Abstract
A new hydrothermal cross-linking chitosan (HCC) was prepared by hydrothermal reaction at a mild temperature (180 °C) to diminish the solubility in acid solution, and the amine groups were almost retained during the hydrothermal reaction characterized by FT-IR and the dissolution rate in pH 3 solution decreased from 89.6 to 12.6 %. The effects of initial pH, contact time, initial concentration and temperature on the sorption capacity are discussed using 24 full-factorial central composite design using response-surface methodology. The HCC showed the highest uranium sorption capacity at initial pH of 7.92 and contact time of 273.6 min with 24 full-factorial central composite design and the maximum adsorption capacity was 273 mg/g. The adsorption process could be well defined by the Langmuir isotherm and the thermodynamic parameters, ∆G°(298 K), ∆H° and ∆S°, demonstrated shown that the sorption process of U(VI) onto HCC was feasible, spontaneous and endothermic in nature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adsorption, due to its high efficiency and ease of handling, has been developed for the removal and recovery of uranium from radioactive wastes in consideration of the dual significance of the potential environmental health threat and a nonrenewable resource of nuclear energy [1]. The carbonaceous materials such as activated carbon [2, 3], carbon nanotubes [4, 5] and carbon fiber [6] have been gradually applied to this area because of their higher thermal and radiation resistance than organic exchanger resins and better acid–base stability compared with familiar inorganic sorbents.
Chitosan, modified from chitin, is the second most abundant naturally polysaccharide in the world, which has good biological features such as non-toxicity, hydrophilicity, biocompatibility, biodegradability and antibacterial properties. On chitosan’s chains, there are lots of free amine groups and hydroxyl groups, which have been reported to be coordination sites with metal ions [7]. Although chitosan has good adsorption capacity, it lucks good mechanical properties and it can be easily dissolved in acid solutions due to the presence of amine groups within its structure. Hence, numerous studies have been devoted to homogeneous or heterogeneous crosslinking of chitosan with di-or polyfunctional agents, such as sodium tripolyphosphate, glutaraldehyde, ethyleneglycol diglycidyl ether and epichlorohydrin [8–12]. However, the chemical crosslinking methods usually require many synthetic steps and in some toxic reagents are employed. Therefore it is necessary to investigate a “green” cross-linking method via a one-step process.
The classical method of optimizing sorption conditions by varying the level of one parameter over a certain range, while holding the rest of the test variables constant. This single-factor-at-a-time strategy is generally time-consuming and requires a large number of experiments to be carried out. The effect of interactions between the variables isn’t considered and the method is inadequate for a full understanding of the response surface.
In this work, a new hydrothermal cross-linking chitosan (HCC) was prepared by hydrothermal reaction at a mild temperature (180 °C). The synthetic procedure is illustrated in Fig. 1, its structural and compositional characteristics were determined by SEM and FT-IR. The effects of initial pH, contact time, initial concentration and temperature on the sorption capacity are discussed using 24 full-factorial central composite design employing response-surface methodology [13–15]. In addition, the adsorption kinetics, isotherm models and thermodynamics were also studied.
Materials and methods
Materials
Chitosan, with a deacetylation percentage of 85 % was purchased from the reagent factory of shanghai of Chinese medicine crop. All the other chemical reagents were of AR grades. For the preparation of a stock uranium(VI) solution, 1.1,792 g U3O8 was put into a 100 mL beaker, and 10 mL hydrochloric acid (ρ = 1.18 g/mL), 2 mL 30 % hydrogen peroxide were added. The beaker was heated to evaporate the solution almost to dryness and then 10 mL hydrochloric acid was added into a solution. The solution was then transferred to a 1,000 mL volumetric flask, diluted with distilled water and shaken to produce the stock solution of 1 mg/mL uranium(VI). The uranium solutions were prepared by diluting the stock solution to appropriate volumes depending upon the experimental requirements.
Preparation of hydrothermal cross-linking chitosan
Typically, 2.0 g of pure chitosan (CTS) was dissolved in 18 mL 2 % (V/V) acetic acid solution and added into a 50 mL Teflon-lined stainless steel autoclave, and then sealed and tempered at 180 °C for 12 h. The products were finally filtered and washed with deionized water, and then dried.
Characterization
The zeta-potential value was obtained by POWEREACH JS94H micro-electrophoresis apparatus. Surface morphology was characterized on a JEOL JSM-5900 scanning electron microscopy. The FT-IR spectra were recorded on Nicolet Nexus 870 Fourier transform infrared spectrometer using the KBr pellet technique (1:50) with the resolution 2/cm. To determinate the dissolution rate of CTS and HCC in aqueous solution, typically, 0.1 g sample was added to the aqueous solution with fixed initial pH varying from 3.0 to 10.0 adjusted by 0.1 mol/L NaOH and HCl solution and then shook for 12 h. The volume of solution was 100 mL and the dissolution temperature was 20 °C. This was followed by filtration and drying for 3 h at 50 °C.The dissolution rate was calculated by Eq. (1)
where m 0 is the initial mass of sample, and m e is the mass of sample after dissolution.
Adsorption experiments
To determinate the U(VI) adsorptive capacity onto samples, typically, 0.010 g of adsorbents was added to U(VI) solution with the fixed initial pH varying from 4.0 to 8.0 (adjusted with 0.1 mol/L HNO3 and 0.1 mol/L NaOH) and with U(VI) concentrations ranging from 20 to 80 mg/L. The batch sorption was performed in a reciprocating water bath shaker with concussion speed of 200 rpm with predetermined time and intervals (60 min, 5 °C). The remaining U(VI) of solution was determined by the method of Arsenazo-III spectrophotometry by 721 spectrophotometer (Shanghai Spectrum Instruments Co., Ltd) [16]. The amounts of U(VI) adsorbed(q, mg/g) and distribution coefficient (K d, mg/L) were calculated according to the Eqs. (2) and (3)
where C 0 and C e are the uranium concentration in the initial and equilibrium solution (mg/L) respectively; V the volume of the U(VI) solution(L), m is the mass of dry adsorbents (g).
Experimental design for adsorption studies
The optimum condition for sorption of UO2 2+ by HCC was determined by means of the central composite design (CCD) under response surface methodology (RSM). Statistical design of experiments refers to the process of planning to experiments so that appropriate data that can be analyzed by statistical methods will be collected resulting in valid and objective conclusions [17]. Response surface methodology is a collection of mathematical and statistical techniques based on the fit of a polynomial equation to the experimental data, which must describe the behavior of a data set with the objective of making statistical previsions [18]. The experiments were carried out by the four independent process variables, initial pH (X 1), temperature (X 2), initial UO2 2+ concentration (X 3) and contact time (X 4) chosen according to the central composite design (CCD). The optimization of UO2 2+ removal was performed using the four chosen independent process variables with seven replicates at centre points, employing a total of 31 experiments in this study. The design matrix for the four variables is varied at five levels (−2, −1, 0, +1, +2). The full model equation with linear and quadratic terms for predicting the optimal response was given as,
In the equation, b 0 represents the intercept. The terms, b i, b ii, and b ij represent the linear effects, second order effects, and dual interaction between the investigated parameters. For the current model investigated in this study, the second-order polynomial equation can be presented as,
The range and the level of experimental variables investigated in this study are shown in Table 1.
The four variables were varied at two levels (+1 and −1). The higher and the lower levels of the variables were designated as +1 and −1, respectively. The centre point was designated as 0, with −2 and +2 showing star points (a) which are calculated using the following equation:
Codification of the variable levels consists of transforming each real value investigated into a coordinate on a scale with dimensionless values proportional to its localization in the experimental space [19].
The following equation can be used to transform a real value (z i ) into a coded value (x i ) for statistical calculations:
where Δz i is the difference between the real values designed for experiments and z 0 the real value at the central point. MatLab 7.1 and Excel were used for regression and graphical analysis of the data obtained. The optimum values of the selected variables were obtained by solving the regression equation and by analyzing the response surface contour plots.
Result and discussion
Characterization
Ft-ir
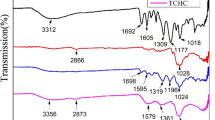
The FT-IR spectra of pristine chitosan (CTS) and HCC are shown in Fig. 2. The broad band at 3,400/cm was assigned to the O–H and –NH2 stretching vibration, and the peak appearing at 2,918 and 2,852/cm were due to the –CH2 symmetric and asymmetric absorption, and the characteristic band at 1,661 and 1,419/cm was attributed to the C=O and C–N vibration in the amide group associated with few remaining acetamide groups present on the chain due to incomplete deacetylation of chitosan. The two peaks at 1,082 and 1,028/cm correspond to stretching vibrations of the primary and secondary –OH groups respectively. The absorption band appearing at 900/cm was a characteristic absorption band of the beta-d-glucose unit. They all demonstrated that amine groups were almost retained during the hydrothermal reaction and HCC was rich in amino groups, which was also confirmed by that the zeta-potential value of HCC that was positive at the pH value below 6.5. These could be interpreted as the degradation of the chitosan chain and decomposition of the pyranose ring through dehydration. Amine groups didn’t react with hydroxyl groups because its protonation in acid environment and hydroxy groups were not connected to strong electron-withdrawing group. Chitosan chain generate oligosaccharides and aromatic compounds in dehydration reaction; Oligosaccharides dehydration and intermolecular crosslinking in higher temperature.
SEM
Figure 3 showed the SEM images of HCC with magnification of 5,000 and 10,000, respectively. It could be seen that HCC has an irregular alveolate surface with some carbon microspheres (2 μm) and loose, porous structure. It demonstrated that hydrothermal carbonization for CTS was similar to hydrothermal carbon spheres (HCSs).However, amino groups tend not to be involved in hydrothermal reaction and cause irregular alveolate surface.
Dissolution rate
The dissolution rate of CTS and HCC are shown in Fig. 4. At lower pH, CTS can be easily dissolved due to its amine groups, dissolution rate reach up to 89.6 % at pH 3.0, then keep this high rate till to pH 6.0. In contrast, HCC has degradation-resistance at low pH, dissolution rate was only 12.6 % at pH 3.0 and decreased to 4.2 % above pH 5.0. All these demonstrate that hydrothermal cross-linking was an effective way to prevent chitosan degradation in acidic medium.
The optimization of adsorption parameters
The effect of experimental variables such as the initial pH, the contact time, the initial concentration and the temperature on UO2 2+ adsorption capacity of the adsorbent were investigated using RSM according to the CCD. The experiments were carried out with 16 factorial points, nine star points (1 at the center) and six replicate points used in the central composite design.
The relationship between the independent variables and the responses are expressed by the following quadratic model:
The ANOVA (analysis of variance) of data was carried out at 96.6 % confidence level to check the fitting of the experimental values to the predicted ones (Table 2). F test for the significance level of data gave P < 0.05 with a model F value of 32.66 revealing that this regression is statistically significant. The correlation coefficient value (R 2) of 93.6 % indicated that there was a high correlation between the observed values and the predicted ones.
Significance of each coefficient present in Eq. (7) is determined by the student’s t test and P-values. The coefficients of independent variables t and P values according to the investigated parameters are given in Table 3. The influences of initial pH, temperature and initial concentration on the adsorption process were considered to be statistically significant as main affects (P < 0.05).
It is known that the larger the coefficient, the larger is the effect of the related parameter. When the effect of a factor is positive, an increase in the value of the removal efficiency is observed if the factor changes from low to high level. In contrast, if the effect is negative, a reduction in removal efficiency occurs for the high level of the same factor [20].
The positive values of coefficients related to the initial concentration (X 3 = 24.015) and temperature (X 2 = 7.620) indicate that the initial concentration and temperature have positive effects on the adsorption. However, initial pH coefficient (X 1 X 1 = −14.875) has a negative cumulative effect on the adsorption of UO2 2+ from aqueous solution by HCC. Furthermore, the observation that the adsorption of UO2 2+ increases between pH 4.0–6.5 and decreases between pH 6.5–8.0 (Fig. 5) should also be taken account when considering the initial pH effect. The optimum experimental values in coded form were found for each parameter for the uptake capacity of UO2 2+ by Mintab: X 1 = 1.96, X 2 = 2.00, X 3 = 2.00, X 4 = 1.56.
These coded values can be transformed to real values using Eq. (6). The optimum values giving the maximum uptake of uranium ions (273 mg/g) were calculated to be at initial pH of 7.92, temperature of 40 °C, initial concentration of 80 mg/L and contact time of 273.6 min.
Effect of initial pH and initial concentration on the UO2 2+ uptake
Effluents from nuclear industry containing uranium have a variable pH (acidic to alkaline) [21]. The effects of initial pH and initial UO2 2+ concentration on the uptake are shown in Fig. 6. The metal uptake increased with increasing initial solution pH ranging from 4.0 to 6.5; pH values higher than 6.5 reduced the UO2 2+ uptake.On the other hand, metal uptake increased with increasing initial metal ions concentration ranging from 20 to 80 mg/L in all pH ranges.
The increase of metal uptake by increasing initial metal ions concentration is due to an increase in the driving force of the concentration gradient, rather than the increase in the initial metal ion concentration. Under the same conditions, if the concentration of metal ions in the solution is higher, the active sites of adsorbent are surrounded by more metal ions, and the process of adsorption is carried out more effectively. Therefore, the value of q increased with increasing initial metal ions concentration [22].
At lower pH conditions, UO2 2+ uptake was decreased due to increasing positive charge of the adsorbent surface. The pH values of aqueous solution influence the precipitation of U(VI) ions and ionization of surface functional groups. At low pH, the HCC surface was expected to be protonated and hence positively charged. Hence, adsorption of UO2 2+ ions was less preferred at low pH.An increase in pH resulted in a less positive HCC surface that adsorbed the cationic uranium species [(UO2)2(OH) 2+2 , UO2OH+,(UO2)3(OH) +5 ]. Around the circumneutral pH, U(VI) speciation was dominated by anionic uranyl carbonate [(UO2)2CO3(OH)3−,UO2(CO3) 4−3 , UO2(CO3) 2−2 ] and hence adsorption onto HCC decreased beyond pH 7.0 [23].
Effect of contact time and temperature on the UO2 2+ uptake
The effect of contact time and temperature on the uptake of UO2 2+ is shown in Fig. 7. The U(VI) uptake increased with increasing temperature ranging from 20 to 40 °C, the adsorption capacity reinforced with the rise of temperature indicating that the process is endothermic. The U(VI) uptake by HCC increased sharply at the beginning, and then gradually reached equilibrium after 180 min, which was deemed sufficient to establish sorption equilibrium. The faster adsorption rate at the beginning would be due to the larger concentration gradient.
Confirmation experiments
Further to support the statistical approach which have applied is suitable for the experimental design used, the confirmatory experiments were conducted with the effects as suggested by the model (pH, initial concentration, contact time, temperature). The effect of pH, initial concentration, contact time and temperature were shown in Fig. 8 and data is in accordance with the results obtained from optimized conditions.
Sorption kinetics studies
In order to explain the controlling mechanism of adsorption processes such as mass transfer and chemical reaction, two different kinetic models, pseudo-first order and pseudo-second order models were applied to test the sorption kinetics.
The pseudo-first order kinetic model and pseudo-second order kinetic model may be represented by Eqs. 9 and 10
where q e and q t refer to the amount of U(VI) adsorbed (mg g−1) at equilibrium time and any time (min), respectively; k 1 (min−1) and k 2 (g mg−1 min−1) are the rate constant of first and second order adsorption.
The adsorption kinetic parameters in Eqs. 9 and 10 were calculated from the slopes and intercepts of the fitted curves, and the results were shown in Table 4. Obviously, the correlation coefficient, R 2 of the pseudo-second-order was better than pseudo-first-order, which indicated that the pseudo-second-order kinetic model was more suitable to describe the adsorption process of U(VI) onto HCC. Therefore, the adsorption process of U(VI) on HCC might be regarded as chemi-sorption, which could be the rate-controlling step.
Determination of thermodynamic parameters
To investigate the effect of temperature on the adsorption of uranium, thermodynamic parameters such as enthalpy (∆H 0), entropy (∆S 0) and Gibbs free energy (∆G 0)must be taken into consideration in order to determine the spontaneity of a process.The adsorption thermodynamic parameters ∆H 0, ∆S 0 and ∆G 0 could be calculated by the following Eqs. (11), (12) and (13) [24, 25]
where K D is the Langmuir constant, ΔS 0 is the change of entropy (J mol−1 K−1), ΔH 0 is the change of enthalpy(kJ/mol), T is the absolute temperature in Kelvin (K) and R is the gas constant (8.314 J mol−1 K−1). ΔH 0 and ΔS 0 can be calculated from the slope and intercept of the straight line (Fig. 8). The change of Gibbs free energy values are calculated from Eq. 10.
The results were shown in the Table 5. The positive value of enthalpy change ΔH 0 indicated that the sorption process was endothermic. The negative values of ΔG 0 indicated that the adsorptive performance of UO2 2+ on the HCC was feasible and spontaneous. Moreover, the decrease of ΔG 0 values with temperature increasing shows that the adsorption process was more favorable at higher temperature, the positive value of ΔS 0 showed the randomness at the solid-solution interface was increasing during the adsorption process [26]. Comparison of the thermodynamic parameters and optimal uranium sorption capacity with previous related studies was shown in Table 6.
Adsorption isotherm
The adsorption isotherms indicate how the adsorption molecules distribute between the liquid phase and the solid phase when the adsorption process reaches equilibrium state [29]. In order to deeply study the sorption process of the adsorbents, the equilibrium data have been subjected different sorption isotherms, namely Langmuir [30], Freundlich [31] models. The Langmuir equation can be described by the linearized Eq. 14 [32]
where C e is the equilibrium concentration (mg/L), q e is the amount of solute sorbed per unit weight of sorbent (mg/g), q m is the Langmuir constant, which represents the saturated monolayer sorption capacity (mg/g). K L is a constant related to the energy of adsorption.
The Freundlich model can be applied to nonideal sorption on heterogeneous surfaces as well as multilayer sorption [33]. The empirical Freundlich equation can also be transformed into linearized Eq. 15
where C e is the equilibrium concentration (mg/L), q e is the amount of solute sorbed per unit weight of sorbent (mg/g), K F is the Freundlich constant related to the adsorption capacity, and n is relevant to the adsorption intensity.
The adsorption constants evaluated from the isotherms with the correlation coefficients (R 2) were given in Table 7 Langmuir isotherm was found to be more suitable to describe the sorption characteristics of U(VI) on HCC when comparing the values of correlation coefficients of both models. The result indicated that the present adsorption process on HCC probably dominated by a monolayer adsorption rather than a multilayer one.
Conclusion
In the present work, a new HCC was prepared by hydrothermal reaction at a mild temperature (180 °C) to diminish the solubility in acid solution, and the amine groups were almost retained during the hydrothermal reaction characterized by FT-IR and the dissolution rate in pH 3.0 solution decreased from 89.6 to 12.6 %. The effects of initial pH, contact time, initial concentration and temperature on the sorption capacity are discussed using 24 full-factorial central composite design using response-surface methodology.
The uranium uptake experiments using 24 full-factorial central composite design showed 96 % confidence level The F test gave P < 0.05 with a model F value of 32.66 which revealed that this regression is statistically significant. The correlation coefficient value (R 2) of 93.6 % indicated that there was a high correlation between the observed values and the predicted ones.The HCC showed the highest uranium sorption capacity at initial pH of 7.92 and contact time of 273.6 min with 24 full-factorial central composite design and the maximum adsorption capacity was 273 mg/g. The adsorption process could be well defined by the Langmuir isotherm and sorption process of U(VI) onto HCC was feasible, spontaneous and endothermic in nature.
References
Rao TP, Metilda P, Gladis JM (2006) Talanta 68:1047–1064
Coleman SJ, Coronado PR, Maxwell RS, Reynold JG (2003) Environ Sci Technol 37:2286–2290
Zhao YS, Liu CX, Feng M, Chen Z, Li SQ, Tian G, Wang L, Huang JB, Li SJ (2010) J Hazard Mater 176:119–124
Schierz A, Zanker H (2009) Environ Pollut 157:1088–1094
Shao DD, Jiang ZQ, Wang XK, Li JX, Meng YD (2009) 113: 860–864
Xu Y, Zondlo JW, Finklea HO, Brennsteiner A (2000) Fuel Process Technol 68:189–208
Wan Ngah WS, Fatinathan S (2008) Chem Eng J 143:62
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Chen AH, Liu SC, Chen CY et al (2008) Comparative adsorption of Cu (II), Zn (II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J Hazard Mater 154(1):184–191
Akkaya R, Ulusoy U (2008) Adsorptive features of chitosan entrapped in polyacrylamide hydrogel for Pb2+, UO22+, and Th4+. J Hazard Mater 151:380–388
Wang G, Liu J, Wang X, Xie Z, Deng N (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Cochran WG, Cox GM (1957) Experimental designs
Khuri AI, Cornell JA (1987) Response surface: design and analysis. Marcell Decker Inc., New York
Montgomery DC (1991) Design and analysis of experiments, 3rd edn. Wiley, New York
Nie B, Zhang Z, Cao X et al (2013) Sorption study of uranium from aqueous solution on ordered mesoporous carbon CMK-3. J Radioanal Nucl Chem 295(1):663–670
Montgomery DC (1996) Design and analysis of experiments, 4th edn. John Wiley & Sons, New York
Bezerra MA, Santelli RE, Oliveira PE, Villar LS, Escaleira LA (2008) Talanta 76:965–977
Cana MY, Yildiz E (2006) J Hazard Mater B135:165–170
Carmona MER, da Silva MAP, Leite SGF (2005) Process Biochem 40:779–788
Bhat SV, Meloa JS, Chaugule BB, D’Souza SF (2008) Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Ghorbania F, Younesia H, Ghasempouria SM, Zinatizadeh AA, Aminia M, Daneshia A (2008) Chem Eng J 145:267–275
Kumar S, Loganathan VA, Gupta RB et al (2011) An assessment of U (VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manag 92(10):2504–2512
Ho YS, Ofomaja AE (2005) Process Biochem 40:3455
Malkoc E, Nuhoglu Y (2005) J Hazard Mater 127:120
Wang YQ, Zhang ZB, Liu YH et al (2012) Adsorption of U (VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem Eng J 198:246–253
Liu YH, Wang YQ, Zhang ZB et al (2013) Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl Surf Sci 273:68–74
Zhang Z, Zhou Z, Cao X et al (2014) Removal of uranium (VI) from aqueous solutions by new phosphorus-containing carbon spheres synthesized via one-step hydrothermal carbonization of glucose in the presence of phosphoric acid. J Radioanal Nucl Chem 299(3):1479–1487
Hameed BH, Din ATM, Ahmad AL (2007) J Hazard Mater 141:819–825
Langmuir I (1918) J Am Chem Soc 38:1361–1403
Freundlich H (1906) J Phys Chem 57(1906):385–470
Hazer O, Kartal S (2010) Talanta 82:1974–1979
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Bioresour Technol 96:1241–1248
Acknowledgments
We gratefully acknowledge the financial support provided by National Natural Science Foundation of China (Grant Nos. 21101024, 21201033,21301028), National Undergraduate Training Programs for Innovation and Entrepreneurship (201210405006), Key Project of Chinese Ministry of Education (Grant No. 211086), the Young Scientists Training Program of Jiangxi Province (Grant No. 20122BCB23023), Natural Science Foundation of Jiangxi Province (Grant Nos. 20114BAB203002, 20122BAB203012,20132BAB203027), China Postdoctoral Science Foundation (Grant No. 20110490857), and Project of Jiangxi Provincial Department of Education (Grant Nos. GJJ13452).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, WL., Zhang, ZB., Cao, XH. et al. Uranium adsorption studies on hydrothermal carbon produced by chitosan using statistical design method. J Radioanal Nucl Chem 301, 197–205 (2014). https://doi.org/10.1007/s10967-014-3128-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3128-8