pan X et al (2013) Alterations of immune response of non-small cell lung cancer with Azacytidine. Oncotarget 4(11):2067–2079
Ma Y, Conforti R, Aymeric L, Locher C, Kepp O, Kroemer G et al (2011) How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev 30(1):71–82
Kumai T, Kobayashi H, Harabuchi Y, Celis E (2017) Peptide vaccines in cancer—old concept revisited. Curr Opin Immunol 45:1–7
Thomas S, Prendergast GC (2016) Cancer vaccines: a brief overview. In: Thomas S (ed) Vaccine design: methods and protocols: vol 1: vaccines for human diseases. Springer, New York, pp 755–761
Wong KK, Li WA, Mooney DJ, Dranoff G (2016) Chapter five—advances in therapeutic cancer vaccines. In: Robert DS (ed) Advances in immunology, vol 130. Academic, New York, pp 191–249
Gulley JL, Mulders P, Albers P, Banchereau J, Bolla M, Pantel K et al (2016) Perspectives on sipuleucel-T: its role in the prostate cancer treatment paradigm. Oncoimmunology 5(4):e1107698. PubMed PMID: PMC4839373
Tse BW-C, Jovanovic L, Nelson CC, de Souza P, Power CA, Russell PJ (2014) From bench to bedside: immunotherapy for prostate cancer. Biomed Res Int 2014:981434. PubMed PMID: PMC4168152
Arenas-Ramirez N, Zou C, Popp S, Zingg D, Brannetti B, Wirth E et al (2016) Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci Transl Med 8(367):367ra166–367ra166
Spurrell EL, Lockley M (2014) Adaptive immunity in cancer immunology and therapeutics. ecancermedicalscience 8:441. PubMed PMID: PMC4096025
Yoshimoto T, Chiba Y, Furusawa J-I, Xu M, Tsunoda R, Higuchi K et al (2015) Potential clinical application of interleukin-27 as an antitumor agent. Cancer Sci 106(9):1103–1110. PubMed PMID: PMC4582978
Liu Y, Zeng G (2012) Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother 35(4):299–308. PubMed PMID: PMC3331796
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Nabavi, N., Roberts, M.E., Crea, F., Collins, C.C., Wang, Y., Bishop, J.L. (2017). Immuno-oncology of Dormant Tumours. In: Wang, Y., Crea, F. (eds) Tumor Dormancy and Recurrence. Cancer Drug Discovery and Development. Humana Press, Cham. https://doi.org/10.1007/978-3-319-59242-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-59242-8_4
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-59240-4
Online ISBN: 978-3-319-59242-8
eBook Packages: MedicineMedicine (R0)