Abstract
Here I briefly review the major stress system of the body, an intrinsic stress system in the cochlea, how stress can be recognized in laboratory animals, and how stress can be induced in animals. Then I discuss the effects of stress on the cochlea and the auditory central nervous system. This leads to the examination of animal models of stress that is causal to or exacerbates tinnitus and briefly also how tinnitus may cause stress. I end with a discussion of behavioral tests that are used to decide whether animals have tinnitus. I also suggest that these various test procedures may cause or exaggerate signs of tinnitus.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Stress
“Stress” can be divided into different levels as “good stress,” “tolerable stress,” and “toxic stress.” Good stress is “characterized by moderate, short-lived increases in heart rate, blood pressure, and stress hormone levels.” Tolerable stress “refers to a physiological state that could potentially disrupt brain architecture, e.g., through cortisol-induced disruption of neural circuits or neuronal death in the hippocampus.” Toxic stress “refers to strong, frequent, and/or prolonged activation of the body’s stress-response systems. The defining characteristic of toxic stress is that it disrupts brain architecture, and affects other organ systems” (Shonkoff et al. 2009).
A stressful situation activates three major systems in the brain that regulate bodily functions. The first of these systems is the voluntary nervous system, which activates the motor system to, e.g., allow behavioral response to auditory information. The second is the autonomic nervous system, which responds to emergencies. The third system is the neuroendocrine system, which maintains the body’s internal functioning and consists of a set of cells secreting amine- and peptide-based hormones/transmitters (Toni 2004). These “stress hormones” are transported through the bloodstream and stimulate the release of other hormones. Major stress hormones are adrenaline and cortisol. When the body is exposed to stressors, adrenaline is quickly released into the bloodstream to put the body into a general state of arousal and enable it to cope with a challenge. The adrenal glands secrete glucocorticoids, i.e., hormones that affect glucose metabolism. In humans, the main glucocorticoid is cortisol, whereas in common animal models such as rodents, it is corticosterone. Glucocorticoids help to mediate the stress response, and some of its slower actions counteract the primary response to stress and help reestablish homeostasis (Brain facts 2015).
5.2 Hypothalamic-Pituitary-Adrenal Axis and the Auditory System
The hypothalamic-pituitary-adrenal (HPA) axis is part of the neuroendocrine system and is the major stress-response system of the body (Fig. 5.1). In response to stress, the hypothalamus releases corticotropin-releasing factor (CRF), which travels via blood circulation to the pituitary, where it binds to its receptor and produces adrenocorticotropic hormone (ACTH). ACTH is then secreted into the systemic blood circulation and travels to the adrenal cortex, where it binds to the melanocortin receptor 2 (MCR2) to stimulate the production and release of glucocorticoids (Toni 2004; Mazurek et al. 2012). HPA-induced glucocorticoids affect their target tissue trough the glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs). GRs are nearly expressed everywhere in the body; however, expression of MR receptors is restricted to selected tissues including the brain and pituitary gland (providing feedback; Fig. 5.1), the eye, kidney, and the inner ear. Mice demonstrate the highest expression level of MR mRNA in the inner ear, as compared to other tissues. MRs regulate the ionic and water transports resulting in the reabsorption of sodium and an excretion of potassium (Basappa et al. 2012; Mazurek et al. 2012).
Simplified representation of the classic hypothalamic–pituitary–adrenal (HPA) axis. ACTH adrenocorticotropic hormone, CRF corticotropin-releasing factor. Redrawn and simplified from Basappa et al. (2012)
The HPA axis involves adaptation to increased demands and maintains homeostasis after stressful challenges, but it also supports normal physiology and homeostasis (Canlon et al. 2007). The overall function of the HPA axis is controlled by several negative feedback loops (Fig. 5.1). A dysfunctional HPA axis is associated with manifestations of psychosomatic and psychiatric disorders. Hyperactivity of the HPA axis is often found in major depression and is associated with increased susceptibility to infection and cardiovascular problems (McEwen 2007). The glucocorticoid receptors, affecting the main targets of the HPA axis, are important regulators for protecting against noise trauma (Canlon et al. 2007).
5.3 Recognizing Stress in Animals
Stress does not occur unless the animal perceives a threat (Carstens and Moberg 2000). Because most stressors are brief, the changes in biological function required to cope with the stressor are minimal and of little consequence to the animal’s well-being (Carstens and Moberg 2000). These brief stressors include stressors associated with the experimental manipulation and handling of the animal (Carstens and Moberg 2000). According to Bali and Jaggi (2015), stress in animals may be assessed (1) at the behavioral level reflected in social interaction, (2) at the biochemical level by measuring plasma corticosterone and ACTH, and (3) at the physiological level by measuring food intake and body weight. Carstens and Moberg (2000) pointed out that the stressor responses of the HPA axis provide an example of the difficulty encountered in measuring stress: “Measuring the secretion of the glucocorticosteroids—cortisol (primates) and corticosterone (rodents)—has been the most popular tool for evaluating stress, and frequently increases in circulating glucocorticosteroids have been used as proof of stress. It is evident that numerous stressors do elicit an increase in circulating steroids but not all stressors elicit an HPA response” (Carstens and Moberg 2000).
5.4 Causing Stress in Animals
The well-described stress models used in research include immobilization, restraint, electric foot shock, and social isolation (Bali and Jaggi 2015). We will first describe restraint stress and foot-shock stress, as they are most used in auditory research. However, we should also realize that noise exposure in itself is a stressor associated with increase in plasma norepinephrine levels in awake animals (Muchnik et al. 1998).
5.4.1 Restraint Stress
Restraint stress is a form of immobilization stress in which animals are not allowed to move for a specified period of time. Restraint stress is induced by placing the test animal in a plastic tube or wire-mesh container. This does allow limb movement but limits the range of overall movement. Based on neural and endocrine responses, restraint stress appears to be less intense than immobilization. Restraint stress is a commonly employed model for the induction of acute as well as chronic stress in rodents (Bali and Jaggi 2015).
5.4.2 Electric Foot Shock-Induced Stress
The electric foot shock paradigm mainly comprises acute or chronic exposures of foot shocks with variable intensity and duration on an electrified grid floor in an electric foot shock apparatus. Electric foot shocks are an integral part of classical conditioning tests used to assess the presence of tinnitus in animals (Sect. 5.8). It is generally not appreciated that foot shocks cause acute stress and may affect the very thing the procedure aims to measure. As Bali and Jaggi (2015) noted: “Electric foot shock stressor includes both physical as well as emotional components and it is used as direct (physical stress) and indirect stressor (psychological stress). It has been mainly used with varying degree to produce mild as well severe stress of both acute and chronic in nature.”
5.4.3 Noise-Induced Stress
Chronic noise-induced activation of the HPA axis might cause a variety of problems because of abnormally high levels of circulating stress hormones. The auditory system connects via the amygdala and other circuits to the HPA axis and can thereby cause the release of stress-related hormones (Fig. 5.2). Henkin and Knigge (1963) suggested that noise-induced corticosterone elevations persist for up to ~12 h after stress induction. Noise-induced stress can affect arousal and startle responses, the latter being more and more used in the gap-startle test for tinnitus in animals. We will expand on this in Sect. 5.8.
Schematic of the various interconnections between the auditory system and the structures involved in noise-induced arousal, startle, and stress. The green boxes and lines represent the auditory system. The purple boxes and lines the HPA stress system. The orange boxes and lines represent the arousal and startle system. CN cochlear nucleus, IC inferior colliculus, SC superior colliculus, HPC hippocampus, NAc nucleus acumbens, PPT pedunculopontine tegmental nucleus, HPA hypothalamic-pituitary-adrenal axis. Modified from Eggermont (2013a)
The mechanism of noise-induced stress on the cochlea briefly can be described as (Eggermont 2013a): noise exposure activates neuroendocrine cells containing corticotropin-releasing hormone (CRH) in the hypothalamic paraventricular nucleus, which stimulates the release of ACTH in the pituitary gland (Fig. 5.1). ACTH release and the resulting secretion of corticosterone (in rodents) in the adrenal gland increase with noise intensity. The increased levels of ACTH as well as corticosterone remained elevated for the duration of noise presentation along with behavioral stress response. As we have seen, corticosterone in turn activates glucocorticoid receptors in target structures such as the inner ear (Kraus and Canlon 2012).
5.5 Stress and the Cochlea
5.5.1 The HPA Axis Signaling System
The effects of noise stress on the cochlea are well studied (Horner 2003). After exposing rats daily to 85 dB SPL white noise for 4 h on 3 consecutive days, Rarey et al. (1995) detected a significant decrease in glucocorticoid receptor protein levels in the organ of Corti, but not in the spiral ligament, together with a significant increase in serum corticosterone levels compared to nonexposed controls. Curtis and Rarey (1995) then used immobilization stress. They observed a significant quadratic trend of GR levels in spiral ligament tissues of rats restrained from 6 h daily. GR levels were elevated by day 2, and by day 21 GR levels had returned to near normal values. There was also a statistically significant decrease in the organ of Corti’s GR levels when the daily restraint stress was applied for up to 7 days, but was again no longer observed after 21 days.
There are several reports demonstrating that acute stress can protect the auditory system. Noise-induced temporary threshold shifts after noise exposure (120 dB, 20 min) were less in stressed guinea pigs than in unstressed controls (Muchnik et al., 1998). Wang and Liberman (2002) showed that two 12-h epochs of mild physical restraint significantly reduced permanent threshold shifts from a subsequent acoustic overexposure, as long as the treatment-trauma interval was short (≤2 h). The period of protection coincided with the period of elevated corticosterone levels. Thus, cochlear protective effects of sound conditioning may be mediated by stress pathways through the activation of glucocorticoid receptors in the inner ear (Canlon et al. 2007; Meltser and Canlon 2011). Kraus and Canlon (2012) summarized this as: “while there are positive correlations between stress and hearing problems, a large body of studies provides evidence that acute stress can enhance hearing or mediate protection against noise-induced hearing loss.”
Mazurek et al. (2010) examined the effect of stress on the auditory system of Wistar rats. Stress was induced by a combination of handling the animals, moving the animals to a new cage and a different room, exposed them to unpleasant sound and vibration, and restrain them. The unstressed control animals were kept in their home cage. Mazurek et al. (2010) found that such induced 24-h stress decreased auditory brainstem response (ABR) thresholds and increased ABR amplitude and strength of distortion product otoacoustic emissions (DPOAEs). The increased ABR and DPOAE amplitudes were most pronounced between 3 and 6 h post-stress, and 1 week later returned to control levels. Corticosterone and tumor necrosis factor alpha concentrations were systemically elevated in stressed animals between 3 and 6 h post-stress, pointing to the activation of the HPA axis. Expression of the HPA-axis-associated GR and hypoxia-inducible factor 1 alpha (Hif1a) genes was modulated in some auditory tissues. In the inferior colliculus (IC), Mazurek et al. (2010) found an upregulation of GR mRNA 3 h post-stress and continuous upregulation of Hif1a up to 24 h post-stress. In the spiral ganglion, there were no differences in gene expression between stressed and control animals. In the organ of Corti, no changes in the expression of GR mRNA were found; however, the expression of Hif1a was significantly downregulated 1 week after stress induction. In addition, the expression of prestin in the OHCs was significantly upregulated 6 h post-stress. Mazurek et al. (2010) concluded “that 24-h stress induces transient hypersensitivity of the auditory system and modulates gene expression in a tissue-specific manner.” Knipper et al. (2013) reported an influence of stress on the IHC synapse in rodents. Two days after stress induction, the number of release sites (ribbons) at IHC synapses was increased in animals that exhibited high corticosterone levels (Singer et al. 2013).
5.5.2 The Local Cochlear CRF-Signaling System
Vetter and colleagues (Basappa et al. 2012) recently discovered “a novel cochlear signaling system that is molecularly equivalent to the classic hypothalamic–pituitary–adrenal (HPA) axis.” This cochlear signaling system balances auditory sensitivity and susceptibility to noise-induced hearing loss and protects against metabolic insults from exposures to ototoxic drugs (Basappa et al. 2012). This local HPA system appears independent of the systemic HPA signaling to the cochlea. It consists of locally produced CRF, a CRF1-receptor, and ACTH (Fig. 5.3). Deletion of the CRF1-receptor gene resulted in auditory impairment of knockout animals (Graham and Vetter 2011). As we have seen, systemic HPA activation also influences hearing via delivery of systemic glucocorticoids through the circulation (Basappa et al. 2012).
The cochlea CRF signaling system (top) expresses an HPA-equivalent signaling system (bottom). ACTH adrenocorticothropin, CRF corticotropin-releasing factor, CRF1 and CRF2 are CRF-receptors, MC2R mineralcorticoid receptor, POMC pro-opiomelanocortin. From Basappa et al. (2012)
5.6 Stress and the Central Nervous System
Mazurek et al. (2015) emphasized that stress causes changes in neuroplasticity. Auditory neural plasticity may be defined as the dynamic changes that occur in the structural and functional characteristics of auditory neurons in response to changes in, or in the significance of, the sound they receive (Irvine 2010). Synaptic plasticity affecting the glutamate postsynaptic system and especially the AMPA and NMDA receptors appears to be regulated by stress (Hubert et al. 2014; Timmermans et al. 2013). A stressful acoustic stimulus, such as noise, causes amygdala-mediated release of stress hormones via the HPA-axis, which may have negative effects on the central nervous system (Fig. 5.2). The hippocampus can affect auditory processing by being able to mediate novelty detection. Noise exposure affects hippocampal neurogenesis and LTP in a manner that affects structural plasticity, learning, and memory (Kraus and Canlon 2012). High stress levels at the time of a moderate auditory trauma led to a “tinnitus-specific” central responsiveness, including more severe IHC ribbon loss, reduction of ABR amplitudes, and the decline of Arc/Arg3.1 expression levels in the hippocampal CA1 or auditory cortex (Singer et al. 2013). In contrast, moderate stress levels at the time of trauma could prevent such a tinnitus-specific central response and restore adaptive central responses (Knipper et al. 2013).
Nava et al. (2017) studied the time course of acute stress by foot shock on dendritic remodeling within the prelimbic (PL) region of the rodent prefrontal cortex (PFC). They analyzed dendritic length and spine density at 1 day, 7 days, and 14 days after inducing stress. At day 1, they found increased small-spine density and dendritic retraction, together with significant atrophy of apical dendrites. After 7 and 14 days recovery, complete normalization of spine density was observed. Nava et al. (2017) concluded that acute stressors may induce rapid and sustained changes of PL neurons. These changes in the PFC could affect the protective gating effect that was hypothesized to prevent tinnitus (Leaver et al. 2011; next section).
5.7 Stress and Tinnitus
Tinnitus is strongly associated with emotional stress, anxiety, and depression (Langguth 2011; Mazurek et al. 2012). Like external environmental noise, the internally generated noise of tinnitus may cause emotional distress resulting in mood disorders like depression. In turn, stress or depression may contribute to the development of tinnitus (Halford and Anderson 1991; Robinson et al. 2007; Canlon et al. 2013). Reciprocal interactions of auditory areas and areas processing emotion seem essential for tinnitus generation (Rauschecker et al. 2010; Langguth et al. 2011; Fig. 5.4). The phantom sound may be caused by disinhibition, increased spontaneous firing rates, increased neural synchronization, and tonotopic reorganization in the central auditory system (Eggermont and Roberts 2004; Roberts et al. 2010). Furthermore, since the auditory and limbic systems are interconnected, tinnitus can affect emotional as well as cognitive properties of the limbic system. In turn, the limbic system may play a role for tinnitus generation or stabilization.
Schematic of putative auditory-limbic interactions in tinnitus. Sensory input originates from the auditory midbrain (purple) and enters both auditory and limbic circuits via the medial geniculate body (MGB; yellow). Under normal circumstances, the limbic system may identify a sensory signal as perceptually irrelevant and inhibit the unwanted signal at the MGB via projections from the ventromedial prefrontal cortex (vmPFC; pink) to the auditory thalamic reticular nucleus (TRN) via the red pathway. In chronic tinnitus, inefficient vmPFC output via the red pathway prevents inhibition of the tinnitus signal at the level of the MGB, resulting in a constant perceptual presence of the tinnitus signal. Cortical structures are indicated in pink, the thalamus is indicated in yellow, the thalamic reticular nucleus in orange, the basal ganglia in light green, and the amygdala in dark green. MDN medial dorsal nucleus, VP ventral pallidum, AC auditory cortex, NAc nucleus accumbens. Based on Leaver et al. (2011)
Canlon et al. (2013) described findings in a cross-sectional study on the association of hyperacusis and stress on tinnitus, assessed by the tinnitus handicap questionnaire (THQ; Kuk et al. 1990) score. They found that the only significant predictors of mean THQ score for the left ear were hyperacusis and stress and only stress for the right ear. Canlon et al. (2013) suggested that stress seems an important predictor of tinnitus severity.
5.7.1 Stress Causing Tinnitus
Hu et al. (2010) used a mild-chronic stress model of depression in Sprague-Dawley rat and evaluated the effects with positron emission tomography (PET) imaging technique. They used both physical stressors (e.g., sleep deprivation, water deprivation, and heat stress) and psychosocial stressors (e.g., crowding, loud sound). After 4 weeks of random mixed stressing, brain PET analysis showed activation of left auditory cortex and deactivation of left inferior colliculus. No changes were detectable in the visual pathway. Changes in the auditory system correlated significantly with the depressive symptoms of experimental animals (Hu et al. 2010). Although this does not directly relates to tinnitus, the comorbidity with depressive symptoms and increased metabolic activation in auditory cortex is suggestive.
Another link to tinnitus may be found in the stress-induced increase in central opioid-dynorphin activity that potentiates the HPA-axis. Opioid peptides are found within and released from neural systems that regulate the body’s overall biologic response to physical/emotional stress. Emotional or physical stress induces potent analgesic effects, and the biologic response to stress is likely to involve multiple opioid systems (Sahley et al. 2013). Naturally occurring opioid dynorphins are also released from lateral efferent olivocochlear axons into the synaptic region beneath the cochlear IHCs during stressful episodes (Sahley and Nodar 2001). This results in altered neural excitability and/or distribution of spontaneous firing rates in type I auditory nerve fibers with low SFRs and high thresholds. Incidentally, the same subset of type I nerve fibers is affected by TTS, via the damaged ribbon synapses (Kujawa and Liberman 2009). Sahley et al. (2013) wondered if a lateral efferent olivocochlear-activated release of endogenous dynorphins may generate increased SFRs in ANFs (in the same way as induced by salicylate; Ruel et al. 2008) that could be processed by the central auditory system as either an acute subjective tinnitus. This so far remains in the domain of speculation.
5.7.2 Tinnitus Causing Stress
Recent reviews by Kraus and Canlon (2012) and Wallhäusser-Franke et al. (2012) connected nonauditory effects of noise and tinnitus respectively to the activity in the limbic system. The sensation of sound and noise, or the absence of sound, not only induces structural or functional changes in the central auditory system but can also affect limbic regions such as the amygdala and hippocampus (Fig. 5.5). The amygdala is particularly sensitive to meaningful sound, such as animal vocalizations or speech, crying, or music. As we have seen, the amygdala plays a central role in auditory fear conditioning, regulation of the acoustic startle response, and can modulate auditory cortex plasticity. A stressful acoustic stimulus, such as noise, causes amygdala-mediated release of stress hormones via the HPA-axis, which may have negative effects on health, as well as on the central nervous system. In contrast, short-term exposure to stress hormones elicits positive effects such as hearing protection. Noise exposure affects hippocampal neurogenesis and LTP in a manner that affects structural plasticity, learning, and memory. Tinnitus, typically induced by NIHL, is associated with emotional stress, depression, and anatomical changes of the hippocampus (Goble et al. 2009). In turn, the limbic system may play a role in the generation as well as the suppression of tinnitus indicating that the limbic system may be an essential target for tinnitus treatment (Eggermont 2013a).
Effects of tinnitus on limbic structures. Tinnitus, just as environmental noise, activates the amygdala, which in turn initiates stress hormone (corticosteroids such as glucocorticoids and, in animals, corticosterone) release through the limbic HPA-axis. Stress hormones as well as neuronal activity in the amygdala or auditory system affect the hippocampus by reducing neuronal activity, modifying synaptic plasticity, memory properties, and inducing long-term changes such as altered cell morphology and decrease of neurogenesis. BNST bed nucleus of stria terminalis, HPA hypothalamic-pituitary-adrenal, PFC prefrontal cortex, SAM sympathetic-adrenal-medullary. From Eggermont (2013a)
Hyperarousal also plays a role in Jastreboff’s (1990) neurophysiological tinnitus model. Besides altered activation in auditory brain regions, there is evidence that tinnitus is associated with increased activity in regions associated with emotion processing and the control of autonomic bodily functions such as the prefrontal cortex and the amygdala (Fig. 5.4; Leaver et al. 2011). This is thought to be a feature that is common to many disorders that are associated with unexplained functional somatic symptoms and that show high comorbidities with depressivity and anxiety such as tinnitus or sleep disorders (De Ridder et al. 2011).
5.8 Recognizing Tinnitus in Animals?
In my discussion of what we might actually measure in animal models of tinnitus (Eggermont 2013b), I wrote “The search for neural substrates of tinnitus requires animal models that show behavioral evidence of tinnitus under conditions similar to those that cause tinnitus in humans. Humans can tell us if they have tinnitus and can describe how loud it is, what it sounds like and whether they are bothered by it. They don’t experience it during sleep and can affect its perception by directing attention away from it (Searchfield et al. 2007); in other words tinnitus is a conscious percept (De Ridder et al. 2011).” The main question is: do animals experience tinnitus in similar ways, including cognitive and emotional aspects, and can it be demonstrated? In some behavioral test protocols, an animal is trained to respond differently to silence than to a presented sound with properties preferably similar to the expected tinnitus. Then the animal receives a tinnitus-inducing drug such as salicylate or is exposed to noise. The animal is subsequently assessed on its behavioral responses to continuous silence and external sound, the dominant idea being that tinnitus abolishes the notion of silence, i.e., the absence of an external sound.
5.8.1 Using Classical Conditioning
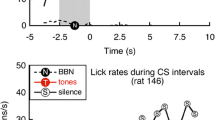
The classical behavioral techniques are based on conditioned response suppression (Estes and Skinner 1941; Fig. 5.6). Jastreboff et al. (1988a, b) introduced these tests into tinnitus research. They deprived rats of water and had them continuously engaged in licking behavior during each experimental session. A constant 24-h background noise functioned as a safe-to-drink signal. The conditioned stimulus consisted of a temporary interruption of the background noise, which was paired with a mild foot shock during the training (note the stressor!). The occurrence of silence thus slowly produced a decreased number of licks. Using this procedure, Jastreboff et al. showed that rats given salicylate after the training were less likely than control animals to stop drinking when the noise was turned off. The interpretation is that the treated animals still hear a sound when no external sound is present, i.e., they have tinnitus. Heffner and Harrington (2002) modified this procedure for use in hamsters. Bauer and Brozoski (2001) trained rats to press a lever in the presence of a 60 dB SPL broadband noise to obtain food, but they had to stop pressing the lever during silent intervals to avoid a foot shock (note the stressor!). After noise exposure, the animals were then tested by a procedure where four intervals containing a tone without shocks were presented, followed by four silent intervals where a shock was administered if the animal did not stop lever pressing. The tone was varied in frequency and intensity with the expectation that animals with tinnitus would respond differently to the tones matching the tinnitus pitch than the control animals. This modification would allow an estimate of “tinnitus pitch.”
Neural circuit for fear conditioning. The crucial point here is that the auditory cortex is not involved in generating the activity that leads to the conditioned response. This crucial role is reserved for the medial part of the medial geniculate body (MGBm)—posterior intralaminar nucleus (PIN) complex—making this behavioral response dominated by subcortical activity. CS conditioned stimulus, IC inferior colliculus, MGBm medial part of the medial geniculate body, MGBv ventral part of the medial geniculate body, PIN posterior intralaminar nucleus, US unconditioned stimulus. From Eggermont (2013b)
Another approach used a shock avoidance (note the stressor!) conditioning procedure in which rats learned to climb a pole during the presentation of a sound to avoid a foot shock. Animals could remain on the cage floor during quiet intervals when the shocks were turned off (Guitton et al. 2003). Following salicylate treatment, rats climbed the pole (false positive) during quiet, which was interpreted as evidence of tinnitus. A schedule-induced polydipsia avoidance conditioning procedure (Lobarinas et al. 2004) also associated shock avoidance (note the stressor!) behavior with the presence of sound. Animals suppressed licking during sound trials. High doses of salicylate suppressed licks-in-quiet; this was interpreted as evidence of tinnitus.
Rüttiger et al. (2003) introduced a (putatively stress-free) positive reinforcement technique in which responses made in the presence of sound were reinforced with a fluid reward, but not during quiet. Salicylates induced a high false response rate in quiet; the false alarm rate was equivalent to the access rate evoked by a 30 dB SPL broadband noise.
5.8.2 Using the Gap-Startle Response
Turner et al. (2006) introduced a completely different and potentially powerful method for tinnitus screening in rats using a modified pre-pulse inhibition of the acoustic startle reflex (Fig. 5.7). This method does not require training but can be made more sensitive by fear conditioning (note the stressor!) on the pre-pulse. The presence of a gap in a continuous acoustic background functioned as the pre-pulse and induced an inhibition or reduction of a very loud noise-burst-induced startle reflex (a putative stressor!). The authors hypothesized that if the background acoustic signal was qualitatively similar to the rat’s tinnitus, poorer detection of a silent gap in this background would be expected, and the startle reflex would not be inhibited.
Simplified pre-pulse startle response circuit. The auditory pathway is indicated with olive-colored boxes and connections. The startle circuit is indicated by pink boxes and red connections. The pre-pulse inhibition modulating circuit includes the path through the superior colliculus but could in addition be affected by the pathway and structures indicated in blue. This latter pathway inhibits the PnC and is potentially affected by auditory cortex and more directly by the MGB via the amygdala (BLA). The arrowheads indicate excitatory connections, and round-dotted endings indicate inhibitory connections. BLA basolateral amygdala, MGBm medial part of the medial geniculate body, NAc nucleus accumbens, PnC nucleus reticularis pontis caudalis, PPT pedunculopontine tegmental nucleus. From Eggermont (2013b)
Animal models of tinnitus require an unambiguous behavioral correlate of the presence of tinnitus. Various conditioned response methods and gap-startle reflex methods as described above are in use, and the outcomes generally correspond with putative electrophysiological substrates of tinnitus. However, for salicylate-induced tinnitus, there is clear discordance between the behavioral and electrophysiological test results. As a result, it is not clear if the various tests reflect tinnitus, hyperacusis, or may be just hearing loss (Eggermont 2013b).
Salloum et al. (2016) may have provided a solution to distinguish the effects of tinnitus and hyperacusis on the gap-startle. They hypothesized that hyperacusis-like enhancements of the acoustic startle response could lead to an apparent reduction of gap suppression, resembling that caused by tinnitus, by altering responses to the startle stimulus or the background noise. Salloum et al. (2016) demonstrated that besides hearing loss, also changes in sensitivity to background noise or to startle stimuli are potential confounds that, when present, can underlie changes in gap detection irrespective of tinnitus.
Could the behavioral techniques to assess tinnitus induce stress and consequently induce or exaggerate tinnitus? Looking back at the standard methods used to induce stress, handling, foot shock, noise exposure, and moving to a different cage, i.e., from cage to startle box, it should not be surprising if it did. If not all animals are similarly sensitive to these types of stress, this idea could explain why typically only ~1/3 to ½ of the animals in the gap-startle test show a “tinnitus” response. This should be investigated.
5.9 Summary
Stress induced in and auditory research context is characterized by moderate, short-lived increases in heart rate, blood pressure, and stress hormone levels. The well-described stress models used in auditory research include immobilization, restraint, and electric foot shocks. Handling is also a common source of stress in laboratory animals. Noise exposure is also a stressor associated with increase in plasma norepinephrine levels in awake animals. Stress induced by noise can protect the auditory system. The cochlea is affected by the glucocorticoids systemically released by the HPA system, but also by a local corticotropin-releasing factor signaling system. Stress may cause tinnitus, but tinnitus may in turn cause or acerbate stress.
No stress occurs unless the animal perceives a threat. Stress in animals may be assessed (1) at the behavioral level reflected in social interaction, (2) at the biochemical level by measuring plasma corticosterone and ACTH, and (3) at the physiological level by measuring food intake and body weight. Tinnitus in animals has been assessed by conditioned response suppression or by the gap-startle reflex. Both methods use stressors, i.e., foot shock and loud noise. I suggest that these may interfere with the outcome of these tests.
References
Bali A, Jaggi AS (2015) Preclinical experimental stress studies: protocols, assessment and comparison. Eur J Pharmacol 746:282–292
Basappa J, Graham CE, Turcan S, Vetter DE (2012) The cochlea as an independent neuroendocrine organ: expression and possible roles of a local hypothalamic-pituitary-adrenal axis-equivalent signaling system. Hear Res 288:3–18
Bauer CA, Brozoski TJ (2001) Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol 2(1):54–64
Brain facts (2015) A primer on the brain and nervous system. Soc Neurosci. https://www.sfn.org/public-outreach/brainfacts-dot-org
Canlon B, Meltser I, Johansson P, Tahera Y (2007) Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear Res 226:61–69
Canlon B, Theorell T, Hasson D (2013) Associations between stress and hearing problems in humans. Hear Res 295:9–15
Carstens E, Moberg GP (2000) Recognizing pain and distress in laboratory animals. ILAR J 41(2):62–71
Curtis LM, Rarey KE (1995) Effect of stress on cochlear glucocorticoid protein. II. Restraint. Hear Res 92(1–2):120–125
De Ridder D, Elgoyhen AB, Romo R, Langguth B (2011) Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA 108(20):8075–8080
Eggermont JJ (2013a) Noise and the brain. Experience dependent developmental and adult plasticity. Academic Press, London
Eggermont JJ (2013b) Hearing loss, hyperacusis, and tinnitus: what is modeled in animal research? Hear Res 295:140–149
Eggermont JJ, Roberts LE (2004) The Neuroscience of tinnitus. Trends Neurosci 27:676–682
Estes WK, Skinner BF (1941) Some quantitative properties of anxiety. J Exp Psychol 29:390–400
Goble TJ, Møller AR, Thompson LT (2009) Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res 253:52–59
Graham CE, Vetter DE (2011) The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci 31(4):1267–1278
Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003) Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci 23(9):3944–3952
Halford JB, Anderson SD (1991) Anxiety and depression in tinnitus sufferers. J Psychosom Res 35:383–390
Heffner HE, Harrington IA (2002) Tinnitus in hamsters following exposure to intense sound. Hear Res 170(1–2):83–95
Henkin RI, Knigge KM (1963) Effect of sound on the hypothalamic-pituitary-adrenal axis. Am J Phys 204:710–714
Horner KC (2003) The emotional ear in stress. Neurosci Biobehav Rev 27:437–446
Hu H, Su L, Xu YQ, Zhang H, Wang LW (2010) Behavioral and [F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression. Neuroscience 169:171–181
Hubert GW, Li C, Rainnie DG, Muly EC (2014) Effects of stress on AMPA receptor distribution and function in the basolateral amygdala. Brain Struct Funct 219:1169–1179
Irvine DRF (2010) Plasticity in the auditory pathway: structural organization of the descending auditory pathway. In: Rees A, Palmer AR (eds) The Oxford handbook of auditory science: the auditory brain, 2nd edn. Oxford University Press, New York, pp 387–415
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:228–251
Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT (1988a) Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci 102:811–822
Jastreboff PJ, Brennan JF, Sasaki CT (1988b) An animal model for tinnitus. Laryngoscope 98:280–286
Knipper M, van Dijk P, Nunes I, Rüttiger L, Zimmermann U (2013) Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol 111:17–33
Kraus KS, Canlon B (2012) Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288(1–2):34–46
Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29:14077–14085
Kuk FK, Tyler RS, Russell D, Jordan H (1990) The psychometric properties of a tinnitus handicap questionnaire. Ear Hear 11:434–445
Langguth B (2011) A review of tinnitus symptoms beyond ‘ringing in the ears’: a call to action. Curr Med Res Opin 27:1635–1643
Langguth B, Landgrebe M, Kleinjung T, Sand GP, Hajak G (2011) Tinnitus and depression. World J Biol Psychiatry 12:489–500
Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP (2011) Dysregulation of limbic and auditory networks in tinnitus. Neuron 69:33–43
Lobarinas E, Sun W, Cushing R, Salvi R (2004) A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC). Hear Res 190(1–2):109–114
Mazurek B, Haupt H, Joachim R, Klapp BF, Stöver T, Szczepek AJ (2010) Stress induces transient auditory hypersensitivity in rats. Hear Res 259(1–2):55–63
Mazurek B, Haupt H, Olze H, Szczepek AJ (2012) Stress and tinnitus-from bedside to bench and back. Front Syst Neurosci 6:47. doi:10.3389/fnsys.2012.00047
Mazurek B, Szczepek AJ, Hebert S (2015) Stress and tinnitus. HNO 63(4):258–265
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904
Meltser I, Canlon B (2011) Protecting the auditory system with glucocorticoids. Hear Res 281(1–2):47–55
Muchnik C, Rosenthal T, Peleg E, Hildesheimer M (1998) Stress reaction to intense sound exposure under different arousal levels in guinea pigs. Acta Otolaryngol 118:646–650
Nava N, Treccani G, Alabsi A, Kaastrup Mueller H, Elfving B, Popoli M, Wegener G, Nyengaard JR (2017) Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cerebral Cortex 27(1):694–705
Rarey KE, Gerhardt KJ, Curtis LM, ten Cate WJ (1995) Effect of stress on cochlear glucocorticoid protein, acoustic stress. Hear Res 82:135–138
Rauschecker JP, Leaver AM, Mühlau M (2010) Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66:819–826
Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA (2010) Ringing ears: the neuroscience of tinnitus. J Neuroscience 30(45):14972–14979
Robinson SK, Viirre ES, Stein MB (2007) Antidepressant therapy in tinnitus. Hear Res 226:221–231
Ruel J, Chabbert C, Nouvian R, Bendris R, Eybalin M, Leger CL, Bourien J, Mersel M, Puel JL (2008) Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci 28:7313–7323
Rüttiger L, Ciuffani J, Zenner HP, Knipper M (2003) A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res 180(1–2):39–50
Sahley TL, Nodar RH (2001) A biochemical model of peripheral tinnitus. Hear Res 152(1–2):43–54
Sahley TL, Hammongs MD, Musiek FE (2013) Endogenous dynorphins, glutamate and N-methyl-D-aspartate(NMDA) receptors may participate in a stress-mediated type-I auditory neural exacerbation of tinnitus. Brain Res 1499:80–108
Salloum RH, Sandridge S, Patton DJ, Stillitano G, Dawson G, Niforatos J, Santiago L, Kaltenbach JA (2016) Untangling the effects of tinnitus and hypersensitivity to sound (hyperacusis) in the gap detection test. Hear Res 331:92–100
Searchfield GD, Morrison-Low J, Wise K (2007) Object identification and attention training for treating tinnitus. Prog Brain Res 166:441–460
Shonkoff JP, Boyce WT, McEwen BS (2009) Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA 301(21):2252–2259
Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Köpschall I, Rohbock K, Varakina K, Verpoorten S, Reinbothe T, Schimmang T, Rüttiger L, Knipper M (2013) Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol 47(1):261–279
Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ (2013) Stress and excitatory synapses: from health to disease. Neuroscience 248:626–636
Toni R (2004) The neuroendocrine system: organization and homeostatic role. J Endocrinol Investig 27(6 Suppl):35–47
Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM (2006) Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120:188–195
Wallhäusser-Franke E, Brade J, Balkenhol T, D-Amelio R, Seegmüller A, Delb W (2012) Tinnitus: distinguishing between subjectively perceived loudness and tinnitus-related distress. PLoS One 7:e34583
Wang Y, Liberman MC (2002) Restraint stress and protection from acoustic injury in mice. Hear Res 165(1–2):96–102
Acknowledgments
This work was supported by the Natural Science and Engineering Research Council (NSERC) of Canada.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Eggermont, J.J. (2017). Animal Models of Stress and Tinnitus. In: Szczepek, A., Mazurek, B. (eds) Tinnitus and Stress. Springer, Cham. https://doi.org/10.1007/978-3-319-58397-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-58397-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58396-9
Online ISBN: 978-3-319-58397-6
eBook Packages: MedicineMedicine (R0)