Abstract
The high concentration of ground-level ozone is a serious problem in many areas. Ozone formation is non-linear process and is produced from the photochemical interactions of volatile organic compounds (VOCs) and oxides of nitrogen (NOx) in the troposphere. Generally, MIR (Maximum Incremental Reactivity) scale is one of the popular ones in the assessment of ozone formation potential of various VOC compounds. In this study, we have taken this scale into consideration for analysing the ranking of benzene, toluene, ethylbenzene and xylene (BTEX) at selected sites which are divided on the basis of near to traffic intersection and less vegetation (Site I) and away from traffic intersection with dense vegetation (Site II) during winter season (Nov’10–Feb’11). The results showed that the average concentration of ozone was found to be higher at Site I than Site II. As per MIR scale, irrespective of site and month, xylenes (m-/p-xylene plus o-xylene) are the most dominant contributor to ozone formation amongst BTEX. Toluene is the second largest contributor to ozone formation. Amongst all, ozone formation potential of benzene is least but it is the most hazardous and carcinogenic species amongst BTEX. Thus, this scale is very useful for examining the relative importance of VOC compounds for their role in photochemical smog formation including production of ozone.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With the increase in the rate of urbanization and industrialization all over the world, large amounts of volatile organic compounds (VOCs ) are released into the air annually (Gee and Sollars 1998; Tonooka et al. 2001; Khoder 2007). The concentration of VOCs in the air is depicted by such processes as emissions, evaporation, deposition, and photochemical reactions under the sunlight. Benzene, toluene, ethylbenzene, and isomers of xylene (Meta, Ortho, and Para—Xylene) (BTEX ) are public health concern; they are major components of automobile exhaust . VOCs are majorly responsible in the processes of smog formation and also act as precursor of ozone. Currently, traffic is a chief anthropogenic source of VOCs along with some indoor sources like tobacco smoke, household cleaning or degreasing, air freshening, domestic heating and cooking, painting, disinfecting, varnishing, etc. Amongst the BTEX compounds, benzene has been generally recognized as a human carcinogen and the others also possess high toxicity, especially to central nervous system in humans (Xianglu and Naeher 2006). Therefore, this group of VOCs has received much attention in exposure assessment studies . Control plans have been established to lower their levels in ambient air. Just like particulate matter, personal exposure of VOCs cannot be accurately estimated (Gonzalez-Flesca et al. 2000). Exposure data from stationary monitoring sites cannot give the real exposure profile in urban areas, since the level of traffic VOCs decreases drastically as the distance from the main traffic roads increases, causing high spatial variations in the distribution of VOCs. Indeed, the influence of industrial sources on VOC levels along traffic road seemed negligible (Batterman et al. 2002), indicating that air VOCs were so much limited to the small area around the source that even curb side levels were frequently found to have lower concentration of VOCs than the middle lanes of the main roads. In several studies comparing VOC exposures in various commuting modes , roughly the same conclusion was reached: private cars or taxis were exposed to higher levels of VOCs than buses or trains (Chan et al. 2003; Lau and Chan 2003). Such factors as traffic density, wind, temperature, and city buildings make the spatial variation even greater (Upmanis et al. 2001). Due to the difficulties in directly measuring these small-scale spatial variations, it is a promising job to investigate whether it is feasible to find some traffic indicators as surrogates for traffic-related VOCs and NO2 exposures . Some good results have been produced but their potential for generalization needs to be validated in further studies (Carr et al. 2002). In one study, traffic volume and the percentage of traffic jam were able to account for 0.76–0.80 of the variability in concentration changes of benzene, toluene, and ethylbenzene. In addition, ambient VOC levels have clear seasonal variation and are higher in the winter season, as was observed in an exposure study in Greece (Kourtidis et al. 2002; Pankow et al. 2003). Traffic-related VOC pollution has frequently been demonstrated to be a more serious problem in the developing countries than in the United States and Europe, as indicated by the VOC data obtained in Karachi, Pakistan (Barletta et al. 2002), a VOC exposure study in India which gave very high levels of VOCs (Mukherjee et al. 2003), and data on BTEX ambient air levels in three cities in Southern China (Wang et al. 2002). A study in Mexico City gave different levels of benzene at different monitoring sites, some of which were rather high (Bravo et al. 1991). Ten year fixed-site monitoring data of VOCs in Mexico City (Arriaga-Colina et al. 2004) showed that total VOC levels in the city may have a decreasing trend due to the effective emission control measures though it was still higher than in many other cities in the world. A personal exposure measurement campaign carried out amongst service station attendants, street vendors, and office workers discovered that BTEX exposures amongst service station attendants were the highest (310/680/110/490 μg/m3, respectively) (Romieu et al. 1999). Study in Australia also reported very high in-vehicle benzene exposures (Duffy and Nelson 1997). A study conducted in the United States on traffic-related exposures amongst highway patrol troopers found low levels of VOC exposures (Riediker et al. 2003). It has to be pointed out that most of these monitoring data are from fixed-site monitoring, while traffic-related exposure in traffic environment may be higher and pose a more serious threat to commuters and traffic-exposed workers. BTEX compounds take part in photochemical processes , even in areas distant from primary emissions. They have a high photochemical ozone creation potential in the atmosphere. The main process of chemical removal of BTEX from the atmosphere is through reaction with the OH radical during daytime. Typical atmospheric lifetimes with respect to reaction with OH radical are 225 h (benzene), 50 h (toluene), 40 h (ethylbenzene), 20 h (o-xylene), 12 h (m-xylene), and 19 h (p-xylene) for a 24-h average OH concentration of 1 × 106 cm−3. The xylenes (m,p-xylene plus o-xylene) are reported to be the dominant contributors to ozone formation amongst BTEX (Tsujino and Kuwata 1993; Na et al. 2005). BTEX ratios can be useful as a tool to investigate photochemical processes (Tsujino and Kuwata 1993). The ratio between m,p-xylene and ethylbenzene (m,p-X/E) is used to investigate the extent of atmospheric photochemical reactivity and is a useful tool for estimating the photochemical age of an air mass (Nelson and Quigley 1984; Monod et al. 2001; Hsieh and Tsai 2003; Hsieh et al. 2011).
Delhi is one of the most polluted cities in the world due to its unrestricted growth (Khare and Kansal 2004). Urban transport, manufacturing industries, and thermal power plants (TPPs) are the major sources of anthropogenic pollution (CPCB 1995). As a consequence, the assimilative capacity of atmosphere is being stressed. To tackle the problem, a number of measures have been adopted in the past for the control of vehicular and manufacturing industry emissions . These include tightening vehicular emission limits, switching to cleaner fuels (i.e. unleaded gasoline, reduction of sulphur in diesel, reduction of benzene content in gasoline), phasing out of old vehicles and maintenance of in-use vehicles, conversion of all buses and public transport vehicles to natural gas, introduction of Metro Rail, and closing or relocating polluting industries and industries operating in non-conforming areas (Khare and Kansal 2004). However, in spite of these measures, the ambient air quality of Delhi does not comply with National Ambient Air Quality Standards (NAAQS ) (Kandlikar 2007). Emissions from diesel internal combustion engine were found to be a dominating source of VOCs in ambient air of Delhi ranging between 26 and 54% (Srivastava et al. 2005). Large number of diesel generator sets is used in Delhi in residential, commercial, and industrial areas as a backup to power supply. A recent study, although centred on urban atmosphere of Delhi, can be generalized for Indian climate, exhibit clear seasonal variations of the inter-species ratios indicating differential reactivity of the VOC species in different seasons (Srivastava 2005). Observed seasonal trends can be addressed by the seasonal characteristics of the prevailing meteorology, variations in the source strength and, most importantly, the availability of OH radical and insolation that take care of the removal process of the VOC species from the atmosphere. The meteorology in Delhi shows an explicit winter and summer characteristics. In the winter months, calm conditions and high stability of the atmosphere prevails, which hinder the pollutants from dissipating faster. Temperature inversion , which is a common phenomenon in the winter months and low mixing heights do restrict dilution process of the pollutants. Thus in the winter months, the pollutants generally show a higher level of concentration. An enhanced emission of aromatics is also reported due to cold start of gasoline powered vehicles in the winter months. In Indian cities during winter, slum dwellers ignite biomass including wood waste and other organic refuse for heating that also contribute to VOC loading of the atmosphere (Padhy and Varshney 2000). In contrast, the summer months in Delhi experience higher mixing height and an unstable atmosphere in addition to which there might be several occasions of sandstorm, locally known as andhi (Hoque et al. 2008). Meteorologically, these factors favour to better mixing and easy dissipation of the pollutants leading to their lower levels in the atmosphere. Delhi records more insolation during summers which helps in the photolysis of species like ozone, aldehydes, etc. and leading to the formation of OH radical. The reaction of terpene with ozone also leads to the formation of OH radical. Thus in the summer months, high level of OH concentration could prevail in the atmosphere of Delhi, which plays the key role in the atmospheric clean up and degradation process of the aromatic VOCs. The study shows a clear seasonal profile with a unimodal pattern for the summer months. In industrial areas, toluene and xylene profiles are bimodal indicative of enhanced evaporation of toluene from industrial units, vehicular service stations, electric motor winding, and waste decomposition at waste dumping lands around the sampling location in hotter months (Srivastava 2005). Thus, meteorological conditions and human activities are two important factors which have high impact on air quality in Delhi city. Therefore, the objectives of study are to (1) assess the concentrations of BTEX compounds at selected sites, viz., I and II in Delhi and (2) evaluate the ozone formation potential of BTEX species.
2 Materials and Methods
2.1 Study Area
Delhi is located at 28°61′N, 77°23′E, and lies in Northern India. The national capital territory (NCT) is stretched over an area of 1483 km2. Delhi is located approximately 213–305 m above sea level. Delhi stands in the middle of the Indian subcontinent, between the Himalayas and Aravalli’s range bordered by Haryana in East and by Uttar Pradesh across the river Yamuna. Yamuna enters Delhi near Palla village after covering a distance of about 400 km from its origin and exits from Delhi (NCT) at the village Jaitpur after traversing a distance of 50 km within the NCT (Report: Knowledge, Attitude, and Practice of Delhiities towards the River Yamuna). Two prominent features of Delhi’s geography are Yamuna flood plain and Delhi ridge. The ridge forms the most dominant feature in this region. Aravallis extend from Gujarat through Rajasthan to Haryana up to Delhi. The spurs of Aravallis are popularly known as ‘Delhi ridge in Delhi’, which is divided into Northern, Central, South central, and Southern ridge.
Delhi sprawls an area of 1500 km2. Its east–west length is approximately 51.9 km and the northwest width approximately 48.48 km. The spread of Delhi is somewhat circular. The transportation network in Delhi is predominately road based. Delhi has the largest road length of 1284 km/100 km2 of area in India. The road network in the city is 22,487 km long. Engineering, clothing, and commercial activities predominate although electrical goods are gaining importance. Most industries are located in the west, south, and southwest of the city. Delhi has two major thermal power plants, which are not sufficient to cater to the total power need of the city. Generator sets are thus used in commercial, residential, and industrial areas as backup power. Cooking, generator sets, various internal combustion engines, burning of organic wastes landfill, sewage treatment plants, slums, and open defecation are some of the sources of VOCs in Delhi.
2.2 Climatology of Delhi
Delhi features an atypical version of the humid subtropical climate. Summers are long and extremely hot, from early April to mid-October, with the monsoon season in between. Early March sees a reversal in the direction of wind, from the north-western direction, to the south-western. These bring the hot waves from Rajasthan, carrying sand and are a characteristic of the Delhi summer, known as ‘loo’. The months of March to May see a time of hot prickling heat. Monsoon arrives at the end of June, bringing some respite from the heat, but increasing humidity at the same time. The brief, mild winter starts in late November and peaks in January and is notorious for its heavy fog or ‘smog’ formation.
Extreme temperatures range from −0.6 °C (30.9 °F) to 46.7 °C (116.1 °F). The annual mean temperature is 25 °C (77 °F); monthly mean temperatures range from 13 °C to 32 °C (56–90 °F). The average annual rainfall is approximately 714 mm (28.1 in.), most of which is during the monsoons in July and August, the average date of the advent of monsoon winds in Delhi is 29 June. The heavy rains of the monsoon act as a ‘scrubber’.
The sampling sites, viz., I and II are located in the north part of Delhi. Site I, around 1.3 km near the Inter State Bus Terminus (ISBT), Kashmiri Gate, is a busy traffic area due to its proximity to a college, a hospital, and a shopping complex and Civil Lines Metro Station (Table 4.1). The area witnesses heavy footfall. It has the latitude of 28°40′39.55″N, longitude of 77°13′30.11″E, and altitude of 719 ft. It, being the only CNG refilling station in approximately 7 km radius, attracts a large number of vehicles for refuelling of CNG and petrol/diesel. It is open for 24 h a day. Approximately, 400–500 vehicles get refuelled every day, consuming around 4000 kg of gas and 3000–4000 L of petrol/diesel. The dominant vegetation of the nearby area are deciduous trees as Azadirachta indica (neem), Ficus religiosa (peepal), Moraceae sp. (mulberry), Mangifera indica (mango), Ficus sp. (banyan), Psidium sp. (guava), bougainvillea, Plumeria sp. (champa/frangipani), Butea monosperma (palash), etc. Site II is situated in the Northern Ridge area inside the north campus of the University of Delhi, away from traffic intersection with dense vegetation (Table 4.1), with 28°41′19.20″N latitude, 77°12′48.5″E longitude, and altitude of 728 ft. Monitoring was done in the Field Nursery of University of Delhi which is opposite the Zoology Department and close to the main road of university area. The dominant vegetation of the area are deciduous trees as Acacia arabica (Babul), Jujube sp. (Ber) Acacia sp. (VilaytiKikar), Azadirachta indica (Neem), Mangifera indica (mango), etc.
2.3 Monitoring of BTEX, NO2, and Ozone
For sampling BTEX, Organic Vapour Sampler (OVS) APM 856 (Envirotech Instruments Pvt. Ltd. India), consisting of fabricated diffusive glass sampling tubes and regulated air suctioning pump, was used. The sampling was done from November 2010 to February 2011 in every 3 days a week. The ambient air was sucked through known amount of activated charcoal (18–35 mesh size) contained in sealed glass tubes procured from Envirotech Instruments Pvt. Ltd. This method is TO-17 Method given by US EPA (1999). The activated charcoal samples were transferred from the sealed tubes into a glass vial and sealed immediately in order to prevent further adsorption of compounds. These were stored at 4 °C until analysis. To the sample vial, 2 mL of carbon disulphide was added and shaken gently for 40 min (Chan et al. 1991). Carbon disulphide was filtered out with Teflon syringe filter (0.22 μm).
Analysis was done by GC–FID (Shimadzu, GC-2010) equipped with Omega SPTm column (30 m × 0.25 mm id). GC was programmed for 50 °C, held for 2 min, and ramped to 260 °C at a rate of 10 °C/min with 12 min held at 230 °C. Nitrogen was used as carrier gas with flow rate of 1.21 mL/min and split ratio of 1:10. The standard calibration mixture containing benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene procured from Supelco was used for calibration. Calibration standards were prepared by diluting the stock standard mixture. The quality assurance and quality control (QA/QC) measures included laboratory and field blank and replicates were taken for measurements of samples. For laboratory blank, unexposed charcoal tubes were analysed for VOCs similar to the exposed ones.
For monitoring ozone in ambient air, a chemical method, known as Method 411 developed by the Central Pollution Control Board, was used (CPCB Report 2011). Ozone was determined quantitatively by passing the gas through a neutral solution of potassium iodide and titrating the free iodine with standardized sodium thiosulphate .
3 Results and Discussion
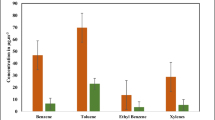
The daily average concentration of BTEX during winter season (Nov–Feb) at both the selected sites, viz., I and II is shown in Fig. 4.1. It has been clearly depicted that m,p-xylene and toluene were the most abundant BTEX compounds at both the sites. The daily average mean concentrations at Site I for benzene was 0.34 ± 0.11 μg/m3, 1.23 ± 0.45 μg/m3 for toluene, 1.11 ± 0.14 μg/m3 for ethylbenzene, 3.01 ± 0.23 μg/m3 for o-xylene, and 2.22 ± 0.42 μg/m3 for m,p-xylene while at Site II, benzene was 0.11 ± 0.21 μg/m3, 0.66 ± 0.17 μg/m3 for toluene, 0.22 ± 0.07 μg/m3 for ethylbenzene, 1.35 ± 0.32 μg/m3 for o-xylene, and 0.87 ± 0.55 μg/m3 for m,p-xylene. This is because of the reason that Site I is CNG/petro/diesel refuelling station site which is situated opposite the hospital, adjacent to Civil Lines metro station, nearby traffic intersection zone, i.e. ISBT and surrounded by so many small eateries. All these factors favoured high concentrations of air pollutants whereas Site II is away from traffic intersection area with dense vegetation; therefore, less concentration of pollutants were expected. In addition to that, the daily mean benzene concentration during winter season at Site I (0.34 ± 0.11 μg/m3) did not exceed the annual threshold concentration (5 μg/m3) set by the European Union (European Commission 2013). Benzene is a carcinogenic compound causing leukaemia. The US EPA (2005), through cancer risk analysis, estimates that an individual exposed to benzene levels between 0.13 and 0.45 μg/m3 for 70 years has a cancer risk probability of 1/1,000,000. Exposure levels between 1.3 and 4.5 μg/m3 raise the risk to 1/100,000 and between 13 and 45 μg/m3, the risk of getting cancer, especially leukaemia, rises to 1/1000. Using the unit risk factor from the WHO (2000), in a polluted and populated city like Delhi, average benzene levels of 0.34 μg/m3, about 22 additional cases of leukaemia would be expected in the city over a 70-year period.
From Fig. 4.2 , it has been shown that higher daily mean concentrations of ozone of 45.58 ± 0.11 μg/m3 were found at Site I as compared to Site II where ozone concentration was found to be lesser, i.e. 22.87 ± 0.01 μg/m3.
3.1 Ranking of BTEX Compounds with Respect to Ozone Formation Potential and Reactivity with OH
Each element in BTEX compounds plays different roles in photochemical smog formation (Carter 1990), especially in the production of ozone (Carter 1994). Ozone formation potential (OFP ) is the most widely used tool for describing the maximum ozone formation capacity in cities where ozone formation is VOC sensitive. It can be evaluated using the maximum incremental reactivity (MIR ) developed by Carter (1994). MIR is a popular scale in the assessment of OFP of various VOC compounds (Hung-Lung et al. 2007). Carter’s MIR is the amount of ozone formed when 1 g of VOC is added to an initial VOC–NOx mixture under relatively high NOx conditions, indicating how much a compound may contribute to the ozone formation in the air mass (Carter 1994). The reactivity of VOC with OH radical determines the ability of the hydrocarbon to form higher oxidized products such as ketones, acids, aldehydes, and organic peroxy radicals. The MIR coefficients were taken from Atkinson (1997) and Carter (1990, 1994) and rate constants of BTEX-OH reactions from Jenkin et al. (2003). The ranking of the BTEX species according to their concentrations, OFP, and reaction with OH during winter season at different sites is given in Tables 4.1 and 4.2. Based on the MIR scale, m,p-xylenes were the biggest contributors to ozone formation followed by o-xylene and toluene, whereas benzene was the lowest contributor during the different seasons and period of study. This is in accordance with Na et al. (2005) and Grosjean et al. (1998), who found that m,p-xylene and o-xylene were the dominant contributor to ozone formation amongst BTEX at Seoul and Porto Alegre, respectively. In the present study, higher o-xylene contribution was found at Site I as compared to Site II. Reaction of BTEX with OH radical, which leads to the formation of oxidized products, followed a similar pattern to that found in the contribution of BTEX to ozone formation at different sites during winter season. The pattern was o-xylene > m,p-xylenes > toluene > ethylbenzene > benzene. In addition, the highest o-xylene contribution was found at both the selected sites.
4 Conclusion
BTEX compounds and ozone were measured during winter season from November 2010 to February 2011 at two different selected sites categorized on the basis of near to traffic intersection area and less vegetation (Site I) and away from traffic intersection area with dense vegetation area (Site II). Site I had reported higher concentrations of BTEX compounds and ozone as compared to Site II. Monthly variation of BTEX concentrations and their potential for ozone formation have been evaluated and discussed. It was found that o-xylene was most abundant amongst BTEX compounds, followed by m,p-xylene, toluene, ethylbenzene, and benzene at both the sites during the study period. Ozone formation potentials for BTEX species were estimated using MIR. Xylenes were the dominant contributor to ozone formation. Benzene had the lowest potential for the formation of ozone. Finally, the annual average benzene level in the study did not exceed the annual limit value set by the European Union but still represents a risk to human health. For a city such as Delhi of 0.34 μg/m3, about 22 additional cases of leukaemia would be expected in the city over a 70-year period using the unit risk factor of 6 × 10−6 (μg/m3)−1 recommended by the WHO (2000).
References
Arriaga-Colina JL, West JJ, Sosa G et al (2004) Measurements of VOCs in Mexico City (1992–2001) and evaluation of VOCs and CO in the emissions inventory. Atmos Environ 38:2523–2533
Atkinson R (1997) Gas-phase tropospheric chemistry of volatile organic compounds 1. Alkanes and alkenes. J Phys Chem 26:215–290
Barletta B, Meinardi S, Simpson IJ et al (2002) Mixing ratios of volatile organic compounds (VOCs) in the atmosphere of Karachi, Pakistan. Atmos Environ 36:3429–3443
Batterman SA, Peng CY, Braun J (2002) Levels and composition of volatile organic compounds on commuting routes in Detroit, Michigan. Atmos Environ 36:6015–6030
Bravo HA, Camancho RC, Roy-Ocotla GR et al (1991) Analysis of the change in atmospheric urban formaldehyde and photochemical activity as a result of using methyl-t-butyl-ether (MTBE) as an additive in gasoline of the metropolitan area of Mexico City. Atmos Environ 25B:285–288
Carr D, Van EOWS, Wagner C et al (2002) Modeling annual benzene, toluene, NO2, and soot concentrations on the basis of road traffic characteristics. Environ Res 90:111–118
Carter WPL (1990) A detailed mechanism for the gas-phase atmospheric reaction of organic compounds. Atmos Environ 24A:481–518
Carter WPL (1994) Development of ozone reactivity scales for volatile organic compounds. J Air Waste Manage Assoc 44:881–899
Central Pollution Control Board (CPCB) (1995) Annual report 1993/94. Central Pollution Control Board, Ministry of Environment & Forests, Government of India, New Delhi, 154
Central Pollution Control Board (CPCB) (2011) Status of the vehicular pollution control programme in Delhi, 1–131
Chan CC, Xi CM, Liu JM (1991) Driver exposure to volatile organic compounds, CO, ozone, and NO2 under different driving conditions. Environ Sci Technol 25:964–972
Chan LY, Lau WL, Wang XM, Tang JH (2003) Preliminary measurements of aromatic VOCs in public transportation modes in Guangzhou, China. Environ Int 29:429–435
Duffy BL, Nelson PF (1997) Exposure to emissions of 1, 3-butadiene and benzene in the cabins of moving motor vehicles and buses in Sidney, Australia. Atmos Environ 31:3877–3885
European Commission, Environment (2013) http://www.ec.Europa.eu/environment /air/quality/standards.htm. Accessed 23 May 2013
Gee IL, Sollars CJ (1998) Ambient air levels of volatile organic compounds in Latin American and Asian cities. Chemosphere 36:2497–2506
Gonzalez-Flesca N, Bates MS, Delmas V, Cocheo V (2000) Benzene exposure assessment at indoor, outdoor, and personal levels. The French contribution to the life MACBETH programme. Environ Monit Assess 65:59–67
Grosjean E, Rasmussen RA, Grosjean D (1998) Ambient levels of gas phase pollution in Porto Alegre, Brazil. Atmos Environ 32:3371–3379
Hoque RR, Khillare PS, Agarwal T et al (2008) Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, India. Sci Tot Environ 392:30–40
Hsieh CC, Tsai JH (2003) VOC concentration characteristics in southern Taiwan. Chemosphere 50:545–556
Hsieh LT, Wang YF, Yang HH, Mi HH (2011) Measurements and correlations of MTBE and BETX in traffic tunnels. Aerosol Air Qual Res 11:763–775
Hung-Lung C, Jiun-Horng T, Shih-Yu C, Kuo-Hsiung L, Sen-Yi M (2007) VOC concentration profiles in an ozone non-attainment area: a case study in an urban and industrial complex metroplex in southern Taiwan. Atmos Environ 41:1848–1860
Jenkin ME, Saunders SM, Wagner V, Pilling MJ (2003) Protocol for the development of the master chemical mechanism, MCMv3 (part B): tropospheric degradation of aromatic volatile organic compounds. Atmos Chem Phys 3:191–193
Kandlikar M (2007) Air pollution at a hotspot location in Delhi: detecting trends, seasonal cycles and oscillations. Atmos Environ 41:5934–5947
Khare M, Kansal A (2004) Sectoral analysis of air pollution control in Delhi. In: Elsom DM, Longhurst JWS (eds) Regional and local aspects of air quality management. WIT Press, Southampton, pp 193–222
Khoder MI (2007) Ambient levels of volatile organic compounds in the atmosphere of Greater Cairo. Atmos Environ 41:554–566
Kourtidis KA, Ziomas I, Zerefos C et al (2002) Benzene, toluene, ozone, NO2 and SO2 measurements in an urban street canyon in Thessaloniki, Greece. Atmos Environ 36:5355–5364
Lau WL, Chan LY (2003) Commuter exposure to aromatic VOCs in public transportation modes in Hong Kong. Sci Tot Environ 308:143–155
Monod A, Sive BC, Avino P et al (2001) Monoaromatic compounds in ambient air of various cities: a focus on correlations between the xylenes and ethylbenzene. Atmos Environ 35:135–149
Mukherjee AK, Bhattacharya SK, Ahmed S et al (2003) Exposure of drivers and conductors to noise, heat, dust and volatile organic compounds in the state transport special buses of Kolkata city. Trans Res Part D Trans Environ 8:11–19
Na K, Moon KC, Kim YP (2005) Source contribution to aromatic VOC concentration and ozone formation potential in the atmosphere of Seoul. Atmos Environ 39:5517–5524
Nelson PF, Quigley SM (1984) The hydrocarbon composition of exhaust emitted from gasoline fueled vehicles. Atmos Environ 18:79–87
Padhy PK, Varshney CK (2000) Total non-methane volatile organic compounds (TNMVOC) in the atmosphere of Delhi. Atmos Environ 34(4):577–584
Pankow JF, Luo W, Bender DA et al (2003) Concentrations and co-occurrence correlations of 88 volatile organic compounds (VOCs) in the ambient air of 13 semi-rural to urban locations in the United States. Atmos Environ 37:5023–5046
Riediker M, Williams R, Devlin R, Griggs T, Bromberg P (2003) Exposure to particulate matter, volatile organic compounds, and other air pollutants inside patrol cars. Environ Sci Technol 37:2084–2093
Romieu I, Ramirez M, Maneses F et al (1999) Environmental exposure to volatile organic compounds among workers in Mexico City as assessed by personal monitors and blood concentrations. Environ Health Pers 107:511–555
Srivastava A (2005) Variability in VOC concentrations in an urban area of Delhi. Environ Monit Assess 107:363–373
Srivastava A, Joseph AE, Patil S et al (2005) Air toxics in ambient air of Delhi. Atmos Environ 39:59–71
Tonooka Y, Kannari A, Higashino H, Murano K (2001) NMVOCs and CO emission inventory in East Asia. Water Air Soil Pollut 130:1–4
Tsujino Y, Kuwata K (1993) Sensitive flame ionization detector for the determination of trace of atmospheric hydrocarbons by capillary gas chromatography. J Chromatogr 642:383–388
Upmanis H, Eliasson I, Andersson-Sköld Y (2001) Case studies of the spatial variation of benzene and toluene concentrations in parks and adjacent built-up areas. Water Air Soil Pollut 129:61–81
US EPA (1999) Compendium method TO-17, EPA/625/R-96/010b
US EPA (2005) Guidelines for carcinogenic risk assessment. Risk Assessment Forum, EPA/630/P-03/001F, March 2005
Wang XM, Sheng GY, Fu JM et al (2002) Urban roadside aromatic hydrocarbons in three cities of the Pearl River Delta, People’s Republic of China. Atmos Environ 36:5141–5148
WHO (2000) Air quality guidelines for Europe, 2nd edn. World Health Organisation, Copenhagen
Xianglu H, Naeher LP (2006) A review of traffic-related air pollution exposure assessment studies in the developing world. Environ Int 32(1):106–120
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Saxena, P., Ghosh, C. (2018). Ranking of BTEX with Respect to Ozone Formation by Development of Ozone Reactivity Scale. In: Jindal, T. (eds) Paradigms in Pollution Prevention. SpringerBriefs in Environmental Science. Springer, Cham. https://doi.org/10.1007/978-3-319-58415-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-58415-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58414-0
Online ISBN: 978-3-319-58415-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)