Abstract
Echocardiography has evolved into an important diagnostic tool in cardiac imaging and is frequently used in preparing for cardiac surgery. In this chapter we will discuss a surgeon’s view on imaging of the tricuspid valve. Generally, cardiac surgeons focus on four different aspects of echocardiography when preparing for tricuspid valve surgery: the size of the tricuspid annulus; the severity of tricuspid regurgitation, the morphology of valve leaflets and the degree of tethering of the tricuspid valve. In this chapter we discuss each of these four aspect separately. The emphasis is based on two dimensional echocardiography used in three clinical cases outlining the advantages and disadvantages of this contemporary technique.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Historically, tricuspid valve disease was believed to be benign and of lesser importance. However, recent studies have shown that tricuspid valve regurgitation leads to impaired survival [1]. These observations have led to renewed interest in the tricuspid valve and subsequently the imaging of the tricuspid valve.
During the last decades, cardiac imaging progressed from infant techniques to a tool which plays a crucial role in clinical practice. Especially echocardiography, being non invasive, easy to handle and relatively inexpensive, has evolved to an important diagnostic tool in cardiac imaging. Furthermore, echo images are used by surgeons to determine indications for operations and more importantly to provide surgeons with an adequate anatomical overview in order to select the suitable technique for the operation ahead. This chapter will discuss the surgeon’s view on this specifiek form of cardiac imaging. The emphasis is based upon two dimensional cardiac echocardiography which is used in three clinical cases which will be presented in this chapter in order to outline the advantages and disadvantages of this technique.
In general cardiac surgeons focus on four different aspects of echocardiography when preparing for tricuspid valve surgery: the size of the tricuspid annulus; the severity of tricuspid regurgitation, the morphology of valve leaflets and the degree of tethering of the tricuspid valve.
Tricuspid Annulus
The tricuspid valve annulus is only partially fibrous in nature. Three leaflets are attached on the annulus: the septal leaflet , the anterior leaflet and the posterior leaflet . The septal part of the annulus is believed to be analogous to the inter-trigonal part of the mitral annulus and the only fibrous part of the annulus. Therefore, it is relatively spared from displacement in annular dilation. The other parts of the tricuspid annulus are more flexible. Hence, dilatation of the annulus occurs in the anterior and posterior direction, which may lead to functional regurgitation due to leaflet malcoaptation. Fukuda and colleagues used real time 3D transthoracic echocardiography to map the tricuspid annulus. They noticed that the tricuspid annulus had a nonplanar elliptical shape, which differs from the more symmetrical saddle shape of the mitral annulus. Therefore, tricuspid annuloplasty rings are non-planar and attempt to resemble the physiological structure (Fig. 7.1). During a cardiac cycle the annulus diameter reduces approximately 19%. They also noticed that in case of dilatation the tricuspid annulus gradually becomes more planar [2]. 2D transthoracic echocardiography (TTE) echocardiography underestimates tricuspid annulus measurements significantly, while 3D echocardiography gives a more correct estimate compared to the golden standard (magnetic resonance imaging) [3]. Current European guidelines (ESC) advise tricuspid valve surgery when patients undergo left sided valve surgery if tricuspid annulus dilation >40 mm (21 mm/m2) is present even in mild/non-severe tricuspid regurgitation (Class IIa evidence) [4].
A Carpentier-Edwards Physio tricuspid annuloplasty ring. The rings are non-planar and resemble the physiological tricuspid valve annulus. The rings have a septal segment opening (indicated by the arrow), in order to avoid damage to the conduction system. Source: http://www.edwards.com/eu/products/rings/pages/physiotricuspid.aspx
Degree of Tricuspid Regurgitation
Both the European and American guidelines agree that tricuspid valve surgery is indicated in patients with severe tricuspid regurgitation undergoing left sided valve surgery, both class I evidence [4, 5]. Nevertheless, assessing the severity of tricuspid regurgitation is still controversial. A recent study identified a modest inter-observer agreement in assessing tricuspid regurgitation, however this variability improved with a new standardized assessing method [6].
Morphology
Tricuspid valve morphology is an important parameter to consider before surgery, since various structural abnormalities in the leaflets generally require different surgical approaches. Leaflets can be fibrotic, calcified, damaged by vaso-active peptides or extra-cardiac (pacemaker, ICD) leads and vegetation’s due to endocarditis can be present. Nevertheless, tricuspid valve morphology is difficult to visualize by 2D echocardiography, since not all three leaflets can be visualized in one single view by using the standardized echographical angles [7].
Tethering
Tethering is a phenomena where the papillary muscles and tendinous chords have become functionally too short resulting in malcoaptation. Leaflet tethering is generally associated with functional tricuspid value regurgitation. Tethering is usually caused by dilatation of right ventricle, but in some cases it may be caused by a diversity of subvalvulair abnormalities, like aberrant chordae [8]. Leaflet tethering is a preoperative predictor of residual tricuspid regurgitation [9].
In the following part of this chapter, we describe three cases in which the tricuspid valve repair has been performed. These cases describe the presenting clinical symptoms, a brief medical history and a summary of the heart team evaluation. The intraoperative findings and performance and the clinical course of the postoperative period will be discussed. Thereafter, all cases will be evaluated and a final take home message will be provided.
Case 1: Functional Tricuspid Regurgitation with a Structural Component
A 53 year old female presents with symptoms of dyspnea and angina pectoris. She is in NYHA class III. Her medical history is significant for hypercholesteremia, hypertension and terminal kidney failure based upon lithium use for a bipolar psychiatric disorder.
Heart Team Evaluation
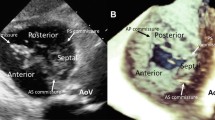
First echocardiogram was made 8 months before the heart team evaluation. Trans-esophageal echocardiography (TEE) shows severe mitral regurgitation, which is likely caused by a restrictive posterior valve. Moderate left atrium dilatation was found. Left ventricular ejection fraction was measured 47%. Two months prior to operation severe tricuspid regurgitation was found on Trans thoracic echocardiography (TTE) , as shown in Fig. 7.2. The annulus of the tricuspid valve measured 48 mm. The heart team concludes that left sided valve intervention is necessary and concomitant tricuspid valve surgery is indicated. This decision is supported by the current ESC-guidelines [4].
(a) TTE 4-chamber view, note the left sided dilatation. The tricuspid annulus measured 48 mm. (b) TTE Doppler of the tricuspid valve demonstrated severe insufficiency. Note the direction of the jet points toward the atrial septum (eccentrical jet). RA right atrium, RV right ventricle, LV left ventricle, LA left atrium
Operation
Patients was electively admitted for mitral and tricuspid valve surgery. Operation was done by median sternotomy. After central bi-caval cannulation the circulation is taken over by cardio pulmonary bypass and after aortic cross clamping, cardioplegia was admitted. The left atrium was opened via Waterston’s groove and the mitral valve is exposed. A Mitral Physio-ring (Edwards Lifescience) size 26 is implanted in the dilated annulus. The left atrium is closed with 4 × 0 Prolene sutures. The right atrium is opened and the tricuspid valve is exposed. The annulus is dilated and septal leaflet tissue is limited. This wasn’t noted on preoperative echocardiography images. A Tricuspid Physioring (Edward Lifescience) size 32 is implanted with Tricon 2 × 0 annular sutures. The right atrium is closed with Prolene 4 × 0 sutures. During removal of the aortic cross clamp and venting the aortic ascendens major ST deviations followed by ventricular fibrillation occurs, most likely due to coronary air embolisms. After defibrillating the rhythm converted to sinus rhythm. Temporary epicardial pacemaker leads were left on the right ventricle and the sternum was closed by steel wires. Subsequently, the skin was closed by staplers. Both the mitral and tricuspid ring are shown in Fig. 7.3a.
(a) TTE 4-chamber view. Both the ring in the tricuspid position as the ring in mitral postistion are visible. When eyeballing the left chamber, a reduction in dilation already has taken place. (b) TTE 4-chamber view of the tricuspid valve. Severe residual tricuspid regurgitation is present. Note that the jet is smaller than the preoperative jet
Postoperative Period
The patient is admitted to the intensive care unit and recovered successfully without any significant events. Postoperative TTE shows a trace of mitral regurgitation and still severe residual tricuspid regurgitation, as shown in Fig. 7.3b. In this particular case, the limited valve tissue could be the reason of persistent malcoaption. Annuloplasty, by implantation of a rigid ring alone, without valve leaflet augmentation has resulted in a failed repair.
Case 2: Cardiac Endovascular Pacemaker-Lead Interference of the Tricuspid Valve
A 79 year old male presents with dyspnea (NYHA class III) and intense fatigue. Physical examination reveals peripheral edema, vesicular breath sounds and bilateral jugular vein engorgment. Blood pressure is 113/61 with a ventricular paced rhythm of 50 beats per minute. His medical history includes complete heart block 5 years ago and subsequently an implantation of endovascular DDD pacemaker system . Shortly after the implantation an acute myocardial infarction occured due to significant lesion of the ramus circumflexus sinister. The patient was treated by a percutaneous coronary intervention. In the following years liver dysfunction developed, probably caused by right heart failure.
Heart Team Evaluation
This patient is discussed twice in a heart team meeting. The first meeting took place after echocardiographic imaging which showed a severely dilated right ventricle, as shown in Fig. 7.4a. Right ventricle dimensions are 87 mm for apex to basis and the annulus measured 59 mm. Systolic function of the right ventricle is graded as moderate. Mean pulmonary artery pressure (mPAP) is mildly increased (30 mmHg). Fig. 7.4b displays massive tricuspid regurgitation which is probably caused by both right ventricular dilatation and cardiac pacemaker lead interference of the tricuspid valve. 3D echocardiography confirms this suspicion (Fig. 7.4c). The left heart has fractional shortening of 40%, which is graded as a moderate systolic function. Only mild mitral regurgitation is noticed.
Panel (a) shows dilatation of the right ventricle and right atrium. RA dimensions are 73 × 59 mm, TAPSE is 21 mm. (b) Massive tricuspid regurgitation is present. (c) With 3d echocardiography the cardiac pacemaker lead can be visualized (the arrow points to the lead). MV mitral valve, TV tricuspid valve
The heart team initially concludes that the congestive right heart failure is caused by tricuspid regurgitation with volume overload, primary right ventricular failure and the lack of AV synchrony. However, the consensus is reached to prescribe a medical therapy in the first place and not to operate this patient because of the unpredictable outcome of and uncertainty of tricuspid valve repair and the expected high operation risks (due to age and pre-existed kidney failure). The consensus is discussed with the patient and he agrees with the proposed medical treatment.
During the following months the right heart failure persisted and the patient needs to be admitted for intravenous diuretic treatment. In the second meeting the heart team decides to perform the high risk operation due to persistent right heart failure on medications. Additionally, the VVI-pacemaker system is to be replaced for an epicardial system, due to interference with tricuspid valve leaflets causing severe tricuspid regurgitation.
Operation
Patient is electively admitted at our center and underwent surgery of the tricuspid valve, a coronary artery bypass graft (CABG) and removal of the endovascular DDD pacemaker system, which is replaced with a epicardial DDD pacemaker system . Operation was done via median sternotomy. After central and bi-caval cannulation, circulation is taken over by cardio pulmonary bypass. Because of pre-existed kidney failure blood pressure is kept at 70 mmHg, resulting in good diuresis during surgery. The operation is performed on beating heart without aorta cross clamping. The distal right coronary artery is calcified and this vessel is grafted by a vena saphenous magna (VSM) graft . On beating heart the right atrium is opened and the tricuspid valve is exposed. The atrial lead is located in the auricle, but the lead had grown into the anterior part of the right atrium and also in the anterior part of the tricuspid annulus, resulting in deformation of the tricuspid annulus. The ventricular lead originates from the VCS following the posterior wall of the atrium to the inter-commissural area of the tricuspid annulus and pushed the septal leaflet laterally, in which the lead also have grown into. Carefully, both pacemaker leads are removed and a small defect on the septal leaflet is sutured by prolene 6 × 0 sutures, without leaflet extention. A Tricuspid Physio (Edwards Lifescience) ring size 34 is implanted and the tricuspid valve appears sufficient with watertest. New epicardial leads are introduced and are connected to a Biotronik pacemaker , which is placed at the dorsal left side of the rectus abdominis. Peri-operative TEE showed signifcant diminished tricuspid valve regurgitation.
Postoperative Period
Posteroperatively the patient is transfered to the intensive care. The intensive care stay was characterized by hemodynamic and respiratory stability. However, the pre-existing kidney failure worsened, which was medically treated. Thereafter, the patients recovered and left the hospital in reasonable good condition.
Post-operative echocardiography 2 weeks after operation showed moderate residual tricuspid regurgitation, as is shown in Fig. 7.5b. Right ventricle size reduced to moderate dilation and its function remained moderate. Despite annuloplasty and valvuloplasty of the septal leaflet, moderate residual tricuspid regurgitation recurred post-operatively. The reappearance of tricuspid regurgitation after 2 weeks after recovery of the right ventricular function suggests structural failure of the valve leaflets. Primary suturing of the destructed valve leaflet may lead to fibrosis and eventually retraction of the valve leaflet and increases residual regurgitation post-operatively.
Case 3: Functional Regurgitation Due to Left Sided Heart Disease
A 78 old male presents with progressive dyspnea (NYHA class IV), orthopneu, lower limbs edema and nocturia. His stomach is slightly distended. He smokes ten cigarettes a day. Physical examination reveals blood pressure of 106/85 with 104 beats per minute and auscultation reveals bibasilar crackles of the lungs. His medical history is significant for atrium fibrillation and instable angina pectoris.
Heart Team Evaluation
Echocardiography shows severe central mitral valve regurgitation and dilated tricuspid valve annulus of >40 mm. Measurement of the tricuspid valve annulus was done by TEE and TTE. TTE measurement of the annulus of the 4-chamber view was found to be 50 mm, as shown in Fig. 7.5b. Measuring by TEE resulted in an annulus size of 48 mm Fig. 7.6a. Consensus was reached to operate on the mitral valve with concomitant tricuspid valve surgery.
Operation
Patient is electively admitted at the Thoraxcenter and underwent surgery of the mitral and tricuspid valves. Operation was done via a median sternotomy. Aftercentral and bi-caval cannulation circulation is taken over by cardio pulmonary bypass and after aortic cross clamping St Thomas cardioplegia was admitted. The left atrium was opened through Waterson’s groove and the mitral valve is exposed. The annulus is dilated, without structural defects of the leaflets. A mitral Phsyio-ring II (Edwards Lifescience) size 30 is implanted with Ticron 2 × 0 sutures. No mitral regurgitation is present after watertesting. The right atrium is opened and the tricuspid valve is exposed. Conform echographical findings, no structural defects of the tricuspid leaflets and sub-valvular apparatus is seen. A tricuspid Physio-ring (Edwards Lifescience) size 32 is implanted with Ticron 2 × 0 sutures. Thereafter, both atria were closed with Prolene sutures and patient was weaned of cardiopulmonary bypass. Peri-operative TEE revealed no residual mitral or tricuspid valve regurgitation, the echographical appearance of both Physio-rings is displayed in Fig. 7.7.
Postoperative Period
Postoperatively the patient was transferred to the intensive care. Here, post-operative bleeding persisted and the patient was re-explored in the operating room the next day and multiple cloths were removed retrosternal and intra-pericardial. Additionally, the postoperative course was complicated by rectal blood loss and delirium, which was treated by lowering anticoagulant dose and haloperidol and clonidine. The next month sternum dehiscence occurred which was re-fixated. Cultures of exudate did not show mediastinitis. Eventually, patient recovered successfully and left the hospital in reasonable good condition. Antibiotics were prescribed to prevent wound infection of the sternum. Postoperative TTE showed good results, as shown in Fig. 7.8a, b. Tricuspid regurgitation is reduced to none or trivial.
Discussion
2D echo imaging plays an important role in clinical decision making regarding tricuspid valve surgery. The four chamber view of trans thoracic echocardiography is especially helpful since it gives an excellent overview of the septal and anterior leaflet. The septal leaflet is seen on the septal side and the anterior leaflet on the free wall [10], although the posterior leaflet is less frequently seen from this angle [11].
The posterior leaflet is surgically of less interest when performing a reduction annuloplasty with an down-sized rigid ring as this technique causes the posterior leaflet to enfold and therefore excluding it from its function. Therefore, structural defects of the posterior valve are of less significance for the post-operative tricuspid valve function when performing ring annuloplasty. The first case is an example in which echocardiographic imaging showed calcification and leaflet thickening of the anterior valve leaflet (Fig. 7.1a). An eccentric jet , pointing towards the septum is also visible, indicating a structural defect of the anterior valve leaflet. Nevertheless in this case, only a ring annuloplasty was performed, resulting in post-operative residual tricuspid regurgitation. Tricuspid valve replacement was not considered an option due to the increased mortality and morbidity risk. The postoperative echocardiography (Fig. 7.2) demonstrated a residual tricuspid regurgitation with an eccentric jet. Even though the tricuspid valve regurgitation has decreased after annuloplasty the residual tricuspid valve regurgitation suggests a persistent structural dysfunction of the valve leaflet. In conclusion, ring annuloplasty alone is not sufficient when valve leaflets are damaged or leaflet tissue is limited.
3D echocardiography might provided a better overview of all three leaflets in one view and more importantly in a dynamic moving manner [10].
The second case illustrates endovascular cardiac pacemaker lead interfering with the tricuspid valve. Lead entanglement, lead perforation, lead impingement and lead adherence to the valve annulus or valve leaflet may result in significant tricuspid regurgitation. In this particular case it is suspected that the right ventricular lead was partially causing the regurgitation, however 2D echocardiography fails to confirm this suspicion, because of the absence of a clear view of the leads, as is showed in Fig. 7.3a, b. On the other hand, 3D echocardiography clearly illustrated the anatomical position and interference of the cardiac pacemaker leads with the tricuspid valve, as shown in Fig. 7.3c. Lin et al. demonstrated in a series of 41 patients that lead inference can be diagnosed preoperatively using 2D echocardiography in a mere 5% of patients [12]. 3D echocardiography may prove to be an excellent tool in visualizing endovascular pacemaker lead interference and subsequent damage of the tricuspid valve. A recent study showed that cardiac leads can be identified in 74% of patients using 3D echocardiography [13]. Additionally, the patient in case two developed moderate tricuspid regurgitation after 2 weeks of surgery, while peri-operative TEE showed sufficient tricuspid valve function. The regurgitation is probably caused by fibroses and subsequent retraction of the sutured defect of the septal leaflet. In retrospect, simply suturing the defect without leaflet augmentation was probably not the best option.
The third case is an example of functional tricuspid regurgitation. Functional regurgitation is regurgitation due to annular dilation, without any structural damage on the leaflets and subvalvular apparatus itself. 2D TTE echocardiography showed annulus dilation (Fig. 7.5). Peri-operatively no structural abnormality was observed macroscopically in the (sub)valvular apparatus except for the expected annulus dilatation and an annuloplasty ring was implanted with a satisfactory outcome. Therefore, this case also underlines the fact that a ring annuloplasty is generally sufficient in cases with functional tricuspid valve regurgitation without any form of structural abnormality of the valve leaflets.
Take Home Message
The septal and anterior leaflets of the tricuspid valve are surgically of more interest than the posterior leaflet. Therefore, routine 2D TTE four chamber view already provides the surgeon with sufficient information regarding annulus diameter and structural leaflet abnormalities. However, when structural deformations of the tricuspid valve are present or are suspected, 3D echocardiography has demonstrated its superiority in evaluating the complex tricuspid valve anatomy. Furthermore, annuloplasty by ring implantation is generally not enough to correct structural tricuspid valve regurgitation.
References
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43(3):405–9.
Fukuda S, Saracino G, Matsumura Y, Daimon M, Tran H, Greenberg NL, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation. 2006;114:I492–I8.
Anwar AM, Soliman OII, Nemes A, Geuns RJM, Geleijnse ML, Cate FJ. Value of assessment of tricuspid annulus: real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Card Imaging. 2007;23(6):701–5.
Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42(4):S1–44.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–185.
Grant AD, Thavendiranathan P, Rodriguez LL, Kwon D, Marwick TH. Development of a consensus algorithm to improve interobserver agreement and accuracy in the determination of tricuspid regurgitation severity. J Am Soc Echocardiogr. 2014;27(3):277–84.
Muraru D, Badano LP, Sarais C, Soldà E, Iliceto S. Evaluation of tricuspid valve morphology and function by transthoracic three-dimensional echocardiography. Curr Cardiol Rep. 2011;13(3):242–9.
Kobza R, Kurz DJ, Oechslin EN, Pretre R, Zuber M, Vogt P, et al. Aberrant tendinous chords with tethering of the tricuspid leaflets: a congenital anomaly causing severe tricuspid regurgitation. Heart. 2004;90(3):319–23.
Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111(8):975–9.
Anwar AM, Geleijnse ML, Soliman OII, McGhie JS, Frowijn R, Nemes A, et al. Assessment of normal tricuspid valve anatomy in adults by real-time three-dimensional echocardiography. Int J Cardiovasc Imaging. 2007;23(6):717–24.
Addetia K, Yamat M, Mediratta A, Medvedofsky D, Patel M, Ferrara P, et al. Comprehensive two-dimensional interrogation of the tricuspid valve using knowledge derived from three-dimensional echocardiography. J Am Soc Echocardiogr. 2016;29(1):74–82.
Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45(10):1672–5.
Cheng Y, Gao H, Tang L, Li J, Yao L. Clinical utility of three-dimensional echocardiography in the evaluation of tricuspid regurgitation induced by implantable device leads. Echocardiography. 2016;33(11):1689–96.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Review Questions
Review Questions
-
45.
Which echocardiography finding is important in the decision making regarding tricuspid valve replacement or valvuloplasty?
-
(a)
Annulus dilation >40 mm
-
(b)
Annulus dilation <40 mm
-
(c)
Structural valve damage
-
(a)
-
46.
Which tricuspid valve leaflet is surgically of less interest when performing an annuloplasty?
-
(a)
Anterior leaflet
-
(b)
Septal leaflet
-
(c)
Posterior leaflet
-
(a)
-
47.
What is the incremental value of 3D echocardiography over in 2D echocardiography in tricuspid valve imaging?
-
(a)
Imaging all tricuspid valve leaflets in one view more easily
-
(b)
It provides real time images
-
(c)
It is cheaper
-
(a)
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Veen, K.M., ten Cate, F.J., Oei, F.B. (2018). A Surgeon’s View on Echocardiographic Imaging of the Tricuspid Valve. In: Soliman, O.I., ten Cate, F.J. (eds) Practical Manual of Tricuspid Valve Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-58229-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-58229-0_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58228-3
Online ISBN: 978-3-319-58229-0
eBook Packages: MedicineMedicine (R0)