Abstract
Pediatric sialadenitis accounts for up to 10% of all salivary gland pathology and is most commonly in the parotid gland. Many factors contribute to salivary gland disease in children such as viral parotitis and juvenile recurrent parotitis being the two most common. Other etiologies include bacterial infections, congenital or traumatic duct obstruction, autoimmune disease, and genetic defects. Viral sialadenitis is most commonly due to paramyxovirus. However, clinicians must distinguish this from a mumps infection in order to monitor for systemic complications. Juvenile recurrent parotitis is typically biphasic in age distribution, occurring between ages 2–6 and at the start of puberty. This is a clinical diagnosis aided with the use of ultrasonography, and patients must have a minimum of two episodes to be diagnosed. The specific etiology is not completely understood, although may be linked to immune deficiency, genetics, or allergy. Bacterial sialadenitis primarily occurs in children younger than 2 months and is distinguished from other diseases of the salivary gland by the presence of pus. The most common bacterial etiology is Staphylococcus areas. Progression to abscess formation, although rare, should be evaluated with ultrasound as this can lead to respiratory compromise. If an abscess is nonresponsive to antimicrobials and remains chronic, mycobacterial infection should be ruled out. Finally, salivary stones occur most frequently in the submandibular gland, are diagnosed via ultrasound, and can be successfully removed with sialendoscopy in pediatric patients.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Key Points
-
1.
In children, sialadenitis is more common in the parotid gland and most commonly caused by viral inflammation or juvenile recurrent parotitis (JRP).
-
2.
Sialolithiasis occurs in children less commonly. When present, the submandibular gland is most commonly involved.
-
3.
Sialendoscopy is a useful diagnostic and potentially therapeutic procedure in children with recurrent or refractory inflammation in the parotid or submandibular gland.
-
4.
Imaging should be limited to ultrasound, unless a tumor is expected, to avoid undue radiation exposure in children.
Introduction
Pediatric sialadenitis accounts for up to 10% of all salivary gland pathology [1]. Viral parotitis and juvenile recurrent parotitis (JRP) are the two most common etiologies. JRP is the most common inflammatory salivary gland disorder in children in the United States and is second only to mumps worldwide [2]. Many factors contribute to salivary gland disease in children, including viral or bacterial infections, congenital or traumatic duct obstruction, autoimmune disease, and genetic defects. In children, parotid sialadenitis is more common than submandibular sialadenitis. Tumors of the salivary glands are rare in children and rarely present with inflammatory symptoms. Salivary stones are a frequent cause of chronic or recurrent obstructive sialadenitis, though much less common in children than adults. Stones are much more common in the submandibular gland than parotid gland, in both populations.
The aim of this chapter is to present a comprehensive review of pathophysiology, clinical presentation, diagnosis, and treatment of pediatric salivary gland disorders and the emerging role of sialendoscopy in the treatment of these disorders.
Etiologies
Viral Sialadenitis
Viral parotitis is generally caused by the paramyxovirus . Mumps is the most common infectious inflammatory condition but has become much less common with immunization. The effectiveness of the vaccine approaches 90% [3, 4]. However, clinicians should distinguish mumps from other causes of sialadenitis in the pediatric population, as outbreaks have occurred among highly vaccinated individuals [3, 4]. Mumps is a systemic illness that infects the salivary glands without producing purulence. Prodromal symptoms include fever, headache, and malaise, with subsequent gland involvement. Additional exocrine glands can be affected, and systemic complications, such as encephalitis, are not uncommon. Serologic assays are useful in confirming the diagnosis. Other viruses (EBV, parainfluenza, HIV) are less commonly associated with salivary gland inflammation.
Bacterial Sialadenitis
Pediatric bacterial sialadenitis most commonly occurs in children younger than 2 months and is usually in the parotid gland [5, 6]. Predisposing factors for pediatric bacterial sialadenitis include chronic tonsillitis, dental abscess, and mumps parotitis [7,8,9]. In the newborn period, it usually presents as an acute single episode; however, after infancy, multiple recurrent episodes can occur and can continue into late adolescence [7, 8, 10]. Bacterial sialadenitis in neonates typically occurs within the first 2 weeks of life and, unlike adult parotitis, generally occurs bilaterally. Generally, neonatal bacterial parotitis occurs in premature infants due to the greater propensity of dehydration, duct stasis, and immune suppression [7, 8, 10].
Bacterial sialadenitis is characterized by acute swelling of the cheek that extends to the angle of the mandible . It is usually distinguished from other inflammatory diseases of the salivary gland by the presence of pus. In the absence of purulence, fever and leukocytosis support the diagnosis. Any purulence should be sent for gram stain as well as aerobic and anaerobic culture. While awaiting the culture results, antistaphylococcal penicillinase-resistant antibiotics should be started. The pathogens recovered in acute bacterial sialadenitis depend on the age group. In the neonate, Staphylococcus aureus , gram-positive cocci, and gram-negative bacilli are the predominant organisms [11, 12]. Unlike the neonate, however, children older than 1 year of age predominately grow Staphylococcus aureus, streptococcus species, and anaerobic pathogens [5, 12, 13].
Progression of bacterial sialadenitis to abscess formation, although rare, should be evaluated with imaging such as ultrasound and often occurs as a result of Streptococcus pnuemoniae [14]. Due to the vertical separation of the parotid fascia , a fluctuant mass is seldom appreciated in acute parotitis, so clinical signs such as progressive edema, induration, and sepsis are usually indicative of a parotid abscess [7]. If progression to abscess formation occurs in the submandibular gland, it may result in floor of mouth edema and respiratory compromise so attentive observation must be initiated.
Mycobacterial Infection
Mycobacterium is known to cause infections of the head and neck; however, they have rarely been reported to involve the parotid gland [6, 15]. Infection of the glandular parenchyma is usually secondarily spread from the intraglandular and periglandular lymph nodes [16]. This is due to the fact that the salivary glands are typically spared from direct mycobacterial infection because of the proteolytic enzymes with antibacterial properties and the continuous flow of saliva preventing stagnation and growth [6, 16]. A mycobacterial abscess presents as a chronic, non-tender salivary gland mass, nonresponsive to antimicrobials, and can often be indistinguishable from a neoplasm [6, 15,16,17,18]. Because of this, culture, histology, chest XR, and PPD are all used to aid in diagnosis; however, FNA proves most valuable as, histologically, granulomas will be present [6, 15, 16]. If histology proves to be noncontributory, parotidectomy is essential to differentiate this infection from other neoplasms [6, 16].
These mycobacterial infections can be caused by Mycobacterium tuberculosis (TB) as well as nontuberculous mycobacteria (NTM) such as mycobacterium avium-intracellulare [12]. In order to differentiate between TB and NTM, a Wade-Fite stain can be performed to detect differences in the glycoprotein coat [15]. It is important to differentiate TB from NTM because the management differs. A diagnosis of TB requires possible treatment of any contacts and initiation of rifampin, isoniazid, ethambutol, and pyrazinamide [15]. NTM on the other hand appears to have nonperson-to-person contact and requires azithromycin, clarithromycin, and ethambutol treatment [15]. For either case, if patients are nonresponsive to or noncompliant with treatment, surgical resection should be initiated. Cooperation between the otolaryngologist and the pediatrician is also extremely important for effective management of other organ systems as it has been reported that 25% of patients who had TB in the parotid gland had concomitant pulmonary infection [6, 15].
Juvenile Recurrent Parotitis
Juvenile recurrent parotitis (JRP) is characterized as recurrent episodes of inflammation of the parotid gland. Symptoms include jaw swelling, pain, and redness, associated with fever and malaise. Most cases are unilateral; however, when bilateral cases occur, one side is usually dominant [19, 20]. The true incidence of JRP is unknown as most reports are case series. Studies show predominance in males, though the sex distribution is thought to flip if events continue into adulthood [20,21,22]. The age distribution is biphasic, typically occurring between ages 2 and 6 and again at the start of puberty [20, 21, 23,24,25]. The natural history is recurrence; however, most authors agree that this is a self-limited disease that resolves sometime after puberty and rarely extends into adulthood [19,20,21].
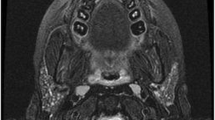
The diagnosis of juvenile recurrent parotitis is made clinically in patients with a history of recurrence and physical exam findings. More recently, ultrasonographic findings are consistently being used to make the clinical diagnosis [19] (Fig. 12.1). The minimum requirement for diagnosis is two episodes, although most patients are only diagnosed after multiple episodes have occurred [26]. Hackett et al. reported an average of 4.7 episodes with a range between two and nine events [26]. Typically, symptoms last 4 to 7 days for each episode [24]. The interval between attacks varies individually, with episodes occurring every 3–4 months to ten times per year [21, 24]. Treatment is based on the frequency and severity of disease. Early recognition of JRP and treatment of this pathology are of utmost importance to prevent further progression along the inflammatory cascade. Each attack may further tissue destruction and function of the gland. For this reason, active and early intervention when the acute inflammation subsides is prudent.
The link between genetics, immunologic disease, allergy, and sialadenitis is not completely understood. Although early studies have excluded a relationship connecting these factors, bilateral or multiglandular disease, especially in a setting of arthritis or atypical rashes, should warrant autoimmune workup and/or rheumatology referral [22, 23, 27]. Autoimmune disease is also less likely, in that autoantibodies are usually absent [23, 27]. However, others have supported such an association based on cytologic and pathologic findings of inflammation, vasculitis, tissue destruction, and stenosis [24, 28]. IgA deficiency could predispose to infection, while genetic factors influence the overall immune response [24, 29, 30].
It has been difficult to identify one specific etiology pertaining to JRP. There are case reports that link it to immune deficiency, genetics, and allergy; however, no causality has been proven because in many early, large studies of JRP, these conditions were not found to contribute to this diease [23, 27, 30,31,32]. Conventional thought had been that an ascending infection was a primary event, while the development of sialectases is a secondary change predisposing to chronic low-grade inflammation with acute exacerbations [21, 23, 27]. Now, the general consensus is that JRP is a multifactorial process that multiple factors, independently or in combination, can result in recurrent inflammation [19, 33].
Clinicians have proposed a specific sequence of events, deemed the “salivary gland inflammatory cycle ” that causes a structural change leading to the recurrent sialadenitis. Predisposing factors of the inflammatory cycle include dehydration, infection, congenital ductal abnormalities, and/or autoimmune factors [21, 23, 27]. The cycle starts with decreased salivary flow, leading to inflammation and tissue destruction. This tissue destruction would then cause ductal dysfunction, metaplasia, and increased mucinous secretion yielding mucus, debris (including desquamated cells), and stenosis [19, 21, 22, 33, 34]. Mucus plugs or stenosis would then cause post-obstructive sialectases and ultimately complete the full circle and return to decreased salivary flow [19, 21, 22, 33]. Support of this theory comes from histologic specimens showing dilated ducts (sialectases) with lymphocytic infiltration in the surrounding tissues and epithelium [23, 27]. Additional components that can result from or add to the cycle include the precipitation of proteins and calculus formation, both leading to further obstruction, decreased salivary flow, and inflammation [33].
Sialolithiasis in Children
Stones in children, as in adults, occur most frequently in the submandibular gland. In fact, 80–90% of stones in children are found in the submandibular gland [35,36,37]. Less than 5% of total cases of sialolithiasis occur in children, so most of the literature on stones pertains to adults [36, 38]. Salivary stones in pediatric cases are smaller, occur distally within the duct, and present with shorter symptom duration [38, 39]. Ultrasound is the diagnostic test of choice to avoid radiation exposure in children. A case could also be made for proceeding directly to surgical intervention in patients with recurrent postprandial pain and swelling. Sialendoscopy has a greater sensitivity than conventional radiology, ultrasound, and MRI.55 Retrospective review of 5-year experience by Martins-Carvalho et al. [20] showed that pre-sialendoscopy US was only successful in predicting pathology in seven of 38 (18%) cases. Of the ten patients with lithiasis found using sialendoscopy, only four had been detected using preoperative ultrasonography.
Clinical Presentation and Diagnosis
The most common presenting symptoms of acute sialadenitis whether due to infection or JRP are pain, fever, and erythema overlying the affected gland(s). Symptoms are usually unilateral; in bilateral cases, symptoms are more prominent on one side [5]. Pain is elicited with salivation, mastication, and/or swallowing. Trismus can be present. The ostium of the duct(s) is erythematous and edematous. Purulence and/or inspissated mucus may be expressed by manual palpation and gentle pressure applied over the salivary gland and duct. In severe cases of infectious sialadenitis, systemic complications can extend regionally into adjacent tissues (cellulitis) or systemically spread to distal sites [5]. Clinical signs vary based on the site of inflammation and an acute or chronic presentation.
Sialadenitis should be differentiated clinically from periodic sialadenosis. Sialadenosis is defined as non-painful, noninflammatory salivary gland prominence or swelling. It can be unilateral or bilateral. It can be found in pediatric patients with diabetes mellitus, insulin resistance syndrome, and bulimia. Sialadenosis management should focus on diagnosing/treating underlying conditions, ruling out underlying or occult tumors, and avoiding surgical intervention or sialendoscopy.
Reports have also described immune deficiency in association with sialadenitis. Several authors have reported IgA deficiency in patients presenting with recurrent parotitis through serology and immunofluorescent studies [29, 31, 32]. Salivary gland involvement in children with human immunodeficiency virus (HIV) is well recognized. Characteristically, one or both glands are firm, nontender, and chronically enlarged. Xerostomia may also be a presenting symptom. Infiltration of CD8-positive lymphocytes, possibly as a result of HIV, Epstein-Barr virus (EBV) , or an interaction between the two, enlarges the gland [40]. The diagnosis of HIV parotitis is usually clinical with typical findings of HIV (multiple parotid cysts).
Management
The treatment of sialadenitis is usually conservative and directed toward its etiology. Acute infections are treated with appropriate antistaphylococcal antibiotics. Viral sialadenitis, or mumps, is managed supportively, as it is a self-limited disease, and no antiviral agent is available for treatment. Sialadenitis in association with autoimmune disease, immune deficiency, and genetic factors is managed conservatively and according to the underlying systemic condition. Chronic sialadenitis and JRP have a multifactorial etiology, and management recommendations have not been uniform [19, 21, 24]. Over the last 20 years, there has been a rising interest in the surgical management of both sialolithiasis and chronic or recurrent acute sialadenitis. Many authors have contributed to the advancements of conventional surgical procedures to nonsurgical and minimally invasive procedures and the development of treatment algorithms [41].
The conservative management of acute sialadenitis consists of analgesics (NSAIDs or systemic steroids), adequate hydration, warm massage, antibiotics (when pus is identified at duct ostium), and sialogogues. The goal of these conservative measures is to provide symptomatic relief and prevent permanent parenchymal damage. Broad antimicrobial therapy is indicated to cover aerobic and anaerobic pathogens [5, 13]. Analgesics are used to provide pain relief. Both have been reported to rapidly decrease swelling and prevent damage to the parenchyma [20, 21, 38]. Rehydration is important as dehydration may exacerbate the inflammatory response [5, 21, 33]. Warm massage and sialogogues are reported to stimulate salivary flow [21, 23]. In cases where conservative management fails to resolve acute symptoms, abscess development should be suspected. CT or ultrasound should be obtained for confirmation and preoperative surgical planning. Abscess formation requires incision and drainage.
Acute infection and inflammation are relative contraindications to surgical intervention. Duct manipulation should not be performed in the setting of acute infection due to concerns about scarring, bleeding, ductal perforation, and exacerbation of the inflammatory process [5, 21]. Thus, medical therapy to decrease swelling, pain, infection, and inflammation should occur prior to surgical intervention.
Recurrent acute sialadenitis of the submandibular gland in children and JRP are far more difficult to manage. Treatment recommendations have ranged from conservative to aggressive and have been not uniformly accepted. This has been, in part, due to its scarcity, uncertain etiology, and natural history. Prevention of sialadenitis by using prophylactic antibiotics has been suggested, but there is little evidence to support this practice [21]. Some authors have suggested expectant management as many patients are known to recover spontaneously [21].
Several techniques have been advocated to control repeated attacks of inflammation. Traditional management involves gland excision, salivary gland duct ligation, blind duct dilation and lavage, and tympanic neurectomy [21, 42]. Complications include nerve damage, asymmetric scarring, hemorrhage, infection, sialocele, hematoma, wound infection, and salivary fistula [42]. Duct ligation and dilation/lavage have variable outcomes [21]. Some studies found that sialography alone resulted in beneficial clinical effects [21, 41]. Recently, there has been a paradigm shift in the management of sialadenitis and sialolithiasis toward gland preservation techniques that employ sialendoscopy.
Through the work of Nahlieli et al., Marchal et al., and that of many others, salivary endoscopy has been validated in pediatrics as a safe and efficacious tool for the diagnosis and treatment of salivary gland disorders [20, 25, 26, 33, 36, 38, 43,44,45,46,47,48]. Shacham et al., Martins-Carvalho et al., and Nahlieli et al. report the largest series of interventional pediatric sialendoscopy [20, 25, 43]. After a single procedure, they describe over 80–90% symptom resolution in 70, 38, and 23 patients, respectively. The other referenced studies describe similar success rates [25, 26, 33, 47, 48].
Direct endoscopic visualization can help identify or confirm a specific pathology. Common findings of chronic sialadenitis include a widened Stenson’s duct; white, avascular appearance of the duct; stenosis; mucus plug/debris; and salivary stones within the duct (Figs. 12.1, 12.2, 12.3, 12.4) [20, 43, 48]. Marchal and colleagues reported a 98% success rate at identifying ductal and parenchymal pathology [49]. While avascularity, debris, and salivary stones are readily visualized, stenosis is diagnosed based on narrowing of the duct under endoscopic control and difficulty introducing and mobilizing the sialendoscope [43]. Recently, duct-dilating balloons have been developed, and the authors have been using very small Fogarty balloons to dilate strictures. Sialendoscopy has been reported to have better sensitivity in diagnosing salivary stones in children than conventional radiology, CT, ultrasonography, and MRI [39, 43, 44, 50]. These same authors found smaller stones in the pediatric population, finding those missed on radiologic evaluation to be present on endoscopy.
In addition to diagnosis, interventional sialendoscopy has advanced to address the variety of factors causing sialadenitis. Inflammatory changes resulting in tissue damage, strictures, and organic debris can successfully be treated with dilation, lavage, and/or corticosteroid application [20, 25, 28, 44, 45]. Dilation of stenosis using the endoscope, lasers, balloon catheters, or high-pressure saline solution has been described [20, 36, 43, 48]. Mucus plugs and other debris are flushed with saline irrigation throughout the procedure. Corticosteroid application is an accepted practice though no formal studies have investigated outcomes of the technique [19, 20, 26, 33, 36, 37, 43, 45]. Hydrocortisone, triamcinolone, and prednisolone have all been applied. In theory, topical steroid applications prevent scarring and restenosis and may decrease inflammation in chronic inflammatory sialadenitis, like JRP.
A 2015 systematic review and meta-analysis by Ramakrishna et al. identified seven papers relevant to sialendoscopy in the management of JRP [51]. Evidence was level 3 and 4 but showed success rates for no further episodes (n = 120) of 73% by patient and 81% by gland. There were no major complications.
Pediatric sialendoscopy is also applied successfully to obstructive symptoms resulting from sialolithiasis. Though the efficacy of sialendoscopy alone is well reported, combined procedures may be required, with similar or improved success rates [26, 37, 43, 50]. Reports have suggested that retrieval success is dependent on size. For stones in children greater than 2–3 mm (parotid and submandibular gland, respectively), most authors employ additional techniques [49, 52]. Stone fragmentation can be applied with a microdrill or laser through the sialendoscope working channel or lithotripsy prior to extraction [36, 43, 45]. Other alternatives to complete sialendoscopic extraction for giant (>15 mm), proximal, or intraglandular stones include endoscopy combined with intraoral sialolithotomy [36, 38, 43, 50, 53]. Lastly, excision of the gland is considered for refractory cases [26, 42].
Postoperative stenting is not a uniform practice [37]. It is considered in cases of significant stenosis or injury. When employed, stents are often left in place for 2–4 weeks to allow adequate healing time [37].
Salivary endoscopy is most commonly performed under general anesthesia. However, in cases of inflammatory disease, older children may tolerate an office-based procedure with local anesthesia. Konstantinidis et al. reported seven out of eight children who underwent sialendoscopy and dilation after topical anesthetic and intraductal injection [46]. No major complications were reported. More than half of these children were symptom-free; two experienced one recurrence, and one required repeat sialendoscopy. Older children frequently tolerate office-based steroid injection, and there is some evidence that ductal corticosteroid infusion (DCI) may yield similar results as sialendoscopy in JRP patients [54]. This study was limited by small number of patients (12) and short follow-up (mean 3.8 months), and all procedures were done under general anesthesia.
Complications of sialendoscopy are uncommon and usually minor, resolving without permanent complication [26, 33, 37, 43, 44, 52]. Major complications are duct avulsion and immediate postoperative airway compromise. Minor complications include duct wall perforation, nerve paresthesia, postoperative infection, traumatic ranula, and iatrogenic duct stenosis.
Procedural Approach
One key difference between pediatric and adult sialendoscopy is concern about volume of irrigation. Pediatric patients have less tolerance of swelling, especially in the submandibular region, before airway compromise becomes a concern. There have been complications of airway compromise [20] due to excessive irrigation, so occasional gland massage and drainage of irrigant are recommended.
Duct lumen caliber in children limits scope size as well. Diagnostic 0.8 mm single port (for irrigation) scopes are occasionally utilized in children but rarely needed in adults. The parotid and submandibular gland duct anatomy is similar in children and adults. The caliber of each duct tends to be about 1 mm smaller in children than adults. As a general rule, the maximum size stone that can be removed in children without fragmentation is 3 mm in the submandibular duct and 2 mm in the parotid duct.
One disadvantage to sialendoscopy in children is the need for general anesthesia. Konstantinidis I, et al. reported that they were successful in performing sialendoscopy with local anesthesia in seven of nine pediatric patients treated [46]. Thus, local and or topical anesthesia should be considered in older and or more mature pediatric patients.
Conclusion
Sialadenitis in the pediatric population accounts for up to 10% of all salivary gland disease. Viral parotitis and juvenile recurrent parotitis (JRP) are the two most common etiologies. Many factors contribute to salivary gland disease in children, including viral or bacterial infections, congenital or traumatic duct obstruction (i.e., after lingual frenulotomy), autoimmune disease, and genetic defects. In children, parotid sialadenitis is more common than submandibular sialadenitis. Tumors of the salivary glands are rare in children and rarely present with inflammatory symptoms. Salivary stones are a frequent cause of chronic or recurrent obstructive sialadenitis, though much less common in children than adults. Stones are much more common in the submandibular gland than parotid gland, in both populations.
In the United States, the most common diagnosis related to sialadenitis in children is juvenile recurrent parotitis. Prior to sialendoscopy, treatment for this morbid and painful condition had been challenging. Sialendoscopy is a diagnostic and potentially therapeutic procedure that is minimally invasive, safe, and effective in reducing the proportion of patients experiencing disease recurrence. This procedure is also very helpful in reducing recurrent disease flares and removing obstructive sialoliths in children, thus preserving gland function without the potential morbidity associated with open gland excision.
References
Francis CL, Larsen CG. Pediatric sialadenitis. Otolaryngol Clin N Am. 2014;47(5):763–8.
Schneider H, Koch M, Kunzel J, et al. Juvenile recurrent parotitis: a retrospective comparison of sialendoscopy versus conservative therapy. Laryngoscope. 2014;124(2):451–5.
Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis. 2007;13(1):12–7.
Mumps outbreak on a University Campus—California, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(48):986–9.
Brook I. Diagnosis and management of parotitis. Arch Otolaryngol Head Neck Surg. 1992;118(5):469–71.
Errami N, Benjelloun A, et al. Tuberculosis of the parotid gland: histology surprise. Pan Afr Med J. 2015;20:343.
Bradley PJ. Microbiology and management of sialadenitis. Curr Infect Dis Rep. 2002;4(3):217–24.
David R, O’Connel EJ. Suppurative parotitis in Childrens. Am J Dis Child. 1970;119:332–5.
Preshall KE, Koopmann JR, Coulthard SW. Sialadenitis in children. Int J Pediatr Otorhinolaryngol. 1986;11(2):199–203.
Boulyana M. Acute neonatal parotitis with late-onset septic shock due to Streptococcus agalactiae. Case Rep Pediatr. 2014;2014:3.
Brook I. Suppurative parotitis caused by anaerobic bacteria in newborns. Pediatr Infect Dis J. 2002;21(1):81–2.
Brook I. The bacteriology of salivary gland infections. Oral Maxillofac Surg Clin North Am. 2009;21(3):269–74.
Brook I, Frazier EH, Thompson DH. Aerobic and anaerobic microbiology of acute suppurative parotitis. Laryngoscope. 1991;101(2):170–2.
Jones HE. Recurrent parotitis in children. Arch Dis Child. 1953;28:182–6.
Jervis PN, Lee JA, Bull PD. Management of non-tuberculous mycobacterial poer-sialadenitis in children: the Sheffield otolaryngology experience. Clin Otolaryngol Allied Sci. 2001;23(3):243–8.
Holmes S, Gleeson MJ, Cawson RA. Mycobacterial disease of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:292–8.
Hankins D, Kelly M, Vijayan V. Mycobacterium simiae infection of the parotid gland in an immunocmopetent child. J Pediatric Infect Dis Soc. 2013;2(4):394–6.
O’Connell JE, George MK, Speculand B, Pahor AL. Mycobacterial infection of the parotid gland: an unusual cause of parotid swelling. J Laryngol Otol. 1993;21(3):561–4.
Katz P, Hartl DM, Guerre A. Treatment of juvenile recurrent parotitis. Otolaryngol Clin N Am. 2009;42(6):1087–91.
Nahlieli O, Shacham R, Shlesinger M, Eliav E. Juvenile recurrent parotitis: a new method of diagnosis and treatment. Pediatrics. 2004;114(1):9–12.
Chitre VV, Premchandra DJ. Recurrent parotitis. Arch Dis Child. 1997;77(4):359–63.
Watkin GT, Hobsley M. Natural history of patients with recurrent parotitis and punctate sialectasis. Br J Surg. 1986;73(9):745–8.
Ericson S, Zetterlund B, Ohman J. Recurrent parotitis and sialectasis in childhood. Clinical, radiologic, immunologic, bacteriologic, and histologic study. Ann Otol Rhinol Laryngol. 1991;100(7):527–35.
Leerdam CM, Martin HC, Isaacs D. Recurrent parotitis of childhood. J Paediatr Child Health. 2005;41(12):631–4.
Shacham R, Droma EB, London D, Bar T, Nahlieli O. Long-term experience with endoscopic diagnosis and treatment of juvenile recurrent parotitis. J Oral Maxillofac Surg. 2009;67(1):162–7.
Hackett AM, Baranano CF, Reed M, Duvvuri U, Smith RJ, Mehta D. Sialoendoscopy for the treatment of pediatric salivary gland disorders. Arch Otolaryngol Head Neck Surg. 2012;138(10):912–5.
Konno A, Ito E. A study on the pathogenesis of recurrent parotitis in childhood. Ann Otol Rhinol Laryngol Suppl. 1979;88(6 Pt 4 Suppl 63):1–20.
Shacham R, Puterman MB, Ohana N, Nahlieli O. Endoscopic treatment of salivary glands affected by autoimmune diseases. J Oral Maxillofac Surg. 2011;69(2):476–81.
Friis B, Karup Pedersen F, Schiodt M, Wiik A, Hoj L, Andersen V. Immunological studies in two children with recurrent parotitis. Acta Paediatr Scand. 1983;72(2):265–8.
Reid E, Douglas F, Crow Y, Hollman A, Gibson J. Autosomal dominant juvenile recurrent parotitis. J Med Genet. 1998;35(5):417–9.
Fazekas T, Wiesbauer P, Schroth B, Potschger U, Gadner H, Heitger A. Selective IgA deficiency in children with recurrent parotitis of childhood. Pediatr Infect Dis J. 2005;24(5):461–2.
Shkalim V, Monselise Y, Mosseri R, Finkelstein Y, Garty BZ. Recurrent parotitis in selective IgA deficiency. Pediatr Allergy Immunol. 2004;15(3):281–3.
Jabbour N, Tibesar R, Lander T, Sidman J. Sialendoscopy in children. Int J Pediatr Otorhinolaryngol. 2010;74(4):347–50.
Chuangqi Y, Chi Y, Lingyan Z. Sialendoscopic findings in patients with obstructive sialadenitis: long-term experience. Br J Oral Maxillofac Surg. 2013;51(4):337–41.
Bodner L, Fliss DM. Parotid and submandibular calculi in children. Int J Pediatr Otorhinolaryngol. 1995;31(1):35–42.
Faure F, Querin S, Dulguerov P, Froehlich P, Disant F, Marchal F. Pediatric salivary gland obstructive swelling: sialendoscopic approach. Laryngoscope. 2007;117(8):1364–7.
Strychowsky JE, Sommer DD, Gupta MK, Cohen N, Nahlieli O. Sialendoscopy for the management of obstructive salivary gland disease: a systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2012;138(6):541–7.
Nahlieli O, Eliav E, Hasson O, Zagury A, Baruchin AM. Pediatric sialolithiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(6):709–12.
Chung MK, Jeong HS, Ko MH, et al. Pediatric sialolithiasis: what is different from adult sialolithiasis? Int J Pediatr Otorhinolaryngol. 2007;71(5):787–91.
Pinto A, De Rossi SS. Salivary gland disease in pediatric HIV patients: an update. J Dent Child (Chic). 2004;71(1):33–7.
Fritsch MH. Sialendoscopy and lithotripsy: literature review. Otolaryngol Clin N Am. 2009;42(6):915–26.
Capaccio P, Torretta S, Pignataro L. The role of adenectomy for salivary gland obstructions in the era of sialendoscopy and lithotripsy. Otolaryngol Clin N Am. 2009;42(6):1161–71.
Martins-Carvalho C, Plouin-Gaudon I, Quenin S, et al. Pediatric sialendoscopy: a 5-year experience at a single institution. Arch Otolaryngol Head Neck Surg. 2010;136(1):33–6.
Faure F, Froehlich P, Marchal F. Paediatric sialendoscopy. Curr Opin Otolaryngol Head Neck Surg. 2008;16(1):60–3.
Bruch JM, Setlur J. Pediatric sialendoscopy. Adv Otorhinolaryngol. 2012;73:149–52.
Konstantinidis I, Chatziavramidis A, Tsakiropoulou E, Malliari H, Constantinidis J. Pediatric sialendoscopy under local anesthesia: limitations and potentials. Int J Pediatr Otorhinolaryngol. 2011;75(2):245–9.
Capaccio P, Sigismund PE, Luca N, Marchisio P, Pignataro L. Modern management of juvenile recurrent parotitis. J Laryngol Otol. 2012;126(12):1254–60.
Quenin S, Plouin-Gaudon I, Marchal F, Froehlich P, Disant F, Faure F. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134(7):715–9.
Marchal F, Dulguerov P. Sialolithiasis management: the state of the art. Arch Otolaryngol Head Neck Surg. 2003;129(9):951–6.
Rahmati R, Gillespie MB, Eisele DW. Is sialendoscopy an effective treatment for obstructive salivary gland disease? Laryngoscope. 2013;123(8):1828–9.
Ramakrishna J, Strychowsky J, Gupta M, Sommer DD. Sialendoscopy for the management of juvenile recurrent parotitis: a systematic review and meta-analysis. Laryngoscope. 2015;125(6):1472–9.
Walvekar RR, Razfar A, Carrau RL, Schaitkin B. Sialendoscopy and associated complications: a preliminary experience. Laryngoscope. 2008;118(5):776–9.
Wallace E, Tauzin M, Hagan J, Schaitkin B, Walvekar RR. Management of giant sialoliths: review of the literature and preliminary experience with interventional sialendoscopy. Laryngoscope. 2010;120(10):1974–8.
Roby BB, Mattingly J, Jensen EL, Gao D, Chan KH. Treatment of juvenile recurrent parotitis of childhood: an analysis of effectiveness. JAMA Otolaryngol Head Neck Surg. 2015;141(2):126–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Larsen, C.G., Francis, C.L., Hamill, C.S. (2018). Pediatric Salivary Disorders. In: Gillespie, M., Walvekar, R., Schaitkin, B., Eisele, D. (eds) Gland-Preserving Salivary Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-58335-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-58335-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58333-4
Online ISBN: 978-3-319-58335-8
eBook Packages: MedicineMedicine (R0)