Abstract
The hydrothermal carbonisation process has attracted great interest in recent years, as it is an eco-friendly method for obtaining carbonaceous materials. This method consists of heating an organic material in the presence of water in a closed vessel at temperatures above 100 °C. The pressure generated by the water vapour next to a heat supply gives rise to the reactions necessary to form hydrothermal carbon (HTC). This chapter describes the manufacture of these materials and their application as adsorbents of contaminants in the aqueous phase. The manufacturing method differs from the classic pyrolysis method because of its lower power consumption, higher yield, and drastic reduction of pollutant emissions. HTCs are chars with high oxygen content, a large number of functional groups, and low porous development. They have been used to remove numerous contaminants of all types, but they are especially suitable for the removal of heavy metals.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Carbonaceous materials have numerous applications that have been known for some time. The two most important purposes (depending on the amount used) are as fuel or as adsorbent. As an adsorbent, the employment of activated carbons is important, which are usually obtained from charcoal (or biochar) that is obtained from the biomass.
The traditional method of carbonisation is pyrolysis of the biomass. In general terms, it consists of heating a lignocellulosic biomass at elevated temperatures (400–1000 °C) for several hours, or even days. This method poses some problems:
-
The use of high temperatures requires a large amount of energy, in addition to the hazardous process and the wear on the materials employed (by both high temperatures and corrosive by-products).
-
Biomass drying also requires energy, as the presence of liquid water considerably increases energy consumption.
-
An environmental problem is the production of high amounts of pyroligneous acid, tar, and contaminating gases, which do not allow the process to be classified as environmental friendly.
-
Lastly, pyrolysis yield, though variable, is usually very low.
This is where hydrothermal carbonisation comes into play. Hydrothermal carbons (HTCs) can be obtained from a biomass and water at mild temperatures (typically 200 °C) under water-generated pressure. Their advantages are:
-
The raw material does not need to be dried because of the fact that it will be mixed with liquid water. Together with mild temperatures, both factors represent significant savings on energy expenditure.
-
Consequently, a “wet” biomass, such as grass, leaves, or a glucose solution can be used.
-
No gases are released, no tar is produced, and the liquid obtained is not particularly polluting and very easy to handle later.
-
In addition, the yield is higher, about twice as high as in pyrolysis (Kumar et al. 2011).
Therefore, it is a method of carbonisation that will be used frequently in the future. This process has been known, as the early 1900s (Bergius and Specht 1913), for making synthetic coal at low temperatures and was recently rediscovered for the preparation of chars.
The product obtained has only one property in which it is expected to be inferior to the pyrolytic coal: its heat capacity, which will be somewhat less per unit mass. But there are many applications (Hu et al. 2008; Titirici and Antonietti 2010; Titirici 2012; Titirici et al. 2012) for which this carbonaceous material has been successfully tested. In this chapter, we will focus on obtaining HTCs and their use in the purification of contaminated water.
4.2 Hydrothermal Carbon Preparation
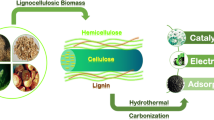
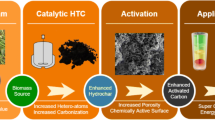
The properties of an HTC depend on factors such as the precursor selected, hydrothermal process conditions, and the use of templates or solids to coat. Subsequently, HTCs can be activated or functionalized in several ways. Figure 4.1 shows a diagram of the possible processes taken into account in the manufacture of HTCs.
4.2.1 Precursors
Several precursors have been employed as a source of carbon in the preparation of these materials, as shown in Fig. 4.2. A typical option is to use pure compounds from the biomass, such as glucose (Sevilla and Fuertes 2009; Chen et al. 2011; Yu et al. 2014; Martín-Jimeno et al. 2015; Roldán et al. 2016), starch, sucrose (Sevilla and Fuertes 2009), or fructose (Alatalo et al. 2016). The selection of these compounds is because of the simplicity of the process, as the reactions involved can be studied more easily. There are numerous bibliographical references that try to understand the hydrothermal process (Titirici 2012), and they usually use a simple compound, such as glucose. One advantage of these substances is that they are very abundant and inexpensive. For example, glucose is the most abundant carbohydrate unit in biomass. When using glucose, HTC is obtained in spherical forms, which grow in size with increasing carbonisation time. Chen et al. (2011) tested several compounds (glucose, sucrose, starch, and cellulose) as precursors, but glucose was finally selected because the result has more carboxylic groups.
An intermediate choice between the use of pure compounds and an unmodified biomass is the use of biomass extracts. For example, bayberry tannin has also been used (Li et al. 2014). Lignocellulosic materials are very common as raw materials for charcoals or activated carbon manufacturing. They are composed of three main components: cellulose, hemicellulose, and lignin. As cited in Sect. 4.1, one advantage of HTC is its ability to utilise biomass feedstock without drying before or during the process. It has an edge in terms of energy savings over pyrolytic carbonisation. Obviously, lignocellulosic materials have been widely used in HTC preparation (Hu et al. 2008). Other materials that have been used include switchgrass Panicum virgatum (Kumar et al. 2011; Regmi et al. 2012), pine needles Salix psammophila K (Liu et al. 2013; Zhu et al. 2014), wood (Laube and Reza 2016), pinewood (Liu et al. 2010), pinewood sawdust, rice husks (Liu and Zhang 2009, 2011), walnut shells, sunflower stems, olive stones (Román et al. 2013), rice straw (Dai et al. 2014), wheat straw, sawdust, corn stalks (Sun et al. 2015), and peanut hulls (Xue et al. 2012). The effect of removing lignin (Liu and Guo 2015) has also been studied, and it has been concluded that lignin, more stable than cellulose and hemicellulose, protects the other two from heat treatment. A sample of lignin-free wood carbonises faster than normal wood. It has been observed (Hu et al. 2008) that soft plant tissues, without an extended crystalline cellulose scaffold, yield globular carbonaceous nanoparticles; however, hard plant tissues with structural crystalline cellulose scaffolds can preserve their outer shape.
A less common option is the use of microorganisms. An industrial microorganism (Saccharomyces cerevisiae) has been used to obtain HTC (Li et al. 2016). Another source of carbonisable biomass is waste products . These can be industrial, such as beer waste (Hao et al. 2014), or livestock waste, such as swine manure (Cao et al. 2011). Another biomass residue of animal origin is prawn shells, which are a source of chitin (poly-β-(1-4)-N-acetyl-d-glucosamine). Chitin can be deacetylated to give rise to chitosan (Zhang et al. 2014a; Shen et al. 2016), which can also be carbonised. These cases have the advantages of eliminating and valuing wastes, which are usually considered zero-cost raw materials, and of creating an extra environmental profit. We can also work with compounds that do not come from a biomass, such as glyoxal and acrylonitrile (Yang et al. 2014).
4.2.2 Hydrothermal Process
The hydrothermal process is characterised by moderate temperatures (<300 °C) and high pressure, as the water included in the reactor increases the pressure when it exceeds 100 °C (autogenous pressure). This reaction can also be performed by raising the pressure externally, for instance, by using a pressurised gas. But if it is done in equipment without a pressure measurement appliance, the temperature must be monitored because of the fact that pressure increases very quickly. Table 4.1 shows pressure data obtained autogenously as a function of temperature, calculated according to the Clausius-Clapeyron Eq. (4.1).
where P1 and P2 are the water vapour pressures at temperatures T1 and T2, respectively, ΔH vap is the water vaporisation enthalpy (40,650 J mol−1), and R is the gas constant (8.314 J K−1 mol−1).
If 374 °C and 22.1 MPa are exceeded, water will behave as a supercritical fluid. In this situation, mainly liquid and gaseous fuels are obtained, and it is denominated by hydrothermal liquefaction or hydrothermal gasification . At very high temperatures (Kruse et al. 2013), the formation of gases like H2 and CH4 is favoured. At intermediate temperatures, hydrolysis of the biomass occurs, and medium molecules, such as ketones, carboxylic acids, or phenols, are obtained, from which bio-oil can be prepared. At lower temperatures, the hydrolysis reaction decreases and dehydration is favoured, so the main product is a solid, HTC. As the solid product (char) will be analysed in this chapter, we will limit ourselves to studying the subcritical conditions of hydrothermal carbonisation below 300 °C.
It should be kept in mind that if substances other than water are used that can reach the vapour state (Yang et al. 2014), the partial pressures of each of these substances will be added together to give rise to a greater pressure than expected. This fact must be considered in order to avoid accidents and to compare different reagents under the same conditions. One factor that can explain this water reactivity, besides the energy supplied by the heating, is the increase of the water ionisation constant when the temperature increases (Bandura and Lvov 2006), causing a higher concentration of H + and OH− that can catalyse the dehydration reaction. This effect is slightly amplified by the increase in pressure. In Table 4.2, some of the parameters of hydrothermal carbonisation used in published works are shown.
As shown in Table 4.2, in most cases, temperatures of 180–200 °C are reached, with varying times between 10 and 24 h. When heating to higher temperatures (Liu and Zhang 2009, 2011; Liu et al. 2010; Kumar et al. 2011; Regmi et al. 2012), the preparation time can be considerably reduced. Although this can be considered an advantage, it must be taken into account that the autogenous pressure produced by raising the temperature from 200 °C to 300 °C is multiplied several times (Table 4.1) and that certain commonly used materials, such as Teflon, cannot be used at that temperature. Higher temperatures mean more energy expenditure, which can be compensated for by the brevity of the process. These issues should be considered if HTC production is intended to reach an industrial scale, but are not the most relevant for laboratory research. More important could be the influence of temperature on the porous and chemical structures of the HTC.
The experiments shown in this study have been done on a scale of a few grammes, except Laube and Reza (2016). Apparently, this kind of study is still in the laboratory phase, and there does not seem to be any interest in obtaining medium- to large-scale adsorbents .
A parameter with greater variation is the biomass/water ratio, which varies from 1:2.4 to 1:40. No exhaustive studies have been done on the influence of this proportion, although there is one reference (Sevilla and Fuertes 2009) in which a higher yield and larger size of the spheres obtained were observed by increasing the concentration of glucose. But this result does not allow conclusions to be drawn when using other materials, such as a ligand-cellulosic biomass . It should be remembered that the presence of water is crucial to this synthesis method, as it generates autogenous pressure and reacts with the parting material to produce carbonisation.
One problem posed by HTCs at the end of the hydrothermal carbonisation process is that they have very low porosity. Several strategies have been put into place to introduce micro- and mesopores and thus increase the specific surface area. The most common method and the one used with pyrolysed carbons is activation (see Sect. 4.2.5), but templates have also been used to improve this property (see Sect. 4.2.3).
Some authors add a small amount of acid, as it favours the dehydration processes of some organic molecules , such as carbohydrates (Cao et al. 2011; Liu et al. 2013; Zhang et al. 2014b). On the other hand, Dai et al. (2014) observed that increasing the hydrothermal treatment time from 4 to 14 h hardly changed the composition of the final product, although it must be considered that they used a solution of LaCl3 and KOH instead of distilled water. The presence of these compounds can catalyse the hydrothermal process (Hu et al. 2008) in such a way that extended time does not make a big difference. Slight carbon enrichment and a decrease in the content of hydrogen and oxygen can be observed. There is a large difference (Dai et al. 2014) when varying the composition of the raw material, but there are only small differences between HTCs prepared at different temperatures from the same precursor.
Sevilla and Fuertes (2009) carried out an extensive study on the effect of preparation conditions (temperature, time, and concentration) on the properties of HTC, using glucose, sucrose, and starch. The yield obtained in the hydrothermal process increased with increased temperature (in the range of 170–240 °C), with longer treatment time (from 0.5 to 15 h), and with higher concentration of the carbohydrate in water. Similarly, the average size of the HTC spheres obtained varied. However, Shen et al. (2016) have reported a decrease in yield from 78.8% to 35.2% when increasing the temperature from 140 °C to 220 °C. The influence of hydrothermal treatment conditions on the properties of the HTC is a complex subject and seems to depend heavily on the raw material used.
Sevilla and Fuertes (2009) detected the existence of a large number of functional groups, but they were unevenly distributed. The nucleus of the spheres is highly aromatic with stable oxygen groups (ether, quinone, pyrone, etc.), while the shell contains a high density of functional groups (hydroxyl, phenol, carboxylic, etc.). Thus, the exterior would be hydrophilic, while the interior would be hydrophobic. This hydrophilic outer surface favours the dispersion of the HTC particles in water.
At the end of the hydrothermal treatment, the product must be ovendried before being used. Numerous authors have previously performed washing with acetone to remove organic substances soluble in this solvent. The reason comes from the fact that the hydrothermal treatment was initially used for gasification and liquefaction of the organic matter, and the liquid produced when washing it with acetone is a biofuel. Except for a decrease in the contribution of functional groups (Cao et al. 2011), no major structural changes in the HTCs thus treated have been described .
4.2.3 Templates
One of the main disadvantages of the use of HTCs in adsorption is that they present both limited porosity and surface area. This is also true of pyrolytic carbons, and activation is used to improve their properties. Activation can also be used in HTCs and will be discussed in Sect. 4.2.5.
Another method to increase porosity is the use of templates in the hydrothermal process. One of the templates employed (Martín-Jimeno et al. 2015) is graphene oxide. It presents several interesting characteristics : good dispersibility in water and organic solvents, greater reactivity than non-oxidized graphene, and a tendency to form monolithic gels. Thus, a suspension of graphene oxide was prepared in which glucose was dissolved. This mixture was heated in an autoclave at 200 °C for 16 h, yielding a carbonaceous hydrogel that was dried and subsequently activated. This method allows to obtain a monolith instead of the usual powder formed by the spheres characteristic of this process. The density of the monolith increases as the glucose concentration increases, indicating that the carbonisation must be incorporated into the template structure. It could also be considered to be an example of coating (see Sect. 4.2.4).
A reagent frequently dealt with in chemical activation is ZnCl2. Roldán et al. (2016) introduced it into the mixture before the hydrothermal process, employing the “salt-templating” methodology . The basic concept is that the hypersaline conditions stabilise the surface of the first small nanoparticles, avoiding Ostwald ripening or excessive particle growth. These particles turn collectively unstable at sufficiently high concentrations, undergoing spinodal phase separation and cross-linking towards the final porous carbon gels. The more salt is added, the smaller the primary particles are and, hence, the higher the surface area is. The HTC prepared with ZnCl2 presents a greater specific surface once the hydrothermal process is finished. But when washing the carbon to remove the Zn and when performing the pyrolysis of the HTC to activate it thermally, the specific surface increases considerably, whether ZnCl2 has been used or not, but with narrower pores in this case. There is a big difference when the HTC is not washed before the pyrolysis because, in that case, a much higher specific surface is obtained because of the activation process. Note that it has already been mentioned that ZnCl2 is usually used in the chemical activation of pyrolytic carbons. This, in fact, does not imply an improvement in the adsorption capacity of dyes , which are the pollutants with which they have been tested, being more effective the introduction of N and S heteroatoms.
The same method of “salt templating” was exercised with a LiCl-ZnCl2 mixture (Alatalo et al. 2016). Micro-/mesoporous HTCs were thus achieved with a specific surface area greater than 400 m2/g obtained with BET method. Another common template (Hu et al. 2008) for pyrolytic carbons that could be brought into play here is silica. By modifying the polarity of the silica surface, porous structures of various sizes and shapes could be synthesised.
4.2.4 Coatin g
One possibility offered by the HTC method, when employing water-soluble substances such as glucose, is the possibility of coating carbonaceous shells on a structured surface. The coating process is dependent (Titirici 2012) on the surface properties, hydrothermal carbonisation time, and concentration. It is mainly used to coat nanostructures and in applications related to energy storage. This technique is not usually applied to make adsorbent materials, although there are some examples.
Zhao et al. (2016) used polyacrylonitrile fibres as a template. First, they reinforced the bond between the fibres with ethylenediamine and ethylene glycol. Then, they added these fibres to a glucose solution and performed the hydrothermal carbonisation of the carbohydrate, which was deposited on the fibre structure.
The opposite process can also be performed: depositing certain compounds on the HTC, for which the existence of oxygenated functional groups is very helpful. This process should not be confused with functionalisation, and sometimes it is difficult to establish a limit between the two. We can consider the process to be coating when it forms a species with its own crystallinity and functionalisation if a molecule binds to the HTC by a covalent bond and acts as an organic functional group of the corresponding graphene molecule. An example of coating is the deposition of noble metal nanoparticles on carbonaceous nanofibers (Quian et al. 2007). These materials are often used in catalysis.
4.2.5 Activation
The activation process is added to improve the porous structure of the carbonaceous materials, i.e., increase the volume of the pores and the specific surface. There are two main types: chemical activation and physical activation. The main difference between the two is the reactivity of the compounds used as activating agents. The activation process is important when HTC adsorbents are prepared because their capacity is often related to their specific surface and their porous structure. This aspect is more important in adsorption than in other applications of HTCs.
4.2.5.1 Chemical Activation
One of the methods used is chemical activation . The compounds employed are more reactive than those employed in physical activation, and the temperature range is wide, ranging from room temperature to about 800 °C. Activation time is usually from 1 to 2 h. It must be taken into account that the use of high temperatures (>400 °C) can destroy existing functional groups, so in many applications, it is necessary to evaluate if it is convenient to increase porosity and specific surface area by modifying the surface chemistry.
One of the most common reagents is KOH . Thus, Martín-Jimeno et al. (2015) activated an HTC obtained with templates of graphene oxide, treating it with KOH at temperatures of 700 °C and 800 °C. Regmi et al. (2012) also performed a treatment with a KOH solution at room temperature. Although the treatment entails an improvement in the adsorption of Cu(II) and Cd(II) , it hardly implies a change when the concentration of the KOH solution oscillates between 0.5 and 2.0 N. Activation under these conditions gives rise to very high yields (Sun et al. 2015) with little loss of mass. But it does change the PZC of the HTCs obtained from acidic to slightly basic. This implies a change in the electrical charge of the surface as a function of pH, which is different from the usual behaviour of the HTCs. Also, a NaOH 1 M solution can be used as an activating agent (Zhao et al. 2016).
On the other hand, Hao et al. (2014) used H3PO4 to activate an HTC obtained from beer waste . In this case, they studied the influence of different variables of the activation process on the product obtained. These variables were the activation temperature (600–700 °C), the activation time, the concentration of H3PO4, and the flow rate of N2.
4.2.5.2 Physical and Thermal Activation
The other habitual method of activation is physical activation , which consists of heating the sample in the presence of a gas, the most common gases being air, carbon dioxide, and water vapour. The observed changes are those usually described in the activation of pyrolytic carbons; however, lower performance (greater burn-off) has been reported as compared to the activation of pyrolytic carbons. This is not a surprise, as hydrothermal carbonisation results in a carbonaceous material richer in oxygen than pyrolytic carbon. This implies that it contains more volatile matter and that a greater proportion is lost than the fixed carbon in the activation treatment.
Thus, by activating an HTC with carbon dioxide at 800 °C during a variable period of time (Liu and Zhang 2011), there is an increase in the ash content and a decrease in the yield as the activation time is increased. At the same time, there is an increase of the specific surface. An increase in the proportion of wide pores (meso- and macropores) also occurs (and this does not always happen with pyrolytic carbons).
When comparing the activation of HTCs from different materials with air and carbon dioxide (Román et al. 2013), it is observed that the specific surface and volume of micropores, which are two related parameters in activated carbon, are always higher when using CO2. In contrast, different results are obtained in the volume of meso- and macropores. In both activation processes , micropores are created, and the specific surface area increases.
A variation of this method is thermal activation , which consists of heating the HTC in the presence of an inert gas. In this case, the activation is produced by the temperature and not by the reaction with the gas, as it only serves as a carrier of the by-products formed. Although some authors (Zhu et al. 2014) use it as an activation method (N2, from 300 °C to 700 °C), one should consider whether a second pyrolysis-type carbonisation or an actual activation is actually being performed. As mentioned above, HTCs differ from pyrolytic carbons because of their low porous development. In the work of Zhu et al. (2014), the evolution of the composition of the chars according to temperature is as expected: an increase of the carbon content and a reduction of the hydrogen and (especially) oxygen content. This variation is important when starting the heat treatment (up to 300 °C) and also between the temperatures of 400 °C and 600 °C. In the first step, it can be assumed that changes in composition are mainly because of water loss (desorption or dehydration). And between 400 °C and 600 °C, changes are because of the pyrolysis process. A consequence of this variation in the oxygen ratio is that the pH changes from slightly acidic in the HTC (5.2) to very alkaline in the sample treated at 700 °C (pH 11.8).
Table 4.3 summarises some of the activation methods found in the literature. Chemical activation methods (with liquid or solid reagents) predominate over physical activation methods (using gaseous reagents). The activation times are, in general, shorter than those of hydrothermal carbonisation. There may be large variations in the conditions under which the activation is carried out .
4.2.6 Functionalisation
There are many methods of changing the surface chemistry of a carbonaceous material, and some of them have been used with HTC. Activation can modify the functional groups of the carbonaceous material, but its main function is to improve the porosity. For this reason, activation methods will not be dealt with in this section.
Before modifying the surface chemistry of an HTC, its properties must be taken into account. If it is obtained from a biomass, it must be assumed that the raw material contains a large amount of oxygen in its composition. Most of the starting materials (carbohydrates and lignocellulosic materials) have an oxygen content approaching 50% by weight, excluding moisture. As high temperatures have not been used, many of the original functional groups have not been destroyed. We have, therefore, a carbonaceous material with a high number of functional groups.
4.2.6.1 Functionalisation During the Hydrothermal Process (One Step)
Functionalisation can begin during the hydrothermal process . Thus, Roldán et al. (2016) used glucose as a source of carbon and used either pyrrole-2-carboxaldehyde to add nitrogen atoms or 2-thiophenecarboxaldehyde atoms to add sulphur atoms. Nitrogen atoms may be included in the HTC if they already exist in the raw material, for example, in chitin (White et al. 2009). Functionalised HTC with fructose and 2-thiophenecarboxaldehyde (Alatalo et al. 2016) has also been prepared. Note that glucose has been mixed with phosphoric acid (Zhang et al. 2014a). We can observe another example in the use of an alkaline solution of LaCl3 instead of water to obtain a lanthanide-doped HTC (Dai et al. 2014). Demir-Cakan et al. (2009) used acrylic acid mixed with glucose to obtain a high number of carboxyl groups in the HTC.
Functionalisation can be carried out by choosing the appropriate starting material. A work has been previously cited (Chen et al. 2011) comparing HTCs prepared from glucose, sucrose, starch, and microcrystalline cellulose. HTCs with different numbers of carboxyl groups were obtained, despite using the same conditions in the hydrothermal process.
4.2.6.2 Post-functionalisation (Two Steps)
Chemical modification of the surface can be done after obtaining the HTC. The methods followed are similar to those employed for activated carbons of pyrolytic origin.
A simple method is oxidation or calcination under air. atmosphere It is usually carried out at temperatures below 400 °C (Chen et al. 2011; Zhang et al. 2013; Yu et al. 2014) because, at this temperature, most of the functional groups begin to be destroyed. But the temperature must be higher than 200 °C, as it is necessary for sufficient oxygen chemisorption of the air to occur so that the functional groups can be formed. The objective in this temperature range is to achieve a greater number of oxygen functional groups , which in many cases improve the adsorption capacity of the HTC. Zhou et al. (2015) have studied the effects of temperature (from 100 °C to 300 °C) and time (from 1 to 5 h) in detail. The oxygen content and the number of carboxyl groups increase linearly with temperature and time, except in the change from 250 °C to 300 °C where the number of carboxyl groups increases significantly. Chen et al. (2011) studied the effect of temperature between 100 °C and 400 °C and analysed the functional groups using the Boehm method . As the temperature increased, the weaker acidic functional groups (type phenol) decreased from 100 °C, while the strongly acidic ones (type carboxyl) increased with temperature. The variation in both cases is especially important when the temperature exceeds 200 °C. In contrast, the increase in oxidation time, although increasing the number of carboxyl groups, does it very weakly. These results have been confirmed using FTIR and XPS data.
Another method of oxidation (Li et al. 2016) is oxidation with potassium permanganate in a strongly acidic medium, according to the well-known Hummers method (Hummers and Offeman 1958), to obtain graphite oxide. Two other oxidation methods frequently used with carbonaceous materials are the use of nitric acid (Liu et al. 2013) and the use of hydrogen peroxide (Xue et al. 2012). In both cases, the oxygen content increases, and at the same time, a decrease of PZC is verified.
As a general rule, mild oxidation methods give rise to the adsorption of oxygen to create functional groups, increasing the mass of the carbon. Aggressive methods produce carbon oxidation and the formation of carbon oxides, reducing the total mass. It is not strange, therefore, that in the described conditions the mass yield exceeds 100% in some cases.
Some uses of functionalisation are highly specific. One example is the use of o-phosphoethanolamine and N,N′-dicyclohexylcarbodiimide (Yu et al. 2014) with the aim of improving the adsorption of (UO2)2+ by the phosphate groups , because the phosphate group has strong tendencies to form complexes with uranyl ions.
Other functionalisations require several stages . For example, Song et al. (2012) used 5-azacytosine to adsorb U(VI), as this multidentate ligand had demonstrated a high affinity for dissolution towards the actinide metals. To bind the 5-azacytosine to the carbon, they first performed calcination in air at 300 °C. The product obtained was reacted with ethane-1,2-diamine in the presence of N,N′-dicyclohexylcarbodiimide. And in the third step, amino groups grafted on the HTC surface reacted with 5-azacytosine.
Another similar case is functionalisation with salicylideneimine (Wang et al. 2012). The first two steps are similar, but in the third step, salicylaldehyde reacts with the amino groups previously formed to anchor to the surface of the HTC as salicylideneimine, which is a good ligand of cations.
Functionalisation may sometimes depend on the raw material. Thus, Liu and Zhang (2011) prepared two HTCs activated from pinewood dust and rice husks. The last is known to have a lot of silicium that converts to silica. When phenol adsorption was probed, there was a large difference in the amount adsorbed between the two coals, which can be explained by the greater specific surface area of the pinewood dust HTC. However, the difference in Cu(II) adsorption was lower, which can be explained by the presence of silicon forming silanol groups, which easily bind Cu, increasing the adsorption capacity.
A two-stage process whose functionalisation begins in the hydrothermal process is HTC preparation (Yang et al. 2014) from glyoxal and acrylonitrile to obtain cyano (-CN) functional groups . These react with hydroxylamine hydrochloride to obtain amidoxime grafted to the HTC surface .
4.2.7 Hydrothermal Versus Pyrolytic Carbonisation
It is interesting to compare the two methods of carbonisation, although there are not many papers dedicated to this question. The first differences we can find are in the preparation conditions. The hydrothermal process is carried out at moderate temperatures (180–300 °C) and high pressure (1–10 MPa), while pyrolytic carbonisation is carried out at a higher temperature (400–1000 °C) and at atmospheric pressure (0.10 MPa). In fact, out of these two factors, temperature seems to be the one that most influences the final properties of the material obtained.
Some papers have been published comparing materials obtained by the two methods of carbonisation. Liu et al. (2010) have studied the properties of HTC obtained at 300 °C and pyrolytic carbon obtained at 700 °C in an atmosphere of N2 from pinewood. Cao et al. (2011) used dried swine manure as raw material. The pyrolytic char was obtained at 620 °C after 2 h, and the HTC was obtained at 250 °C after 20 h. Falco et al. (2011) prepared HTC at 180 °C for 12 h and pyrolytic char in an atmosphere of nitrogen at various temperatures. In this experiment, the HTCs were later pyrolysed.
The composition obtained by elemental analysis is very different between the two carbons used in the work of Liu et al. (2010). Pyrolytic carbon contains a large amount of carbon while reducing the content of hydrogen, nitrogen, and oxygen. This is because the higher the temperature, the degraded the heteroatom-containing functional groups (i.e., the more labile groups). The composition obtained suggested (Durán-Valle 2006) that the aromatisation process progressed more in the pyrolytic carbon than in the HTC.
The proximate analysis is related to these data . A larger amount of heteroatoms is related to functional groups less stable when heated, and therefore, the HTC contains a greater amount of volatile matter and less fixed carbon. The amount of ash is also lower in the HTC, but this can be mainly attributed to the higher yield of this carbonisation, as it can be considered that the amount of ash in the raw material is usually kept approximately stable during both processes of carbonisation. That is, the variation observed is because of the decrease in the fixed carbon content and especially in a volatile matter. Some authors also proposed (Liu et al. 2010) that the presence of water in the hydrothermal method can dissolve part of the mineral matter.
Another difference found (Liu et al. 2010) is in the acidic/basic properties. As it contains more oxygen, the HTC has more acidic functional groups, and its zero point charge (PZC) is lower than in pyrolytic carbons.
As for the surface structure, both carbons have poor porosity development, which is even lower in HTC. The specific surface obtained is low, mainly because of the small volume of micropores formed. For many applications in adsorption of these materials, it is necessary to develop porosity, especially when apolar pollutants are removed. This can be done by using templates in the hydrothermal process or by activating both types of carbons, as discussed above.
The degree of graphitisation is usually very low in HTCs. Demir-Cakan et al. (2009) have measured this parameter using Raman spectroscopy. The data of the proximate and ultimate analyses and of the physical structure support this conclusion.
Sun et al. (2011) compared two pyrolytic carbons (obtained at 400 °C) and two HTCs (obtained at 250 °C) from poultry litter and wheat straw. The differences between the coals obtained are not as big as has been described in other works (Liu et al. 2010). This can be explained by the low temperature and limited pyrolysis time (2–4 h) in the work of Sun et al. (2011), suggesting that pyrolytic carbons are on the limit of what can be considered a carbonaceous material and not a roasting material. Thus, the elemental composition is similar, although the small differences observed support what has been indicated in other works: higher carbon content and lower nitrogen, hydrogen , and oxygen content in the pyrolytic carbon compared to HTC. It is also observed that the specific surface is lower in the case of thermal coals than in HTCs, which is not usually the case. However, the measure of aromaticity by 13C-NMR indicates that it is more developed in pyrolytic carbons.
A similar result is observed when comparing an HTC obtained at 180 °C with a pyrolytic carbon prepared at 350 °C; both are rich in oxygen and hydrogen when compared to a pyrolytic carbon obtained at 750 °C, which is most common (Falco et al. 2011). The change in composition depends on temperature, and it is observed that pyrolysis of the HTC increases the carbon content and decreases hydrogen and oxygen content, obtaining an elemental analysis similar to that of a carbon obtained by pyrolysis at the same temperature.
The yields depend on both the raw material and the carbonisation conditions and can reach very different values. In general, it can be stated that the hydrothermal method achieves a yield double that of the pyrolytic method .
4.3 Adsorption
Among the other applications cited in the introduction, HTCs have been employed in adsorption processes, especially in the removal of contaminants from aqueous solutions.
4.3.1 Dye Adsorption
As stated in Chap. 1, dyes are pollutants that are difficult to remove from wastewater because they are non-biodegradable as well as toxic to plants, animals, and humans. The greatest environmental problem with dyes is that their absorption of sunlight entering the water interferes with the growth of bacteria, which dips to an insufficient level for biologically degrading impurities in the water. Also, the growth of algae decreases, as sunlight does not reach them; some algae even die, affecting the food chain. The dye molecules are distinguished by their large size, which is why adsorbents with a larger pore size, between the broad microporous and the narrow mesoporous, are preferable.
Alatalo et al. (2016) used a ZnCl2-LiCl template , using fructose as the carbon source organic material. When 2-thiophene carboxaldehyde was added, a solid with a larger surface area and pore volume was obtained. Both materials were tested as methylene blue adsorbents. The material without sulphur is more effective, probably because of higher oxygen content. In addition, it maintains its adsorption capacity better when performing several consecutive cycles.
Martín-Jimeno et al. (2015) adsorbed three dyes (rhodamine B, fuchsin basic, and methyl orange; see Fig. 4.3) on HTCs made on templates, some of them activated with KOH. The best-absorbed dye was methyl orange, because it is the narrowest of the three, and these basically prepared templates show mainly narrow microporosity. Those HTCs activated with high KOH ratios developed broader porosity (broad micropores and narrow mesopores), so they adsorbed a greater amount of rhodamine B and fuchsin basic than the other HTCs.
Roldán et al. (2016) prepared HTC using glucose, doping the HTC with N or S by adding pyrrolecarboxaldehyde, thiopenecarboxaldehyde, or both. These change the porosity before pyrolysis , with a higher percentage of mesopores. Also, the sample contains ZnCl2. In this experiment, these authors did not wash the salts, because ZnCl2 is an activation agent activated to develop microporosity. These HTCs were used as adsorbents of methylene blue and rhodamine B. This dye is adsorbed to a lesser extent, and the authors explain it as having a somewhat larger size than methylene blue. It is interesting to note that non-pyrolysed HTCs, despite their smaller surface, adsorb dyes better. This can be explained by the lower oxygen content of the pyrolysed materials, which interact less well with the cationic dyes.
Hao et al. (2014) studied the adsorption of methylene blue dye from aqueous solutions using activated carbons produced by chemical activation of hydrothermally carbonised (HTC) beer waste. By studying the adsorption isotherms, it was concluded that activated carbon has a good adsorption capacity for the dye studied (341 mg/g). The pH value affects the adsorption process, so at a low pH, the adsorption is lower than at a high pH. This is attributed to the fact that at pH values above the PZC, the surface of the adsorbent is negatively charged, which increases the adsorption capacity of the positively charged methylene blue molecule.
4.3.2 Pesticides
One of the activities that produces a lot of water pollution is agriculture, for example, with the discharge of pesticides by agricultural irrigation systems. There are few published works about the adsorption of pesticides with HTCs. However, the removal of paraquat (1,1′-dimethyl-4,4′-bipyridyl dichloride) from water has been studied (Zhao et al. 2016). This herbicide is banned in several countries because of its high toxicity but is still used in many regions of the world. To test the removal, they prepared PAN fibres that were mixed with a solution of glucose to later submit them to the hydrothermal process. An HTC in the form of fibres was thus obtained, which was activated with 1M NaOH. The specific surface area obtained was low (<12 m2/g). The hydrothermal treatment increases the tensile strength, and although it decreases the elongation at break, it has good flexibility. Such enhanced mechanical strength is beneficial for applications in wastewater treatment. The adsorbed amount is practically zero, only with PAN fibres. It increases about 50 times after the hydrothermal treatment and is multiplied by three after activation with NaOH. This shows that adsorbent capacity comes from the presence of a porous material and is enhanced by the creation of oxygenated functional groups. As the pH decreases, the adsorption capacity of paraquat decreases. This is because of electrostatic interactions. Paraquat is a cation (Fig. 4.4) and is repelled by the positive charge of the protonated HTC surface at a low pH. As the pH increases, this repulsion decreases, and the effect disappears at a pH above 7. Also, the adjustment to kinetic models (pseudo second order) and isotherms (Langmuir) is in agreement with the idea that the limiting step of adsorption occurs at the surface, which could be explained by the electrostatic interactions .
4.3.3 Drugs
There are not any publications on the adsorption of drugs with HTCs. In particular, tetracycline (Fig. 4.5) is an antibiotic that is widely used in aquaculture and veterinary medicine to improve growth rates and feed efficiencies. It is excreted as an unmetabolized compound, and the greatest known risk is the development of multiresistant bacterial strains that will no longer be able to be treated with current antibiotics. Zhu et al. (2014) have studied their adsorption with heat-activated HTC, obtaining the best results for treated char at the highest temperatures (700 °C). In this case, it must be assumed that because functional groups are not abundant in HTC, it is the specific surface that drives adsorption.
4.3.4 Endocrine Disrupting Chemicals
These compounds, as drugs, have biological activity. They can mimic the activity of natural hormones. They have been found in the effluents of municipal sewage treatment plants. For example, Sun et al. (2011) have tested HTCs as adsorbents of bisphenol A, 17α-ethinylestradiol, and phenanthrene. They deduced that compared to pyrolytic carbons, more polar HTC favours adsorption of the more polar compounds.
4.3.5 Metal Ions
Water pollution caused by heavy metals is a serious worldwide environmental problem with significant impacts on human health and on nature. One common method of removing these contaminants is adsorption. Although HTCs have been employed to adsorb many different contaminants, they have certain properties that make them useful for the adsorption of metals. In fact, they can compete with activated carbons with a specific surface area 10 times greater. Among these properties are the following:
-
They have a large number of oxygenated functional groups originating from the raw material. These functional groups are polar and, therefore, more favourable for ion adsorption.
-
These functional groups in the HTC can be used to give rise to other distinct functionalities, increasing or making the interaction with the metallic ions more specific.
-
They are acidic. This means that in most situations where there is a metal in a solution, it is found as a cation, and the HTC has given protons to the solution and is negatively charged. The negative charge on its surface attracts positively charged metal ions (see Fig. 4.6).
-
Some metals, especially in a high oxidation state, are dissolved as anions. The high number of functional groups on the HTC makes it possible to easily change the charge on its surface by modifying the pH of the medium. Thus, at a pH < PZC, the surface will be positively charged and will prefer to adsorb anions.
As mentioned above, the pH of the solution may affect the adsorption capacity, as it modifies the surface of the HTC. An example with a metal cation (Mn+) and a surface with a determined PZC are shown in Fig. 4.6. When pH < PZC, the HTC accepts protons, which positively charge its surface, repelling the cations. When pH > PZC, the HTC yields protons from its functional groups, remaining negatively charged and attracting the cations. The situation may be more complex than shown in Fig. 4.6, as the metal may form other species, such as anions or precipitated solids.
4.3.5.1 p-Block and d-Block Metals
Cr(VI) is considered to be one of the top priority toxic pollutants because of its mutagenicity and carcinogenicity properties. Chromium mainly comes from processes such as textile dyeing, leather tanning, and the chromic salt industry. Shen et al. (2016) prepared HTC from chitosan obtaining better results than those published with other adsorbent materials. Chitosan is a copolymer of 2-glucosamine and N-acetyl-2-glucosamine. Hydroxyl and amine groups are interesting substances to adsorb metals, and chitosan is a good scavenger from Pb(II) or Cd(II) at a high pH. Cr(VI) adsorption is favoured at low pH, but chitosan is not stable in an acidic solution. In principle, chitosan cannot be used to remove Cr(VI). But a chitosan carbonaceous adsorbent can exhibit good chemical stability. To maintain amino and hydroxyl groups, carbonisation must be carried out at low temperatures, which is possible with the HTC method. HTCs were prepared at different temperatures (140–220 °C). The HTC prepared at 140 °C does not appear to be completely charred, and those prepared at 200 °C and 220 °C had lower adsorption capacity than those prepared at 160 °C and 180 °C. This may be because of the destruction of functional groups, as lower N and O contents were observed when raising the preparation temperature. As with other metallic cations, the adsorption is fast, as equilibrium is reached in 30 min. The kinetic model that best fits the experimental results is that of pseudo-first order.
Cr(VI) adsorption is strongly pH dependent. This is because this metal is in the form of anions (CrO4 2−, HCrO4 −, Cr2O7 2−), which are attracted electrostatically to the positive charges of the surface. These are more abundant as pH decreases in the solution. In addition, the presence of amino groups favours this increase in positive charge, as they protonate more easily than the oxygenated functional groups.
The maximum adsorption capacity obtained by Shen et al. (2016) at a pH of 4.0 was 343 mg/g and showed a saturation effect, which can be explained by the existence of a limited number of active sites on the adsorbent. The adsorption capacity of the non-carbonized chitosan is slightly higher, but, as stated above, it cannot be used in highly acidic solutions. This also supports the idea that there is a limited number of active sites, so the isotherm data are better fitted to the Langmuir model than to Freundlich equation. The enthalpy and entropy values are negative, as well as free energy, which indicates an exothermic and spontaneous process. The adsorbent is easy to recycle: a centrifugation and a wash with NaOH is enough. After five cycles, more than 92% of the initial adsorption capacity is still maintained.
Copper and cadmium are heavy metals that are involved in several industrial processes. Regmi et al. (2012) have studied their adsorption obtaining excellent results in some experimental conditions. They observed, as with other cations in solutions, that at an acidic pH level (less than 5), the adsorption is very low, as the surface of the HTC is protonated and repels the positive charges of the cations (see Fig. 4.6). The adsorption increases above a pH of 5 because the surface groups are not protonated; therefore, the negative charge that attracts the cations predominates. It also influences the precipitation of metals. Thus, copper precipitates at a pH of 7 and cadmium at a pH of 10. But both are removed at a pH of 5, which can be due only to adsorption. There are big differences in the adsorption capacities whether we use HTC activated with KOH, inactivated HTC, or the starting biomass (switchgrass).
Liu and Zhang (2011) have also studied the adsorption of Cu(II) on activated HTC. Likewise, they have observed that the adsorbed amount increases with pH. This occurs for both pyrolytic carbons and HTCs, but with the difference that in the latter, having a PZC in the study range (pH of 1–6), the influence of pH is greater, rapidly increasing the adsorbed amount when pH > PZC, as in that situation the predominant charge at the surface is negative (Liu et al. 2010). It should be added that the HTCs present a greater number of functional groups, so the number of charges of any type on the surface will be greater than in pyrolytic carbons. A negative charge attracts the cation Cu(II).
Sun et al. (2015) studied the adsorption of Cd(II) with non-modified HTCs and HTCs modified with KOH. The modified carbons showed a higher adsorption capacity, as they reduced about 90% of the Cd(II) in the solution, while the unmodified HTCs barely removed 10%. This is because of the greater number of oxygen groups on the surface of the modified carbon. These isotherms are better fitted to the Langmuir model than the Freundlich equation, which indicates homogeneity at the surface. As described in previous paragraphs (Regmi et al. 2012), when the pH is lower than the PZC, the surface of the HTC is positively charged, as is the cadmium cation, so adsorption is low. By using a pH higher than the PZC, the surface charge is negative and the adsorption increases sharply.
Demir-Cakan et al. (2009) studied the adsorption of Cd(II) and Pb(II) in HTCs prepared from glucose and acrylic acid to obtain a large number of carboxyl functional groups. The greater the amount of acrylic acid used in the hydrothermal carbonisation, the greater the adsorption of both metals. The capacity of the best HTC is superior to that of other adsorbents commonly used for the adsorption of Pb(II) and similar to them in the adsorption of Cd(II). The adsorption isotherm of both metals is better fitted to the Freundlich model than Langmuir equation, which the authors attribute to the heterogeneity of the surface. A similar work has been published on the adsorption of Cd(II) and Pb(II), but the carboxyl groups were obtained by oxidation of the HTC at 300 °C (Chen et al. 2011). The adsorbed amount is increased by releasing Pb(II) instead of Cd(II), and if oxidised HTC is used, the adsorbed amount is multiplied several times. In addition, in this experiment, the oxidised HTC was found to be superior to other common adsorbents when Pb(II) is used and competitive with them when using Cd(II).
The adsorption of Pb(II) with HTCs has been studied by Liu and Zhang (2009). The adsorbed amount is low at very acidic pH levels, but improves, reaching a maximum near pH = 5 and decreasing with a higher pH. Above pH = 5, hydroxylated compounds of Pb(II) are formed in which the interaction with the negatively charged surface weakens as the charge decreases. Pb(II) adsorption can be greatly improved by oxidising the HTC with hydrogen peroxide (Xue et al. 2012). In addition, the adsorption equilibrium is reached faster. Note that a change in adsorption has been observed: the HTC isotherm fits better to the Freundlich model and the modified HTC to the Langmuir model. The authors proposed that this is because the oxidation decreases the heterogeneity of the surface. They also pointed out that in previous work, they detected that Pb(II) is removed when a pyrolytic coal is used, by the precipitation mechanism. Crystalline compounds are formed which can be detected by XRD. But with the HTCs, these crystals are not observed. From published studies on Pb(II) adsorption, it can be concluded that the predominant interaction is that of the metal with oxygen atoms on the surface of the HTC.
4.3.5.2 f-Block Metals
A metallic element that is very interesting to adsorb is uranium. And it is probably this element that is the most studied using HTCs as adsorbents. Interest is because of two reasons. Uranium is used for the generation of electricity and is a nonrenewable resource, so it is valuable and worth the time and effort to recover it. But it is also an element that can affect the ecosystem because of the radiochemical and toxic effects that it presents. Carbonaceous materials are suitable adsorbents, as they have a great stability against radiation, heat, acids, and bases. Specifically, against radiation, the effect of gamma irradiation with doses from 1 to 100 kGy has been studied (Yang et al. 2014). The adsorption capacity decreases slightly, and the selectivity towards the U(VI) is hardly affected, even at the highest doses of radiation. The only problem it presents is that they usually have few functional groups so that unmodified carbons exhibit relatively low selectivity and poor adsorption capacity towards uranium. An advantage of HTCs is that, although they have a lower specific surface area than other carbon materials, they have a greater number of oxygenated surface groups, which is an advantage in the case of adsorbates such as U(VI), as we will see below.
Several HTCs have been used to adsorb U(VI). It is soluble in moderate concentrations only at acidic pH levels, up to pH = 4.5. Under these conditions, it is found as uranyl cation (UO2 2+).
With respect to synthesis of HTCs for uranium removal, Li et al. (2016) prepared carbon microspheres from Saccharomyces cerevisiae cells by hydrothermal methods, followed by chemical modification using the Hummers method to increase the number of fungal groups. Li et al. (2014) used another approach. Some of the compounds used as an antidote in cases of acute poisoning by uranyl cations are polyphenols. As a result of this, they used a type of polyphenols (bayberry tannins) with glyoxal introduced as a cross-linking agent, with the target of obtaining a large amount of phenol groups on a stable material. They obtained more than 10 mmol/g between phenol and carboxyl groups. In addition, they achieved greater porous development when using glyoxal than when using only tannins. Other authors have also used glyoxal (Yang et al. 2014), not as a cross-linking agent but as a carbon source. They mixed glyoxal with acrylonitrile to have -CN groups that could react with NH2OH·HCl to obtain amidoxime groups at the surface and avoid the formation of carboxyl groups. Amidoxime is known to be an effective bidentate ligand of U(VI). Yu et al. (2014) tested another strategy (taking advantage of the tendency of uranium to form complexes with phosphates) to prepare HTCs with these functional groups. For this, they obtained HTC from glucose and oxidised them in air at moderate (i.e., 300 °C). They then reacted the HTC with o-phosphoethanolamine and N,N′-dicyclohexylcarbodiimide to functionalize the surface. Even simpler was the method of Zhang et al. (2014a), who prepared HTC with phosphate groups in a single step mixing glucose and phosphoric acid. Song et al. (2012) followed a similar strategy opting for a multidentate ligand that usually has good selectivity towards actinides in the 5-azacytosine column-actinide/lanthanide separation processes. After the hydrothermal process and calcination in air at a moderate temperature, the HTC was first reacted with ethylenediamine and subsequently with 5-azacytosine. The oxidation process increased the number of carboxyl groups, unlike for Li et al. (2014). Wang et al. (2012) chose another ligand, the salicylideneimine, known for its ability to coordinate with hexavalent cations. For this, they performed a synthesis similar to that of Song et al. (2012). The difference is in using salicylaldehyde after the reaction with ethylenediamine. Liu et al. (2013) opted for a two-stage synthesis, in which after obtaining HTC from pine needles, this was oxidised with 2 M HNO3 to obtain a greater number of carboxyl groups. In this case, HTC porosity dramatically decreases, but in spite of this, a considerably better adsorption of U(VI) is obtained. Zhang et al. (2013) prepared HTC from glucose and performed oxidation in air at temperatures between 150 °C and 300 °C, without further surface modification. The number of carboxyl groups obtained depended mainly on the oxidation temperature and not on time. This amount of functional groups influences the adsorption capacity. It is also possible to use simpler processes such as those proposed by Kumar et al. (2011) that used lignocellulosic material (switchgrass, Panicum virgatum) to obtain HTCs in a single stage without further chemical modification. A synthesis was also performed with chitosan (Zhang et al. 2014b), a material chosen for its abundance, low cost, and numerous amino groups in its composition.
When adsorption of U(VI) was carried out, a great influence of pH (from 1 to 4.5) was observed, with a low adsorption at very acidic pH levels and higher adsorption at a pH of 4. It was explained (Fig. 4.6) by the fact that at low pH levels, functional groups are protonated, and so there is electronic repulsion with U(VI) in its UO2 2+ form. But as the pH increases, the positive charge on the surface decreases, as well as the repulsion. Some authors increased the range of study to pH = 8. It is possible that the uranyl cation is soluble if it forms a CO3 2− coordination compound (Kumar et al. 2011). In this case, the maximum amount of adsorption is obtained at pH = 6.0. It should be noted that above this pH, the U predominant species in a solution have a negative charge, whereas at pH levels <6.0, they have a positive charge. As for ionic strength, its effect is not clear, and with some of the adsorbents cited, it does not produce an effect, while with some others it does. Overall, the adsorption process of U(VI) is very fast. Some of the published results are shown in Table 4.4. Two factors might be responsible: the absence of developed porosity, which prevents the existence of a prolonged diffusion process, and the strong interaction between the cation and the HTC’s active sites. This quickness in adsorption is habitual when metallic cations are used.
The kinetics conform to a pseudo-second-order model, which is based on the fact that the limiting step of the reaction is because of a process of chemisorption on the surface, which includes the formation of bonds. In addition, the intraparticle diffusion model presents a poor fit, confirming that the slow step proceeds on the surface. This adsorption mechanism also confirms the adsorption isotherm data, which are better fitted to the Langmuir model than the Freundlich model. The Langmuir model assumes monolayer adsorption, which would be closer to a chemisorption process. It also supports the model that shows an increase of the quantity adsorbed with an increase in temperature. Li et al. (2014) and Wang et al. (2012) adjusted the isotherm data to the Dubinin-Radushkevich model and obtained a binding energy whose positive value (+12.54 kJ/mol) also suggesting chemisorption. This data is supported by positive values of enthalpy and entropy and the negative value of the free energy. The adsorption of U(VI), therefore, is spontaneous and endothermic. Liu et al. (2013) prepared two materials with very different specific surfaces and found that the one with a larger surface area saturated before the other material, demonstrating that the number of active sites for U(VI) adsorption is limited and does not depend directly on the value of the specific surface.
The results obtained for the adsorption of U(VI) in several adsorption works about this metal cation are shown in Table 4.5. It can be seen that the amount adsorbed strongly depends on the relative concentrations of HTC and U(VI), presenting very different values. Better results are obtained when the pH level is less than 5.0. It should be noted that when both data have been measured and published, there is a close relationship between the capacity measured experimentally and that indicated for the monolayer by the Langmuir model. This fact indicates that adsorption, as indicated above, must be in a single layer and probably as a chemisorption.
Another similar ion (actinide and presenting environmental problems resembling U(VI)) is Th(IV). It appears in many minerals for industrial use (Syed 1999), so it is frequently found in sewage. In addition, it is an element used as a nuclear fuel and in alloys, so it is also interesting to recover. Zhou et al. (2015) used a similar char that they had already used to adsorb U (VI) (Zhang et al. 2013), which was oxidised in air at a moderate temperature to increase the number of oxygenated functional groups. The maximum amount removed from Th (IV) is achieved at pH = 3.5, but the authors speculated that it may be because of two factors: higher adsorption (because of the decrease in positive charge on the HTC) and the beginning of precipitation as Th(OH)4. The effect of the treatment temperature in air is clearer. As temperature rises, the HTCs have higher oxygen content, and this increases the amount of adsorbed thorium. The data on the effects of contact time (fast adsorption), application of mathematical models to kinetics (adjusted to the pseudo-second-order model) and isotherms (where Langmuir model represents the phenomenon better than the Freundlich model), and the effect of temperature (increasing adsorption by raising the temperature) indicate that the adsorption process, as with U(VI), is chemical adsorption .
4.3.5.3 Mixture of Metals
Few studies have been done on simultaneous metal adsorption with HTCs (Sun et al. 2015). It has been observed that the smaller the radii of the hydrated cations are, the greater the adsorption; this is also described with other adsorbents. Adsorption is greater when using HTCs with high oxygen content. In addition, it has been measured that the adsorption of a metal is lower if it is adsorbed from a mixture than if it is adsorbed from a pure solution. This is because of the competition between several metals for active adsorption sites; in other words, the number of active adsorption sites on the surface of the HTC is limited.
Xue et al. (2012) have studied the adsorption of a mixture of heavy metals in an aqueous solution using HTC in columns. Conclusions are similar to work on batch adsorption (Sun et al. 2015), which reinforces the idea that there are a limited number of active sites in HTCs.
As general remark for metal adsorption, it can be deduced that this process is governed by the existence of active sites, where a specific bond occurs. This fact is supported by kinetic, adsorption isotherms and thermodynamics, in which the Langmuir model, which supposes a limited number of active sites, is more adequate than the Freundlich model. In several cases, the existence of chemisorption may be assumed. It can also be generally deduced that the adsorption of metals depends heavily on pH levels. This can influence the charge of the species containing the metal and also the charge of the HTC surface. The priority mechanism seems to be similar to ion exchange .
4.3.6 Phosphorus
Phosphorus-containing minerals are a limited resource, and they are essential for agriculture as a nutrient. In addition, when phosphorus compounds enter an aquatic environment, they may cause eutrophication problems. Therefore, there is a high level of interest in removal (to reduce environmental problems) and recovery (to use in agriculture) of phosphorus. HTCs doped with lanthanum have been proposed as phosphorus adsorbents (Dai et al. 2014). Some authors have found that efficiency of this HTC is higher than La(OH)3 and that HTC without lanthanum showed no phosphorus removal activity. The lanthanum content and phosphorus removal efficiency were increased with the hydrothermal carbonisation time. Lanthanum must be bonded to functional surface groups, because no lanthanum compounds were detected with XRD. The pH levels of the solution and coexisting anions have a limited effect on the activity of La-HTC, which can be used in a wide range of situations. As is the case for other adsorbates that bind to specific surface sites, the Langmuir model better describes the adsorption isotherm than the Freundlich model .
4.3.7 Phenol s
Liu and Zhang (2011) have studied the adsorption of phenol on activated HTC. They have worked with different pH levels, and the adsorption has not deflected. This result was attributed to the fact that the phenol is in molecular form in that range of pH and is not affected by the positive charges on the surface of the HTC.
4.3.8 Wastewater
In general, adsorption studies have been performed in simple solutions. But it is also possible to study the adsorption on HTCs in more complex mixtures. The purification of water resulting from fermentation of lactic acid has been studied (Laube and Reza 2016). In this work, several different adsorbents were used, and it was concluded that HTCs prepared from willow and poplar are the most effective, combining good adsorption capacity and high physical and chemical endurance.
4.3.9 Reusability
To reduce the overall cost of practical applications, adsorbent reusability is significant. Depending on the adsorbent and adsorbate, regeneration methods are diverse. To date, some studies have analysed the reusability of HTCs.
For adsorption of U(VI: Li et al. 2016), the adsorbed amount was found to be very low at pH = 1.0 and higher when the pH level was raised to pH = 4.5. A strong acid treatment method was used to regenerate the HTC, and after five cycles no significant loss of adsorbent capacity had been observed. The same method was employed by Zhou et al. (2015) for the regeneration of HTC used to adsorb Th (IV). The same technique was used to regenerate an HTC fibre on which paraquat had been adsorbed. In this case, the manipulation produced the breakage of the external HTC separating it from the internal fibre, which resulted in a loss of efficiency (Zhou et al. 2015). Even so, it remained at 83% after five cycles. Yu et al. (2014) compared this method with the use of EDTA to recover the adsorbed U (VI) and concluded that the use of EDTA is preferable, even at concentrations lower than those of the acid. Shen et al. (2016) studied the adsorption of an anion (CrO4 2−) in place of the cations studied by the previously cited authors so that their regeneration method was the opposite: they used a 2 M NaOH solution. The loss of the adsorption capacity was 7.7% after five cycles, indicating that it is a good method for regeneration. This ease of regeneration is because of the operation of HTCs as ion exchangers, thanks to a large number of functional groups on their surfaces .
4.4 Conclusions
The hydrothermal carbonisation method is an alternative to the classical pyrolysis method. It has several advantages, among which are a lower production of pollutants, better yield, and lower energy consumption, as a lower temperature is used and wet raw material can be used. These characteristics allow for the inclusion of this procedure within Green Chemistry.
The product obtained is characterised by low porous development, high oxygen content, and a large number of functional groups on its surface. It can be activated or prepared by using templates to increase its porosity. It can also be functionalized in very different ways, which is favoured by the abundance of preexisting functional groups, as well as being used to coat other materials.
It has been used in several applications, for instance, the adsorption of contaminants in water. In this field, it can be said that research is just beginning, as the number of bibliographical references found in the preparation of this document (year: 2016) is low.
HTCs have been used as adsorbents for many different substances, with good results. But to date, they appear to be especially useful for the adsorption of ions in a solution, whether organic or inorganic. This is because the existence of a large number of functional groups also allows for a large number of positive or negative charges as a function of the pH of the solution. These charges attract the ions with the opposite charge. Functional groups also facilitate the regeneration of the adsorbent by using acids or alkalis.
In summary, HTCs are materials with a wide range of possibilities for employment, and they are expected to be investigated and used frequently in the future.
References
Alatalo SM, Mäkilä E, Repo E, Heinonen M, Salonen J, Kukk E, Sillanpääa M, Titirici MM (2016) Meso- and microporous soft templated hydrothermal carbons for dye removal from water. Green Chem 18:1137–1146
Bandura AV, Lvov SN (2006) The ionization constant of water over wide range of temperature and density. J Phys Chem Ref Data 35:15–30
Bergius F, Specht H (1913) Die Anwendung hoher Drucke bei chemischen Vorgngen und eine Nachbildung des Entstehungsprozesses der Steinkohle, vol 58. Verlag Wilhelm Knapp, Halle an der Saale
Cao X, Ro KS, Chappell M, Li Y, Mao J (2011) Chemical structures of swine-manure chars produced under different carbonization conditions investigated by advanced solid-state 13C nuclear magnetic resonance (NMR) spectroscopy. Energ Fuel 25:388–397
Chen Z, Ma L, Li S, Geng J, Song Q, Liu J, Wang C, Wang H, Li J, Qin Z, Li S (2011) Simple approach to carboxyl-rich materials through low-temperature heat treatment of hydrothermal carbon in air. Appl Surf Sci 257:8686–8691
Dai L, Wu B, Tan F, He M, Wang W, Qin H, Tang X, Zhu O, Pan K, Hu Q (2014) Engineered hydrochar composites for phosphorus removal/recovery: lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw. Bioresour Technol 161:327–332
Demir-Cakan R, Baccile N, Antonietti M, Titirici MM (2009) Carboxylate-Rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem Mater 21:484–490
Durán-Valle CJ (2006) Geometrical relationship between elemental composition and molecular size in carbonaceous materials. Appl Surf Sci 252:6097–6101
Falco C, Perez Caballero F, Babonneau F, Gervais C, Laurent G, Titirici MM, Baccile N (2011) Hydrothermal carbon from biomass: structural differences between hydrothermal and pyrolyzed carbons via 13C solid state NMR. Langmuir 27:14460–14471
Hao W, Björkman E, Lilliestråle M, Hedin N (2014) Activated carbons for water treatment prepared by phosphoric acid activation of hydrothermally treated beer waste. Ind Eng Chem Res 53:15389–15397
Hu B, Yu SH, Wang K, Liu L, Xu XW (2008) Functional carbonaceous materials from hydrothermal carbonization of biomass: an effective chemical process. Dalton Trans 40:5414–5423
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Kruse A, Funke A, Titirici MM (2013) Hydrothermal conversion of biomass to fuels and energetic materials. Curr Opin Chem Biol 17:515–521
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An assessment of U (VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manag 92:2504–2512
Laube H, Reza MT (2016) Application of biosorbents for ion removal from sodium lactate fermentation broth. J Environ Chem Eng 4:10–19
Li B, Ma L, Tian Y, Yang X, Li J, Bai C, Yang X, Zhang S, Li S, Jin Y (2014) A catechol-like phenolic ligand-functionalized hydrothermal carbon: one-pot synthesis, characterization and sorption behaviour toward uranium. J Hazard Mater 271:41–49
Li F, Li D, Li X, Liao J, Li S, Yang J, Yang Y, Tang J, Liu N (2016) Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem Eng J 284:630–639
Liu F, Guo M (2015) Comparison of the characteristics of hydrothermal carbons derived from holocellulose and crude biomass. J Mater Sci 50:1624–1631
Liu Z, Zhang FS (2009) Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J Hazard Mater 167:933–939
Liu Z, Zhang FS (2011) Removal of copper (II) and phenol from aqueous solution using porous carbons derived from hydrothermal chars. Desalination 267:101–106
Liu Z, Zhang FS, Wu J (2010) Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 89:510–514
Liu YH, Wang YQ, Zhang ZB, Cao XH, Nie WB, Li Q, Hua R (2013) Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl Surf Sci 273:68–74
Martín-Jimeno FJ, Suárez-García F, Paredes JI, Martínez-Alonso A, Tascón JMD (2015) Activated carbon xerogels with a cellular morphology derived from hydrothermally carbonized glucose-graphene oxide hybrids and their performance towards CO2 and dye adsorption. Carbon 81:137–147
Quian HS, Antonietti M, Yu SH (2007) Hybrid “golden fleece”: synthesis and catalytic performance of uniform carbon nanofibers and silica nanotubes embedded with a high population of noble-metal nanoparticles. Adv Funct Mater 17:637–643
Regmi P, Garcia Moscoso JL, Kumar S, Cao X, Maob J, Schafran G (2012) Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J Environ Manag 109:61–69
Roldán L, Marco Y, García-Bordejé E (2016) Bio-sourced mesoporous carbon doped with heteroatoms (N,S) synthesised using one-step hydrothermal process for water remediation. Micropor Mesopor Mat 222:55–62
Román S, Valente Nabais JM, Ledesma B, González JF, Laginhas C, Titirici MM (2013) Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Micropor Mesopor Mat 165:127–133
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem Eur J 15:4195–4203
Shen F, Su J, Zhang X, Zhang K, Qi X (2016) Chitosan-derived carbonaceous material for highly efficient adsorption of chromium (VI) from aqueous solution. Int J Biol Macromol 91:443–449
Song Q, Ma L, Liu J, Bai C, Geng J, Wang H, Li B, Wang L, Li S (2012) Preparation and adsorption performance of 5-azacytosine-functionalized hydrothermal carbon for selective solid-phase extraction of uranium. J Colloid Interf Sci 386:291–299
Sun K, Ro K, Guo M, Novak J, Mashayekhi H, Xing B (2011) Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour Technol 102:5757–5763
Sun K, Tnag J, Gong Y, Zhang H (2015) Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ Sci Pollut R 22:16640–16651
Syed HS (1999) Comparison studies adsorption of thorium and uranium on pure clay minerals and local Malaysian soil sediments. J Radioanal Nucl Chem 241:11–14
Titirici MM (2012) Hydrothermal carbons: synthesis, characterization, and applications. In: Tascon JMD (ed) Novel carbon adsorbents. Elsevier Ltd, Amsterdam, pp 351–399
Titirici MM, Antonietti M (2010) Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem Soc Rev 39:103–116
Titirici MM, White RJ, Falco C, Sevilla M (2012) Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci 5:6796–6822
Wang H, Ma L, Cao K, Geng J, Liu J, Song Q, Yang X, Li S (2012) Selective solid-phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J Hazard Mater 229–230:321–330
White RJ, Antonietti M, Titirici MM (2009) Naturally inspired nitrogen doped porous carbon. J Mater Chem 19:8645–8650
Xue Y, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR, Ro KS (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200:673–680
Yang X, Li J, Liu J, Tian Y, Li B, Cao K, Liu S, Hou M, Li S, Ma L (2014) Simple small molecule carbon source strategy for synthesis of functional hydrothermal carbon: preparation of highly efficient uranium selective solid phase extractant. J Mater Chem A 2:1550–1559
Yu XF, Liu YH, Zhou ZW, Xiong GX, Cao XH, Li M, Zhang ZB (2014) Adsorptive removal of U(VI) from aqueous solution by hydrothermal carbon spheres with phosphate group. J Radioanal Nucl Chem 300:1235–1244
Zhang ZB, Nie WB, Li Q, Xiong GX, Cao XH, Liu YH (2013) Removal of uranium(VI) from aqueous solutions by carboxyl-rich hydrothermal carbon spheres through low-temperature heat treatment in air. J Radioanal Nucl Chem 298:361–368
Zhang ZB, Zhou ZW, Cao XH, Liu YH, Xiong GX, Liang P (2014a) Removal of uranium(VI) from aqueous solutions by new phosphorus-containing carbon spheres synthesized via one-step hydrothermal carbonization of glucose in the presence of phosphoric acid. J Radioanal Nucl Chem 299:1479–1487
Zhang WL, Zhang ZB, Cao XH, Ma RC, Liu YH (2014b) Uranium adsorption studies on hydrothermal carbon produced by chitosan using statistical design method. J Radioanal Nucl Chem 301:197–205
Zhao R, Wang Y, Li X, Sun B, Li Y, Ji H, Qiu J, Wang C (2016) Surface activated hydrothermal carbon-coated electrospun PAN Fiber membrane with enhanced adsorption properties for herbicide. ACS Sustain Chem Eng 4:2584–2592
Zhou ZW, Xiong GX, Liu YH, Cao XH, Zhang ZB (2015) Removal of thorium(IV) from aqueous solutions by carboxyl-rich hydrothermal carbon spheres through low-temperature heat treatment in air. Desalin Water Treat 54:2516–2529
Zhu X, Liu Y, Zhou C, Luo G, Zhang S, Chen J (2014) A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 77:627–636
Acknowledgements
Authors acknowledge the support provided by Universidad de Extremadura and Junta de Extremadura/FEDER ref. GRU15123.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Durán-Valle, C.J., Botet-Jiménez, A.B., Omenat-Morán, D. (2017). Hydrothermal Carbonisation: An Eco-Friendly Method for the Production of Carbon Adsorbents. In: Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Ávila, H. (eds) Adsorption Processes for Water Treatment and Purification . Springer, Cham. https://doi.org/10.1007/978-3-319-58136-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-58136-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58135-4
Online ISBN: 978-3-319-58136-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)